Abstract
Background
Diagnosis and treatment of renal cell carcinoma (rcc) might be different in Indigenous Canadians than in non-Indigenous Canadians. In this cohort study, we compared rcc presentation and treatments in Indigenous and non-Indigenous Canadians.
Methods
Patients registered in the Canadian Kidney Cancer Information System treated at 16 institutions between 2011 and 2018 were included. Baseline patient, tumour, and treatment characteristics were compared between Indigenous and non-Indigenous Canadians. The primary objective was to determine if differences in rcc stage at diagnosis were evident between the groups. The secondary objective was to determine if treatments and outcomes were different between the groups.
Results
During the study period, 105 of the 4529 registered patients self-identified as Indigenous. Those patients were significantly younger at the time of clinical diagnosis (57.9 ± 11.3 years vs. 62.0 ± 12.1 years, p = 0.0006) and had a family history prevalence of rcc that was double the prevalence in the non-Indigenous patients (14% vs. 7%, p = 0.004). Clinical stage at diagnosis was similar in the two groups (p = 0.61). The disease was metastatic at presentation in 11 Indigenous Canadians (10%) and in 355 non-Indigenous Canadians (8%). Comorbid conditions that could affect the management of rcc—such as obesity, renal disease, diabetes mellitus, and smoking—were more common in Indigenous Canadians (p < 0.05). Indigenous Canadians experienced a lower rate of active surveillance (p = 0.01). Treatments and median time to treatments were similar in the two groups.
Conclusions
Compared with their non-Indigenous counterparts, Indigenous Canadian patients with rcc are diagnosed at an earlier age and at a similar clinical stage. Despite higher baseline comorbid conditions, clinical outcomes are not worse for Indigenous Canadians than for non-Indigenous Canadians.
Keywords: Renal cell carcinoma, Indigenous Canadians, diagnosis, treatment, outcomes
INTRODUCTION
In Canada, kidney cancer is the 10th most common cancer, resulting in an estimated 6600 new diagnoses and 1900 deaths in 20171. Most kidney cancers are classified as renal cell carcinoma (rcc), which accounts for more than 90% of all kidney cancer cases2. Established risk factors for rcc include male sex, older age, family history of rcc, smoking, hypertension, and obesity3. Differences in the rcc incidence by ethnic group have been reported in the literature4. The incidence of rcc is higher in Indigenous populations in Canada and internationally, including in Native Americans, Aboriginal peoples of Chukotka, and Aboriginal peoples of New South Wales5–8. The higher incidence of renal tumours might be the result of higher prevalences of risk factors and genetic differences in Indigenous groups6.
Indigenous Canadians are a heterogeneous group that includes the First Nations, Inuit, and Métis9. According to the 2016 Canadian census, 2.1 million people reported Indigenous ancestry, of whom 1.5 million were First Nations10. Indigenous Canadians comprise 6.2% of the total Canadian population and are the fastest growing ethnic group10. Because of a lack of centralized reporting methods, regional variation in rcc incidence rates have been cited historically in the literature9,11–14. Further, Indigenous Canadians could possibly experience barriers (for example, cultural and geographic) to health care and to extents that vary with regional factors on-reserve or in urban areas6.
Despite being the fastest growing ethnicity in Canada15, Indigenous Canadians remain largely underrepresented in medical research. Although disparities in the incidence of kidney cancer have previously been reported for the Indigenous Canadian population9, the reasons for those differences are unclear. Further, the available evidence is outdated, and details about rcc diagnosis, treatment, and survival in Indigenous Canadians are lacking.
METHODS
Study Design
Data from the Canadian Kidney Cancer Information System (ckcis) were extracted for 1 January 2011 to 30 June 2018. The ckcis is a prospective cohort database encompassing 16 academic centres across Canada, all of which have institutional research ethics board approval for the use of ckcis data. Self-identification as an Indigenous Canadian can occur through a ckcis-specific voluntary questionnaire about baseline demographic data that patients complete, or through demographic data already collected in the health record.
