Abstract
Background
Chemoradiation with curative intent is considered the standard of care in patients with locally advanced, stage iii non-small-cell lung cancer (nsclc). However, some patients with stage iii (N2 or N3, excluding T4) nsclc might be eligible for surgery. The objective of the present systematic review was to investigate the efficacy of surgery after chemoradiotherapy compared with chemoradiotherapy alone in patients with potentially resectable locally advanced nsclc.
Methods
A search of the medline, embase, and PubMed databases sought randomized controlled trials (rcts) comparing surgery after chemoradiotherapy with chemoradiotherapy alone in patients with stage iii (N2 or N3, excluding T4) nsclc.
Results
Three included rcts consistently found no statistically significant difference in overall survival between patients with locally advanced nsclc who received surgery and chemoradiotherapy or chemoradiotherapy alone. Only one rct found that progression-free survival was significantly longer in patients treated with chemoradiation and surgery (hazard ratio: 0.77; 95% confidence interval: 0.62 to 0.96). In a post hoc analysis of the same trial, the overall survival rate was higher in the surgical group than in matched patients in a chemoradiation-only group if a lobectomy was performed (p = 0.002), but not if a pneumonectomy was performed. Furthermore, fewer treatment-related deaths occurred in patients who underwent lobectomy than in those who underwent pneumonectomy.
Conclusions
For patients with locally advanced nsclc, the benefits of surgery after chemoradiation are uncertain. Surgery after chemoradiation for patients who do not require a pneumonectomy might be an option.
Keywords: Non-small-cell lung cancer, locally advanced, chemotherapy, radiation therapy, lobectomy, pneumonectomy
INTRODUCTION
More than 80% of patients with lung cancer in Canada have non-small-cell lung cancer (nsclc), and they are often diagnosed at an advanced stage1. The 5-year relative overall survival (os) rates for patients with stage iiia and iiib disease are approximately 14% and 5% respectively2. The generally accepted standard therapy for patients with stage iii locally advanced nsclc is combined-modality chemoradiation. In 2005, the Program in Evidence-Based Care at Cancer Care Ontario produced a guideline to determine the most effective chemoradiotherapy regimens for patients with unresectable stage iii nsclc3. However, some patients with stage iii (N2 or N3, excluding T4) cancers might be eligible for surgical resection. That scenario was not covered in the 2005 guideline.
To make recommendations as part of a clinical practice guideline about the effectiveness of surgery after chemoradiotherapy in patients with locally advanced nsclc4, the Program in Evidence-Based Care, together with Cancer Care Ontario’s Lung Cancer Disease Site Group, developed the evidentiary base reported here. Based on the objectives of the present review, the authors derived this research question: Does surgery after chemoradiotherapy compared with chemoradiotherapy alone for patients with potentially resectable stage iii (N2 or N3, excluding T4) nsclc improve patient outcomes?
METHODS
The American College of Chest Physicians guideline was used as a base for this systematic review5. That guideline was considered to include all evidence published before December 2011 that was relevant to the study question. Our evidence review was conducted in two planned stages, including a search for systematic reviews and a primary literature review.
Search for Existing Systematic Reviews
The medline (2011 to 20 October 2016), embase (2011 to 20 October 2016), and PubMed (1 January 2016 to 20 October 2016) databases were searched for published systematic reviews or meta-analyses of phase ii or iii randomized controlled trials (rcts). Search terms indicative of nsclc and chemoradiotherapy were used. The full search strategy is available in supplemental Appendix 1.
Search for Primary Literature
In the absence of systematic reviews addressing the research question, other methods were used to search for primary literature. The medline (2011 to 20 October 2016), embase (2011 to 20 October 2016), and PubMed (1 January 2016 to 20 October 2016) databases were searched for rcts. Reference lists of relevant papers and review articles were scanned for additional citations. Search terms indicative of nsclc, chemoradiotherapy, and rcts were used. The full search strategy is available in supplemental Appendix 1.
