Abstract
Introduction
Total pancreatectomy for pancreatic ductal adenocarcinoma has historically been associated with substantial patient morbidity and mortality. Given advancements in perioperative and postoperative care, evaluation of the surgical treatment options for pancreatic adenocarcinoma should consider patient outcomes and long-term survival for total pancreatectomy compared with partial pancreatectomy.
Methods
The U.S. National Cancer Database was queried for patients undergoing total pancreatectomy or partial pancreatectomy for pancreatic adenocarcinoma during 1998–2006. Demographics, tumour characteristics, operative outcomes, 30-day mortality, 30-day readmission, additional treatment, and Kaplan–Meier survival curves were compared.
Results
The database query returned 807 patients who underwent total pancreatectomy and 5840 who underwent partial pancreatectomy. More patients who underwent total pancreatectomy than a partial pancreatectomy had a margin-negative resection (p < 0.0001). Mortality and readmission rates were similar in the two groups, as was long-term survival on Kaplan–Meier curves (p = 0.377). A statistically significant difference in the rate of surgery only (without additional treatment) was observed for patients in the total pancreatectomy group (p = 0.0003).
Conclusions
Although total compared with partial pancreatectomy was associated with a higher rate of margin-negative resection, median survival was not significantly different for patients undergoing either procedure. Patients who underwent total pancreatectomy were significantly less likely to receive adjuvant therapy.
Keywords: Pancreatectomy, pancreaticoduodenectomy, pancreatic neoplasms, morbidity, mortality, databases, postoperative care
INTRODUCTION
Pancreatic adenocarcinoma is increasing in incidence and is the 4th leading cause of cancer-related mortality in the United States1. Surgical resection is the primary intervention and the only potentially curative treatment option, but fewer than 20% of patients are surgical candidates2–4. Most patients undergo partial pancreatectomy (distal pancreatectomy or pancreaticoduodenectomy, depending on the site of the tumour), and most studies report a positive resection margin in about 30% of patients who undergo a partial pancreatectomy5,6.
Total pancreatectomy is typically reserved for patients with large or locally advanced tumours wherein complete removal of the pancreas is needed to obtain a macroscopically and microscopically negative (R0) surgical margin7. Some patients with multifocal or infiltrating disease and others with intraductal papillary mucinous neoplasms are candidates for total pancreatectomy8. Total pancreatectomy is sometimes used when a partial pancreatectomy with or without reconstruction is technically impractical7. Compared with residual disease (margin-positive resection), complete tumour removal with negative resection margins is associated with more favourable outcomes in terms of survival9.
Partial pancreatectomy for pancreatic adenocarcinoma is considered a safe operation, with mortality rates of about 2% at high-volume centres; however, morbidity rates are substantial, especially for pancreaticoduodenectomy10. Total pancreatectomy has historically been considered a highly morbid operation, with poor overall acceptance by most surgeons; the procedure was previously associated with mortality rates of about 10%–15% and morbidity rates of about 40%–50%2,11,12. An early report by Ihse et al.13 of outcomes after total pancreatectomy cited 27% operative mortality and 52% morbidity compared with 3% mortality and 28% morbidity for partial pancreatectomy.
In contrast, contemporary data from several single institutions show improved patient outcomes after total pancreatectomy3,4,14. Those recent studies suggest that total pancreatectomy can be performed safely, but small sample sizes limit applicability12. Management of insulin-dependent diabetes mellitus, a sequela of total pancreatectomy, has improved with the introduction of newer insulin regimens and delivery devices15. Therapeutic advances such as better pancreatic enzyme supplementation and antihypertensive drugs have led to improved quality of life for patients with pancreatic exocrine insufficiency16.
Nathan et al.2 reviewed U.S. Surveillance, Epidemiology, and End Results program data and reported mortality rates after total pancreatectomy ranging from 5.8%–9.3%, depending on the primary tumour site. In contrast, patients who underwent partial pancreatectomy experienced a 30-day mortality rate of less than 2% and a morbidity rate of 23% for pancreaticoduodenectomy; for distal pancreatectomy, mortality was less than 0.5%, and morbidity was 18%2. Compared with patients who undergo a partial pancreatectomy, those who undergo total pancreatectomy experience a higher incidence of perioperative morbidity and a higher re-operation rate17.
Because of lower mortality and morbidity rates associated with partial pancreatectomy, more patients with locally advanced tumours are being offered a larger surgical resection involving venous and arterial resection18; however, large comparative studies of outcomes after partial and total pancreatectomy to inform patient communication are lacking. We queried the U.S. National Cancer Database (ncdb) to compare outcomes for readmission, mortality, and long-term survival in patients with pancreatic adenocarcinoma who underwent a total or partial pancreatectomy.
METHODS
Data Source
We queried the ncdb for all patients who underwent a total or partial pancreatectomy for pancreatic adenocarcinoma during 1998–2006. The ncdb, a joint project between the Commission on Cancer of the American College of Surgeons and the American Cancer Society, collects clinical oncology outcomes from more than 1500 hospitals with Commission on Cancer–accredited programs19. Patient data files in the ncdb have been de-identified. The American College of Surgeons and the Commission on Cancer have neither verified nor are responsible for the analytic or statistical methodology used in the present study, including the conclusions drawn by the investigators.
Study Population
Patients were identified based on the International Classification of Diseases for Oncology, 3rd edition. Histology codes were used to select pancreatic adenocarcinoma not otherwise specified (8140) and infiltrating ductal carcinoma not otherwise specified (8500). To establish a homogeneous cohort of patients, we excluded patients with other histologic variants of adenocarcinoma. Site codes were used to establish comparison groups as follows: tumours of pancreatic head (C250), tumours of pancreatic body (C251), tumours of pancreatic tail (C252), and other pancreatic tumours of neck (C257), duct (C253), overlapping lesions (C258), or unspecified location (C259). The primary tumour site was defined on final pathology by its relationship to the left or right of the superior mesenteric vessel groove (pancreatic neck) as pancreatic body/tail or pancreatic head respectively. When not fitting into one of those two categories, patients were placed into a third group (tumour unspecified or nonspecific).