Patients who were diagnosed with rcc of any type after 1 January 2011 were included in the analysis. Some of the patients presented after 2011 with metastatic disease, and the medical record was reviewed to capture their information. Patients who self-identified as Indigenous were stratified in the Indigenous Canadian cohort; all other self-identified ethnicities, including Arab, East Asian, black, white, Hispanic, South Asian, Southeast Asian, Native Hawaiian and other Pacific Islanders, and racially mixed were stratified in the non-Indigenous Canadian cohort.
Outcomes and Statistical Analysis
The primary outcome of the study was rcc stage at diagnosis. The primary objective was to determine if Indigenous and non-Indigenous Canadian patients show differences in rcc stage at diagnosis. The secondary outcome was treatments received by patients for rcc management. The secondary objective was to determine if treatments and outcomes were different between Indigenous and non-Indigenous Canadian patients.
Baseline patient, tumour, and treatment characteristics were collected and compared between the Indigenous and non-Indigenous Canadian cohorts. Results are presented using descriptive statistics, the t-test for independent samples, and the chi-square test. Time to treatment was compared using the Wilcoxon rank sum test. Overall survival was modelled using the Kaplan–Meier method and compared using the log-rank test. A Cox proportional hazards model was used to adjust for potential confounders.
RESULTS
Patient and Tumour Characteristics
Of the 4529 patients identified, 105 self-identified as Indigenous. At the time of analysis, the Indigenous Canadian cohort had been followed for a median of 1.8 years [interquartile range (iqr): 0.78–3.23 years], and the non-Indigenous Canadian cohort, for 2.0 years (iqr: 0.86–3.47 years; p = 0.19). Compared with the non-Indigenous Canadian cohort, the Indigenous Canadian cohort was significantly younger at the time of diagnosis (mean age: 57.9 ± 11.3 years vs. 62.0 ± 12.1 years; p = 0.0006). Most patients (66%) were men.
The rate of comorbidities was higher in the Indigenous than in the non-Indigenous Canadian cohort, including hypertension (85% vs. 77%, p = 0.05), diabetes mellitus (81% vs. 65%, p = 0.0006), and renal disease (71% vs. 59%, p = 0.009). Additionally, lifestyle factors that are associated with rcc risk were elevated in the Indigenous Canadian cohort, including obesity (mean body mass index: 31.9 ± 7.2 vs. 29.3 ± 9.1 in the non-Indigenous Canadian cohort; p = 0.007) and smoking (80% vs. 60%, p < 0.0001). A family history of rcc was significantly more frequent in the Indigenous Canadian cohort than in the non-Indigenous Canadian cohort (14% vs. 7%, p = 0.004), but despite that finding, tumour bilaterality was infrequent in both groups.
At clinical diagnosis, disease stage was similar in the cohorts (p = 0.61). Most patients had cT1 stage disease (68%), with no evidence of nodal or distant metastases. Metastatic disease was diagnosed in 11 Indigenous Canadian patients (10%) and in 355 non-Indigenous Canadian patients (8%, p = 0.36). Table I presents complete baseline and tumour characteristics for the study patients.
TABLE I.
Baseline characteristics of the study patients
| Characteristic | Patient group | p Value | |
|---|---|---|---|
|
| |||
| Indigenous | Non-Indigenous | ||
| Patients (n) | 105 | 4424 | |
|
| |||
| Mean age (years) | 57.9±11.3 | 62.0±12.1 | 0.0006 |
|
| |||
| Male sex [n (%)] | 75 (71.4) | 2900 (65.6) | 0.21 |
|
| |||
| Mean BMI | 31.9±7.2 | 29.3±9.1 | 0.007 |
|
| |||
| Hypertension [n (%)] | 89 (84.8) | 3392 (76.7) | 0.052 |
|
| |||
| Renal disease [n (%)] | 75 (71.4) | 2598 (58.7) | 0.009 |
|
| |||
| Diabetes mellitus [n (%)] | 85 (81.0) | 2868 (64.8) | 0.0006 |
|
| |||
| Smoker [n (%)] | 79 (79.8) | 2389 (59.8) | <0.0001 |
|
| |||
| Bilateral tumours [n (%)] | 136 (3.1) | 5 (4.8) | 0.33 |
|
| |||
| Family history of RCC [n (%)] | 13 (14.1) | 221 (6.5) | 0.004 |
|
| |||
| Clinical T stage [n (%)] | |||
| 1 | 70 (66.7) | 2995 (67.7) | 0.61 |
| 2 | 15 (14.3) | 717 (16.2) | |
| 3 | 19 (18.1) | 629 (14.2) | |
| 4 | 1 (1.0) | 83 (1.9) | |
|
| |||
| Metastases at diagnosis [n (%)] | |||
| Nodal | 7 (6.7) | 328 (7.4) | 0.77 |
| Distant | 11 (10.5) | 355 (8.0) | 0.36 |
BMI = body mass index; RCC = renal cell carcinoma.
