Abstract
Introduction
Radiation-induced brain necrosis [“radionecrosis” (rn)] is a relatively uncommon but potentially severe adverse effect of stereotactic radiosurgery (srs) for brain metastasis. Although dose, volume, and hypofractionation have been suggested to affect rn rates, patient and treatment variability in this population make it difficult to clearly delineate the risk. We set out to establish the effect of fractionation on rn rates by reviewing patients receiving simultaneous multi-fraction and single-fraction treatment at our centre.
Methods
Patients receiving simultaneous (within 1 month) 1-fraction (ssrs) and 3-fraction (fsrs) radiosurgery treatments during 2012–2015 were identified in our institution’s database. Serial post-srs magnetic resonance imaging (mri) was reviewed to determine rn and local recurrence. The effect of maximum dose, volume, whole-brain radiotherapy (wbrt), and fractionation on rn development was assessed using logistic regression for paired data. Results are reported using odds ratios (ors) and corresponding 95% confidence intervals (cis).
Results
Of 90 patients identified, 22 had at least a 6-month mri follow-up. Median follow-up was 320 days. The most common primary tumour type was non-small-cell lung cancer, followed by breast and rectal cancer. Radionecrosis developed in 16 patients [21 of 62 lesions (34%), with 4 being symptomatic (20%)]. Of the 21 lesions in which rn developed, 11 received 3 fractions, and 10 received 1 fraction. The or for the association between the incidence of rn and maximum dose was 1.0 (95% ci: 0.9 to 1.1); for fractionation it was 1.0 (95% ci: 0.3 to 3.6); for previous wbrt, it was 0.4 (95% ci: 0.2 to 1.2); and for a 10-unit increase in volume, it was 3.1 (95% ci: 1.0 to 9.6). Local recurrence developed in 8 patients (12%), 6 of whom belonged to the ssrs group.
Conclusions
Our results indicate that patients receiving srs for multiple brain metastases experience a higher rate of rn than is reported in the literature and poorer survival despite having equivalent local control. Maximum dose did not appear to be associated with rn risk in our cohort, but volume was significantly associated with rn risk. Although fractionated treatment did not directly lower the rate of rn in this population, it might have played a role in reducing the magnitude of the rn risk in large-volume lesions. Further investigation will help to delineate optimal dose and fractionation so as to minimize rn while maintaining local control in this group.
Keywords: Stereotactic radiosurgery, brain metastasis, radionecrosis
INTRODUCTION
Stereotactic radiosurgery (srs) achieves excellent control of brain metastasis (70%–90%) and spares patients many of the toxicities of whole-brain radiotherapy (wbrt)1–3. Stereotactic radiosurgery might be particularly useful in the modern era of targeted systemic treatment and immunotherapies for some cancers, with patients potentially benefiting from improved prognosis and durable control of brain metastasis. Consequently, those patients might also be at risk for late radiotherapy-induced complications.
Radiation-induced brain necrosis [“radionecrosis” (rn)] is a relatively uncommon but potentially severe adverse effect of srs that occurs in approximately 5%–25% of patients3–5. Radionecrosis generally occurs at 6 months to 2 years after srs treatment and can range from an incidental radiographic finding on magnetic resonance imaging (mri) to debilitating neurologic symptoms or seizures6. The pathophysiology is thought to be secondary to endothelial or glial cell damage and perilesional edema6. Typically, rn is managed with steroids; however, in some cases, surgical management is indicated for uncontrolled symptoms7. In fact, rn has become a significant clinical problem such that current trials are evaluating novel agents—for example, bevacizumab (see NCT02490878 at http://ClinicalTrials.gov/)—in attempt to obtain better control of rn and its associated symptoms.
Many factors, such as radiotherapy dose, treated volume, and previous radiation, have been suggested to affect rn rates8–10. However, clearly delineating the risk is difficult given the variability in patient and disease factors in this population, in whom tumour size, proximity to systemic therapy, and previous wbrt can vary significantly. The number of fractions, the treatment technique, and prescription variances (including prescription dose and isodose line) are also quite variable in reports of srs for brain metastasis3–5,9,10.
Hypofractionation has been suggested to have a favourable therapeutic ratio compared with single-fraction srs (ssrs) treatment and has been used in attempt to minimize the incidence of rn. We set out to establish the impact of fractionated radiosurgery (fsrs) compared with ssrs on rn rates by reviewing patients who received simultaneous multifraction and single-fraction treatment for multiple brain metastases at our centre.