The code for the primary lesion site was used to establish comparison groups as follows: total pancreatectomy (codes 40 and 60), pancreaticoduodenectomy (codes 35, 36, and 37), distal pancreatectomy (code 30), and an all-encompassing partial pancreatectomy group (codes 30, 35, 36, and 37). Patients with a procedure discordant to the primary lesion site were not excluded, given that surgeon technical preference was not discoverable. Patients undergoing less-specific procedures were excluded from the analysis (local excision, enucleation), as were patients who underwent procedures with non-specified codes.
If an unplanned total pancreatectomy (because of persistently positive pancreatic neck margins on frozen section) was performed during an intended partial pancreatectomy, that procedure was included as a total pancreatectomy. Positive margins refer to an R1 or R2 resection at the usual resection margins, which refers to any of 3 revisable margins (pancreatic transection margin, common bile duct margin, and proximal bowel margin—either duodenum or stomach depending on technique) and 2 unrevisable margins (vascular groove margin and retroperitoneal margin).
Study Variables
Demographics included age, sex, race, comorbidities, primary payer, median income, regional location, facility type, adjuvant therapy, and year of diagnosis. Tumour characteristics included tumour location, operative procedure at the primary site, histologic grade, clinical stage group and pathologic tumour stage group [American Joint Committee on Cancer (ajcc), 7th edition], the number of regional lymph nodes examined, regional lymph node positivity, and margin status. Operative outcomes included readmission within 30 days of discharge and 30-day mortality. Additional therapy included chemotherapy, radiation, or combinations thereof.
Statistical Analysis
Summary statistics for continuous (mean ± standard deviation) and categorical variables (count and percentage) were calculated by type of operative procedure. Chi-square or Fisher exact tests for categorical variables and t-tests or Wilcoxon rank-sum tests for continuous variables were performed to compare patient demographics, pathology, additional treatments, and postoperative morbidity between the two groups, as well as to compare 30-day mortality and 30-day readmission. Survival was calculated in months from the date of diagnosis to the date on which the patient was last contacted or died. Overall survival was estimated by the Kaplan–Meier method. Median survival time and 95% confidence intervals (cis) are reported. The log-rank test was used to analyze the statistical significance of the Kaplan–Meier estimates. All tests were 2-sided with a significance level of 0.05. Subgroup analyses were performed to explore differences in outcomes and additional treatment for total pancreatectomy compared with distal pancreatectomy and for total pancreatectomy compared with pancreaticoduodenectomy. Statistical analyses were performed using the SAS Enterprise Guide software application (version 6.1: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Study Population
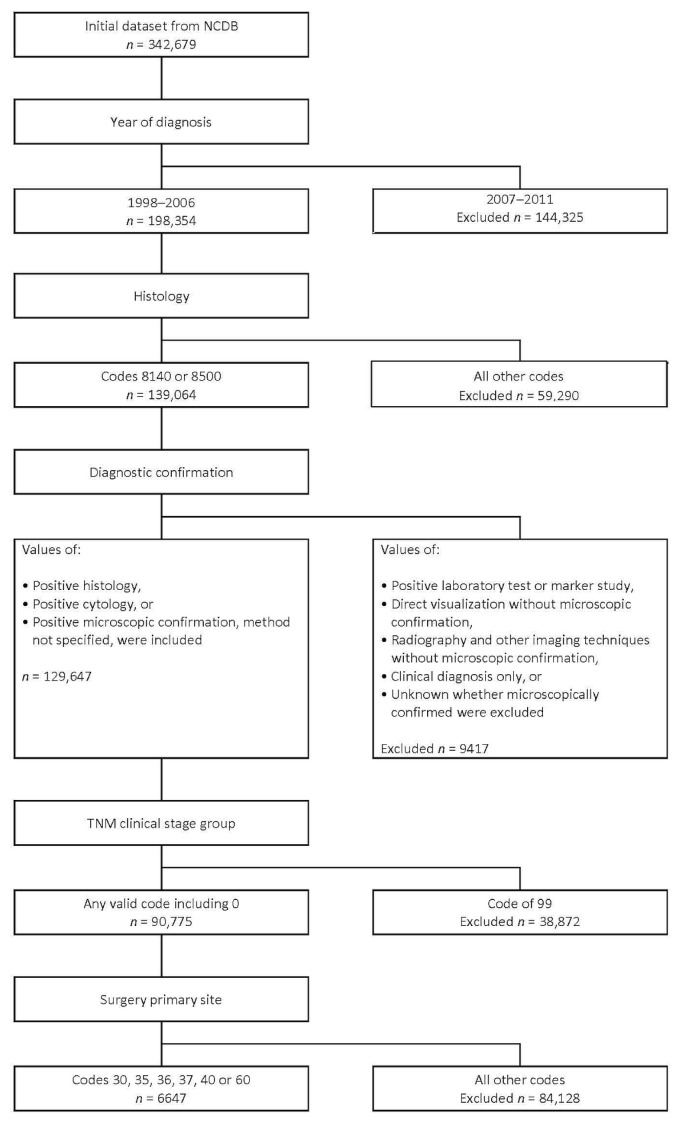
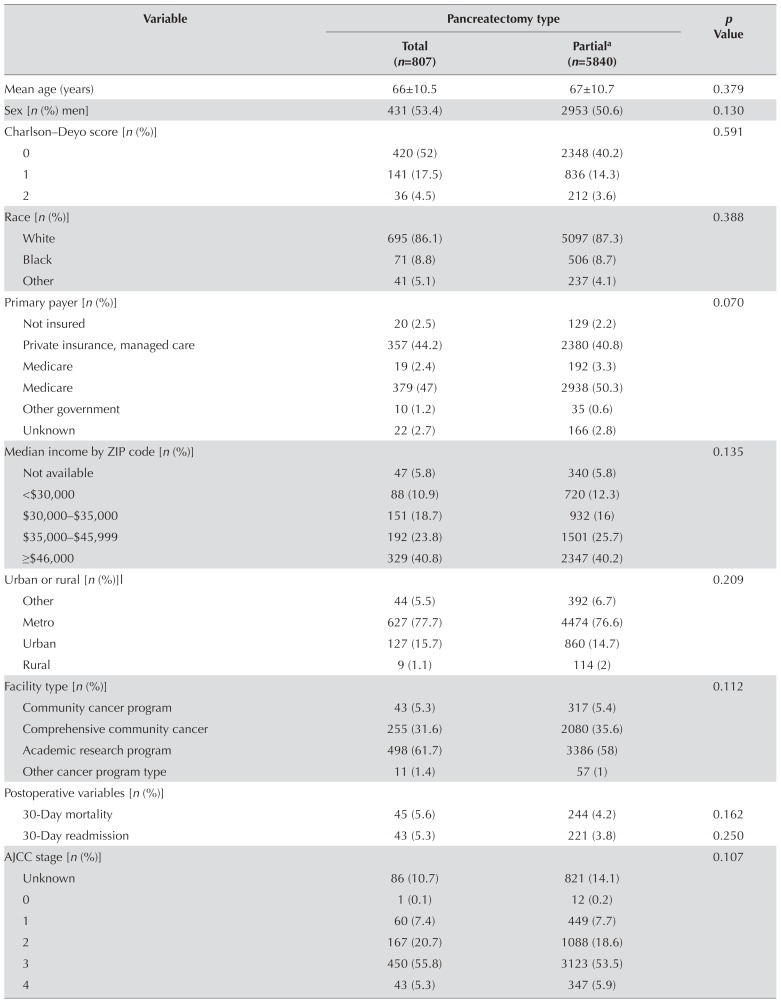
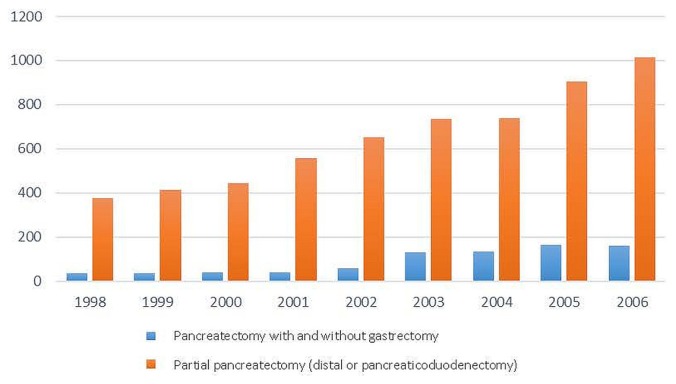
Of 342,679 patients identified from the ncdb, 198,354 were included for the analysis. Of those 198,354 patients, 90,775 had a histologically confirmed diagnosis of pancreatic cancer. Surgical resection of the primary tumour (site) was performed in 6647 patients (Figure 1). Of 6647 patients who underwent surgical resection, 807 underwent total pancreatectomy, and 5840 underwent partial pancreatectomy. Of the 5840 patients who underwent partial pancreatectomy, 5058 underwent pancreaticoduodenectomy, and 782 underwent distal pancreatectomy. Table I shows the patient demographics, tumour characteristics, operative outcomes, and additional treatment for the study cohort. During the study period, 1998–2006, the number of total pancreatectomy procedures increased from 38 to 161 and the number of partial pancreatectomy procedures increased from 375 to 1016 (Figure 2).