Treatment and Outcome Characteristics
The rate of renal mass biopsies was similar in the two groups (23% vs. 30%, p = 0.14). Starting at clinical diagnosis, the median time to biopsy was similar in the Indigenous and non-Indigenous Canadian cohorts [2.4 months (iqr: 1.1–11.5 months) vs. 2.8 months (iqr: 1.4–6.6 months), p = 0.99]. Renal surgery was performed in 81% of the Indigenous Canadian cohort and in 75% of the non-Indigenous Canadian cohort (p = 0.19), with 55% and 52% of those procedures being radical nephrectomy (p = 0.59). The median time to surgery from clinical diagnosis was similar in the Indigenous and non-Indigenous Canadian cohorts [3.9 months (iqr: 2.8–5.5 months) vs. 3.5 months (iqr: 1.8–6.2), p = 0.17]. Less-common therapies used for rcc management in the Indigenous and non-Indigenous Canadian cohorts included cryoablation (1% vs. 0.4%, p = 0.36), radiofrequency ablation (1% vs. 2%, p = 0.52), bone-targeted therapy (1% vs. 1%, p = 1.0), and postsurgical adjuvant therapy (1% vs. 1%, p = 1.0). The rate of active surveillance of small renal masses was significantly lower in the Indigenous Canadian cohort than in the non-Indigenous Canadian cohort (5% vs. 13%, p = 0.01).
All pathology findings were similar in the two groups. Most patients had pathologic T1 tumours and did not experience metastatic spread or receive systemic therapy after nephrectomy (Tables II and III). After nephrectomy, metastasis occurred in 18% of the Indigenous Canadian cohort and in 17% of the non-Indigenous Canadian cohort. At the most recent follow-up, almost all of the patients with metastases had received systemic treatment (18% vs. 16%, p = 0.63), and selected patients had received metastasectomy (2% vs. 5%, p = 0.33). The median time to start of systemic therapy was similar in the Indigenous and non-Indigenous Canadian cohorts [6.1 months (iqr: 2.3–21.4 months) vs. 5.6 months (iqr: 2.7–14.7 months), p = 0.96].
TABLE II.
Treatment and outcome characteristics for the study patients
| Characteristic | Patient group | p Value | |
|---|---|---|---|
|
| |||
| Indigenous (n=105) | Non-Indigenous (n=4424) | ||
| Alive at last follow-up [n (%)] | 97 (92.4) | 3856 (87.2) | 0.11 |
|
| |||
| Duration of follow up (years) | |||
| Median | 1.8 | 2.0 | 0.19 |
| IQR | 0.8–3.2 | 0.9–3.5 | |
|
| |||
| Renal mass biopsy [n (%)] | 24 (22.9) | 1308 (29.6) | 0.14 |
|
| |||
| Time to renal mass biopsy (months) | |||
| Median | 2.4 | 2.8 | 0.99 |
| IQR | 1.1–11.5 | 1.4–6.6 | |
|
| |||
| Surgery [n (%)] | 85 (81.0) | 3334 (75.4) | 0.19 |
|
| |||
| Nephrectomy [n (%)] | |||
| Partial | 38 (44.7) | 1588 (47.6) | 0.59 |
| Radical | 47 (55.3) | 1746 (52.4) | |
|
| |||
| Time to surgery (months) | |||
| Median | 3.9 | 3.5 | 0.17 |
| IQR | 2.8–5.5 | 1.8–6.2 | |
|
| |||
| Active surveillance [n (%)] | 5 (4.8) | 589 (13.3) | 0.01 |
|
| |||
| Cryoablation [n (%)] | 1 (1.0) | 18 (0.4) | 0.36 |
|
| |||
| Radiofrequency ablation [n (%)] | 1 (1.0) | 105 (2.4) | 0.52 |
|
| |||
| Adjuvant therapy [n (%)] | 1 (1.0) | 48 (1.1) | 1.0 |
|
| |||
| Palliative care referral [n (%)] | 4 (14.3) | 205 (19.4) | 0.50 |
|
| |||
| Systemic therapy [n (%)] | 19 (18.1) | 722 (16.3) | 0.63 |
|
| |||
| Time to systemic therapy (months) | |||
| Median | 6.1 | 5.6 | 0.96 |
| IQR | 2.3–21.4 | 2.7–14.7 | |
|
| |||
| Metastasectomy [n (%)] | 2 (1.9) | 201 (4.5) | 0.33 |
|
| |||
| Bone-targeted therapy [n (%)] | 1 (1.0) | 58 (1.3) | 1.0 |
|
| |||
| Clinical trial [n (%)] | 3 (2.9) | 231 (5.2) | 0.28 |
IQR = interquartile range.
