Abstract
Background
Patients with limited-stage (ls) or extensive-stage (es) small-cell lung cancer (sclc) are commonly given platinum-based chemotherapy as first-line treatment. Standard chemotherapy for patients with ls sclc includes a platinum agent such as cisplatin combined with the non-platinum agent etoposide. The objective of the present systematic review was to investigate the efficacy of adding radiotherapy to chemotherapy in patients with es sclc and to determine the appropriate timing, dose, and schedule of chemotherapy or radiation for patients with sclc.
Methods
The medline and embase databases were searched for randomized controlled trials (rcts) comparing treatment with radiotherapy plus chemotherapy against treatment with chemotherapy alone in patients with es sclc. Identified rcts were also included if they compared various timings, doses, and schedules of treatment for patients with es sclc or ls sclc.
Results
Sixty-four rcts were included. In patients with ls sclc, overall survival was greatest with platinum–etoposide compared with other chemotherapy regimens. In patients with es sclc, overall survival was greatest with chemotherapy containing platinum–irinotecan than with chemotherapy containing platinum–etoposide (hazard ratio: 0.84; 95% confidence interval: 0.74 to 0.95; p = 0.006). The addition of radiation to chemotherapy for patients with es sclc showed mixed results. There was no conclusive evidence that the timing, dose, or schedule of thoracic radiation affected treatment outcomes in sclc.
Conclusions
In patients with ls sclc, cisplatin–etoposide plus radiotherapy should remain the standard therapy. In patients with es sclc, the evidence is insufficient to recommend the addition of radiotherapy to chemotherapy as standard practice to improve overall survival. However, on a case-by-case basis, radiotherapy might be added to reduce local recurrence. The most commonly used chemotherapy is platinum–etoposide; however, platinum–irinotecan can be considered.
Keywords: Cisplatin, etoposide, irinotecan, systemic therapy, platinum agents, radiation
INTRODUCTION
Because of the aggressive nature and early metastatic spread of small-cell lung cancer (sclc), chemotherapy is the most common treatment for affected patients. Platinum-based chemotherapy is the standard of care for first-line therapy in limited-stage (ls) and extensive-stage (es) sclc. The platinum agents most commonly used are cisplatin and carboplatin, which are often combined with the non-platinum agent etoposide. For patients with ls sclc, the addition of thoracic radiation therapy to standard combination chemotherapy improves both local control and overall survival (os) and reflects the current standard of care1,2.
To be able to make recommendations as part of a clinical practice guideline about the initial management of sclc, Cancer Care Ontario’s Program in Evidence-Based Care, together with the Lung Cancer Disease Site Group, developed the evidentiary base presented here. The objective of the review was to investigate radiotherapy and first-line chemotherapy in the treatment of patients with non-resected sclc. Given that objective, the authors derived these research questions:
-
■ Thoracic radiation
For patients with non-resected es sclc only, what are the benefits and harms in terms of os, quality of life (qol), and toxicity for chemotherapy and radiotherapy compared with chemotherapy alone?
For patients with non-resected ls sclc or es sclc undergoing chemotherapy, what are the benefits and harms in terms of os, qol and toxicity for
▪ early compared with late radiotherapy, or
▪ sequential compared with concurrent radiotherapy, or
▪ various doses and schedules of radiotherapy?
-
■ Chemotherapy
For patients with non-resected ls sclc or es sclc undergoing chemotherapy, what are the benefits and harms in terms of os, qol, and toxicity for
▪ various chemotherapy combinations, or
▪ various doses and schedules of chemotherapy?
METHODS
The Program in Evidence-Based Care produces evidence-based and evidence-informed guidance documents using the methods of the practice guidelines development cycle3,4. The evidentiary base presented here replaces two older practice guidelines that targeted ls sclc and extends its scope to include es sclc. The literature searches for the two original practice guidelines for ls sclc were conducted for 1996–2002 and are described elsewhere1,5. For the present work, the evidence in ls sclc was updated for 2002 to June 2016, and the search was extended to include evidence in es sclc for 1996 to June 2016.
Literature Search Strategy
A search of the Cochrane Library and the medline and embase databases for systematic reviews and randomized controlled trials (rcts) was conducted for the period 1996 to June 2016 (Table I). Before the primary studies were screened, the systematic reviews were evaluated based on their clinical content and relevance. Abstracts from conferences of the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the World Lung Cancer Conference were searched for the years 1996 through June 2016.
TABLE I.
Literature search strategy
| Step | Query |
|---|---|
| 1 | Carcinoma, Non-Small-Cell Lung/ or NSCLC.ti. or (non adj small).ti. or nonsmall.ti. or non small cell lung cancer/ |
| 2 | ((small adj cell adj lung adj2 (tumo?r$ or adenocarcinoma$ or cancer$ or carcinoma$ or neoplasm$)) or SCLC or (oatcell or oat-cell or oat cell)).tw. |
| 3 | 2 not 1 |
| 4 | small cell lung carcinoma/ or small cell lung cancer/ |
| 5 | 3 or 4 |
| 6 | exp Randomized Controlled Trial/ or Clinical Trial, Phase III/ or Clinical Trial, Phase IV/ or Phase 3 Clinical Trial/ or Phase 4 Clinical Trial/ or ((exp Clinical Trial/ or Prospective Study/ or Prospective Studies/) and Random$.tw.) or exp Randomized Controlled Trials as topic/ or Clinical Trials, Phase III as Topic/ or Clinical Trials, Phase IV as Topic/ or exp “Randomized Controlled Trial (Topic)”/ or “Phase 3 Clinical Trial (Topic)”/ or “Phase 4 Clinical Trial (Topic)”/ or ((exp Clinical Trials as Topic/ or exp “Clinical Trial (Topic)”/) and random$.tw.) or Random Allocation/ or Randomization/ or Single-Blind Method/ or Double-Blind Method/ or Single Blind Procedure/ or Double Blind Procedure/ or Triple Blind Procedure/ or Placebos/ or Placebo/ or ((singl$ or doubl$ or tripl$) adj3 (blind$3 or mask$3 or dummy)).tw. or (random$ control$ trial? or rct or phase III or phase IV or phase 3 or phase 4).tw. or (((phase II or phase 2 or clinic$) adj3 trial$) and random$).tw. or (placebo? or (allocat$ adj2 random$)).tw. or (random$ adj3 trial$).mp. or “clinicaltrials.gov”.mp. |
| 7 | (exp evidence based practice/ or exp practice guideline/ or exp consensus development conference/ or guideline.pt. or practice parameter$. tw. or practice guideline$.mp. or (guideline: or recommend: or consensus or standards).ti. or (guideline: or recommend: or consensus or standards).kw.) not 6 |
| 8 | (exp meta analysis/ or exp “meta analysis (topic)”/ or exp meta-analysis as topic/ or exp “systematic review”/ or exp “systematic review (topic)”/ or ((exp “review”/ or exp “review literature as topic”/ or review.pt.) and ((systematic or selection criteria or data extraction or quality assessment or jaded scale or methodologic$ quality or study) adj selection).tw.) or meta-analysis.mp. or (meta-analy: or metaanaly: or meta analy:).tw. or (systematic review or systematic overview).mp. or ((cochrane or medline or embase or cancerlit or hand search$ or hand-search$ or manual search$ or reference list$ or bibliograph$ or relevant journal$ or pooled analys$ or statistical pooling or mathematical pooling or statistical summar$ or mathematical summar$ or quantitative synthes?s or quantitative overview$ or systematic) adj2 (review$ or overview$)).tw. or (medline or med-line or pubmed or pub-med or embase or cochrane or cancerlit).ab.) not (6 or 7) |
| 9 | 5 and 6 |
| 10 | 5 and 7 |
| 11 | 5 and 8 |
| 12 | remove duplicates from 9 |
| 13 | remove duplicates from 10 |
| 14 | remove duplicates from 11 |
| 15 | 12 or 13 or 14 |
Study Selection Criteria and Process
Studies were included if they were full reports or abstracts of meta-analyses or rcts with more than 30 participants and if they addressed at least one of the research questions. Studies were excluded if the data were not reported separately for patients with ls sclc or es sclc; if they used chemotherapy regimens containing procarbazine or lomustine (or both), or another nitrosourea; if they involved palliative treatment; if they were studying granulocyte colony–stimulating factor, and the dose or administration schedule of the chemotherapy was the same in both arms; and if they did not use an appropriate contemporary standard of care as the control arm. Papers not written in the English language were excluded.
