Abstract
Background
CervixCheck, Manitoba’s cervical cancer screening program, conducted a pilot study to assess whether screening participation could be improved in unscreened women by offering a mailed self-sampling kit for human papillomavirus (hpv) testing instead of a Pap test.
Methods
In a prospective cohort study design, a sample of unscreened women (n = 1052) who had been sent an invitation letter from CervixCheck in the past but who did not respond were randomized to either an intervention group or a control group. The intervention group received a mailed hpv self-sampling kit; the control group received no additional communication. Returned hpv self-sampling swabs were analyzed by a provincial laboratory. After 6 months, screening participation in the two study groups was compared using a logistic regression model adjusted for age and area of residence (urban or rural). Secondary outcomes included hpv positivity, specimen inadequacy, compliance with follow-up, and time to colposcopy.
Results
Screening participation was significantly higher in the intervention group than in the control group (n = 51, 9.6%, vs. n = 13, 2.5%; odds ratio: 4.7; 95% confidence interval: 2.56 to 8.77). Geographic area of residence (urban or rural) and age were not statistically significant.
Conclusions
The study demonstrated that hpv self-sampling kits can enhance screening participation in unscreened non-responder women in the setting of an organized screening program. Next steps should include additional research to determine the best implementation strategy for hpv self-sampling in Manitoba.
Keywords: Human papillomavirus, dna tests, cervical cancer, cancer screening
INTRODUCTION
The Pap (Papanicolaou) test is a standard screening test that examines cervical cells for abnormal cellular changes indicating cervical dysplasia or cervical cancer. Pap tests have been used effectively in Canada and Manitoba to reduce the incidence of and mortality from cervical cancer. CervixCheck, Manitoba’s organized cervical cancer screening program, was initiated in 2001. In Manitoba, more than 40% of cervical cancers are found in women who are unscreened (women who have not been registered in the screening program for at least 5 years and who have never had a Pap test)1.
Between 2014 and 2016, cervical cancer screening participation in Manitoba was 66.2% in eligible women, which is well below the participation target of 80% set by the Pan-Canadian Cervical Cancer Screening Network2. Currently, more than 20,000 women in Manitoba between the ages of 30 and 65 years were sent an invitation letter by CervixCheck, but have no record of a Pap test or colposcopy in the CervixCheck registry. Those unscreened non-responder women are at increased risk for cervical cancer.
Research shows that many barriers prevent women from having a Pap test, including factors at the personal, clinical, and test levels3. Test-level barriers include factors such as physical pain and discomfort or the anticipation of discomfort with a Pap test. Embarrassment and anxiety are other reasons cited as barriers to having a Pap test4. Although the Pap test is an effective screening test, one of its limitations is low sensitivity—particularly for the detection of non–squamous cell cancers, which constituted approximately 40% of all cervical cancers diagnosed in Manitoba between 2009 and 20161,2.
Strong evidence supports hpv testing as an alternative to the Pap test. The sensitivity of the hpv test is greater than that of the Pap test, detecting persistent hpv infections that can lead to cervical cancer for women more than 30 years of age5,6. Molecular dna testing for hpv detects oncogenic hpv types that can cause cervical cancer and its precursors. The technology can also be used as part of a screening program in the form of a self-sampling kit for hpv that might be more appealing to under-screened and unscreened women in hard to reach, underserved, or marginalized populations. A self-sampling kit for hpv might also address some test-level barriers to having a Pap test, such as the anticipation of discomfort or pain, or anxiety about having a Pap test in a clinic.
The purpose of the present study was to compare cervical cancer screening participation in unscreened non-responder women in Manitoba who were mailed and not mailed a self-sampling kit for hpv.
METHODS
Study Design and Population
The study used a prospective cohort study design and included 1052 randomly selected unscreened non-responder women in Manitoba. The sample size was based on an initial screening participation rate of 3%, an estimated 3% participation rate in the control group, and an estimated 9% participation rate in the intervention group7,8. Approximately 500 women were required for each group to find a significant difference between the groups with a 2-sided alpha of 0.05 and a power of 80%.