Study Selection Criteria and Process
Studies were required to be phase iior iii rcts that compared surgery after chemoradiotherapy with chemoradiotherapy alone. Studies had to have included at least 50 patients with stage iii (N2 or N3, excluding T4) nsclc and to have reported at least one of the following outcomes by treatment group: os, progression-free survival (pfs), quality of life, local control, or adverse effects. Studies had to be fully published papers or published abstracts in English. Letters and editorials that reported clinical trial outcomes were excluded. A review of the titles and abstracts that resulted from the search was conducted by a single author (ETV). Items that warranted full-text review were each reviewed by ETV in collaboration with the other authors.
Data Extraction and Assessment of Study Quality and Potential for Bias
Data extraction was performed independently by ETV and reviewed by the other authors. Hazard ratios less than 1.0 indicate improved efficacy for the chemoradiotherapy plus surgery arm, and hazard ratios greater than 1.0 indicate improved efficacy for the chemoradiotherapy-only arm. All extracted data and information were audited by an independent auditor. The Cochrane Risk of Bias Tool was used to assess the risk of bias of the individual studies6.
Synthesizing the Evidence
The presence of clinical heterogeneity because of the various chemoradiotherapy regimens used prevented the performance of a meta-analysis. The rcts used a variety of chemotherapy drugs, radiation doses, and schedules (concurrent or sequential). The outcomes were rated for their importance for decision-making by the authors. Only outcomes that were considered critical or important were included in the present review. Five factors taken from the grade (Grading of Recommendations, Assessment, Development and Evaluations) method for assessing the quality of aggregate evidence7—risk of bias, inconsistency, indirectness, imprecision, and publication bias—were assessed.
RESULTS
Search for Existing Systematic Reviews
No systematic reviews that addressed the research question were found.
Search for Primary Literature
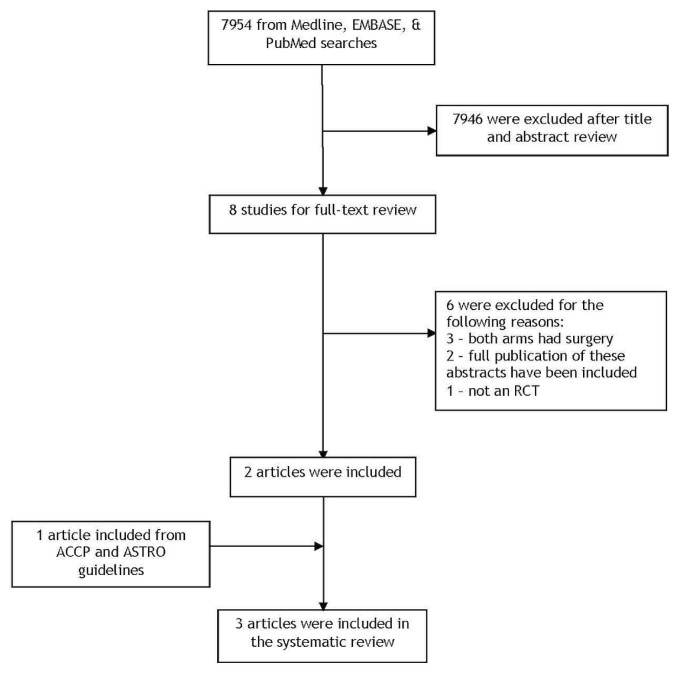
Of 7954 citations identified, 8 were selected for full-text review. Of those, one fully published rct8 and one rct published in abstract form9 met the predefined eligibility criteria for the systematic review. In addition, one article from the evidence base of the American College of Chest Physicians guideline that met the selection criteria for the systematic review was included10. Thus, three relevant articles were included. Figure 1 presents the search flow diagram.
FIGURE 1.
PRISMA flow diagram.
Study Design and Quality
All three rcts were phase iii studies 8–10, but one was published in abstract form9. Tables I–III show the characteristics and outcomes of the included rcts. The authors considered os and quality of life to be critical outcomes for recommendation development. Important secondary outcomes of interest considered were pfs, adverse effects, and local control. Table IV shows the results of the quality assessment of the studies. Risk of bias for the abstract was unclear because most details were not reported9. The Intergroup trial10 had a high risk of bias because allocation to the two arms was unconcealed, and fewer patients in the surgical group received consolidation chemotherapy. Furthermore, the Intergroup10 and espatue trials8 did not report their blinding procedures. Also, the espatue trial included patients with T4 nsclc and might therefore not be directly generalizable to our patient population8.