Data Extraction and Synthesis and Assessment of Study Quality
All eligible studies underwent data extraction independently by a research methodologist (LDDA), and the data were independently audited. Hazard ratios (hrs) are expressed as a ratio less than 1.0 when they indicate benefit for the investigational treatment compared with the control. The quality of the primary studies was assessed using the Cochrane Risk of Bias tool6. The grade (Grading of Recommendations, Assessment, Development and Evaluation) method for assessing the quality of aggregate evidence was used for each comparison7. The Kaplan–Meier curve from each study was visually inspected for os at 12 months, and the median was calculated8.
If appropriate, a meta-analysis was conducted using the Review Manager software application (RevMan 5.3: The Cochrane Collaboration, Copenhagen, Denmark). For time-to-event outcomes, if the hr or its standard error was not reported, the missing value was derived from other information reported in the study, using the methods described by Parmar et al.9. The generic inverse variance model with random effects was used. A probability level for the chi-square statistic less than or equal to 10% (p ≤ 0.10) or an I2 greater than 50% (or both) was considered indicative of statistical heterogeneity.
RESULTS
Search for Existing Systematic Reviews
Of the 563 systematic reviews or meta-analyses found, none addressed the pre-defined research questions and adhered to the study eligibility criteria. They were therefore used only as a source of references.
Search for Primary Literature
Literature Search Results
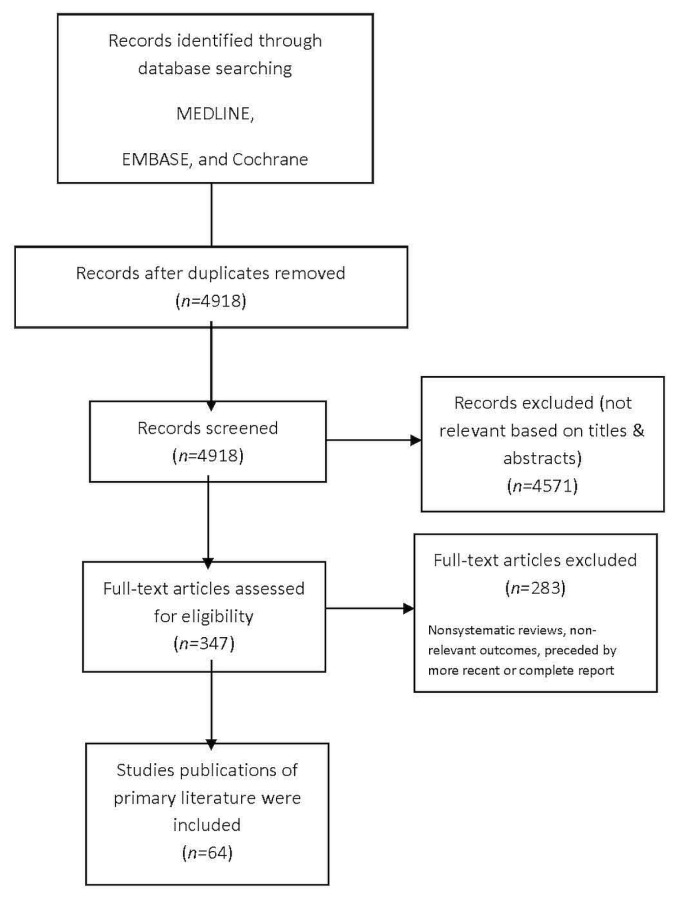
Of 3626 English and foreign-language studies identified, 296 were selected for full-text review, with 64 being found to meet the pre-defined eligibility criteria for this systematic review10–73 (Figure 1).
FIGURE 1.
PRISMA flow diagram.
Study Design and Quality
Approximately one third of the fully published papers gave details of the randomization process, suggesting allocation concealment. There was no indication that allocation was not concealed or that researchers influenced the treatment received. In most trials, the baseline patient and disease characteristics were well balanced, with some exceptions: more than 5% weight loss10, slightly older patients25, difference in median body mass index45, and more brain and lung metastases26,68 in one group. Although not routinely reported, most trials appeared to use an open design without blinding of investigators or participants. The power and required sample size were calculated and reported in most studies, but were not calculated in four trials68,70–72. Fifteen trials were partly terminated early (that is, one arm in the study) or fully terminated early because of slow accrual13,24,34,38,52,56, unacceptable toxicity17,36,39,60, an interim analysis that showed benefit for one group over another or no meaningful difference between groups43,70, negative effects observed in another trial27, or futility after a planned interim analysis19,63.
OS, QOL, and Toxicity Outcomes (Benefits and Harms)
Patients with Non-resected ES SCLC Only
Chemotherapy and Radiotherapy Compared with Chemotherapy Alone
Four moderate aggregate quality rcts reported on os. One study28 showed an improved 1-year os with the addition of hyperfractionated radiat ion to chemotherapy in pat ients with es sclc (p = 0.041), and three studies did not19,41,62. Slotman et al.62 reported that, for the primary endpoint of 1-year os, the addition of thoracic radiotherapy to standard chemotherapy did not improve os; however, a secondary analysis did find significant improvements in 18-month (p = 0.03) and 2-year os (p = 0.004). Similarly, Narayan et al.41 reported a significant improvement for 3-year os [hr: 0.83; 95% confidence interval (ci): 0.72 to 1.08; p = 0.047], but the difference in 5-year os was nonsignificant.
Three low aggregate quality rcts reported on adverse effects. One study showed significantly more grade 4 nausea or vomiting (p = 0.0038) and alopecia (p < 0.001) for patients undergoing chemotherapy alone compared with chemotherapy and thoracic radiotherapy28. Although the differences were nonsignificant, patients also showed more leucopenia, thrombocytopenia, and anemia. Slotman et al.62 reported slightly higher, but nonsignificantly different, rates of fatigue, insomnia, and headache in the chemotherapy plus radiation group. Gore et al.19 reported similar rates of grade 4 toxicity in both groups.
No trial reported on qol.
Patients with Non-resected LS SCLC and ES SCLC Undergoing Chemotherapy
Early Compared with Late Radiotherapy
LS SCLC: In terms of os, the aggregate quality of the trials was moderate. Overall survival was comparable in both the early and the late thoracic radiotherapy arms65,66.
The aggregate quality of the rcts reporting on toxicity was moderate. Sun et al.66 found that patients undergoing early thoracic radiotherapy experienced greater hematologic toxicities such as febrile neutropenia, neutropenia, and anemia (p values not reported). Similarly, Spiro et al.65 found that nonhematologic toxicities were significantly greater in those undergoing early thoracic radiotherapy (p = 0.001) and that hematologic toxicities were similar.
No trial reported qol outcomes.
ES SCLC: No evidence was found for patients with es sclc.
Sequential Compared with Concurrent Radiotherapy
No trials comparing sequential with concurrent radiotherapy for patients with non-resected ls sclc and es sclc undergoing chemotherapy met the inclusion criteria.
Various Doses and Schedules of Radiotherapy
LS SCLC: Five trials reported data for os13,15,16,20,53 and ranged from low to medium in quality. No trial showed a significant survival advantage for one dose or schedule over another. Most trials were small and not powered to answer questions about os.
ES SCLC: No evidence was found for patients with es sclc.
Various Chemotherapy Combinations
Platinum Plus Another Agent Compared with Platinum–Etoposide
LS SCLC: Two moderate-quality trials reported os and toxicity10,30. In Artal-Cortes et al.10, patients received either cisplatin–epirubicin or cisplatin–etoposide, and median os in the two groups was comparable. However, a significantly elevated rate of neutropenia was seen in the cisplatin–etoposide group (p = 0.005). Kubota et al.30 compared cisplatin–irinotecan with cisplatin–etoposide and found that patients in the cisplatin–etoposide group had slightly but nonsignificantly higher median 3-year os and 5-year os. Patients receiving cisplatin–etoposide had higher rates of leucopenia and neutropenia (p value not reported).