TABLE III.
Pathology characteristics
| Characteristic | Patient group | p Value | |
|---|---|---|---|
|
| |||
| Indigenous (n=105) | Non-Indigenous (n=4424) | ||
| Histology [n (%)] | 0.20 | ||
| Clear cell | 65/84 (77.4) | 2354/3316 (71.0) | |
| Non-clear cell | 19/84 (22.6) | 962/3316 (29.0) | |
|
| |||
| Pathologic T stage [n (%)] | 1.0 | ||
| 1 | 49/84 (58.3) | 1876/3274 (57.3) | |
| 2 | 4/84 (4.8) | 264/3274 (8.0) | |
| 3 | 30/84 (35.7) | 1063/3274 (32.5) | |
| 4 | 1/84 (1.2) | 71/3274 (2.2) | |
|
| |||
| Pathologic N1 [n (%)] | 3 (3.6) | 147 (4.5) | 1.0 |
|
| |||
| Pathologic M1 [n (%)] | 7 (8.6) | 218 (7.0) | 0.56 |
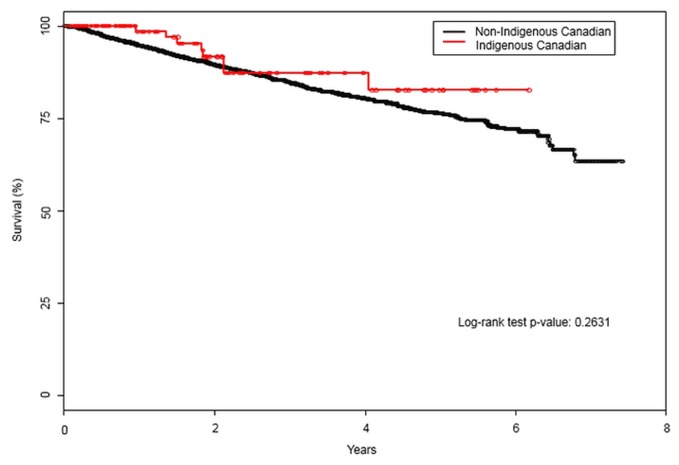
Participation in clinical trials was similar in the two groups (3% vs. 5%, p = 0.28). The rate of palliative care referral for patients with metastatic disease was similar in the two groups (14% vs. 19%, p = 0.50). The 5-year overall survival rate was similar for the Indigenous Canadian cohort [83%; 95% confidence interval (ci): 70.4% to 94.8%] and the non-Indigenous Canadian cohort (76%; 95% ci: 74.2% to 78.4%; p = 0.26; Figure 1). Compared with the Indigenous Canadian group, the non-Indigenous Canadian group had a hazard ratio for death of 1.36 (95% ci: 0.68 to 2.74; p = 0.39). Adjusted for age, the hazard ratio for death in the non-Indigenous Canadian group was 1.02 (95% ci: 1.02 to 1.03; p < 0.0001).
FIGURE 1.
Five-year overall survival for Indigenous compared with non-Indigenous Canadians with renal cell carcinoma.