FIGURE 1.
CONSORT flow diagram for patients included in the study. NCDB = U.S. National Cancer Database.
TABLE I.
Total compared with partial pancreatectomy for pancreatic adenocarcinoma: demographics, tumour characteristics, surgical outcomes, and additional treatment
| Variable | Pancreatectomy type | p Value | |
|---|---|---|---|
|
| |||
| Total (n=807) | Partiala (n=5840) | ||
| Mean age (years) | 66±10.5 | 67±10.7 | 0.379 |
|
| |||
| Sex [n (%) men] | 431 (53.4) | 2953 (50.6) | 0.130 |
|
| |||
| Charlson–Deyo score [n (%)] | 0.591 | ||
| 0 | 420 (52) | 2348 (40.2) | |
| 1 | 141 (17.5) | 836 (14.3) | |
| 2 | 36 (4.5) | 212 (3.6) | |
|
| |||
| Race [n (%)] | 0.388 | ||
| White | 695 (86.1) | 5097 (87.3) | |
| Black | 71 (8.8) | 506 (8.7) | |
| Other | 41 (5.1) | 237 (4.1) | |
|
| |||
| Primary payer [n (%)] | 0.070 | ||
| Not insured | 20 (2.5) | 129 (2.2) | |
| Private insurance, managed care | 357 (44.2) | 2380 (40.8) | |
| Medicare | 19 (2.4) | 192 (3.3) | |
| Medicare | 379 (47) | 2938 (50.3) | |
| Other government | 10 (1.2) | 35 (0.6) | |
| Unknown | 22 (2.7) | 166 (2.8) | |
|
| |||
| Median income by ZIP code [n (%)] | 0.135 | ||
| Not available | 47 (5.8) | 340 (5.8) | |
| <$30,000 | 88 (10.9) | 720 (12.3) | |
| $30,000–$35,000 | 151 (18.7) | 932 (16) | |
| $35,000–$45,999 | 192 (23.8) | 1501 (25.7) | |
| ≥$46,000 | 329 (40.8) | 2347 (40.2) | |
|
| |||
| Urban or rural [n (%)]l | 0.209 | ||
| Other | 44 (5.5) | 392 (6.7) | |
| Metro | 627 (77.7) | 4474 (76.6) | |
| Urban | 127 (15.7) | 860 (14.7) | |
| Rural | 9 (1.1) | 114 (2) | |
|
| |||
| Facility type [n (%)] | 0.112 | ||
| Community cancer program | 43 (5.3) | 317 (5.4) | |
| Comprehensive community cancer | 255 (31.6) | 2080 (35.6) | |
| Academic research program | 498 (61.7) | 3386 (58) | |
| Other cancer program type | 11 (1.4) | 57 (1) | |
|
| |||
| Postoperative variables [n (%)] | |||
| 30-Day mortality | 45 (5.6) | 244 (4.2) | 0.162 |
| 30-Day readmission | 43 (5.3) | 221 (3.8) | 0.250 |
|
| |||
| AJCC stage [n (%)] | 0.107 | ||
| Unknown | 86 (10.7) | 821 (14.1) | |
| 0 | 1 (0.1) | 12 (0.2) | |
| 1 | 60 (7.4) | 449 (7.7) | |
| 2 | 167 (20.7) | 1088 (18.6) | |
| 3 | 450 (55.8) | 3123 (53.5) | |
| 4 | 43 (5.3) | 347 (5.9) | |
|
| |||
| Tumour location [n (%)] | 0.026 | ||
| Pancreatic body and tail | 106 (13.1) | 784 (13.4) | |
| Pancreatic head | 586 (72.6) | 4412 (75.5) | |
| Unspecified or not specific | 115 (14.3) | 644 (11) | |
|
| |||
| Histologic grade [n (%)] | 0.347 | ||
| Well differentiated | 78 (9.7) | 539 (9.2) | |
| Moderately or intermediately differentiated | 403 (49.9) | 2737 (46.9) | |
| Poorly differentiated | 241 (29.9) | 1832 (31.4) | |
| Undifferentiated, anaplastic | 4 (0.5) | 40 (0.7) | |
| Unknown, not determined, not stated | 81 (10) | 692 (11.8) | |
|
| |||
| Regional lymph nodes examined [n (%)] | 0.004 | ||
| 0 | 62 (7.7) | 534 (9.1) | |
| 1–3 | 59 (7.3) | 635 (10.9) | |
| 4–89 | 653 (80.9) | 4356 (74.6) | |
| No nodes removed, aspiration done | 2 (0.2) | 5 (0.1) | |
|
| |||
| Regional nodes positive [n (%)] | 0.288 | ||
| All nodes negative | 273 (33.8) | 2086 (35.7) | |
| 1–3 | 322 (39.9) | 2097 (35.9) | |
| 4–89 | 126 (15.6) | 932 (16) | |
| Unknown | 18 (2.2) | 139 (2.4) | |
| None examined | 62 (7.7) | 534 (9.1) | |
|
| |||
| Margins [n (%)] | <0.0001 | ||
| Negative | 609 (75.5) | 3930 (67.3) | |
| Positive (microscopic) | 171 (21.2) | 1432 (24.5) | |
| Not evaluable | 2 (0.2) | 78 (1.3) | |