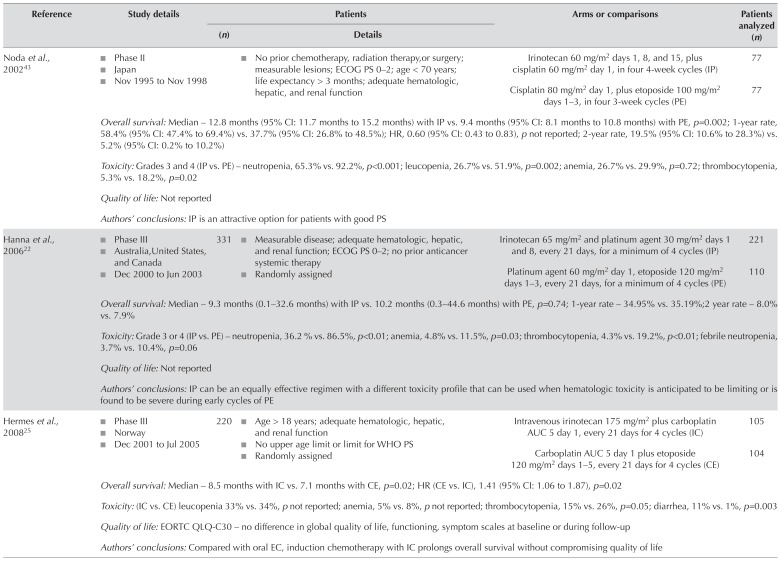
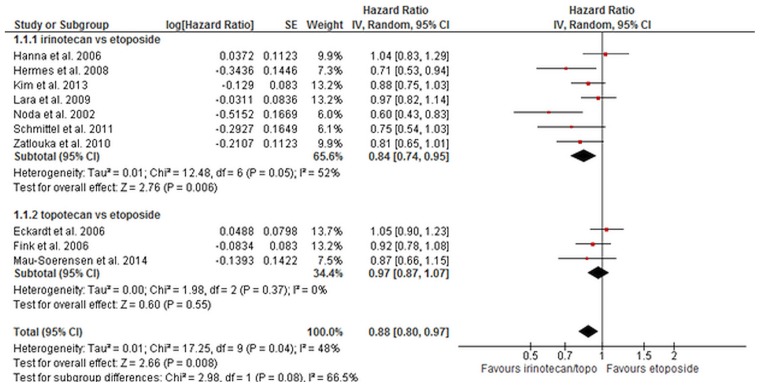
ES SCLC: Eight trials compared platinum–irinotecan with plat inum–etoposide for os in pat ients with es sclc22,25,29,32,43,55,61,73. Data for os from seven trials of moderate aggregate quality were included in a meta-analyses (Table II). The os duration was longer in patients who received irinotecan than in those who received etoposide (hr: 0.84; 95% ci: 0.74 to 0.95; p = 0.006; Figure 2). There was, however, evidence of statistical heterogeneity (I2 = 52%, chi-square = 12.48, p = 0.05). A sensitivity analysis that omitted the Noda et al. trial43 (because of an a priori suspicion that pharmacogenomics differences in the Japanese population might result in different outcomes with irinotecan) still demonstrated a significant benefit for irinotecan and eliminated the statistical heterogeneity (hr: 0.88; 95% ci: 0.79 to 0.98; p = 0.02; I2 = 31%, chi-square[5] = 7.24, p = 0.20). In an exploratory analysis excluding Asian trials29,43, the hr was 0.87 (95% ci: 0.76 to 1.00; p = 0.05; I2 = 45%, chi-square[4] = 7.23, p = 0.12).
TABLE II.
Included studies comparing platinum–irinotecan with platinum–etoposide in extensive-stage small-cell lung cancer
| Reference | Study details | Patients | Arms or comparisons | Patients analyzed (n) | |
|---|---|---|---|---|---|
|
| |||||
| (n) | Details | ||||
| Noda et al., 200243 |
|
|
Irinotecan 60 mg/m2 days 1, 8, and 15, plus cisplatin 60 mg/m2 day 1, in four 4-week cycles (IP) | 77 | |
| Cisplatin 80 mg/m2 day 1, plus etoposide 100 mg/m2 days 1–3, in four 3-week cycles (PE) | 77 | ||||
| Overall survival: Median – 12.8 months (95% CI: 11.7 months to 15.2 months) with IP vs. 9.4 months (95% CI: 8.1 months to 10.8 months) with PE, p=0.002; 1-year rate, 58.4% (95% CI: 47.4% to 69.4%) vs. 37.7% (95% CI: 26.8% to 48.5%); HR, 0.60 (95% CI: 0.43 to 0.83), p not reported; 2-year rate, 19.5% (95% CI: 10.6% to 28.3%) vs. 5.2% (95% CI: 0.2% to 10.2%) | |||||
| Toxicity: Grades 3 and 4 (IP vs. PE) – neutropenia, 65.3% vs. 92.2%, p<0.001; leucopenia, 26.7% vs. 51.9%, p=0.002; anemia, 26.7% vs. 29.9%, p=0.72; thrombocytopenia, 5.3% vs. 18.2%, p=0.02 | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: IP is an attractive option for patients with good PS | |||||
|
| |||||
| Hanna et al., 200622 |
|
331 |
|
Irinotecan 65 mg/m2 and platinum agent 30 mg/m2 days 1 and 8, every 21 days, for a minimum of 4 cycles (IP) | 221 |
| Platinum agent 60 mg/m2 day 1, etoposide 120 mg/m2 days 1–3, every 21 days, for a minimum of 4 cycles (PE) | 110 | ||||
| Overall survival: Median – 9.3 months (0.1–32.6 months) with IP vs. 10.2 months (0.3–44.6 months) with PE, p=0.74; 1-year rate – 34.95% vs. 35.19%;2 year rate – 8.0% vs. 7.9% | |||||
| Toxicity: Grade 3 or 4 (IP vs. PE) – neutropenia, 36.2 % vs. 86.5%, p<0.01; anemia, 4.8% vs. 11.5%, p=0.03; thrombocytopenia, 4.3% vs. 19.2%, p<0.01; febrile neutropenia, 3.7% vs. 10.4%, p=0.06 | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: IP can be an equally effective regimen with a different toxicity profile that can be used when hematologic toxicity is anticipated to be limiting or is found to be severe during early cycles of PE | |||||
|
| |||||
| Hermes et al., 200825 |
|
220 |
|
Intravenous irinotecan 175 mg/m2 plus carboplatin AUC 5 day 1, every 21 days for 4 cycles (IC) | 105 |
| Carboplatin AUC 5 day 1 plus etoposide 120 mg/m2 days 1–5, every 21 days for 4 cycles (CE) | 104 | ||||
| Overall survival: Median – 8.5 months with IC vs. 7.1 months with CE, p=0.02; HR (CE vs. IC), 1.41 (95% CI: 1.06 to 1.87), p=0.02 | |||||
| Toxicity: (IC vs. CE) leucopenia 33% vs. 34%, p not reported; anemia, 5% vs. 8%, p not reported; thrombocytopenia, 15% vs. 26%, p=0.05; diarrhea, 11% vs. 1%, p=0.003 | |||||
| Quality of life: EORTC QLQ-C30 – no difference in global quality of life, functioning, symptom scales at baseline or during follow-up | |||||
| Authors’ conclusions: Compared with oral EC, induction chemotherapy with IC prolongs overall survival without compromising quality of life | |||||
|
| |||||
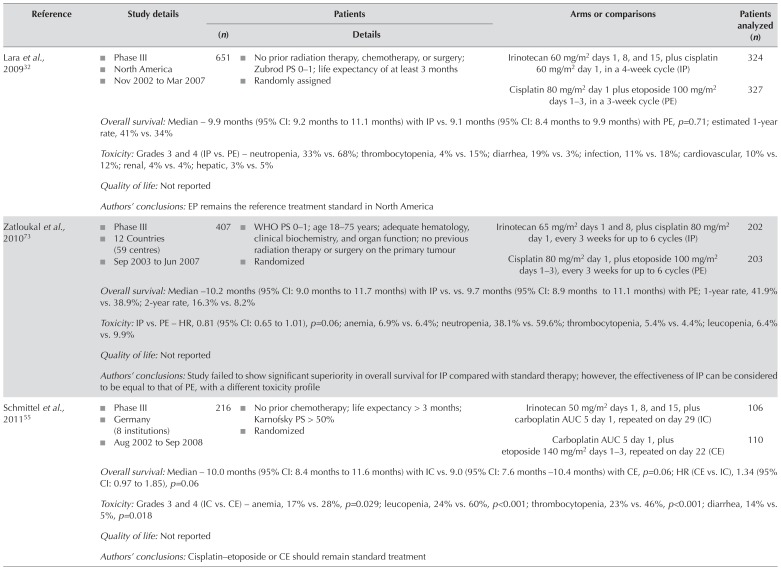
| Lara et al., 200932 |
|
651 |
|
Irinotecan 60 mg/m2 days 1, 8, and 15, plus cisplatin 60 mg/m2 day 1, in a 4-week cycle (IP) | 324 |
| Cisplatin 80 mg/m2 day 1 plus etoposide 100 mg/m2days 1–3, in a 3-week cycle (PE) | 327 | ||||
| Overall survival: Median – 9.9 months (95% CI: 9.2 months to 11.1 months) with IP vs. 9.1 months (95% CI: 8.4 months to 9.9 months) with PE, p=0.71; estimated 1-year rate, 41% vs. 34% | |||||
| Toxicity: Grades 3 and 4 (IP vs. PE) – neutropenia, 33% vs. 68%; thrombocytopenia, 4% vs. 15%; diarrhea, 19% vs. 3%; infection, 11% vs. 18%; cardiovascular, 10% vs. 12%; renal, 4% vs. 4%; hepatic, 3% vs. 5% | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: EP remains the reference treatment standard in North America | |||||
|
| |||||
| Zatloukal et al., 201073 |
|
407 |
|
Irinotecan 65 mg/m2 days 1 and 8, plus cisplatin 80 mg/m2 day 1, every 3 weeks for up to 6 cycles (IP) | 202 |
| Cisplatin 80 mg/m2 day 1, plus etoposide 100 mg/m2 days 1–3), every 3 weeks for up to 6 cycles (PE) | 203 | ||||
| Overall survival: Median – 10.2 months (95% CI: 9.0 months to 11.7 months) with IP vs. vs. 9.7 months (95% CI: 8.9 months to 11.1 months) with PE; 1-year rate, 41.9% vs. 38.9%; 2-year rate, 16.3% vs. 8.2% | |||||
| Toxicity: IP vs. PE – HR, 0.81 (95% CI: 0.65 to 1.01), p=0.06; anemia, 6.9% vs. 6.4%; neutropenia, 38.1% vs. 59.6%; thrombocytopenia, 5.4% vs. 4.4%; leucopenia, 6.4% vs. 9.9% | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: Study failed to show significant superiority in overall survival for IP compared with standard therapy; however, the effectiveness of IP can be considered to be equal to that of PE, with a different toxicity profile | |||||
|
| |||||
| Schmittel et al., 201155 |
|
216 |
|
Irinotecan 50 mg/m2 days 1, 8, and 15, plus carboplatin AUC 5 day 1, repeated on day 29 (IC) | 106 |
| Carboplatin AUC 5 day 1, plus etoposide 140 mg/m2 days 1–3, repeated on day 22 (CE) | 110 | ||||