The study was approved by the Hamilton Integrated Research Ethics Board.
METHODS
Patients with multiple brain metastases receiving both 1 single-fraction and 1 three-fraction srs course simultaneously (within 1 month during 2012–2015) were identified in the CyberKnife (Accuray, Sunnyvale, CA, U.S.A.) database maintained by the Juravinski Cancer Centre (Hamilton, ON). To be included in the analysis, patients were required to have had a biopsy-proven malignancy with a non–central nervous system cause and at least a 6-month mri follow-up (corresponding to the minimum time for rn development). Those patients therefore served as their own matched cohort, because patient and disease characteristics and timing of radiotherapy were consistent for each patient so as to control for any influence of those factors on the rate of rn.
Patients were simulated using computed tomography imaging and fusion of diagnostic or planning mri for contouring. A 1 mm planning target volume margin was used; no margin was applied for the clinical target volume. The prescription isodose line was chosen at the time of planning and was generally between 69%–86%, with the goal of covering 95% of the planning target volume with 100% of the prescription dose.
Treatments were delivered by photon-based robotic radiosurgery on a CyberKnife unit. The srs fractionation was determined by the treating physician based on target volume. Whole-brain radiotherapy was prescribed at the discretion of the physician before (n = 6) or after (n = 6) srs based on number of brain metastases or recurrent metastases respectively.
Serial mri follow-up was performed at approximately 3-month intervals after the srs treatments. The imaging was reviewed by a radiation oncologist (EKD) to determine the lesion quotient (lq), defined as the maximum cross-sectional area on T1 with Gd divided by the maximal cross-sectional area on T2 flair (fluid-attenuated inversion recovery) sequences. Radionecrosis was defined as a lq less than 0.3 with no growth of the lesion on T1 with Gd; a lq greater than 0.6 with corresponding growth on T1 with Gd was considered recurrence. The lq has been used to define rn based on mri findings in multiple radiosurgery studies8,11. Perfusion mri images were also reviewed when available for correlation (determined by the radiologist to indicate likely rn or recurrence). Treatment and clinical data were also collected.
The cohort was assessed using a paired design, with each patient representing their own matched control for the fsrs and ssrs treatments. Associations of previous wbrt, maximum point dose, volume, and fraction size with risk of rn were assessed using logistic regression for paired data, with an unstructured correlation matrix. Results are reported as odds ratios (ors) and their corresponding 95% confidence intervals (cis). The statistical analysis was performed in the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Of 90 patients identified, 22 met the inclusion criteria. Median follow-up was 320 days (range: 167–1982 days), and median survival was 352 days (range: 180–2012 days). All patients had died by the time of data collection (July–August 2017). The most common primary tumour type was non-small-cell lung cancer (46%), followed by breast cancer (18%), melanoma (14%), renal cell carcinoma (14%), and rectal cancer (9%). Table I presents the characteristics of the patients.
TABLE I.
Characteristics of the patients
| Characteristic | Value |
|---|---|
| Patients receiving 1 or 3 fractions (n) | 90 |
|
| |
| Survival [n (%)] | |
| ≥6 Months | 22 (24.4) |
| <6 Months | 68 (75.6) |
|
| |
| Number of lesionsa [n (%)] | |
| 2 | 11 (50) |
| 3 | 5 (23) |
| 4 | 5 (23) |
| 5 | 1 (4) |
|
| |
| Median lesion volume (mL) | 0.67 |
|
| |
| Received systemic therapy (n) | 20 |
|
| |
| Follow-up (days) | |
| Median | 320 |
| Range | 180–2012 |
|
| |
| Age (years) | |
| Median | 59 |
| Range | 42–78 |
|
| |
| Histologya [n (%)] | |
| NSCLC | 10 (45.5) |
| Breast cancer | 4 (18.2) |
| Melanoma | 3 (13.6) |
| Renal cell carcinoma | 3 (13.6) |
| Rectal cancer | 2 (9.1) |
|
| |
| Previous WBRTa (n) | |
| Yes | 12 |
| No | 10 |
In patients who survived for 6 months or more.
NSCLC = non-small-cell lung cancer; WBRT = whole-brain radiotherapy.