DISCUSSION
The Indigenous population of Canada is one of the youngest and fastest-growing ethnic groups in Canada10. To date, few studies have examined the presentation and treatment of rcc in the Indigenous population of Canada9. Analysis of historical data relating to the Indigenous Canadian population raised speculation that differences in rcc stage and treatment might exist. The goal of our study was therefore to investigate whether such differences exist in a contemporary cohort.
Several observational studies have attempted to shed light on the kidney cancer incidence in Indigenous peoples in Canada, reporting age-standardized incidence rates double those in non-Indigenous Canadians; however, those studies are exclusive to provinces in Canada and to certain Indigenous groups9,11–14. Furthermore, those studies often relied on cancer codes from the International Classification of Diseases (9th revision), which includes benign renal masses and non-rcc masses such as urothelial carcinoma of the kidney. Those studies might therefore overestimate the incidence of rcc.
Our study is one of the first to investigate rcc, the most common type of kidney cancer, in the Indigenous Canadian population. We found that the Indigenous Canadian cohort was, on average, 4 years younger at the time of diagnosis and more frequently had a family history of rcc, which raised concerns about a possible association with hereditary renal cancer. Although genetic testing is recorded in the ckcis database, further analysis was not possible because of missing values. However, we found that tumour bilaterality was rare in both the Indigenous and non-Indigenous Canadian cohorts. Future studies should address genetic testing for Indigenous Canadians diagnosed with rcc.
We also found that, at presentation, the Indigenous Canadian cohort had a significantly higher prevalence of comorbid diseases, including obesity, renal disease, diabetes mellitus, and smoking. Those findings are well established in the literature6 and could affect the management of rcc in the Indigenous Canadian population. However, we found that the rates of and median time to biopsy, surgery, and systemic therapy were similar in the Indigenous and non-Indigenous Canadian groups. Despite a greater burden of comorbidities, the rate of active surveillance was lower in the Indigenous Canadian cohort. That finding is somewhat contradictory to the notion that patients with a high burden of comorbid conditions typically opt for active surveillance in the context of localized disease. Further follow-up is required to better understand that association.
Surveillance for rcc in the Indigenous population of Canada is a pertinent issue. We found that the 5-year overall survival rate and clinical outcomes were similar in the Indigenous and non-Indigenous Canadian cohorts. Those findings contrast with the results of recent studies similarly investigating rcc in the Native Alaskan population, which suggested a mortality rate nearly twice that for Americans with European ancestry16. However, it is important to note that regional differences in reporting make it difficult to make valid comparisons. We therefore suggest that, based on our findings, ongoing follow-up and study of rcc in the Indigenous Canadian population is warranted; however, we found that Indigenous and non-Indigenous Canadians received similar care for their rcc.
In the absence of a centralized reporting system for ethnicity and rcc, our study leveraged the strengths of the ckcis database to compare the diagnosis and treatment of rcc in Indigenous and non-Indigenous Canadian cohorts. However, that method has limitations. All patients included in our study received care at a participating academic centre, which is a potential source of selection bias. Thus, because we did not capture patients who received care in a community or rural setting, our findings might not reflect all Canadians diagnosed with rcc. Furthermore, population-based databases have inherent limitations and biases. Most of our findings are also limited to descriptive analyses. A multivariable analysis would have helped in understanding the disparities we identified, but because of the small sample size in the Indigenous Canadian cohort, such an analysis was not feasible. The survival analysis should also be interpreted with caution because of the high proportion of patients who were alive at the time of analysis.
Further, outcomes in the present study relied on self-identification of ethnicity by the patients, based on a standardized ckcis questionnaire or through review of the health record. Inaccuracies with those methods are possible, including misinterpretation of ethnicity by the patient, patient choice not to self-identify as Indigenous, classification as mixed race, or ethnicity not being recorded in the health record. Because ckcis does not capture details about mixed-race ethnicity, patients who were of mixed racial background were included in the non-Indigenous Canadian cohort. However, given that few patients (n = 36) self-identified as mixed-race, exclusion of those patients from the appropriate cohort is unlikely to be a significant source of misclassification bias. Overall, our findings serve as a foundation for continued follow-up and suggest the need for further studies of Indigenous Canadians with rcc in both the community and academic settings.