| Overall survival: Median – 10.0 months (95% CI: 8.4 months to 11.6 months) with IC vs. 9.0 (95% CI: 7.6 months –10.4 months) with CE, p=0.06; HR (CE vs. IC), 1.34 (95% CI: 0.97 to 1.85), p=0.06 | |||||
| Toxicity: Grades 3 and 4 (IC vs. CE) – anemia, 17% vs. 28%, p=0.029; leucopenia, 24% vs. 60%, p<0.001; thrombocytopenia, 23% vs. 46%, p<0.001; diarrhea, 14% vs. 5%, p=0.018 | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: Cisplatin–etoposide or CE should remain standard treatment | |||||
|
| |||||
| Kim et al., 201329 (abstract) |
|
362 |
|
Intravenous cisplatin 70 mg/m2 day 1 plus intravenous irinotecan 65 mg/m2 days 1 and 8, every 3 weeks for a maximum of 6 cycles (IP) | 173 |
| Intravenous cisplatin 70 mg/m2 day 1 plus intravenous etoposide 100 mg/m2 days 1–3, every 3 weeks for a maximum of 6 cycles (PE) | 189 | ||||
| Overall survival: Median – 10.9 months with IP vs. 10.3 months with PE; HR (IP vs. PE), 0.879 (95% CI: 0 to 1.054), p=0.1207 | |||||
| Toxicity: Grades 3 or 4 anemia, nausea, and diarrhea more frequent with IP (no values reported); no difference observed for neutropenia, thrombocytopenia, neutropenic fever, or infection | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: in Korean patients, overall survival was not superior for IP compared with EP | |||||
|
| |||||
| Shi et al., 201561 |
|
62 |
|
Irinotecan 65 mg/m2 days 1 and 8, plus cisplatin 75 mg/m2 day 1, every 3 weeks | 30 |
| Cisplatin 75 mg/m2 day 1, plus etoposide 100 mg/m2 days 1–3), every 3 weeks | 32 | ||||
| Overall survival: Median –18.1 months with IP vs. 15.8 months with PE, p not reported | |||||
| Toxicity: Grades 3 and 4 (IP vs. PE) – neutropenia, 53.3% vs. 71.9%, p=0.057; leucopenia, 43.3% vs. 53.1%, p=0.291; anemia, 30.0% vs. 31.3%, p=0.114; thrombocytopenia, 6.7% vs. 18.8%, p=0.035 | |||||
| Quality of life: Not reported | |||||
| Authors’ conclusions: Compared with PE in this regimen, IP failed to show significant superiority in efficacy | |||||
ECOG = Eastern Cooperative Oncology Group; PS = performance status; CI = confidence interval; HR = hazard ratio; WHO = World Health Organization; AUC = area under the curve; EORTC = Organisation for Research and Treatment of Cancer; QLQ-C30 = 30-question core Quality of Life Questionnaire.
FIGURE 2.
Overall survival for irinotecan compared with etoposide and for topotecan compared with etoposide for extensive-stage small-cell lung cancer. SE = standard error; IV = instrumental variable; CI = confidence interval.
Three trials compared platinum–topotecan with plat inum–etoposide. Data for os from those trials (moderate aggregate quality) were included in a meta-analysis14,17,38. The os duration was not significantly longer in patients who received topotecan than in those who received etoposide (hr: 0.97; 95% ci: 0.87 to 1.07; p = 0.55). There was no evidence of heterogeneity (chi-square[2] = 1.98, p = 0.37). A test for subgroup differences between irinotecan and topotecan revealed no statistically significant differences (chi-square = 1.68, p = 0.19). Overall, a benefit was shown for irinotecan–topotecan compared with etoposide (hr: 0.88; 95% ci: 0.80 to 0.97; p = 0.008). There was evidence of statistical heterogeneity (I2 = 48%, chi-square[9] = 17.25, p = 0.04).
Four trials comparing other chemotherapy combinations with platinum–etoposide were not included in the os meta-analyses10,45,63,67. In one trial, pemetrexed–carboplatin was compared with carboplatin–etoposide and found to be significantly inferior to carboplatin–etoposide (hr: 1.56; 95% ci: 1.27 to 1.92; p < 0.01)63. Sun et al. compared amrubicin–cisplatin with cisplatin–etoposide and found that median survival was greater, but nonsignificantly so, in the amrubicin–cisplatin group67. Lastly, two trials found survival in their experimental cisplatin–epirubicin10 and belotecan–cisplatin45 groups to be comparable to that in the cisplatin–etoposide group.
In terms of toxicity, eight trials compared platinum–irinotecan with platinum–etoposide for patients with es sclc22,25,29,32,43,55,61,73. Significantly fewer incidences of neutropenia22,43, anemia22,55, thrombocytopenia22,25,43,55,61, and febrile neutropenia22, and significantly more incidences of diarrhea25,29,32,55 were reported in patients receiving irinotecan–platinum. A large study conducted by Kim et al.29 found that grade 3 or 4 anemia and nausea were significantly more frequent in their irinotecan–platinum group. Three trials comparing topotecan–cisplatin with cisplatin–etoposide reported on toxicity14,17,38. In one trial, patients received oral topotecan with intravenous cisplatin, and higher rates of leucopenia, thrombocytopenia, and anemia were found in the oral topotecan group (p values not reported)14. In two large studies in which patients received topotecan–cisplatin, significantly fewer incidences of neutropenia (p = 0.004)17, anemia (p = 0.03)17, and leucopenia (p < 0.01)38 were observed. More incidences of thrombocytopenia were observed in one trial (p = 0.006)17, and fewer in the other trial (p < 0.01)38. In addition, four trials compared toxicities between other chemotherapy combinations and platinum–etoposide10,45,63,67. A large trial conducted by Socinski and colleagues63 compared pemetrexed–carboplatin with carboplatin–etoposide and found that patients in the pemetrexed group experienced significantly less neutropenia (p < 0.001), leucopenia (p = 0.01), and febrile neutropenia (p = 0.009), and significantly more anemia (p = 0.049). Another large trial by Sun et al.67 compared amrubicin–cisplatin with cisplatin–etoposide and found higher rates of leucopenia, neutropenia, and thrombocytopenia in patients receiving amrubicin–cisplatin (p values not reported). Oh et al.45 found significantly higher rates of anemia (p = 0.003) and thrombocytopenia (p < 0.001) in patients receiving belotecan–cisplatin compared with those receiving cisplatin–etoposide.
Lastly, two trials reporting on qol found no difference between the groups, suggesting that qol was not compromised based on the arm to which patients were randomized14,25.