Radionecrosis developed in 16 patients (73%) and in 21 of 62 lesions (34%). Of the 21 lesions showing necrosis, 4 were symptomatic (19% of rn lesions, 7% of all lesions) based on correlation of symptoms with location. Of the 21 rn lesions, 11 had been treated with fsrs (52%), and 10 (48%), with ssrs. With respect to lesion location, 14 were in the frontal lobe (67%), 6 were in the occipital region (29%), and 1 was in the cerebellum (5%).
Perfusion mri was completed in 7 of the 22 patients. In 3 cases, the perfusion results were in accordance with rn, and in 2 cases, they were in accordance with recurrence. In the remaining 2 cases, the result was not in agreement with the lq results, and the lesions were designated “recurrence,” in keeping with the interpretation of perfusion results. Table II presents the characteristics of rn and recurrence by fraction size, and Table III shows the associations of treatment variables with rn.
TABLE II.
Treatment characteristics by fractionation group
| Variable | Fractions | |
|---|---|---|
|
| ||
| 1 | 3 | |
| Lesions treated (n) | 39 | 24 |
|
| ||
| Lesion size (mL) | ||
| Median | 0.13 | 8.1 |
| Range | 0.07–17.9 | 0.52–70.4 |
|
| ||
| Prescription dose (cGy) | ||
| Median | 2400 | 2100 |
| Range | 1800–2400 | 2100–2400 |
|
| ||
| Maximum point dose (cGy) | ||
| Median | 2862 | 3020 |
| Range | 2132–3750 | 2470–3380 |
|
| ||
| Radionecrosis (RN) | ||
| Patients experiencing RN (n) | 10 | 11 |
| Median time to RN (days) | 265.5 | 323.3 |
|
| ||
| Recurrences | ||
| Patients experiencing recurrence (n) | 6 | 2 |
| Median time to recurrence (days) | 241.5 | 198.5 |
TABLE III.
Association of factors with radionecrosis risk
| Factor | Comparator | OR | 95% CI | p Value |
|---|---|---|---|---|
| Fractions given | 1 vs. 3 | 1.0 | 0.3 to 3.6 | 0.98 |
| Volume | Factor of 10 increase | 3.1 | 1.0 to 9.6 | 0.04 |
| Whole-brain radiotherapy | Yes vs. no | 0.4 | 0.2 to 1.2 | 0.12 |
| Maximum dose | 1 Gy increase | 1.0 | 0.9 to 1.1 | 0.71 |
The incidence of rn was not associated with maximum radiotherapy dose (or: 1.0; 95% ci: 0.9 to 1.1) and had inconclusive associations with fractionation (or: 1.0; 95% ci: 0.3 to 3.6) and with previous wbrt (or: 0.4; 95% ci: 0.2 to 1.2). A larger volume was associated with an increased risk of rn (or: 3.1; 95% ci: 1.0 to 9.6) for every factor-of-10 increase in volume.
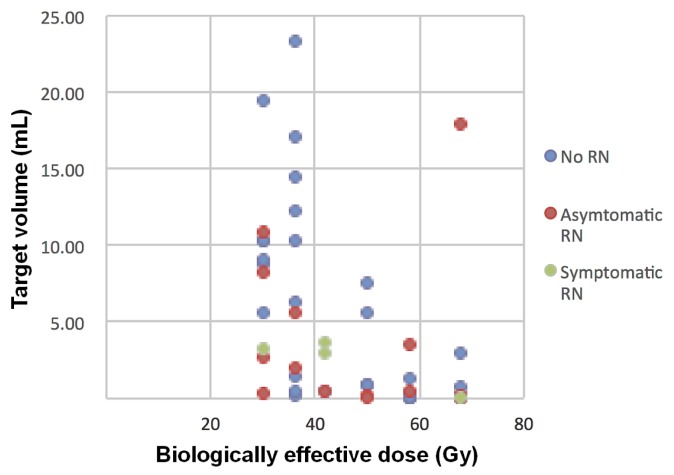
All patients with symptomatic rn were treated with dexamethasone, and symptoms resolved in 2 of the 4 affected patients (50%). However, radiographic rn did not resolve in any patients who developed it. Of the 8 patients who developed a local recurrence (12%), 6 were in the ssrs group, and 2 were in the fsrs group. Those patients were treated either with wbrt or with palliation, often because of simultaneous distant brain progression. Figures 1 and 2 provide examples of patients with rn and recurrence. Figure 3 shows a plot of treated volume against the prescribed dose (presented as the biologically effective dose), with symptomatic and asymptomatic rn labelled.