| Unknown, not applicable | 25 (3.1) | 400 (6.8) | |
|
| |||
| Additional treatment [n (%)] | 0.0009 | ||
| Surgery and CTxb | 389 (48.2) | 2752 (47.1) | |
| Surgery only | 73 (9) | 338 (5.8) | 0.0003 |
| Surgery, CTx, and RTc | 257 (31.8) | 2126 (36.4) | 0.0114 |
| Other | 88 (10.9) | 624 (10.7) | |
Distal pancreatectomy, pancreaticoduodenectomy, pancreatectomy not otherwise specified.
Unknown type or agents, single-agent therapy, multi-agent therapy.
Beam, radioactive implants, radioisotopes, radiation not otherwise specified.
AJCC = American Joint Committee on Cancer; CTx = chemotherapy; RT = radiation therapy.
FIGURE 2.
Total number of procedures performed per year during the study period.
No differences between the total and partial pancreatectomy groups were observed with regard to age (p = 0.379), sex (p = 0.13), race (p = 0.387), insurance status (p = 0.070), or median income (p = 0.135). The proportion of patients listed as “not insured” was similar in the total and partial pancreatectomy groups: 20 of 807 patients (2.5%) and 129 of 5840 patients (2.2%) respectively. Although more total pancreatectomy and partial pancreatectomy procedures were performed in urban and metropolitan regions (77% and 76% respectively), no statistically significant difference in regional distribution was evident between the two groups (p = 0.209). A similar result was found for the type of hospital in which the procedures were performed. Most procedures were performed at major academic research centres (62% for total pancreatectomy and 58% for partial pancreatectomy), but no differences in the overall facility distribution were evident (p = 0.112). The Charlson–Deyo score for comorbidities was similar in the two groups: 17.5% for patients undergoing total pancreatectomy and 14.3% for patients undergoing partial pancreatectomy (p = 0.591). Missing data for the Charlson–Deyo scores for patient comorbidities were excluded from the univariate analysis.
Tumour Characteristics
Compared with patients who underwent a partial pancreatectomy, those who underwent a total pancreatectomy had a significantly higher ajcc clinical stage (p < 0.0001). The distribution of ajcc pathologic tumour stages was similar between the groups (p = 0.107). Pathologic tumour stage iii was the most predominant finding; 55.8% for the total pancreatectomy group, and 53.5% for the partial pancreatectomy group. Tumour grade was also similar between the groups (p = 0.347). Tumour location for the two groups was primarily in the head of the pancreas: 72% for the total pancreatectomy group and 75% for the partial pancreatectomy group (p = 0.026).
The percentage of negative margins (R0 resection) was significantly higher in the total pancreatectomy group (75.5% vs. 67.3% for the partial pancreatectomy group, p < 0.0001). As to the number of regional lymph nodes examined, the most predominant finding was more than 4 nodes: 80.9% for the total pancreatectomy group and 74.6% for the partial pancreatectomy group. When 4 or more lymph nodes were examined, the difference in the number of positive lymph nodes was minimal: 15.6% for the total pancreatectomy group and 16% for the partial pancreatectomy group (Table I).
In the subgroup analysis of total pancreatectomy compared with distal pancreatectomy, statistically significant differences were observed for tumour margins (p < 0.0001) and the number of positive regional lymph nodes (p < 0.0001, Table II). In the total pancreatectomy group, more patients had negative tumour margins (75.5% vs. 66.4% for the distal pancreatectomy group). When 4 or more lymph nodes were examined, more patients in the total pancreatectomy group had regional node positivity (15.6% vs. 8.8% in the distal pancreatectomy group).
TABLE II.