Non-platinum Compared with Platinum–Etoposide Regimens
LS SCLC: Two moderate-quality trials reported on non-platinum compared with platinum–etoposide regimens12,68. One trial compared doxorubicin–cyclophosphamide–etoposide with cisplatin–etoposide and found that the median os duration was slightly but nonsignificantly greater in the patients receiving cisplatin–etoposide12. Sundstrom et al.68 compared epirubicin–cyclophosphamide–vincristine with cisplatin–etoposide and found that patients receiving cisplatin–etoposide experienced a significantly longer median survival duration (p = 0.001). Neither study reported on toxicity or qol outcomes.
ES SCLC: The aggregate os scores of trials comparing amrubicin with cisplatin–etoposide or carboplatin–etoposide were of moderate quality. In one study, the median os was slightly but nonsignificantly greater for patients receiving carboplatin–etoposide60. O’Brien et al.44 conducted a 3-arm study comparing amrubicin alone with amrubicin–cisplatin and with cisplatin–etoposide, finding that os for patients in the amrubicin arms was slightly but nonsignificantly greater. In the trials comparing other chemotherapy combinations (moderate quality), Baka et al.12 compared doxorubicin–cyclophosphamide–etoposide with cisplatin–etoposide and found that median os was slightly but nonsignificantly greater in patients receiving doxorubicin–cyclophosphamide–etoposide. However, the trial by Sundstrom et al.68 found that the median os duration was longer in patients receiving cisplatin–etoposide than in those receiving cyclophosphamide–etoposide–vincristine (p value not reported). The evidence does not support the use of non-platinum-based regimens over platinum–etoposide combinations.
Platinum–Etoposide Plus Another Agent Compared with Platinum–Etoposide
LS SCLC: Aggregate scores for the trials were not possible because the two trials reported on different types of chemotherapy. One trial with high-quality evidence comparing tamoxifen–cisplatin–etoposide with cisplatin–etoposide found that median and 3-year os were significantly improved for patients receiving cisplatin–etoposide (p values not reported)40. Another trial of moderate quality that compared the addition of paclitaxel to cisplatin–etoposide with cisplatin–etoposide alone demonstrated a slightly better but nonsignificantly different median os in the paclitaxel–cisplatin–etoposide arm (p value not reported)39. In addition, one trial reported on toxicity, finding that the toxicity profiles were relatively similar for patients receiving tamoxifen plus cisplatin–etoposide and for those receiving cisplatin-etoposide40. No trial reported on qol.
ES SCLC: Two trials of moderate quality compared paclitaxel–cisplatin–etoposide with cisplatin–etoposide27,39. One trial showed that the median os was slightly but nonsignificantly higher in the cisplatin–etoposide group than in the paclitaxel–cisplatin–etoposide group39. Results from both Mavroudis et al.39 and Niell et al.42 suggested that the addition of paclitaxel to standard doses of cisplatin–etoposide did not improve os. Similarly, another study compared palifosfamide–carboplatin–etoposide with carboplatin–etoposide alone and found that the addition of palifosfamide to carboplatin–etoposide did not improve os27. On the other hand, Pujol et al.46 found that os was significantly better with the addition of 4′-epidoxorubicin–cyclophosphamide to cisplatin–etoposide than with cisplatin–etoposide alone (p = 0.0067). The available evidence does not support the addition of a third agent to platinum–etoposide.
Platinum–Etoposide Plus a Targeted Agent Compared with Platinum–Etoposide
LS SCLC: In one high-quality study, patients received carboplatin–etoposide plus either thalidomide or a placebo33. Median os was slightly but nonsignificantly higher for patients in the group receiving added thalidomide. No trials reported on toxicity or qol.
ES SCLC: Two moderate-quality trials with os scores compared bevacizumab with chemotherapy alone. In both trials, the median survival duration was shown to be slightly but nonsignificantly longer in patients receiving chemotherapy alone (carboplatin–etoposide or cisplatin–etoposide) than in those receiving chemotherapy and bevacizumab, suggesting that the addition of bevacizumab was not associated with any os benefit47,64. Four other trials compared different types of chemotherapy. Langer et al.31 found that the addition of obatoclax to carboplatin–etoposide did not yield a significant improvement in os. Lee et al.33 found that the addition of thalidomide to carboplatin–etoposide was also not associated with a significant os benefit. Lu et al.35 reported that the addition of recombinant human endostatin to carboplatin–etoposide does not improve os in patients with es sclc. Similarly, Rudin et al.50 found no additional os benefit with the addition of oblimersen to carboplatin–etoposide.
Current evidence does not support the addition of a targeted agent to platinum–etoposide therapy.
Maintenance Compared with No Maintenance Therapy
Four moderate-quality rcts compared maintenance therapy with no maintenance therapy. Han et al.21 compared irinotecan maintenance with observation and found that the median os was lower for patients in the maintenance group (p value not reported). Similarly, Schiller et al.54 found that topotecan maintenance therapy did not result in a significant os benefit. A phase ii study using sunitinib as maintenance therapy found that os was greater, but nonsignificantly so, in the group receiving maintenance therapy49. Hanna et al.23 reported similar results for etoposide maintenance therapy, with os being slightly longer, but nonsignificantly so, than that in the observation group.
Four moderate-quality studies reported on toxicity21,23,49,54. Depending on the type of maintenance therapy used, the percentages of fatigue, neutropenia, anemia, and thrombocytopenia were increased in patients who received maintenance treatment (p values not reported). In addition, one trial reported on qol, finding, over a period of 4 months, no significant difference in qol between patients receiving topotecan as maintenance therapy and those in the observation group54.
Platinum–Topoisomerase Inhibitor Compared with Another Regimen
The aggregate os scores of trials comparing amrubicin–cisplatin with irinotecan–cisplatin were of moderate quality18,51. In both trials, median survival was shown to be longer, but nonsignificantly so, in patients receiving irinotecan–cisplatin. Similarly, a trial by Sekine et al.59 found that os duration was slightly but nonsignificantly longer for patients receiving irinotecan–cisplatin than for those receiving irinotecan–cisplatin and etoposide. Tamiya et al.69 found that median and 1-year os were similar for patients receiving amrubicin–irinotecan and for those receiving irinotecan–cisplatin. Quoix et al.48 found that patients receiving either topotecan–etoposide or topotecan–cisplatin experienced a similar median os. Lyss et al.36 found that median os duration was longer for patients receiving paclitaxel–topotecan than for those receiving either paclitaxel–topotecan or topotecan–cisplatin (p values not reported). The trials were all small and underpowered for survival outcomes and therefore should not influence practice.
Various Doses and Schedules of Chemotherapy
LS SCLC: Two moderate-quality trials that examined various chemotherapy doses reported on os. In a phase iii trial conducted by Leyvraz et al.34, the conventional doses of ifosfamide, carboplatin, etoposide, and uromitexan were compared with high doses of the same drugs. No difference in os was observed34. Sculier et al.56 evaluated standard-dose cisplatin–etoposide plus thoracic radiotherapy against daily low-dose cisplatin plus standard-dose etoposide and again found no difference in os. Patients receiving daily cisplatin–etoposide experienced significantly more thrombocytopenia (p < 0.001)56. No trial reported on qol.
ES SCLC: One moderate-quality trial compared chemotherapy doses for best os and least toxicity24. In that trial, patients were randomized to conventional carboplatin–etoposide or dose-intensified therapy with carboplatin–etoposide. No significant differences for os were observed between the groups. Compared with the group of patients receiving dose-intensified treatment, the group receiving conventional carboplatin–etoposide experienced significantly more neutropenia (p = 0.009) and less thrombocytopenia (p = 0.03).
Nine moderate-to high-quality trials looking at varying schedules reported on os11,26,37,52,57,58,70–72. Some trials demonstrated no difference in os; others demonstrated improvements in os. Most trials were small and not powered to answer questions about os.
DISCUSSION
When platinum–etoposide was compared with other regimens in patients with ls sclc, platinum–etoposide was found to be associated with the greatest os and the fewest adverse effects. Those findings suggest that platinum–etoposide in combination with radiotherapy should remain the standard therapy for ls sclc.
In patients with es sclc, platinum–etoposide remains an effective treatment compared with other regimens. However, in our meta-analysis of seven trials involving patients with es sclc, os duration was longer after treatment with platinum–irinotecan than with platinum–etoposide. Based on an a priori suspicion (raised by evidence from previous studies) that the Japanese population might respond differently to irinotecan32, a sensitivity analysis that omitted the Noda et al. trial43 was conducted. In that analysis, platinum–irinotecan still demonstrated a significant os benefit. Based on those findings, platinum–irinotecan should be considered to be an option for patients with es sclc. Whether the benefit is greater in Asian subpopulations cannot be determined at this time. The small survival benefit and lower myelosuppression seen with irinotecan should be balanced against the greater incidence of diarrhea.