TABLE I.
Studies selected for inclusion
| Reference | Design | Target sample size | Follow-up (months) |
|---|---|---|---|
| Albain et al., 200910 (Intergroup 0139) | ▪ Phase III RCT | 510 (484 eligible), | 22.5 |
| ▪ Closed early | randomized before induction | (range: 0.9–125.1) | |
| Inclusion criteria: | Potentially resectable stage IIIA (pN2) disease: T1–3 primary NSCLC with pathology proof of N2 involvement | ||
| Comparison: | (A) Concurrent induction CTx (cisplatin–etoposide) plus RT (45 Gy) and continued RT if no progression; consolidation CTx (cisplatin–etoposide) given | ||
| (B) Concurrent induction CTx (cisplatin–etoposide) plus RT (45 Gy) and resected if no progression; consolidation CTx (cisplatin–etoposide) given | |||
| Patients analyzed: | (A) 213 randomized; 19 excluded in analysis; 50 did not complete consolidation CTx | ||
| (B) 216 randomized; 14 excluded in analysis; 91 did not complete consolidation cCTx | |||
|
| |||
| Sorensen et al., 20139 (Nordic, abstract) | ▪ Phase III RCT | 406 | Not reported |
| ▪ Closed early | |||
| Inclusion criteria: | Previously untreated, histologically verified NSCLC, stages T1–3N2M0 | ||
| Comparison: | (A) Sequential CTx (paclitaxel–carboplatin) followed by RT (60 Gy) | ||
| (B) CTx (paclitaxel–carboplatin), followed by surgery, followed by RT (60 Gy) | |||
| Patients analyzed: | (A) 171 randomized | ||
| (B) 170 randomized; 132 underwent surgery | |||
|
| |||
| Eberhardt et al., 20158 (ESPATUE) | ▪ Phase III RCT | 300, | Median: |
| ▪ Closed early | randomized after induction | 78 | |
| Inclusion criteria: | Potentially resectable stage IIIA (N2) or select stage IIIB NSCLC with pathology proof of N2 involvement; select stage IIIB patients included those with N3 disease with contralateral mediastinal nodes and proven T4 disease with involvement of the pulmonary artery, carina, left atrium, vena cava, or mediastinum | ||
| Comparison: | (A) Induction CTx (cisplatin–paclitaxel), then concurrent CTx (cisplatin–vinorelbine) plus RT (45 Gy), and chemoRT boost, risk-adapted to between 65 Gy and 71 Gy | ||
| (B) Induction CTx (cisplatin–paclitaxel), then concurrent CTx (cisplatin–vinorelbine) plus RT (45 Gy), and resected | |||
| Patients analyzed: | (A) 80 randomized; 76 received chemoRT boost | ||
| (B) 81 randomized; 70 underwent surgery | |||
RCT = randomized controlled trial; NSCLC = non-small-cell lung cancer; CTx = chemotherapy; RT = radiation therapy; chemoRT = chemoradiotherapy.
TABLE II.
Outcomes analyses for the included trials
| Reference | Comparison | PFS | OS |
|---|---|---|---|
| Albain et al., 200910 (Intergroup 0139) | Concurrent induction chemoRT and continued RT if no progression | Median: 10.5 months | Median: 22.2 |
| IQR: 4.8–20.6 months | IQR: 9.7–52.7 months | ||
| Concurrent induction chemoRT and resected if no progression | Median: 12.8 months | Median: 23.6 months | |
| IQR: 5.3–42.2 months | IQR: 9.0 months to not reached | ||
| HR: 0.77; 95% CI: 0.62 to 0.96 | HR: 0.87; 95% CI: 0.70 to 1.10 | ||
| p=0.017 | p=0.24 | ||
Other analyses:
| |||
|
| |||
| Sorensen et al., 20139 (Nordic, abstract) | Sequential chemoRT | Median: 8 months | Median: 15 months |
| CTx, followed by surgery, followed by RT | Median: 10 months | Median: 17 months | |
| p=0.144 | p=0.172 | ||
|
| |||
| Eberhardt et al., 20158 (ESPATUE) | Induction CTx followed by concurrent chemoRT, and chemoRT boost | 5-Year: 35% | 5-Year: 40% |
| 95% CI: 25% to 46% | 95% CI: 29% to 52% | ||
| Induction CTx followed by concurrent chemoRT, and resected | 5-Year: 32% | 5-Year: 44% | |
| 95% CI: 22% to 43% | 95% CI: 32% to 56% | ||
| p=0.75 | p=0.34 | ||
PFS = progression-free survival; OS = overall survival; chemoRT = chemoradiotherapy; IQR = interquartile range; HR = hazard ratio; CI = confidence interval; CTx = chemotherapy; RT = radiation therapy.