Total compared with distal pancreatectomy for pancreatic adenocarcinoma: demographics, tumour characteristics, surgical outcomes, and additional treatment
| Variable | Pancreatectomy type | p Value | |
|---|---|---|---|
|
| |||
| Total (n=807) | Distal (n=782) | ||
| Mean age (years) | 65±10.5 | 66±10.9 | 0.069 |
|
| |||
| Sex [n (%) men] | 431 (53.4) | 359 (45.4) | 0.003 |
|
| |||
| Mean Charlson–Deyo score | 0.36±0.59 | 0.40±0.63 | 0.256 |
|
| |||
| Postoperative variables [n (%)] | |||
| 30-Day mortality | 45 (67.2) | 22 (32.8) | 0.007 |
| 30-Day readmission | 43 (58.9) | 30 (41.1) | 0.952 |
|
| |||
| AJCC stage [n (%)] | <0.0001 | ||
| Unknown | 86 (10.7) | 133 (17) | |
| 0 | 1 (0.1) | 2 (0.3) | |
| 1 | 60 (7.4) | 56 (7.2) | |
| 2 | 167 (20.7) | 196 (25.1) | |
| 3 | 450 (55.8) | 315 (40.3) | |
| 4 | 43 (5.3) | 80 (10.2) | |
|
| |||
| Tumour location [n (%)] | <0.0001 | ||
| Pancreatic body and tail | 106 (13.1) | 539 (68.9) | |
| Pancreatic head | 586 (72.6) | 73 (9.3) | |
| Unspecified or not specific | 115 (14.3) | 170 (21.7) | |
|
| |||
| Histologic grade [n (%)] | 0.161 | ||
| Well differentiated | 78 (9.7) | 64 (8.2) | |
| Moderately or intermediately differentiated | 403 (49.9) | 386 (49.4) | |
| Poorly differentiated | 241 (29.9) | 221 (28.3) | |
| Undifferentiated, anaplastic | 4 (0.5) | 9 (1.2) | |
| Unknown, not determined, not stated | 81 (10) | 102 (13) | |
|
| |||
| Regional lymph nodes examined [n (%)] | <0.0001 | ||
| 0 | 62 (7.7) | 132 (16.9) | |
| 1–3 | 59 (7.3) | 116 (14.8) | |
| 4–89 | 653 (80.9) | 498 (63.7) | |
| None removed, aspiration done | 2 (0.2) | — | |
|
| |||
| Regional nodes positive [n (%)] | <0.0001 | ||
| All negative | 273 (33.8) | 324 (41.4) | |
| 1–3 | 322 (39.9) | 237 (30.3) | |
| 4–89 | 126 (15.6) | 69 (8.8) | |
| Unknown | 18 (2.2) | 14 (1.8) | |
| None examined | 62 (7.7) | 132 (16.9) | |
|
| |||
| Margins [n (%)] | <0.0001 | ||
| Negative | 609 (75.5) | 519 (66.4) | |
| Positive (microscopic) | 171 (21.2) | 199 (25.4) | |
| Not evaluable | 2 (0.2) | 8 (1) | |
| Unknown, not applicable | 25 (3.1) | 56 (7.2) | |
|
| |||
| Additional treatment [n (%)] | 0.288 | ||
| Surgery and CTxa [n (%)] | 389 (48.2) | 386 (49.8) | |
| Surgery only | 73 (9) | 61 (7.8) | |
| Surgery, CTx, and RTb | 257 (31.8) | 268 (34.3) | |
| Other | 88 (10.9) | 67 (8.6) | |
Unknown type or agents, single-agent therapy, multi-agent therapy.
Beam, radioactive implants, radioisotopes, radiation not otherwise specified.
AJCC = American Joint Committee on Cancer; CTx = chemotherapy; RT = radiation therapy.
In the subgroup analysis of total pancreatectomy compared with pancreaticoduodenectomy, a statistically significant difference for tumour margins was observed (p < 0.0001, Table III).
TABLE III.
Total pancreatectomy compared with pancreaticoduodenectomy (Whipple) for pancreatic adenocarcinoma: demographics, tumour characteristics, surgical outcomes, and additional treatment
| Variable | Pancreatectomy type | p Value | |
|---|---|---|---|
|
| |||
| Total (n=807) | Whipplea (n=5058) | ||
| Mean age (years) | 65.1±10.5 | 65.4±10.7 | 0.263 |
|
| |||
| Sex [n (%) men] | 431 (53.4) | 2594 (51.3) | |
|
| |||
| Mean Charlson–Deyo score | 0.36±0.59 | 0.37±0.59 | 0.719 |
|
| |||
| Postoperative variables [n (%)] | |||
| 30-Day mortality | 45 (5.6) | 222 (4.4) | |
| 30-Day readmission | 43 (7.2) | 191 (6.6) | |
|
| |||
| AJCC staging [n (%)] | 0.131 | ||
| Unknown | 86 (10.7) | 688 (13.6) | |
| 0 | 1 (0.1) | 10 (0.2) | |
| 1 | 60 (7.4) | 393 (7.8) | |
| 2 | 167 (20.7) | 892 (17.6) | |
| 3 | 450 (55.8) | 2808 (55.5) | |
| 4 | 43 (5.3) | 267 (5.3) | |
|
| |||
| Tumour location [n (%)] | <0.0001 | ||
| Pancreatic body and tail | 106 (13.1) | 245 (4.8) | |
| Pancreatic head | 586 (72.6) | 4339 (85.8) | |
| Unspecified or not specific | 115 (14.3) | 474 (9.4) | |
|
| |||
| Histologic grade [n (%)] | 0.346 | ||
| Well differentiated | 78 (9.7) | 475 (9.4) | |
| Moderately or intermediately differentiated | 403 (49.9) | 2351 (46.5) | |
| Poorly differentiated | 241 (29.9) | 1611 (31.9) | |
| Undifferentiated, anaplastic | 4 (0.5) | 31 (0.6) | |
| Unknown, not determined, not stated | 81 (10) | 590 (11.7) | |
|
| |||
| Regional lymph nodes examined [n (%)] | 0.039 | ||
| 0 | 62 (7.7) | 402 (7.9) | |
| 1–3 | 59 (7.3) | 519 (10.3) | |
| 4–89 | 653 (80.9) | 3858 (76.3) | |
| None removed, aspiration done | 2 (0.2) | 5 (0.1) | |
|
| |||
| Regional nodes positive [n (%)] | 0.594 | ||
| All negative | 273 (33.8) | 1762 (34.8) | |
| 1–3 | 322 (39.9) | 1860 (36.8) | |
| 4–89 | 126 (15.6) | 863 (17.1) | |
| Unknown | 18 (2.2) | 125 (2.5) | |
| None examined | 62 (7.7) | 402 (7.9) | |
|
| |||
| Margins [n (%)] | <0.0001 | ||
| Negative | 609 (75.5) | 3411 (67.4) | |
| Positive (microscopic) | 171 (21.2) | 1233 (24.4) | |
| Not evaluable | 2 (0.2) | 70 (1.4) | |
| Unknown, not applicable | 25 (3.1) | 344 (6.8) | |
|
| |||
| Additional treatment [n (%)] | 0.0002 | ||
| Surgery and CTx | 389 (48.2) | 2366 (46.8) | |
| Surgery only | 73 | (9) 277 (5.5) | |
| Surgery, CTx, and RTc | 257 (31.8) | 1858 (36.7) | |
| Other | 88 (10.9) | 557 (11) | |
Pancreaticoduodenectomy.