Systemic therapy recommendations in sclc have not changed since the end of the 1990s. However, since the present systematic review was completed, new data evaluating atezolizumab, a PD-L1 immune checkpoint inhibitor, have been presented and published74. The Impower 133 trial randomized 403 patients with es sclc, a good performance status, and no history of autoimmune disease to first-line carboplatin–etoposide plus either atezolizumab or placebo. Median os was improved in patients randomized to atezolizumab (12.3 months vs. 10.3 months; hr: 0.70; 95% ci: 0.54 to 0.91; p = 0.007). Response rates did not differ between the arms, and immune adverse events were as expected. Multiple trials evaluating immune checkpoint inhibitors are ongoing, and the role of those agents in sclc will become clearer over the next few years.
The use of chemotherapy and thoracic radiation therapy reflects the current standard of care for patients with ls sclc1,2. In the present review, we investigated the addition of thoracic radiotherapy to chemotherapy for patients with es sclc. The addition of thoracic radiotherapy was shown to be associated with a significant improvement in median os in one trial; however, that small trial was conducted more than 15 years ago, and the thoracic radiotherapy was given in higher doses and to larger volumes than are typically used in North America28. A phase iii trial reported that the addition of thoracic radiotherapy showed a trend toward improved 1-year os (primary endpoint) that did not reach statistical significance62. The secondary endpoints of 18-month and 2-year os did reach statistical significance62. Another randomized phase ii trial did not show a difference in os, although that trial also included thoracic radiotherapy to oligometastatic sites in addition to thoracic radiotherapy19. The foregoing data suggest that the addition of thoracic radiotherapy to chemotherapy in es sclc should be considered on a case-by-case basis (for example, low-volume extrathoracic disease with residual intrathoracic disease or high-volume pre-treatment disease), but cannot be considered to be the standard of care.
The administration of thoracic radiotherapy and the optimal timing, dose, and schedule has been of interest in many studies. With respect to the optimal timing of radiotherapy (early vs. late), the literature search revealed conflicting evidence and no new evidence for an optimal schedule (concurrent vs. sequential) for patients with ls sclc. It was the consensus of the Working Group members that, for pragmatic reasons, thoracic radiotherapy should be started as early as feasible and administered concurrently (for example, early consultation with radiation oncology). Although an optimal dose of thoracic radiotherapy has not yet been established, trials demonstrating superior os have generally used a total dose of at least 40 Gy in 15 fractions given daily over 3 weeks or 45 Gy in 30 fractions given twice daily (or a biologically equivalent dose). In patients with es sclc, no evidence about the optimal timing, dose, and schedule of thoracic radiotherapy has currently been developed.
SUMMARY
In patients with non-resected ls sclc (stages i–iii), there is evidence to suggest that cisplatin–etoposide in combination with thoracic radiotherapy should remain the standard therapy. The evidence is insufficient to recommend an optimal timing (early vs. late) or an optimal schedule (concurrent vs. sequential) of radiotherapy. Based on the consensus of the Working Group members, thoracic radiotherapy should be started as early as feasible and concurrently. Furthermore, the evidence was insufficient to define an optimal dose of thoracic radiotherapy; however, it is suggested that a total dose of at least 40 Gy in 15 fractions over 3 weeks or 45 Gy in 30 fractions given twice daily (or a biologically equivalent dose) be used.
In patients with non-resected es sclc (stage iv), the evidence is currently insufficient to recommend the addition of thoracic radiotherapy to standard combination chemotherapy as standard practice. The addition of thoracic radiotherapy could, however, be considered on a case-by-case basis. The evidence was insufficient to recommend optimal timing, schedule, or dose of thoracic radiotherapy. The most commonly used induction chemotherapy is platinum–etoposide; however, based on new evidence, platinum–irinotecan has been added as an option. A randomized trial now supports atezolizumab in combination with carboplatin–etoposide.
ACKNOWLEDGMENTS
The authors thank Melissa Brouwers, Nadia Coakley, Glenn Fletcher, Sheila McNair, Hans Messersmith, and Emily Vella for providing feedback on draft versions of this article, and Max Cheung for conducting the data audit.
The Program in Evidence Based Care (pebc) is a provincial initiative of Cancer Care Ontario, supported by the Ontario Ministry of Health and Long-Term Care (mohltc). All work produced by the pebc is editorially independent from the mohltc.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: JRG has received honoraria from Amgen, Boehringer Ingelheim, and Bristol–Myers Squibb. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Okawara G, Gagliardi A, Evans WK and members of the Lung Cancer Disease Site Group. The Role of Thoracic Radiotherapy as an Adjunct to Standard Chemotherapy in Limited-Stage Small Cell Lung Cancer: A Clinical Practice Guideline. Toronto, ON: Cancer Care Ontario; 2003. (Evidence-based series 7-13-3). [Google Scholar]
- 2.Payne DG, Murray N, Warde P. Small cell lung cancer: the role of thoracic irradiation and its timing in relation the chemotherapy [French] Bull Cancer. 1994;81:119–28. [PubMed] [Google Scholar]
- 3.Browman GP, Newman TE, Mohide EA, et al. Progress of clinical oncology guidelines development using the practice guidelines development cycle: the role of practitioner feedback. J Clin Oncol. 1998;16:1226–31. doi: 10.1200/JCO.1998.16.3.1226. [DOI] [PubMed] [Google Scholar]
- 4.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 5.Laurie SA, Logan D, Markman BR, Mackay JA, Evans WK and the Lung Cancer Disease Site Group. The Role of Combination Chemotherapy in the Initial Management of Limited-Stage Small-Cell Lung Cancer. Toronto, ON: Cancer Care Ontario; 2012. (Evidence-based series 7-13-1). [Google Scholar]
- 6.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schünemann H, Brożek J, Guyatt G, Oxman AD, editors. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. n.l.: The Grade Working Group; 2013. [Available online at: https://gdt.gradepro.org/app/handbook/handbook.html; cited 1 June 2017] [Google Scholar]
- 8.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Artal-Cortes A, Gomez-Codina J, Gonzales-Larriba JL, et al. Prospective randomised phase iii trial of etoposide/cisplatin versus high-dose epirubicin/cisplatin in small-cell lung cancer. Clin Lung Cancer. 2004;6:175–83. doi: 10.3816/CLC.2004.n.031. [DOI] [PubMed] [Google Scholar]
- 11.Baka S, Agelaki S, Kotsakis A, et al. Phase iii study comparing sequential versus alternate administration of cisplatin–etoposide and topotecan as first-line treatment in small cell lung cancer. Anticancer Res. 2010;30:3031–8. [PubMed] [Google Scholar]
- 12.Baka S, Califano R, Ferraldeschi R, et al. Phase iii randomised trial of doxorubicin-based chemotherapy compared with platinum-based chemotherapy in small-cell lung cancer. Br J Cancer. 2008;99:442–7. doi: 10.1038/sj.bjc.6604480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blackstock AW, Bogart JA, Matthews C, et al. Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: final report of a randomized phase iii trial. Clin Lung Cancer. 2005;6:287–92. doi: 10.3816/CLC.2005.n.007. [DOI] [PubMed] [Google Scholar]
- 14.Eckardt JR, Von Pawel J, Papai Z, et al. Open-label, multicenter, randomized, phase iii study comparing oral topotecan/cisplatin versus etoposide/cisplatin as treatment for chemotherapy-naive patients with extensive-disease small-cell lung cancer. J Clin Oncol. 2006;24:2044–51. doi: 10.1200/JCO.2005.03.3332. [DOI] [PubMed] [Google Scholar]
- 15.Faivre-Finn C, Blackhall F, Ashcroft L, Thatcher N, Taylor P, Lorigan P on behalf of The Christie nhs Foundation Trust. Long-term toxicity report from a phase ii study of accelerated twice-daily (bd) versus high dose once-daily (od) thoracic radiotherapy (rt) with concurrent chemotherapy for limited-stage small cell lung cancer (ls-sclc) [abstract 2789] Int J Radiat Oncol Biol Phys. 2011;81(suppl):S589. doi: 10.1016/j.ijrobp.2011.06.1104. [DOI] [Google Scholar]