TABLE III.
Adverse events
| Adverse event (grades 3–5) | Patients experiencing the event [n (%)] | ||||
|---|---|---|---|---|---|
|
| |||||
| Albain et al., 200910 (Intergroup 0139) | Sorensen et al., 20139 (Nordic, abstract) | Eberhardt et al., 20158 (ESPATUE) | |||
|
|
|
||||
| ChemoRT plus RT (n=194) | ChemoRT plus resection (n=202) | ChemoRT plus chemoRT boost (n=80) | ChemoRT plus resection (n=81) | ||
| Leucopenia | 107 (55) | 97 (48) | Not reported | 48 (60) | 49 (60) |
|
| |||||
| Neutropenia | 80 (41) | 77 (38) | |||
|
| |||||
| Anemia | 47 (25) | 26 (13) | 7 (9) | 10 (12) | |
|
| |||||
| Thrombocytopenia | 23 (12) | 14 (7) | 8 (11) | 9 (11) | |
|
| |||||
| Worst hematologic toxicity per patient | 125 (65) | 117 (58) | |||
|
| |||||
| Nausea or emesis, or both | 26 (13) | 29 (14) | 7 (9) | 11 (13) | |
|
| |||||
| Neuropathy | 7 (4) | 10 (5) | 5 (6) | 5 (6) | |
|
| |||||
| Esophagitis | 44 (23) | 20 (9) | 21 (26) | 11 (14) | |
| p= 0.0006 | |||||
|
| |||||
| Stomatitis or mucositis, or both | 5 (3) | 6 (3) | 2 (3) | 3 (4) | |
|
| |||||
| Pulmonary | 31 (16) | 31 (15) | 5 (6) | 9 (11) | |
|
| |||||
| Other gastrointestinal or renal | 7 (4) | 10 (5) | 5 (6) | 8 (10) | |
|
| |||||
| Cardiac | 9 (5) | 10 (5) | 2 (3) | 4 (5) | |
|
| |||||
| Miscellaneous infection | 8 (4) | 6 (3) | 3 (3) | 7 (9) | |
|
| |||||
| Hemorrhage | 1 (1) | 1 (1) | |||
|
| |||||
| Fatigue | 9 (5) | 11 (5) | 8 (10) | 5 (6) | |
|
| |||||
| Pain | 16 (20) | 19 (23) | |||
|
| |||||
| Anorexia | 7 (4) | 3 (1) | |||
|
| |||||
| Allergy | 3 (2) | 1 (1) | |||
|
| |||||
| Treatment-related death | 4 | 14 of 16 after pneumonectomy | 2 | 4 of 5 after lobectomy | |
| 1 of 16 after lobectomy | 1 of 5 after bilobectomy | ||||
| 1 of 16 after no thoracotomy | |||||
ChemoRT = chemoradiotherapy; RT = radiation therapy.
TABLE IV.