Unscreened women were those who had no record of a Pap test in the CervixCheck registry in Manitoba and who had been registered for a minimum of 5 years. Non-responders were women who had been sent an invitation letter to be screened, but who remained unscreened. Unscreened and non-responder woman were included in the pilot study provided they were also currently alive and between 30 and 65 years of age, with valid health coverage in Manitoba. Women were not eligible for the study if they had an out-of-province address; a record of a Pap test, colposcopy, or hysterectomy; or an invasive gynecologic cancer diagnosis in the registry. Eligible women (n = 1100) were randomized to either the intervention group (hpv self-sampling mailing) or the control group (no mailing). Because the hpv self-sampling kit was mailed, each woman’s address was verified; 48 women whose mail had previously been returned to the screening program were excluded.
Each study arm was stratified by area of residence to ensure that approximately half the participants resided in urban areas and half resided in rural areas of Manitoba. Area of residence was defined using the first two characters of an individual’s postal code. “Urban” was defined as postal codes in the cities of Winnipeg and Brandon (first two characters: R1 or R9). All other postal codes were considered “rural” (first two characters: R0).
Setting
CervixCheck manages the population-based cervical cancer screening registry for the province of Manitoba. Based on the CervixCheck registry, the province had a population of 682,577 women in 2017. Two thirds of the population lives in the capital city of Winnipeg.
CervixCheck recommends that women between the ages of 21 and 69 years who have ever been sexually active have a Pap test every 3 years. CervixCheck operates a successful correspondence strategy that includes sending an invitation letter to women who have never had a Pap test or have not had a Pap test in at least 5 years, recall letters to women who are due for a Pap test, result letters to women with high-grade cytology results, letters to providers who have clinical responsibility for a woman’s last Pap test if the women did not receive appropriate follow-up after an abnormal test, and letters to women who have not received appropriate follow-up to remind them of the recommended action.
Study Groups
Women in the intervention group were mailed a self-sampling kit for hpv through Canada Post. The control group received no additional communication from Cervix Check, per current program operations. Both study groups were followed for a period of 6 months to determine screening participation. The 6-month follow-up period was selected so as to compare outcomes with outcomes from a previous study that examined screening participation after an invitation letter7. However, CervixCheck continued followup coordination and collection of follow-up information for women who were hpv-positive and women who had a high-grade Pap test beyond the 6-month study duration.
The self-sampling kits for hpv sent to women in the intervention group included an invitation letter with a translated phrase on the reverse that appeared in 21 languages (giving a contact number to review the information with a service provider and an interpreter), instructions for completing the hpv test, an educational brochure, a study response form with statement of consent, an hpv self-sampling device, a biohazard bag to return the sample, and a postage-paid return envelope. A reminder letter was sent 8 weeks after the initial mail-out to any woman in the intervention group who did not respond to the invitation. The hpv self-sampling device used in the study was the FLOQSwab (Copan Diagnostics, Murrieta, CA, U.S.A.), a Health Canada approved medical device. The FLOQSwab was selected after informal focus groups had been held with 56 women from various Manitoba community groups to compare the acceptability of 3 different hpv self-sampling devices [the FLOQSwab, the HerSwab (Eve Medical, Toronto, ON), and the Evalyn Brush (Rovers Medical Devices, Oss, Netherlands)]. These four questions were asked:
■ After reading the instructions, I understand how to do this test.
■ The test unit looks easy to use.
■ I could see myself using this test.
■ Rank the tests from 1 to 3 (1 being most likely to use and 3 being the least likely to use).
Self-samples for hpv that were completed and sent to CervixCheck by women in the intervention group within 6 months of the mail-out date were forwarded by medical courier to the Shared Health Manitoba laboratory (formerly called Diagnostic Services Manitoba) for processing. In consultation with the Shared Health Manitoba laboratory, the FLOQSwab was determined to be compatible with Roche Molecular Diagnostics equipment [the Cobas 4800 System (Roche Molecular Systems, Branchburg, NJ, U.S.A.) using the Cobas HPV Test]. The hpv test results were then communicated by secure e-mail message from the Shared Health Manitoba laboratory to CervixCheck per existing program operations. Women who had an invalid hpv result were sent up to 3 new hpv self-sampling kits for completion. If the third swab came back invalid, women were referred to their health care provider for a Pap test.