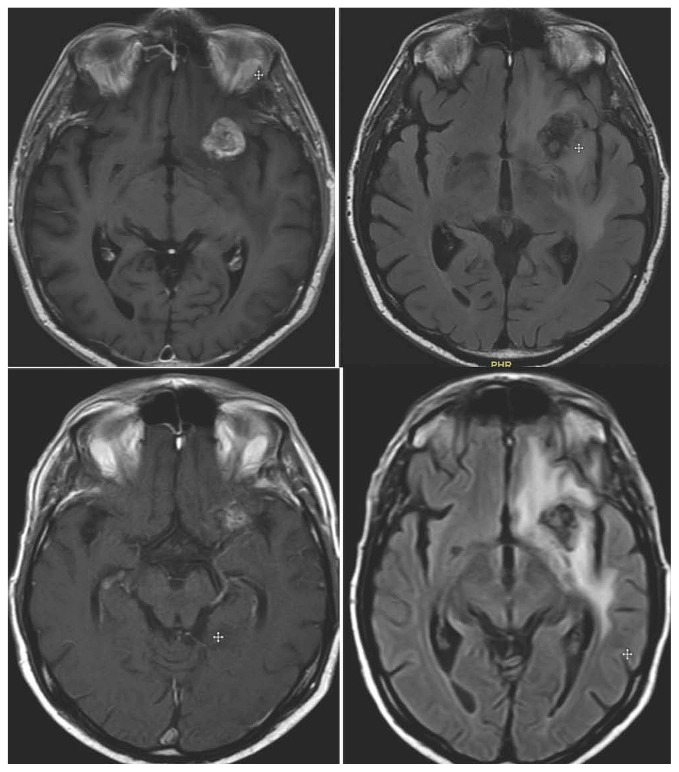
FIGURE 1.
Top panel: Patient with left frontal lesion before stereotactic radiosurgery (SRS). Bottom panel: Development of radionecrosis after SRS. Both panels: T1 plus gadolinium magnetic resonance imaging (MRI), and T2 FLAIR (fluid-attenuated inversion recovery) MRI (left and right respectively).
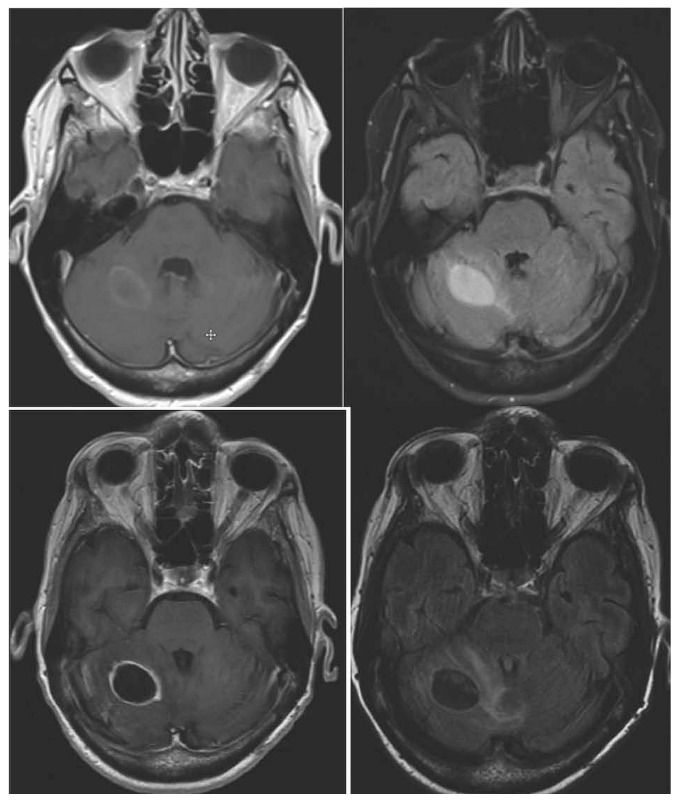
FIGURE 2.
Top panel: Patient with right cerebellar lesion before stereotactic radiosurgery (SRS). Bottom panel: Recurrence after SRS. Both panels: T1 plus gadolinium magnetic resonance imaging (MRI), and T2 FLAIR (fluid-attenuated inversion recovery) MRI (left and right respectively).