Risk of bias for the included studies
| Reference | Comparison | Randomization method | Blinding | Incomplete outcomes data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|
| Albain et al., 200910 (Intergroup 0139) | Concurrent chemoRT plus RT vs. concurrent chemoRT plus surgery | High risk Unmasked assignment Stratified by primary T designation, performance status, and contralateral mediastinal nodal sampling |
Unclear risk Blinding not reported |
High risk Completed consolidation therapy: ChemoRT plus resection, 111/216 ChemoRT plus radiation, 144/213 |
Low risk Primary outcome OS |
Low risk |
|
| ||||||
| Sorensen et al., 20139 (Nordic) | Sequential chemoRT vs. CTx plus surgery plus RT | Unclear risk Abstract |
Unclear risk Abstract |
Unclear risk Abstract |
Unclear risk Abstract |
Unclear risk Abstract |
|
| ||||||
| Eberhardt et al., 20158 (ESPATUE) | Concurrent chemoRT plus chemoRT boost vs. concurrent chemoRT plus surgery | Low risk Stratified by tumour–node subgroup, intended prophylactic cranial irradiation, and country of participating institution |
Unclear risk Blinding not reported |
Low risk | Low risk Primary outcome OS |
Low risk |
ChemoRT = chemoradiotherapy; RT = radiation therapy; OS = overall survival; CTx = chemotherapy.
Outcomes
Two rcts (Intergroup 0139 and espatue) delivered concurrent chemoradiation8,10 and one (Nordic) used sequential chemoradiation9. The overall quality of the evidence was considered to be low because of the potential risk of bias associated with lack of allocation concealment, potential lack of blinding of the outcome assessors, and incomplete outcomes data. Furthermore, issues of imprecision were present because all the studies did not reach their target sample size—although the Intergroup study reported achieving sufficient events (target number of events not reported). Studies were stopped early because of slow accrual or changes in standard chemoradiotherapy treatment.
The rcts consistently found no statistically significant difference in os between their treatment groups. Only the Intergroup study found a significantly longer pfs in patients treated with chemoradiation and surgery (hazard ratio: 0.77; 95% confidence interval: 0.62 to 0.96) compared with chemoradiation alone (p = 0.017)10. The other two trials found no significant differences in pfs between the treatment arms8,9. In a post hoc analysis of the Intergroup study, the os rate was higher for the surgical group than for matched patients in the chemoradiation-only group if a lobectomy was performed (p = 0.002), but not if a pneumonectomy was performed (p value not reported)10. The other two trials did not describe any differences in survival based on extent of surgery; however, in the espatue trial, no treatment-related deaths occurred in patients who underwent pneumonectomy (n = 23), compared with 5 deaths in 47 patients who underwent lobectomy, bilobectomy, or segmentectomy8.
In the Nordic trial9, patients with a diagnosis of adenocarcinoma and those with T1N2 disease (that is, lower T-stage primary tumours) experienced improved survival in the chemoradiation plus surgery arm compared with the chemoradiation-only arm (p = 0.002 and p = 0.0010 respectively). In the Intergroup trial, female patients with an absence of severe weight loss and only 1 involved nodal station were more likely to experience longer os10. However, it is unclear if the regression analysis in that study was performed using data from all patients or only from patients who underwent surgery.
In the Intergroup trial, most of the adverse effects were found to be comparable in the two treatment groups, but a higher proportion of esophagitis was observed in patients treated with chemoradiation alone compared with those who received chemoradiation and surgery (p = 0.0003)10. Treatment-related deaths occurred more often in patients who underwent surgery.
Quality of life and local control outcomes were not reported in any of the trials.
DISCUSSION
In this systematic review, no included trial consistently found a statistically significant difference in os between patients with stage iii (N2 or N3, excluding T4) nsclc who received chemoradiation compared with those who received chemoradiation plus surgery. However, one important aspect to consider is that all three trials were closed early for various reasons, and so overall, each trial might have been underpowered to detect a true difference in os and pfs with the addition of surgery after chemoradiation.