CervixCheck notified women with a positive hpv test of their result by telephone and worked with the women and their health care providers to coordinate all follow-up care required after a positive hpv result. If a woman did not have a health care provider, CervixCheck arranged follow-up. All women in the intervention group with a positive hpv test result were referred to colposcopy.
Data Sources
The CervixCheck registry was used to obtain Pap test dates, colposcopy procedure dates and results, cervical histology results, hysterectomy status, and gynecologic cancer diagnoses. Electronic study records were used to extract all hpv test–related data for the intervention group, including program correspondence dates, responses, hpv test results from the laboratory, and follow-up data for individuals with a positive hpv test result.
Outcomes
The primary outcome of interest was screening participation (hpv test or Pap test). Because area of residence in Manitoba might influence a woman’s access to health care services and because participation varies by age, we investigated whether residence (urban or rural) and age had an effect on screening participation rates in the intervention and control groups.
Screening participation in the intervention group was calculated in three parts:
■ Those who completed the hpv self-sampling test
■ Those who had a Pap test within 6 months of the initial mail-out
■ A combination of those who completed the hpv self-sampling test or who had a Pap test within 6 months of initial mail-out
The denominator of each calculation was the number of women in the intervention group. For the control group, screening participation was calculated as the number of women who had a Pap test within the 6-month study period, divided by the number of women in the control group. Screening participation was also calculated for rural and urban residence in both the intervention and the control group.
Secondary study outcomes were positivity (hpv test and Pap test), specimen inadequacy, compliance with follow-up after abnormal results, and time to colposcopy for women with a positive hpv result or high-grade Pap test result. Positivity was calculated as the number of hpv-positive self-sample tests or high-grade Pap tests divided by, respectively, the number of hpv self-sample tests or Pap tests completed. Inadequacy of testing samples (for example, invalid, indeterminate, or unsatisfactory result) was calculated by determining the number of tests in each group with an inadequate result divided by all tests completed. Compliance with follow-up was determined for each group and calculated as the number of women who underwent colposcopy within 1 year of their positive hpv test, divided by the number of hpv-positive women who were referred for colposcopy in the intervention group, or the number of women who underwent colposcopy within 1 year of their Pap test divided by the number of women with a high-grade Pap result who were referred for colposcopy in the control group. Time to colposcopy in each group was calculated as the number of days between the positive hpv test report date and the date of colposcopy for all women in the intervention group who had a colposcopy (subdivided for hpv16- and hpv18-positive, and hpv-other–positive) or the number of days between the index Pap test report date and the date of colposcopy for all women in the control group with a high-grade Pap result who had a colposcopy.
Statistical Analyses
A generalized estimating equation was used to check for geographic clustering based on the first three letters of the women’s postal codes. The intraclass correlation coefficient of the generalized estimating equation model was extremely low at 0.003, very likely because of the homogeneity of the cohort. That result suggested that clustering of participation by geographic area was not present. Screening participation in the intervention and control groups was therefore compared using multivariable logistic regression, with area of residence (urban or rural) and age as covariates. The percentage of positive and inadequate tests, follow-up compliance, and time to colposcopy were calculated and compared for the two groups. Because of small numbers, data for positivity, inadequacy, compliance to follow-up, and time to colposcopy were suppressed and are therefore not presented in the Results section.
Statistical tests were 2-sided with p < 0.05 considered statistically significant. Analyses were performed on an intention-to-treat basis. All data analyses were completed using the SAS software application (version 9.2: SAS Institute, Cary, NC, U.S.A.). This pilot study was approved by the University of Manitoba’s Health Research Ethics Board [no. HS19764 (H2016:205)], CancerCare Manitoba’s Research Impact Committee, the Health Sciences Centre’s Research Impact Committee, and Roche Diagnostics.