FIGURE 3.
Plot of the biologically effective dose against the target volume, with cases of asymptomatic and symptomatic radionecrosis labelled.
Additional brain metastasis developed in 16 patients (73%). Ultimately, 7 patients appeared to die of neurologic progression (32%); the cause of death for others was systemic progression or a cause that could not be ascertained.
DISCUSSION
Many studies have examined risk factors for rn, with variable results because of variability in the prescription dose and isodose line, the number of fractions, and the variability of patient and disease factors. We attempted to minimize many of those confounding factors by reviewing only patients who received both ssrs and fsrs simultaneously (within 1 month). In this unique patient group, ssrs was paired with fsrs in a single patient, meaning that tumour histology, history of chemotherapy and wbrt, and other predisposing factors inherent to individual patients were identical, and facilitating a more authentic comparison of the effect of fractionation and volume on rn.
Investigators have previously hypothesized a radiobiologic advantage of fractionation in achieving adequate control while minimizing damage to the normal brain parenchyma12. Such an approach might be particularly important when delivering treatment to eloquent locations or large volumes and might minimize rn risk in such areas13. For example, Minniti et al.5 reported rn rates that were significantly different between single- and multiple-fraction srs (20% and 8% respectively), with local control also being improved in patients receiving multiple-fraction treatment (p = 0.01). Kohutek et al.4 reviewed 271 brain metastases receiving single-fraction srs and noted the development of rn in 25.8% and, in patients still alive at 2 years, an increase in the rate to 34%.
In comparison, some studies have reported a lower incidence of rn (7%–9%) with dose delivery in 3–5 fractions14,15. Zhuang et al.16 reviewed 94 patients receiving srs in 1–8 fractions and found that rn occurred only in patients receiving 1–4 fractions, with an overall risk of 11.5%. Finally, Kim et al.17 treated patients with 3–4 fractions and found a necrosis risk of 2.7% while maintaining a local control rate of 90%.
Nevertheless, results in other studies using fsrs have been variable. Fahrig et al.18 found a rn rate of 22% when 5–6 fractions were used for brain metastasis compared with only 7% when 7–fraction radiotherapy was used. Another study prospectively followed patients with resistant primaries, including renal cell carcinoma or melanoma, and found that, although local control rates were similar (p = 0.38) with ssrs and fsrs regimens, rn rates were also no different at 7.2% and 9.6% respectively (p = 0.55)3. In our analysis, the effect of fractionation on rn was also indeterminate, with a possible range of protective to harmful effect (95%% ci: 0.3 to 3.6).
Volume has previously been acknowledged to be an independent risk factor for rn in some studies. Minniti et al.5 reported a volume receiving 10–16 Gy (V10–V16) to be an independent risk factor for rn (and particularly symptomatic rn). Specifically, V10 greater than 12.6 mL and V12 greater than 10.9 mL carried a rn risk greater than 10%. Similarly, Kohutek et al.4 found that increasing tumour diameter correlated with risk of rn (hazard ratio: 3.10; p < 0.001), and Korytko et al.19 found that 12 Gy to a volume greater than 10 mL correlated with symptomatic necrosis of 55.3% compared with 22.5% for the same dose to just 10 mL. Some evidence suggests that fractionating treatment might lower the increased risk of rn that is seen in larger lesions15.
In our cohort, a volume effect was not definitively demonstrated, possibly because of the small sample size. However, given that the volume was significantly larger in the fsrs group, an even larger rate of rn might be expected, and we are therefore optimistic that fractionation might have a protective effect. That observation requires prospective investigation to better clarify its effect.
We also did not show an association between rn and maximum radiosurgery dose in our patients. The observed effect of wbrt appeared to be protective, although given the wide ci, is difficult to interpret and might be related to the variable timing of srs in relation to the disease process in the cohort. Although we acknowledge that other factors could be related to rn development, we limited our analysis to just 4 variables so as to maintain statistical integrity. Those variables were carefully selected based on their consideration in previous studies4,16.
Survival in our cohort of patients with 2 or more synchronous brain metastases was extremely poor compared with the expected survival of 10–12 months in patients with limited brain metastasis1. That observation has also been emphasized in many prospective trials of patients with multiple brain metastases, in which median survival ranged from 4 months to 6 months20–23. More concerning in those studies is the poor survival despite an excellent performance status at time of radiosurgery (>70% Karnofsky performance status), making it difficult to predict which patients might live long enough to benefit from srs.