Resectable stage iii nsclc was defined in the Intergroup trial as good performance status, weight loss less than 10% in the preceding 3 months, single nodal station 2 cm or less in size, with a predicted postoperative forced expiratory volume greater than 800 mL in 1 second. In a post hoc analysis of the Intergroup trial, fewer treatment-related deaths occurred in the patients who underwent lobectomy than in those who underwent pneumonectomy10. In that trial, pfs was also improved with the addition of surgery. Based on that finding, coupled with the increased mortality associated with pneumonectomy, it seems reasonable to consider a lobectomy after chemoradiation in patients with resectable stage iii nsclc, although in the espatue trial, no treatment-related deaths occurred after a pneumonectomy8. Other factors—including patient comorbidities, extent of disease, surgical or technical expertise, and patient preference—are clearly important to consider when discussing the role of surgery. Based on the regression analysis in the Intergroup trial, female patients with an absence of severe weight loss and only 1 involved nodal station might experience better os, but whether the analysis included all patients or only patients who underwent surgery was unclear10. In the absence of subgroup data, it is therefore unclear whether those characteristics should be used to select patients for surgery. The espatue trial included select patients with T4 and N3 disease (that is, stage iiib), constituting about one third of its patients, based on previous experience in phase i/ii trials using trimodality therapy in such cases11. Such patients would be traditionally considered unresectable (although they were considered resectable in espatue). Nonetheless, questions arise about generalizability and surgical expertise in managing more bulky or advanced-stage iii nsclc.
Given the heterogeneity of the studies, including differences in the induction regimens described earlier, a formal meta-analysis could not be carried out. Additionally, the Intergroup trial did not include positron-emission tomography (pet) staging in its entry criteria10, whereas the espatue trial did8. It is unclear whether the Nordic trial included pet staging9. Imaging by pet is known to up-stage approximately 15% of patients with stage iii nsclc. Differences in the use of pet imaging for staging will result in some stage migration and could explain the better os seen in the patients overall in espatue compared with the other trials8. In fact, median survival in the chemoradiation-only arm was similar to that in other contemporaneous trials (using pet) of chemoradiation in nonresectable patients, such as in the standard arm of the Radiation Therapy Oncology Group 0617 study12 and the standard arm of the proclaim trial13.
Additionally, the optimal standard chemoradiation regimen differed between the studies. The Intergroup trial used a once-daily combined-modality approach to 61 Gy total10; the espatue trial used a twice-daily combined modality approach to 45 Gy, followed by a once-daily boost to 65–71 Gy8; and the Nordic trial used a sequential approach of chemotherapy followed by radiation therapy to 60 Gy once daily9. In the Intergroup trial, esophagitis was observed more often in the chemoradiation-only arm, which is not surprising given the increased radiation dose given in the nonsurgical patients10; and yet the rate of esophagitis was similar to that seen in the other trials reviewed here, as well as in other studies of concurrent chemoradiation in stage iii nonresectable nsclc. With the use of more modern radiation techniques and improvements in supportive care, those risks should be mitigated and not be a basis for dose de-escalation. Given the results from the Radiation Therapy Oncology Group 0617 study and proclaim, standard chemoradiation should likely follow guidelines similar to those for patients with nonresectable stage iii nsclc—that is, concurrent platinum-based chemoradiation to a dose of 60–66 Gy in once-daily fractions4.
The findings of the present systematic review are applicable only to patients with potentially resectable stage iii nsclc and cannot be generalized to unresectable nsclc. A recent trial in unresectable stage iii nsclc has demonstrated benefit from the addition of the immune checkpoint inhibitor durvalumab (a monoclonal antibody against PD-L1). The pacific trial randomized patients who had not progressed after chemoradiation for unresectable stage iii nsclc to 1 year of therapy with durvalumab or placebo14,15. A significant improvement in both pfs and os was observed for patients randomized to durvalumab. However, it is unclear whether the findings of pacific can be generalized to patients with potentially resectable nsclc.
The management of stage iii nsclc is diverse, with patient, tumour, and treatment factors all guiding appropriate decision-making. Given the heterogeneity of resectable stage iii nsclc and the difficulty of interpreting the results of randomized trials in this patient population, decisions about the appropriateness of single-modality, bimodality, and trimodality approaches are best made within the context of multidisciplinary thoracic oncology clinics and tumour boards. Patients included in the randomized trials would have been staged according to the 6th edition of the TNM staging system. Since that time, refinements have been made to the definition of stage iii disease (7th and 8th edition staging). However, the concept of resectable stage iii nsclc has not changed significantly. The same issues all require consideration in the context of multidisciplinary care.