RESULTS
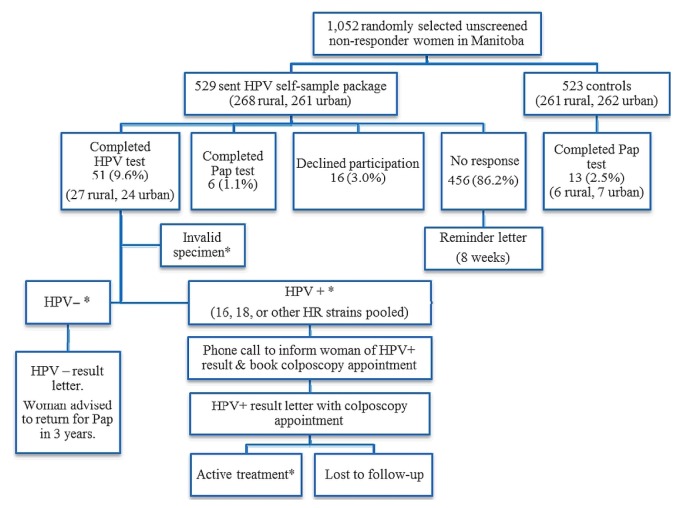
During randomization, 529 women were allocated to the intervention group, and 523 women, to the control group (Table I). By the end of the 6-month study period, 51 women in the intervention group (9.6%) had completed an hpv test; 6 (1.1%) had completed a Pap test; 16 (3.0%) had declined participation; and 456 (86.2%) had not responded or participated in screening. Of the 51 women who completed the hpv self-sample test, 24 lived in an urban area, and 27 lived in a rural area. The combined (hpv and Pap test) screening participation was 10.8% in the intervention group. In the control group, 13 women (2.5%) had a Pap test during the study period. Figure 1 shows the flow of participants in the pilot study. Table II present study response and screening participation results.
TABLE I.
Demographics of the pilot study groups
| Variable | Patient group | ||
|---|---|---|---|
|
| |||
| Intervention | Control | Overall | |
| Patients (n) | 529 | 523 | 1052 |
|
| |||
| Age (years) | |||
| Mean | 52 | 53 | |
| Median | 53 | 54 | |
|
| |||
| Age group [n (%)] | |||
| 30–39 Years | 36 (6.8) | 42 (8.0) | 78 (7.4) |
| 40–49 Years | 149 (28.2) | 142 (27.1) | 291 (27.7) |
| 50–59 Years | 225 (42.5) | 206 (39.4) | 431 (41.0) |
| 60–69 Years | 119 (22.5) | 133 (25.4) | 252 (23.9) |
|
| |||
| Residence | |||
| Urban | 268 (50.7) | 261 (49.9) | 529 (50.3) |
| Rural | 261 (49.3) | 262 (50.1) | 523 (49.7) |
FIGURE 1.
Flow chart for the human papillomavirus (HPV) self-sampling study. *Data suppressed because of small numbers. HR = high risk.
TABLE II.
Participation in screening by the women in the study
| Variable | Patient group | |
|---|---|---|
| Intervention | Control | |
| Denominatora (n) | 529 | 523 |
| HPV self-sampling test completed [n (%)] | 51 (10.1) | NA |
| Pap test completed [n (%)] | 6 (1.2) | 13 (2.5) |
| Combined HPV or Pap test | 57 (11.3)b | 13 (2.5) |
Total study sample.
Excludes Pap test where an HPV test was also completed.
HPV = human papillomavirus; NA = not applicable.
Of the completed hpv self-sampling tests, 86.3% were negative for high-risk hpv. All women who received a positive high-risk hpv test result attended colposcopy. In the control group, most women had a negative cytology result, and no specimens were invalid.