Of the 90 patients identified in our institution’s CyberKnife database, 68 (75.6%) died or were lost to decline before undergoing follow-up mri at 6 months. Because of the very poor survival in this cohort, it is therefore possible that the effect of hypofractionation was not truly appreciated. In other words, many patients did not survive long enough to develop rn, and the benefits of fractionated radiosurgery in protecting against rn might become more apparent in patients who survive longer. Furthermore, our cohort of assessed patients who survived past 6 months was composed primarily of patients with non-small-cell lung, breast, and renal cell cancer or melanoma. Compared with other cancer types that lead to brain metastasis (for example, small-cell lung, gastrointestinal, and bladder cancers), those cancer histologies have been identified as potentially being associated with improved survival durations24. Affected patients experienced benefits in local control from the stereotactic treatment they received, as well as rates of distant brain progression and neurologic death that were similar to those in reports in the literature. That group should therefore be given careful consideration for srs treatment because it could include patients who might survive long enough to benefit. That possibility is particularly important given the potential for improvement in cognitive effects with radiosurgery compared with wbrt and the recognized correlation of neurocognitive function with quality of life25.
Our study has a number of limitations. To select a group of patients with simultaneously treated lesions (1 and 3 fractions, both within 1 month) to serve as their own controls, the sample size was inherently small. Additionally, the diagnosis of radiographic rn is still controversial, and the accuracy of our report is limited to the specific definition used here. Although the lq was chosen for our study, in keeping with the ongoing NCT02490878 trial (available by a search at http://ClinicalTrials.gov/), other studies have demonstrated limitations in the sensitivity and specificity of the lq in predicting rn26. Finally, because the prescription dose and fractionation might have been influenced by the volume of the lesions being treated, it is difficult to conduct a truly independent examination of the effect of fractionation on the patients in the present study.
But despite those limitations, valuable information can still be ascertained for this patient group. An association between increasing lesion volume and rn risk was observed despite the fractionation being used for the srs (and given the much larger volumes seen in the fsrs group). That observation should be carefully considered when prescribing srs for large-volume lesions, and patients should be closely monitored for symptoms of rn. However, we believe that, despite the limitation of small cohort size (precluding more definitive results), we have demonstrated that fractionation could still have a protective effect, lowering the magnitude of the rn risk in large lesions. Secondly, the overall risk of rn appears to be higher than expected when multiple treatments are given simultaneously, which is an important clinical consideration in this patient group. Finally, although prognosis in this group is very poor compared with the group of patients who have solitary or perhaps synchronous metastasis, some patients might survive longer and benefit from locally ablative treatments.
When prescribing ssrs or fsrs, clinicians and researchers must be mindful of the effect of volume, variability in prognosis, and higher rates of rn in the patient group with larger-volume tumours. It appears that fractionation has a protective effect; however, based on our small sample of patients, we cannot estimate the size of that effect. Further prospective research in this group of patients is critical to better delineate the effects of volume and fractionation on rn and to develop strategies to mitigate rn risk.
CONCLUSIONS
In radiosurgery for brain metastasis, rn is a serious adverse event that can result in significant clinical symptoms. Our study confirms the association between larger tumour volume and rn risk, despite fractionation of srs. Fractionation might have a role in lowering the magnitude of the rn risk in large-volume lesions; however, the small number of patients in our study, the potential limitations in our definition of rn, and the large number of asymptomatic rn patients precluded definitive conclusions. Additionally, no effect of maximum dose on rn was observed. Our results indicate that patients receiving srs for multiple brain metastases experience a higher rate of rn than is reported in the literature and poorer survival despite equivalent local control. Further investigation will help to delineate the optimal dose and fractionation for minimizing rn while maintaining local control in this group.