Unknown type or agents, single-agent therapy, multi-agent therapy.
Beam, radioactive implants, radioisotopes, radiation not otherwise specified.
AJCC = American Joint Committee on Cancer; CTx = chemotherapy; RT = radiation therapy.
Mortality and Readmission
The 30-day mortality rates were similar for the total and partial pancreatectomy groups: 5.6% compared with 4.2% respectively. No data for the outcome of 30-day mortality were missing.
Unplanned 30-day readmission rates were similar in the total and partial pancreatectomy groups: 5.3% compared with 3.8% respectively. A higher proportion of patients who underwent partial pancreatectomy than of those who underwent total pancreatectomy had missing data for 30-day readmission: 2444 of 5840 patients (41.8%) compared with 210 of 807 patients (26%) respectively. Patients whose data for 30-day readmission were missing were excluded from the analysis of partial compared with total pancreatectomy (p = 0.245).
Additional Treatment
External-beam radiation was used treat 39.7% of the patients who underwent total pancreatectomy and 45.1% of the patients who underwent partial pancreatectomy, which was a statistically significant difference (chi-square p = 0.0034). An additional form of radiation therapy (unspecified) was used to treat 2% of the patients who underwent total pancreatectomy and 1.1% of those who underwent partial pancreatectomy. A larger proportion of patients who underwent total pancreatectomy received no radiation therapy (57% vs. 53.2% of those who underwent partial pancreatectomy).
With respect to chemotherapy, a statistical difference between the total pancreatectomy and partial pancreatectomy groups was observed: 29.6% and 34% respectively for a single agent administered as first course; 17% and 16.3% respectively for a multi-agent regimen administered as first course; and 6.9% and 6.7% respectively for chemotherapy as first-course therapy with an unknown type or number of agents (p = 0.049). The greatest proportion of patients received combination therapy consisting of surgery and chemotherapy: 48.2% for the total pancreatectomy group and 47.1% for the partial pancreatectomy group. Surgery and chemoradiotherapy were received by 31.8% of patients undergoing total pancreatectomy and by 36.4% of patients undergoing partial pancreatectomy—a statistically significant difference (chi-square p = 0.0114). Patients in the total pancreatectomy group had a statistically higher likelihood of receiving surgery only (9% vs. 5.8% for patients who underwent partial pancreatectomy, p = 0.0003).
In the subgroup analysis of total pancreatectomy and pancreaticoduodenectomy, additional treatment showed a statistically significant difference (p = 0.0002). Surgery, chemotherapy, and radiation were all received by 31.8% of patients undergoing total pancreatectomy and by 36.7% of patients undergoing pancreaticoduodenectomy (Table III).
Survival
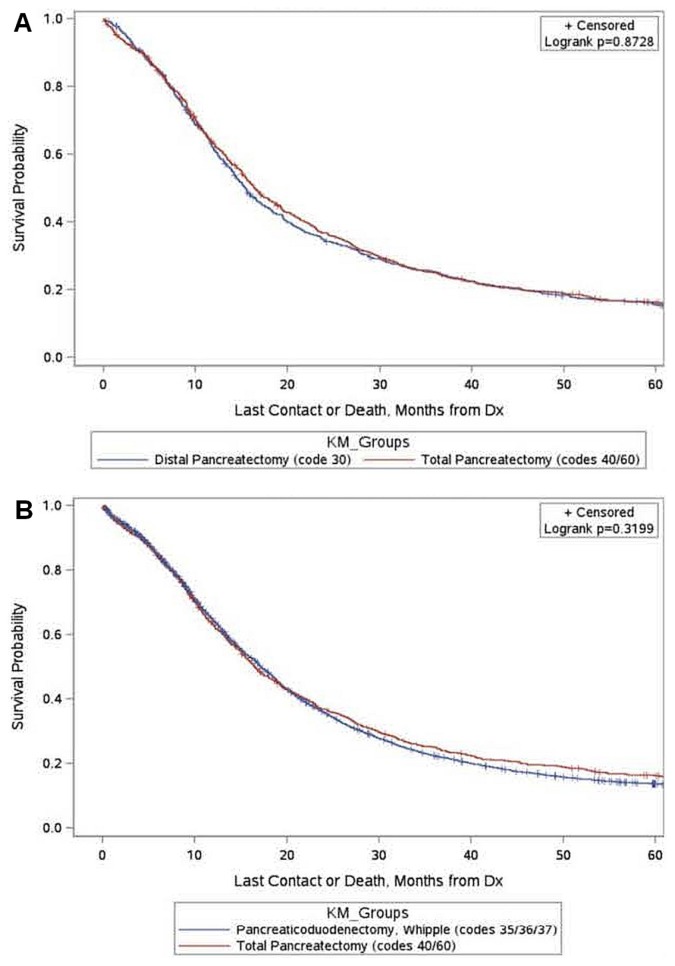
Median survival was not significantly different in patients undergoing total pancreatectomy and in patients undergoing partial pancreatectomy (distal pancreatectomy or pancreaticoduodenectomy): 16.5 months (95% ci: 15.4 months to 18.1 months) and 16.9 months (95% ci: 16.5 months to 17.4 months) respectively (log-rank p = 0.3776). Median survival was not significantly different for patients undergoing distal pancreatectomy (code 30) compared with those undergoing total pancreatectomy (codes 40 and 60): 15.4 months (95% ci: 14.3 months to 16.6 months) and 16.5 months (95% ci: 15.4 months to 18.1 months) respectively (log-rank p = 0.8728, Figure 3). Median survival was not significantly different for patients undergoing pancreaticoduodenectomy (codes 35, 36, or 37) compared with those undergoing total pancreatectomy (codes 40 and 60): 17.1 months (95% ci: 16.7 months to 17.7 months) and 16.5 months (95% ci: 15.4 months to 18.1 months) respectively (log-rank p = 0.3199, Figure 3).
FIGURE 3.
Kaplan–Meier (KM) estimate of survival for patients undergoing (A) total pancreatectomy and distal pancreatectomy, or (B) total pancreatectomy and pancreaticoduodenectomy. Dx = diagnosis.
DISCUSSION
Patient outcomes after distal pancreatectomy and pancreaticoduodenectomy for pancreatic ductal adenocarcinoma are well described, but the role of total pancreatectomy for this disease remains less clear2,3. Total pancreatectomy might be appropriate for select patients who are treated at high-volume centres, but large cohort studies comparing total pancreatectomy with partial pancreatectomy are lacking. Our study aimed to assess operative and oncologic outcomes in patients undergoing those procedures.