CONCLUSIONS
There is uncertainty about the benefit of surgery in patients with stage iii nsclc (N2 or N3, excluding T4) in addition to standard chemoradiation. A consideration of surgery in select patients based on the extent of that surgery (lobectomy vs. pneumonectomy) might be a possibility; however, other factors could be important in guiding decision-making. Further research is needed to elucidate those factors, both prognostic and predictive, to optimize treatment for patients with resectable stage iii nsclc.
Supplemental Materials
ACKNOWLEDGMENTS
The authors thank the following individuals for their assistance in developing this report: Melissa Brouwers, Craig Earle, Sheila McNair, Hans Messersmith, Raymond Poon, Shailendra Verma, and Cindy Walker-Dilks for providing feedback on draft versions of the systematic review. Ananya Nair conducted a data audit.
The Program in Evidence-Based Care (pebc) is a provincial initiative of Cancer Care Ontario supported by the Ontario Ministry of Health and Long-Term Care (mohltc). All work produced by the pebc is editorially independent from the mohltc.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2018 Special Report on Cancer Incidence by Stage. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- 2.Canadian Cancer Society. Prognosis and survival for lung cancer [Web page] Toronto, ON: Canadian Cancer Society; 2019. [Avai lable at: http://www.cancer.ca/en/cancer-information/cancer-type/lung/prognosis-and-survival/; cited 20 April 2019] [Google Scholar]
- 3.Okawara G, Mackay JA, Evans WK, Ung YC on behalf of the Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Management of unresected stage iii non–small cell lung cancer: a systematic review. J Thorac Oncol. 2006;1:377–93. doi: 10.1097/01243894-200605000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Swaminath A, Vella ET, Ramchandar K, et al. on behalf of the Lung Cancer Disease Site Group. Treatment of Patients with Stage III (N2 or N3) Non–Small Cell Lung Cancer [in review at press time] Toronto, ON: Cancer Care Ontario; 2017. [Google Scholar]
- 5.Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage iii non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl):e314S–40S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyatt G, Oxman AD, Akl EA, et al. grade guidelines: 1. Introduction—grade evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Eberhardt WE, Pottgen C, Gauler TC, et al. Phase iii study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage iiia (N2) and selected iiib non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (espatue) J Clin Oncol. 2015;33:4194–201. doi: 10.1200/JCO.2015.62.6812. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen JB, Ravn J, Pilegaard HK, et al. Surgery for nsclc stages T1–3N2M0 having preoperative pathologically verified N2 involvement: a prospect ive randomized multinational phase iii trial by the Nordic Thoracic Oncology Group [abstract 7504] J Clin Oncol. 2013;31 doi: 10.1200/JCO.2012.43.9190. [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2013.31.15_suppl.7504; cited 29 April 2019] [DOI] [Google Scholar]
- 10.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage iii non-small-cell lung cancer: a phase iii randomised controlled trial. Lancet. 2009;374:379–86. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberhardt W, Wilke H, Stamatis G, et al. Preoperative chemotherapy followed by concurrent chemoradiation therapy based on hyperfractionated accelerated radiotherapy and definitive surgery in locally advanced non-small-cell lung cancer: mature results of a phase ii trial. J Clin Oncol. 1998;16:622–34. doi: 10.1200/JCO.1998.16.2.622. [DOI] [PubMed] [Google Scholar]
- 12.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage iiia or iiib non-small-cell lung cancer (rtog 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–99. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senan S, Brade A, Wang LH, et al. proclaim: randomized phase iii trial of pemetrexed–cisplatin or etoposide–cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non–small-cell lung cancer. J Clin Oncol. 2016;34:953–62. doi: 10.1200/JCO.2015.64.8824. [DOI] [PubMed] [Google Scholar]
- 14.Antonia SJ, Villegas A, Daniel D, et al. on behalf of the pacific investigators. Durvalumab after chemoradiotherapy in stage iii non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 15.Antonia SJ, Villegas A, Daniel D, et al. on behalf of the pacific investigators. Overall survival with durvalumab after chemoradiotherapy in stage iii nsclc. N Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.