- 16.Faivre-Finn C, Snee M, Ashcroft L, et al. on behalf of the convert study team. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (convert): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–25. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink TH, Huber RM, Heigener DF, et al. on behalf of Aktion Bronchialkarzinom. Topotecan/cisplatin compared with cisplatin/etoposide as first-line treatment for patients with extensive disease small-cell lung cancer: final results of a randomized phase iii trial. J Thorac Oncol. 2012;7:1432–9. doi: 10.1097/JTO.0b013e318260de75. [DOI] [PubMed] [Google Scholar]
- 18.Fujita Y, Morikawa N, Sugawara S, et al. Final results of randomized phase ii study of carboplatin plus irinotecan vs. carboplatin plus amrubicin for ed-sclc [abstract P1.07-006] J Thorac Oncol. 2015;2:S498. [Available online at: https://www.jto.org/article/S1556-0864(16)30012-0/fulltext (scrolling required); cited 17 May 2019] [Google Scholar]
- 19.Gore EM, Hu C, Sun AY, et al. Randomized phase ii study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extracranial irradiation for extensive-disease small cell lung cancer (ed sclc): nrg Oncology rtog 0937. J Thorac Oncol. 2017;12:1561–70. doi: 10.1016/j.jtho.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronberg BH, Halvorsen TO, Flotten O, et al. on behalf of the Norwegian Lung Cancer Study Group. Randomized phase ii trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol. 2016;55:591–7. doi: 10.3109/0284186X.2015.1092584. [DOI] [PubMed] [Google Scholar]
- 21.Han JY, Kim HT, Lim KY, Yoon SJ, Lee DH, Lee JS. Randomized phase ii study of maintenance irinotecan therapy versus observation following induction chemotherapy with irinotecan and cisplatin in extensive disease small cell lung cancer. J Thorac Oncol. 2008;3:1039–45. doi: 10.1097/JTO.0b013e3181834f8e. [DOI] [PubMed] [Google Scholar]
- 22.Hanna N, Bunn PA, Jr, Langer C, et al. Randomized phase iii trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol. 2006;24:2038–43. doi: 10.1200/JCO.2005.04.8595. [DOI] [PubMed] [Google Scholar]
- 23.Hanna NH, Sandler AB, Loehrer PJ, Sr, et al. Maintenance daily oral etoposide versus no further therapy following induction chemotherapy with etoposide plus ifosfamide plus cisplatin in extensive small-cell lung cancer: a Hoosier Oncology Group randomized study. Ann Oncol. 2002;13:95–102. doi: 10.1093/annonc/mdf014. [DOI] [PubMed] [Google Scholar]
- 24.Heigener DF, Manegold C, Jager E, Saal JG, Zuna I, Gatzemeier U. Multicenter randomized open-label phase iii study comparing efficacy, safety, and tolerability of conventional carboplatin plus etoposide versus dose-intensified carboplatin plus etoposide plus lenograstim in small-cell lung cancer in “extensive disease” stage. Am J Clin Oncol. 2009;32:61–4. doi: 10.1097/COC.0b013e31817be954. [DOI] [PubMed] [Google Scholar]
- 25.Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: a randomized phase iii trial. J Clin Oncol. 2008;26:4261–7. doi: 10.1200/JCO.2007.15.7545. [DOI] [PubMed] [Google Scholar]
- 26.Ignatiadis M, Mavroudis D, Veslemes M, et al. Sequential versus alternating administration of cisplatin/etoposide and topotecan as first-line treatment in extensive-stage small-cell lung cancer: preliminary results of a phase iii trial of the Hellenic Oncology Research Group. Clin Lung Cancer. 2005;7:183–9. doi: 10.3816/CLC.2005.n.034. [DOI] [PubMed] [Google Scholar]
- 27.Jalal SI, Einhorn LH, Lo G, Lavin P, Lebel FM. Results from a randomized study of carboplatin and etoposide (ce) with or without palifosfamide (pa) in extensive stage small cell lung cancer (es-sclc): the matisse study [abstract 7504] J Clin Oncol. 2015;33 doi: 10.1200/jco.2015.33.15_suppl.7504. [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.7504; cited 29 April 2019] [DOI] [PubMed] [Google Scholar]
- 28.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. 1999;17:2092–9. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 29.Kim DW, Kim HG, Kim JH, et al. Phase iii trial comparing irinotecan plus cisplatin (ip) with etoposide plus cisplatin (ep) in Korean patients with extensive disease (ed) small cell lung cancer (sclc) [abstract O21.02] J Thorac Oncol. 2013;8:S219–20. [Available online at: https://www.jto.org/article/S1556-0864(15)33626-1/fulltext (scrolling required); cited 17 May 2019] [Google Scholar]
- 30.Kubota K, Hida T, Ishikura S, et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited-stage small-cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (jcog0202): a randomised phase 3 study. Lancet Oncol. 2014;15:106–13. doi: 10.1016/S1470-2045(13)70511-4. [DOI] [PubMed] [Google Scholar]
- 31.Langer CJ, Albert I, Ross HJ, et al. on behalf of the gem017 investigators. Randomized phase ii study of carboplatin and etoposide with or without obatoclax mesylate in extensive-stage small cell lung cancer. Lung Cancer. 2014;85:420–8. doi: 10.1016/j.lungcan.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Lara PN, Jr, Natale R, Crowley J, et al. Phase iii trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from swog S0124. J Clin Oncol. 2009;27:2530–5. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SM, Woll PJ, Rudd R, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2009;101:1049–57. doi: 10.1093/jnci/djp200. [DOI] [PubMed] [Google Scholar]
- 34.Leyvraz S, Pampallona S, Martinelli G, et al. on behalf of the Solid Tumors Working Party of the European Group for Blood and Marrow Transplantation. A threefold dose intensity treatment with ifosfamide, carboplatin, and etoposide for patients with small cell lung cancer: a randomized trial. J Natl Cancer Inst. 2008;100:533–41. doi: 10.1093/jnci/djn088. [DOI] [PubMed] [Google Scholar]
- 35.Lu S, Li L, Luo Y, et al. A multicenter, open-label, randomized phase ii controlled study of rh-endostatin (Endostar) in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer. J Thorac Oncol. 2015;10:206–11. doi: 10.1097/JTO.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 36.Lyss AP, Herndon JE, 2nd, Lynch TJ, Jr, et al. Novel doublets in extensive-stage small-cell lung cancer: a randomized phase ii study of topotecan plus cisplatin or paclitaxel (calgb 9430) Clin Lung Cancer. 2002;3:205–10. doi: 10.3816/CLC.2002.n.004. [DOI] [PubMed] [Google Scholar]
- 37.Masutani M, Ochi Y, Kadota A, et al. Dose-intensive weekly alternating chemotherapy for patients with small cell lung cancer: randomized trial, can it improve survival of patients with good prognostic factors? Oncol Rep. 2000;7:305–10. [PubMed] [Google Scholar]
- 38.Mau-Soerensen M, Hansen O, Holm B, et al. Randomized phase iii trial in extensive-disease small cell lung cancer comparing first-line etoposide to topotecan in combination with platinum [abstract 7519] J Clin Oncol. 2014;32 [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.7519; cited 27 April 2019] [Google Scholar]
- 39.Mavroudis D, Papadakis E, Veslemes M, et al. on behalf of the Greek Lung Cancer Cooperative Group. A multicenter randomized clinical trial comparing paclitaxel–cisplatin–etoposide versus cisplatin–etoposide as first-line treatment in patients with small-cell lung cancer. Ann Oncol. 2001;12:463–70. doi: 10.1023/A:1011131303391. [DOI] [PubMed] [Google Scholar]
- 40.McClay EF, Bogart J, Herndon JE, 2nd, et al. on behalf of the Cancer and Leukemia Group B Study 9235. A phase iii trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B study 9235. Am J Clin Oncol. 2005;28:81–90. doi: 10.1097/01.coc.0000139940.52625.d0. [DOI] [PubMed] [Google Scholar]
- 41.Narayan S, Singhal M, Beniwal S, et al. A prospective randomized phase iii study of continuum chemotherapy versus chemo-radiotherapy in es-sclc in Asian Indian [abstract Oral10.02] J Thorac Oncol. 2015;2:S193. [Available online at: https://www.jto.org/article/S1556-0864(16)30010-7/fulltext (scrolling required); cited 17 May 2019] [Google Scholar]