We found that there was no statistical difference in the 30-day mortality or 30-day readmission between patients who underwent total or partial pancreatectomy. The number of positive regional lymph nodes was similar in the two groups, but a statistical difference in the rate of margin-negative resection was observed in patients who underwent total pancreatectomy. Despite the latter observation, no difference in median survival was evident.
Compared with previously reported outcomes, mortality rates after total pancreatectomy have improved. The 30-day mortality rate for patients undergoing total pancreatectomy in our cohort was 5.6%, which was similar to the 30-day mortality rate of 5.5% and the 90-day mortality rate of 6.8% reported by others20,21. That decrease in the mortality rate after total pancreatectomy corresponds with an increase in the number of those procedures performed during our study period. Our finding of no statistical difference in mortality or readmission rates after total or partial pancreatectomy could reflect improvements both in operative technique and in the management of patients with diabetes mellitus and pancreatic insufficiency22,23.
Compared with patients who undergo partial pancreatectomy, those who undergo total pancreatectomy might have a bias for larger tumour size. Large tumours located in the body and tail could be associated with advanced-stage disease and delay in diagnosis, whereas symptoms generally appear earlier with tumours located in the pancreatic head24,25. In a multivariate analysis of 2582 patients from the ncdb who underwent total pancreatectomy, Johnston et al.20 reported a survival difference between patients with tumours of varying size (<2 cm, 2–5 cm, >5 cm). Tumour size and tumour grade were significant predictors of os; survival benefit was more often observed in younger patients with smaller node-negative tumours and a margin-negative resection20. The authors noted that the ajcc tumour staging guidelines for pancreatic cancer, which designate tumours as either smaller than 2 cm or larger than 2 cm, should consider the prognostic advantage related to smaller tumour size20.
In our subgroup analysis of total pancreatectomy and distal pancreatectomy, more patients in the total pancreatectomy group had pathologic stage iii tumours. The total pancreatectomy group showed a statistically significant difference for negative tumour margins and a higher number of positive regional lymph nodes, but median survival was similar in the two groups. In a large retrospective review of the ncdb, Mirkin et al.26 reported that the number of regional lymph nodes examined has steadily increased for patients undergoing all surgery subtypes, but that the median number of lymph nodes examined remains higher for patients undergoing total pancreatectomy than for those undergoing distal pancreatectomy.
In our subgroup analysis of total pancreatectomy and pancreaticoduodenectomy, proportions of pathologic stage ii and stage iii tumours were similar in the two groups, and no statistical difference in the number of positive regional lymph nodes was evident. However, despite a statistically significant difference for negative tumour margins in the total pancreatectomy group, median survival was similar in the two groups.
With respect to additional treatment, patients in our total pancreatectomy group were significantly less likely than patients in our partial pancreatectomy group to receive chemoradiotherapy or radiation. The observed difference in systemic treatment in the total pancreatectomy group might be related to the presence of more patients with advanced-stage tumours, functional reserve in the patients, and patient selection. Because of serious complications, many potentially eligible patients do not receive adjuvant therapy after pancreatic adenocarcinoma resection27,28. Some subsets of patients, such as those with high-grade tumours or positive margins, do not achieve benefit from adjuvant therapy. Our review did not allow us to determine the timing of surgery and systemic treatment or the reasons for delay or omission thereof. In a propensity-score analysis of a large cohort of patients from the ncdb, Kooby et al.6 evaluated the effect on os of adjuvant chemotherapy with radiotherapy after resection for pancreatic adenocarcinoma and encouraged its use.
Although we found that total pancreatectomy, compared with partial pancreatectomy, might offer patients a higher likelihood of a margin-negative resection, os did not differ between those groups. That observation might reflect the more advanced nature of the tumours in patients who underwent total pancreatectomy that were thus more likely to have aggressive tumour biology that could offset any benefit of margin-negative resection or lymph node status.
Limitations of our study include a patient cohort that was retrospective and not population-based. We were unable to account for variability in patient treatment, observational bias, cause–effect bias, missing data, and a lack of clinical granularity with respect to operative decision-making, especially for planned compared with unplanned total pancreatectomy. Given that our results reflect a pooled analysis from multiple ncdb participating institutions, we cannot confirm a uniform sectioning technique. Combining the 5 relevant margins into one group is another limitation. The 2 most commonly positive margins (and those most important in determining resectability) are the 2 unrevisable margins (vascular groove and retroperitoneum). If tumour has invaded either of those 2 positions, total pancreatectomy provides no advantage over partial pancreatectomy in achieving an R0 resection.
We encountered limitations with the length of patient follow-up because a proportion of the patients were censored after 6 months. That censoring limited our ability to assess variations in the early survival curves in relation to long-term survival data, which did not demonstrate any difference. Other surrogates for survival in oncology, such as local recurrence rates, were unavailable. The long-term consequences of pancreatic endocrine and exocrine insufficiency after total pancreatectomy were not addressed in this study12,15,22.
Johnston et al.20 noted that a negative tumour margin is one of the few outcomes in resectable pancreatic cancer that surgeons might control. Therefore, if a total pancreatectomy is necessary to achieve a margin-negative resection for advanced-stage tumours located in the pancreatic tail, then progression from partial to total pancreatectomy should be considered, given that total pancreatectomy has been associated with a higher likelihood of negative tumour margins. The higher number of positive regional lymph nodes in the total pancreatectomy group might result in better patient classification, staging, and informed outcomes26. Contrarily, for patients with tumours of the pancreatic head, survival outcomes were observed to be similar for patients undergoing total pancreatectomy or pancreaticoduodenectomy. Despite a statistically significant difference in negative-margin resections in our subgroup analysis, total pancreatectomy might not provide an acceptable benefit and should be avoided.
The decision about whether to perform total or partial pancreatectomy for pancreatic adenocarcinoma remains challenging. The present study provides insight for surgeons planning to perform total pancreatectomy for multifocal, diffuse, or anatomically challenging lesions, and also for those having to convert to unplanned total pancreatectomy because of persistently positive frozen margins. Despite a statistical difference with respect to negative tumour margins in the total pancreatectomy group, the similarity in survival characteristics between that group and the partial pancreatectomy group awaits further explanation. An effort to obtain negative tumour margins with progression to total pancreatectomy could be appropriate for select patients.