ACKNOWLEDGMENTS
Knowledge translation costs for this study were funded by Accuray. Part of this work was previously published as an abstract: Donovan E, Parpia S, Greenspoon J. Is hypofractionation safer than single-fraction radio surgery? The effect of fractionation on radionecrosis [abstract]. Can J Neurol Sci 2018;45(suppl 3):S6–7.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: EKD received a stereotactic radiotherapy fellowship grant from Accuray (July 2018–June 2019). The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40:48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Lesueur P, Lequesne J, Barraux V, et al. Radiosurgery or hypofractionated stereotactic radiotherapy for brain metastases from radioresistant primaries (melanoma and renal cancer) Radiat Oncol. 2018;13:138. doi: 10.1186/s13014-018-1083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125:149–56. doi: 10.1007/s11060-015-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minniti G, Scarigini C, Paolini S, et al. Single-fraction versus multifraction stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95:1142–8. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Miyatake SI, Nonoguchi N, Furuse M, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo) 2015;55:50–9. doi: 10.2176/nmc.ra.2014-0188. [DOI] [PubMed] [Google Scholar]
- 7.Telera S, Fabi A, Pace A, et al. Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol. 2013;113:313–25. doi: 10.1007/s11060-013-1120-8. [DOI] [PubMed] [Google Scholar]
- 8.Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase ii trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2008;81:18–24. doi: 10.1016/j.radonc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Shehata MK, Young B, Reid B, et al. Stereotactic radiosurgery of 468 brain metastases < or =2 cm: implications for srs dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys. 2004;59:87–93. doi: 10.1016/j.ijrobp.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63:898–903. doi: 10.1227/01.NEU.0000333263.31870.31. [DOI] [PubMed] [Google Scholar]
- 12.Manning M, Cardinale R, Benedict SH, et al. Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:603–8. doi: 10.1016/S0360-3016(00)00475-2. [DOI] [PubMed] [Google Scholar]
- 13.Inoue HK, Seto K, Nozaki A, et al. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14) J Radiat Res. 2013;54:727–35. doi: 10.1093/jrr/rrt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doré M, Martin S, Delpon G, Clement K, Campion L, Thilllays F. Stereotactic radiotherapy following surgery for brain metastasis: predictive factors for local control and radionecrosis. Cancer Radiother. 2017;21:4–9. doi: 10.1016/j.canrad.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Eaton BR, LaRiviere MJ, Kim S, et al. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol. 2015;123:103–11. doi: 10.1007/s11060-015-1767-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhuang H, Zheng Y, Wang J, et al. Analysis of risk and predictors of brain radiation necrosis after radiosurgery. Oncotarget. 2016;7:7773–9. doi: 10.18632/oncotarget.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Park HR, Lee JM, et al. Fractionated stereotactic Gamma Knife radiosurgery for large brain metastases: a retrospective, single center study. PLoS One. 2016;11:e0163304. doi: 10.1371/journal.pone.0163304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahrig A, Ganslandt O, Lambrecht U, et al. Hypofractionated stereotactic radiotherapy for brain metastasis—results from three different dose concepts. Strahlenther Onkol. 2007;183:625–30. doi: 10.1007/s00066-007-1714-1. [DOI] [PubMed] [Google Scholar]
- 19.Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy Gamma Knife radiosurgical volume is a predictor for radiation necrosis in non-avm intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–24. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 20.Salvetti DJ, Nagaraja TG, McNeill IT, Xu Z, Sheehan J. Gamma Knife surgery for the treatment of 5 to 15 metastases to the brain: clinical article. J Neurosurg. 2013;118:1250–7. doi: 10.3171/2013.2.JNS121213. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi AM, Recinos PF, Barnett GH, et al. Role of Gamma Knife surgery in patients with 5 or more brain metastases. J Neurosurg. 2012;117(suppl):5–12. doi: 10.3171/2012.8.GKS12983. [DOI] [PubMed] [Google Scholar]
- 22.Grandhi R, Kondziolka D, Panczykowski D, et al. Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg. 2012;117:237–45. doi: 10.3171/2012.4.JNS11870. [DOI] [PubMed] [Google Scholar]
- 23.Lim TK, Kim WK, Yoo CJ, Kim EY, Kim MJ, Yee GT. Fractionated stereotactic radiosurgery for brain metastases using the Novalis Tx system. J Korean Neurosurg Soc. 2018;61:525–9. doi: 10.3340/jkns.2018.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long term survival with metastatic cancer to the brain. Med Oncol. 2000;17:279–86. doi: 10.1007/BF02782192. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 26.Stockham AL, Tievsky AL, Koyfman SA, et al. Conventional mri does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109:149–58. doi: 10.1007/s11060-012-0881-9. [DOI] [PubMed] [Google Scholar]