- 42.Niell HB, Herndon JE, 2nd, Miller AA, et al. on behalf of the Cancer and Leukemia Group. Randomized phase iii Intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony–stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B trial 9732. J Clin Oncol. 2005;23:3752–9. doi: 10.1200/JCO.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 43.Noda K, Nishiwaki Y, Kawahara M, et al. on behalf of the Japan Clinical Oncology Group. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91. doi: 10.1056/NEJMoa003034. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien ME, Konopa K, Lorigan P, et al. Randomised phase ii study of amrubicin as single agent or in combination with cisplatin versus cisplatin etoposide as first-line treatment in patients with extensive stage small cell lung cancer—eortc 08062. Eur J Cancer. 2011;47:2322–30. doi: 10.1016/j.ejca.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Oh IJ, Kim KS, Park CK, et al. Belotecan/cisplatin versus etoposide/cisplatin in previously untreated patients with extensive-stage small cell lung carcinoma: a multi-center randomized phase iii trial. BMC Cancer. 2016;16:690. doi: 10.1186/s12885-016-2741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pujol JL, Daures JP, Riviere A, et al. Etoposide plus cisplatin with or without the combination of 4′-epidoxorubicin plus cyclophosphamide in treatment of extensive small-cell lung cancer: a French Federation of Cancer Institutes multicenter phase iii randomized study. J Natl Cancer Inst. 2001;93:300–8. doi: 10.1093/jnci/93.4.300. [DOI] [PubMed] [Google Scholar]
- 47.Pujol JL, Lavole A, Quoix E, et al. on behalf of the French Cooperative Thoracic Intergroup. Randomized phase ii–iii study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the ifct-0802 trial. Ann Oncol. 2015;26:908–14. doi: 10.1093/annonc/mdv065. [DOI] [PubMed] [Google Scholar]
- 48.Quoix E, Breton JL, Gervais R, et al. A randomised phase ii study of the efficacy and safety of intravenous topotecan in combination with either cisplatin or etoposide in patients with untreated extensive disease small-cell lung cancer. Lung Cancer. 2005;49:253–61. doi: 10.1016/j.lungcan.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Ready NE, Pang HH, Gu L, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small-cell lung cancer: a randomized, double-blind, placebo-controlled phase ii study—calgb 30504 (Alliance) J Clin Oncol. 2015;33:1660–5. doi: 10.1200/JCO.2014.57.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudin CM, Salgia R, Wang X, et al. Randomized phase ii study of carboplatin and etoposide with or without the Bcl-2 anti-sense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: calgb 30103. J Clin Oncol. 2008;26:870–6. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satouchi M, Kotani Y, Shibata T, et al. Phase iii study comparing amrubicin plus cisplatin with irinotecan plus cisplatin in the treatment of extensive-disease small-cell lung cancer: jcog 0509. J Clin Oncol. 2014;32:1262–8. doi: 10.1200/JCO.2013.53.5153. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer PL, Marks RS, Mahoney MR, et al. Randomized phase ii study of daily versus continuous-infusion schedules of topotecan in the treatment of extensive-stage small cell lung cancers. Am J Clin Oncol. 2003;26:236–40. doi: 10.1097/01.COC.0000018038.28645.46. [DOI] [PubMed] [Google Scholar]
- 53.Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase iii trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943–51. doi: 10.1016/j.ijrobp.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 54.Schiller JH, Adak S, Cella D, DeVore RF, 3rd, Johnson DH. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593—a phase iii trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:2114–22. doi: 10.1200/JCO.2001.19.8.2114. [DOI] [PubMed] [Google Scholar]
- 55.Schmittel A, Sebastian M, Fischer von Weikersthal L, et al. on behalf of the Arbeitsgemeinschaft Internistische Onkologie Thoracic Oncology Study Group. A German multicenter, randomized phase iii trial comparing irinotecan–carboplatin with etoposide–carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol. 2011;22:1798–804. doi: 10.1093/annonc/mdq652. [DOI] [PubMed] [Google Scholar]
- 56.Sculier JP, Lafitte JJ, Efremidis A, et al. on behalf of the European Lung Cancer Working Party. A phase iii randomised study of concomitant induction radiochemotherapy testing two modalities of radiosensitisation by cisplatin (standard versus daily) for limited small-cell lung cancer. Ann Oncol. 2008;19:1691–7. doi: 10.1093/annonc/mdn354. [DOI] [PubMed] [Google Scholar]
- 57.Sculier JP, Paesmans M, Lecomte J, et al. on behalf of the European Lung Cancer Working Party. A three-arm phase iii randomised trial assessing, in patients with extensive-disease small-cell lung cancer, accelerated chemotherapy with support of haematological growth factor or oral antibiotics. Br J Cancer. 2001;85:1444–51. doi: 10.1054/bjoc.2001.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekine I, Nishiwaki Y, Noda K, et al. on behalf of the Japan Clinical Oncology Group Lung Cancer Study Group. Randomized phase ii study of cisplatin, irinotecan and etoposide combinations administered weekly or every 4 weeks for extensive small-cell lung cancer (jcog9902-DI) Ann Oncol. 2003;14:709–14. doi: 10.1093/annonc/mdg213. [DOI] [PubMed] [Google Scholar]
- 59.Sekine I, Nokihara H, Takeda K, et al. Randomised phase ii trial of irinotecan plus cisplatin vs. irinotecan, cisplatin plus etoposide repeated every 3 weeks in patients with extensive-disease small-cell lung cancer. Br J Cancer. 2008;98:693–6. doi: 10.1038/sj.bjc.6604233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sekine I, Okamoto H, Horai T, et al. A randomized phase iii study of single-agent amrubicin vs. carboplatin/etoposide in elderly patients with extensive-disease small-cell lung cancer. Clin Lung Cancer. 2014;15:96–102. doi: 10.1016/j.cllc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Shi Y, Hu Y, Hu X, Li X, Lin L, Han X. Cisplatin combined with irinotecan or etoposide for untreated extensive-stage small cell lung cancer: a multicenter randomized controlled clinical trial. Thorac Cancer. 2015;6:785–91. doi: 10.1111/1759-7714.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slotman BJ, Van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 63.Socinski MA, Smit EF, Lorigan P, et al. Phase iii study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27:4787–92. doi: 10.1200/JCO.2009.23.1548. [DOI] [PubMed] [Google Scholar]
- 64.Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase ii study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the salute trial. J Clin Oncol. 2011;29:2215–22. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 65.Spiro SG, James LE, Rudd RM, et al. on behalf of the London Lung Cancer Group. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24:3823–30. doi: 10.1200/JCO.2005.05.3181. [DOI] [PubMed] [Google Scholar]
- 66.Sun JM, Ahn YC, Choi EK, et al. Phase iii trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer. Ann Oncol. 2013;24:2088–92. doi: 10.1093/annonc/mdt140. [Erratum in: Ann Oncol 2014;25:1672] [DOI] [PubMed] [Google Scholar]
- 67.Sun Y, Cheng Y, Hao X, et al. Randomized phase iii trial of amrubicin/cisplatin versus etoposide/cisplatin as first-line treatment for extensive small-cell lung cancer. BMC Cancer. 2016;16:265. doi: 10.1186/s12885-016-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sundstrom S, Bremnes RM, Kaasa S, et al. on behalf of the Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase iii trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665–72. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 69.Tamiya A, Yoshioka H, Nishimura T, et al. Randomized phase ii trial of amrubicin plus irinotecan versus cisplatin plus irinotecan in chemo-naïve patients with extensive disease small-cell lung cancer: results of the Japan Multinational Trial Organization (jmto) lc 08-01. Eur J Cancer. 2015;51(suppl 3):S623–4. [Google Scholar]
- 70.Ueoka H, Kiura K, Tabata M, et al. A randomized trial of hybrid administration of cyclophosphamide, doxorubicin, and vincristine (cav)/cisplatin and etoposide (pvp) versus sequential administration of cav-pvp for the treatment of patients with small cell lung carcinoma: results of long term follow-up. Cancer. 1998;83:283–90. doi: 10.1002/(SICI)1097-0142(19980715)83:2<283::AID-CNCR12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 71.Veslemes M, Polyzos A, Latsi P, et al. Optimal duration of chemotherapy in small cell lung cancer: a randomized study of 4 versus 6 cycles of cisplatin-etoposide. J Chemother. 1998;10:136–40. doi: 10.1179/joc.1998.10.2.136. [DOI] [PubMed] [Google Scholar]
- 72.Xiao XG, Wang S, Xia S, et al. Retrospective study of irinoecan/cisplatin followed by etoposide/cisplatin or the reverse sequence in extensive-stage small cell lung cancer. Onco Targets Ther. 2015;8:2209–14. doi: 10.2147/OTT.S89606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zatloukal P, Cardenal F, Szczesna A, et al. A multicenter international randomized phase iii study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21:1810–6. doi: 10.1093/annonc/mdq036. [DOI] [PubMed] [Google Scholar]
- 74.Horn L, Mansfield AS, Szczęsna A, et al. on behalf of the IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]