Table III presents the results of the multivariable logistic regression comparing screening participation in the intervention and control groups. Screening participation was significantly higher in the intervention group than in the control group (odds ratio: 4.7; 95% confidence interval: 2.56 to 8.77). Area of residence (rural vs. urban) and age were not significant predictors of screening participation. The attributable risk was 8.3%.
TABLE III.
Multivariable logistic regression model
| Variable | Comparison | OR | 95% CI | p Value |
|---|---|---|---|---|
| Study group | Intervention (HPV test) | 4.70 | 2.56 to 8.77 | <0.0001 |
| Control | 1.00 | |||
|
| ||||
| Area of residence | Rural | 1.16 | 0.71 to 1.90 | 0.56 |
| Urban | 1.00 | |||
|
| ||||
| Age | 0.99 | 0.96 to 1.02 | 0.58 | |
OR = odds ratio; CI = confidence interval; HPV = human papillomavirus.
DISCUSSION
Our study found that, in the context of an organized screening program, cervical cancer screening participation was significantly higher for unscreened non-responder women who were sent an hpv self-sampling kit than for women who were not sent a kit. The baseline cervical cancer screening participation rate for unscreened women who have never been sent an invitation letter is 3%. Participation increases to about 6% after an invitation letter from CervixCheck7. We predicted a 3% participation rate in the control group of the present study, similar to the 3% baseline participation. Based on previous research that included persistent non-responders, we expected a 9% screening participation rate for the intervention group8. The participation rate for the unscreened non-responder women sent an hpv self-sampling kit in the present study is consistent with the results from other studies that have examined the effect of hpv self-sampling.
Previous studies of hpv self-sampling have shown that women have positive attitudes toward hpv self-sampling and are more likely to participate in cervical cancer screening when offered an hpv self-sample test, and have confirmed that self-collected vaginal samples are of high quality9–11. A systematic review and meta-analysis conducted in 2013 assessed 10 hpv self-sampling studies from around the world and found that unscreened and under-screened women offered hpv self-sampling were significantly more likely to participate in cervical cancer screening4. The two Canadian studies that investigated hpv self-sampling in under-screened women in rural settings showed significant increases in cervical cancer screening participation12,13. The study from Newfoundland and Lab-rador found that offering hpv self-sampling and regular Pap tests with intense education and promotions led to a statistically significant increase in cervical cancer screening participation in a rural community12. Another study conducted in a rural Ontario community mailed hpv self-sampling kits directly to women; screening participation was 32% in the intervention group compared with 9% in a control group who were not sent hpv self-sampling kits13.
We expected that the screening participation in our study would be lower than in other studies because the intervention group in our study included only unscreened non-responders and those women represent a very difficult-to- reach population.
The main strengths of our study are that it was carried out in a programmatic setting in Canada and that it focused only on unscreened women. As an organized cervical cancer screening program, the CervixCheck registry contains the demographic data, provincial health billing data for relevant tests and procedures (for example, Pap test, hysterectomy, colposcopy), and diagnoses of gynecologic cancers necessary to enable the program to accurately identify women who were eligible to be screened according to the provincial screening guidelines, but who have not participated in cervical cancer screening to date. The main limitation of the study is that it did not address how varied implementation strategies might affect screening participation by unscreened women who are offered an hpv self-sampling kit. For example, how factors other than the hpv self-sampling device itself might have affected screening participation is unknown.
Because a large proportion of women did not complete the hpv test (86.2%), a high number of the hpv self-sampling kits were unused. Although a cost–benefit analysis is beyond the scope of the present study, such an analysis is an important step in determining the feasibility of offering hpv self-sampling kits in Manitoba in the future. The hpv self-sampling kits that were completed and returned to CervixCheck for processing placed a short-term increased demand on the program’s human resources because the follow-up efforts required were significant and beyond the usual scope of CervixCheck’s program operations. The service delivery model used in our study would not be achievable with the current structure and resources of the CervixCheck program. Additionally, greater participation would have to be demonstrated for the program to advocate for more resources to facilitate this model of service delivery.