CONCLUSIONS
Compared with partial pancreatectomy, total pancreatectomy for pancreatic adenocarcinoma is associated with similar mortality and readmission rates. A higher rate of margin-negative tumour resections in the total pancreatectomy group was not associated with improved os, which was similar in the two groups. Total pancreatectomy could be a considered for patients whose resection margins after a partial pancreatic resection remain positive for malignancy.
ACKNOWLEDGMENTS
We thank Geraldine M. Chadwick, aud, who provided medical writing services on behalf of the Department of Surgery, Carolinas Medical Center. An abstract of this work was published by the Journal of the American College of Surgeons (Scientific Forum—2017;225(suppl 1):S139).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Sirri E, Castro FA, Kieschke J, et al. Recent trends in survival of patients with pancreatic cancer in Germany and the United States. Pancreas. 2016;45:908–14. doi: 10.1097/MPA.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 2.Nathan H, Wolfgang CL, Edil BH, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87–92. doi: 10.1002/jso.21189. [DOI] [PubMed] [Google Scholar]
- 3.Nayar P, Chandak A, Gupta N, et al. Postoperative mortality following multi-modality therapy for pancreatic cancer: analysis of the seer–Medicare data. J Surg Oncol. 2017;115:158–63. doi: 10.1002/jso.24472. [DOI] [PubMed] [Google Scholar]
- 4.Yeo TP. Demographics, epidemiology, and inheritance of pancreatic ductal adenocarcinoma. Semin Oncol. 2015;42:8–18. doi: 10.1053/j.seminoncol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–6. doi: 10.1016/j.jamcollsurg.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Kooby DA, Lad NL, Squires MH, 3rd, et al. Value of intraoperative neck margin analysis during Whipple for pancreatic adenocarcinoma: a multicenter analysis of 1399 patients. Ann Surg. 2014;260:494–501. doi: 10.1097/SLA.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 7.Sarr MG, Behrns KE, van Heerden JA. Total pancreatectomy. An objective analysis of its use in pancreatic cancer. Hepatogastroenterology. 1993;40:418–21. [PubMed] [Google Scholar]
- 8.Wang X, Hong X, Pang H, et al. Selecting optimal surgical procedures for intraductal papillary mucinous neoplasm (ipmn): an analysis based on the Surveillance, Epidemiology, and End Result registry database. Eur J Surg Oncol. 2016;42:1526–32. doi: 10.1016/j.ejso.2016.06.402. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015;102:1459–72. doi: 10.1002/bjs.9892. [DOI] [PubMed] [Google Scholar]
- 10.Clancy TE. Surgery for pancreatic cancer. Hematol Oncol Clin North Am. 2015;29:701–16. doi: 10.1016/j.hoc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Barbier L, Jamal W, Dokmak S, et al. Impact of total pancreatectomy: short- and long-term assessment. HPB (Oxford) 2013;15:882–92. doi: 10.1111/hpb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–7. doi: 10.1097/SLA.0b013e3181ae9f93. [DOI] [PubMed] [Google Scholar]
- 13.Ihse I, Anderson H, Andrén-Sandberg Total pancreatectomy for cancer of the pancreas: is it appropriate? World J Surg. 1996;20:288–93. doi: 10.1007/s002689900046. [DOI] [PubMed] [Google Scholar]
- 14.Dudekula A, Munigala S, Zureikat AH, Yadav D. Operative trends for pancreatic diseases in the U.S.A.: analysis of the nationwide inpatient sample from 1998–2011. J Gastrointest Surg. 2016;20:803–11. doi: 10.1007/s11605-015-3067-x. [DOI] [PubMed] [Google Scholar]
- 15.de la Iglesia-Garcia D, Huang W, Szatmary P, et al. on behalf of the nihr Pancreas Biomedical Research Unit Patient Advisory Group. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut. 2017;66:1354–5. doi: 10.1136/gutjnl-2016-312529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246:966–75. doi: 10.1097/SLA.0b013e31815c2ca3. [DOI] [PubMed] [Google Scholar]
- 17.Crippa S, Tamburrino D, Partelli S, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79–86. doi: 10.1016/j.surg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Kasumova GG, Conway WC, Tseng JF. The role of venous and arterial resection in pancreatic cancer surgery. Ann Surg Oncol. 2018;25:51–8. doi: 10.1245/s10434-016-5676-3. [DOI] [PubMed] [Google Scholar]
- 19.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 20.Johnston WC, Hoen HM, Cassera MA, et al. Total pancreatectomy for pancreatic ductal adenocarcinoma: review of the National Cancer Data Base. HPB (Oxford) 2016;18:21–8. doi: 10.1016/j.hpb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zakaria HM, Stauffer JA, Raimondo M, Woodward TA, Wallace MB, Asbun HJ. Total pancreatectomy: short- and long-term outcomes at a high-volume pancreas center. World J Gastrointest Surg. 2016;8:634–42. doi: 10.4240/wjgs.v8.i9.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makuc J. Management of pancreatogenic diabetes: challenges and solutions. Diabetes Metab Syndr Obes. 2016;9:311–15. doi: 10.2147/DMSO.S99701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casadei R, Monari F, Buscemi S, et al. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg. 2010;62:41–6. doi: 10.1007/s13304-010-0005-z. [DOI] [PubMed] [Google Scholar]
- 24.Ruess DA, Makowiec F, Chikhladze S, et al. The prognostic influence of intrapancreatic tumor location on survival after resection of pancreatic ductal adenocarcinoma. BMC Surg. 2015;15:123. doi: 10.1186/s12893-015-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artinyan A, Soriano PA, Prendergast C, Low T, Ellenhorn JD, Kim J. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371–6. doi: 10.1080/13651820802291233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirkin KA, Hollenbeak CS, Wong J. Greater lymph node retrieval and lymph node ratio impacts survival in resected pancreatic cancer. J Surg Res. 2017;220:12–24. doi: 10.1016/j.jss.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 27.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg. 2014;260:372–7. doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 28.Xai BT, Habib DA, Dhar VK, et al. Early recurrence and omission of adjuvant therapy after pancreaticoduodenectomy argue against a surgery-first approach. Ann Surg Oncol. 2016;23:4156–64. doi: 10.1245/s10434-016-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]