CONCLUSIONS
In the present study, Indigenous Canadians with rcc were found, compared with their non-Indigenous counterparts, to be diagnosed at an earlier age but at a similar clinical stage. Although Indigenous Canadian patients had more comorbid diseases at baseline, they experienced clinical outcomes comparable to those in non-Indigenous Canadian patients. We advocate for ongoing rcc surveillance in the Indigenous Canadian population so as to monitor and optimize care.
ACKNOWLEDGMENTS
We thank the Kidney Cancer Research Network of Canada.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society, Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- 2.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi SH, Klatte T, Usher-Smith J, Stewart GD. Epidemiology and screening for renal cancer. World J Urol. 2018;36:1341–53. doi: 10.1007/s00345-018-2286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stafford HS, Saltzstein SL, Shimasaki S, Sanders C, Downs TM, Sadler GR. Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. J Urol. 2008;179:1704–8. doi: 10.1016/j.juro.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahoney MC, Michalek AM. A meta-analysis of cancer incidence in United States and Canadian native populations. Int J Epidemiol. 1991;20:323–7. doi: 10.1093/ije/20.2.323. [DOI] [PubMed] [Google Scholar]
- 6.Batai K, Bergersen A, Price E, Hynes K, Ellis NA, Lee BR. Clinical and molecular characteristics and burden of kidney cancer among Hispanics and Native Americans: steps toward precision medicine. Clin Genitourin Cancer. 2018;16:e535–41. doi: 10.1016/j.clgc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Dudarev AA, Chupakhin VS, Odland JØ. Cancer incidence and mortality in Chukotka, 1997–2010. Int J Circumpolar Health. 2013;72:20470. doi: 10.3402/ijch.v72i0.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell S, You H, Baker D. Estimates of cancer incidence, mortality and survival in Aboriginal people from NSW, Australia. BMC Cancer. 2012;12:168. doi: 10.1186/1471-2407-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong ECL, Kapoor A. Epidemiology of prostate and kidney cancer in the Aboriginal population of Canada: a systematic review. Can Urol Assoc J. 2017;11:E222–32. doi: 10.5489/cuaj.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics Canada. Census in Brief. Ethnic and Cultural Origins of Canadians: Portrait of a Rich Heritage. Ottawa, ON: Minister of Industry; 2017. [Available online at: https://www12.statcan.gc.ca/census-recensement/2016/as-sa/98-200-x/2016016/98-200-x2016016-eng.pdf; cited 9 May 2019] [Google Scholar]
- 11.Louchini R, Beaupré M. Cancer incidence and mortality among Aboriginal people living on reserves and northern villages in Quebec, 1988–2004. Int J Circumpolar Health. 2008;67:445–51. doi: 10.3402/ijch.v67i5.18355. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Ramirez DC, Colquhoun A, Parker S, Randall J, Svenson LW, Voaklander D. Cancer incidence and mortality among the Métis population of Alberta, Canada. Int J Circumpolar Health. 2016;75:30059. doi: 10.3402/ijch.v75.30059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrett LD, Chaudhry M. Cancer incidence and mortality in Ontario First Nations, 1968–1991 (Canada) Cancer Causes Control. 2003;14:259–68. doi: 10.1023/A:1023632518568. [DOI] [PubMed] [Google Scholar]
- 14.Young TK, Frank JW. Cancer surveillance in a remote Indian population in northwestern Ontario. Am J Public Health. 1983;73:515–20. doi: 10.2105/AJPH.73.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Canada. Aboriginal Peoples Highlight Tables, 2016 Census [Web page] Ottawa, ON: Statistics Canada; 2016. [Available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/abo-aut/Table.cfm?Lang=Eng&T=101&S=99&O=A; cited 12 May 2019] [Google Scholar]
- 16.Li J, Weir HK, Jim MA, King SM, Wilson R, Master VA. Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990–2009. Am J Public Health. 2014;104(suppl 3):S396–403. doi: 10.2105/AJPH.2013.301616. [DOI] [PMC free article] [PubMed] [Google Scholar]