Based on the results of the present study, our future research will examine the effect of an opt-out letter notifying women that they are overdue for cervical cancer screening, that CervixCheck will be mailing them an hpv self-sampling kit, and that they should notify the program if they do not wish to participate; and the impact of partnering with a community health clinic or nursing station to provide an hpv self-sampling kit that women can use at home or at the clinic. Education and support will be provided by the clinic.
CONCLUSIONS
The present study illustrates that mailing hpv self-sampling kits is a viable screening option for women most at risk for cervical cancer and supports the growing evidence and trend to move from Pap tests to hpv tests for cervical cancer screening.
ACKNOWLEDGMENTS
The project team acknowledges the support of Roche Diagnostics and Copan Flock Technologies srl. They also thank the staff of the Screening Department at CancerCare Manitoba, including Lesley Baldry, Allyson Neaustaeter, Ginny Dimaculangan, Mae Reyes, Clarissa Arcilla, Carmela Villaflor, and Josie Kshymensky.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.CervixCheck. Cervical Cancer Screening in Manitoba, 2012–2014 Report. Winnipeg, MB: CancerCare Manitoba; 2016. [Available online at: http://www.getcheckedmanitoba.ca/files/x-rep-annl-12-14.pdf; cited 29 April 2019] [Google Scholar]
- 2.CervixCheck. Cervical Cancer Screening in Manitoba 2014–2016 Report. Winnipeg, MB: CancerCare Manitoba; 2018. [Google Scholar]
- 3.Racey S, Withrow DR, Gesink DC. Self-collected hpv testing improves participation in cervical cancer screening: a systematic review and meta-analysis. Can J Public Health. 2013;104:e159–66. doi: 10.1007/BF03405681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waller J, Jackowska M, Marlow L, Wardle J. Exploring age differences in reasons for non-attendance for cervical screening: a qualitative study. BJOG. 2011;119:26–32. doi: 10.1111/j.1471-0528.2011.03030.x. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103:368–83. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical hpv testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the hpv focal randomized clinical trial. JAMA. 2018;320:43–52. doi: 10.1001/jama.2018.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker K, Turner D, Demers AA, Martens PJ, Lambert P, Chateau D. Evaluating the effectiveness of cervical cancer screening invitation letters. J Womens Health (Larchmt) 2013;22:687–93. doi: 10.1089/jwh.2012.4203. [DOI] [PubMed] [Google Scholar]
- 8.Szarewski A, Cadman L, Mesher D, et al. hpv self-sampling as an alternative strategy in non-attenders for cervical screening—a randomised controlled trial. Br J Cancer. 2011;104:915–20. doi: 10.1038/bjc.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zehbe I, Moeller H, Severini A, et al. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in First Nation women from Northwest Ontario, Canada: a pilot study. BMJ Open. 2011;1:e000030. doi: 10.1136/bmjopen-2010-000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wikstrom I, Stenvall H, Wilander E. Attitudes to self-sampling of vaginal smear for human papillomavirus analysis among women not attending organized cytology screening. Acta Obstet Gynecol Scand. 2007;86:720–5. doi: 10.1080/00016340701303747. [DOI] [PubMed] [Google Scholar]
- 11.Gok M, Heideman DAM, van Kemenade FJ, et al. Offering self-sampling for human papillomavirus testing to non-attendees of cervical screening programme: characteristics of responders. Eur J Cancer. 2012;48:1799–808. doi: 10.1016/j.ejca.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Duke P, Godwin M, Ratnam S, et al. Effect of vaginal self-sampling on cervical cancer screening rates: a community-based study in Newfoundland. BMC Women’s Health. 2015;15:47. doi: 10.1186/s12905-015-0206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racey S, Gesink DC, Burchell AN, Trivers S, Wong T, Rebbapragada A. A randomized intervention of self-collected samples for human papillomavirus testing in under-screened rural women: uptake of screening and acceptability. J Womens Health (Larchmt) 2015;25:489–97. doi: 10.1089/jwh.2015.5348. [DOI] [PubMed] [Google Scholar]