Abstract
Liver biopsy is the current reliable way of evaluating liver fibrosis. However, no specific sera biomarker could be applied in clinical diagnosis. As the pivotal role of osteopontin (OPN) reported in numerous liver diseases, thrombin‐cleaved OPN (Thr‐OPN) exposes an integrin‐binding motif that promoted biological functions. Herein, we investigated the potential of Thr‐OPN in liver fibrosis. Using patient samples, mouse models and hepatic stellate cells (HSCs), we analyzed the involvement of Thr‐OPN in liver fibrosis. The result showed that, first, Thr‐OPN level was significantly higher in patients with liver cirrhosis than that in patients with chronic hepatitis B and healthy controls. Thr‐OPN level was positively correlated with liver fibrosis degree in clinical samples. Then in mouse models, it showed a similar correlation between hepatic Thr‐OPN levels and liver fibrosis degree. Thr‐OPN peptides exacerbated liver fibrosis in OPN‐deficient mice, whereas the neutralization of Thr‐OPN alleviated liver fibrosis in wild‐type mice. Furthermore, when compared with full‐length OPN (FL‐OPN), Thr‐OPN exhibited a greater ability to promote HSC activation, proliferation, and migration via mitogen‐activated protein (MAP) kinase and nuclear factor (NF)‐κB pathways. In conclusion, Thr‐OPN, not FL‐OPN, was critically involved in the exacerbation of liver fibrosis by α9 and α4 integrins via MAP kinase and NF‐κB signaling pathway, thus representing a novel diagnostic biomarker and treatment target for liver cirrhosis.
Keywords: full‐length osteopontin (FL‐OPN), hepatic stellate cells (HSCs), integrins, liver fibrosis, thrombin‐cleaved osteopontin (Thr‐OPN)
1. INTRODUCTION
Liver cirrhosis (LC) is the end stage of chronic liver disease that results from liver injury of a variety of etiologies. Cirrhosis remains a leading cause of mortality among adults worldwide and is associated with increasing economic burden and social impact (Lozano et al., 2012). The transition from chronic liver disease to cirrhosis is caused by repeated liver injury and wound‐healing. This process is associated with the necrosis or apoptosis of hepatocytes, regeneration of hepatocytes, and non–parenchymal cells and extracellular matrix (ECM) deposition (Bataller & Brenner, 2005). Local macrophages and hepatic stellate cells (HSCs) are activated after necrosis or apoptosis. HSC is the critical non–parenchymal cell and also a target of various fibrogenic factors. After stimulation of inflammatory secreted from neighboring cells, the quiescent HSCs transdifferentiate and proliferate into myofibroblast‐like cells that adopt proinflammatory and profibrogenic phenotypes (Friedman, 2008;Tsukamoto, Zhu, Asahina, Mann, & Mann, 2011). Activated HSCs have been implicated in liver fibrosis by producing profibrogenic factors as well as factors that modulate hepatocyte proliferation and by remodeling the ECM (Yin, Evason, Asahina, & Stainier, 2013), also with immunocytes regulating both the progression and regression of liver fibrosis (Pellicoro, Ramachandran, Iredale, & Fallowfield, 2014). Transforming growth factor‐β (TGF‐β) is considered to be the most potent fibrogenic cytokine in the liver, stimulating ECM protein synthesis and inhibiting its degradation (Friedman, 2008; Tsukamoto et al., 2011). But to date, none have been validated for clinical use, and potentially promising sera biomarkers are only rarely tested in prediagnostic samples. Thus, to date, liver biopsy remains the most reliable way of evaluating liver fibrosis in patients with hepatitis. However, its inherent risks limit its widespread use.
Osteopontin (OPN) is a multifunctional protein and has been linked to many physiological and pathological events, such as cell migration, cell survival, intractable inflammation, and tumor metastasis. OPN can be found in body fluids including plasma, urine, and breast milk under normal physiological conditions and is secreted by macrophages, T cells, and natural killer T cells. A variety of inflammatory diseases, including multiple sclerosis (Chabas et al., 2001; Steinman & Zamvil, 2003), rheumatoid arthritis (RA) (Ohshima et al., 2002; Yumoto et al., 2002), and atherosclerosis (Bruemmer et al., 2003; Matsui et al., 2003), are associated with the high OPN expression. Importantly, OPN overexpression is associated with various liver diseases (Cui et al., 2015; Diao et al., 2004). In nonalcoholic steatohepatitis, OPN has been implicated in the promotion of cirrhosis (Syn et al., 2011). Binding of OPN to surface receptors stimulates cell adhesion, migration, tissue remodeling, and tumor metastasis (Senger, Brown, Perruzzi, Papadopoulossergiou, & Vandewater, 1995; Uchinaka et al., 2015; Uede, 2011; Yamamoto et al., 2003). The full‐length form of OPN (FL‐OPN), which contains an Arg‐Gly‐Asp (RGD) sequence, has diverse functions, including activation of T cells and macrophages, cell adhesion, chemotaxis, and immunomodulation through interaction with integrins such as αvβ3 and αvβ5 (Uchinaka et al., 2015; Uede, 2011; Yamamoto et al., 2003). However, cleavage of OPN by thrombin exposes an additional integrin‐binding motif, SVVYGLR (SLAYGLR in mice), promoting the cell adherence of RGD‐independent cells and a survival advantage to cells expressing α4β1 and α9β1 integrins (Senger et al., 1995; Smith & Giachelli, 1998; Smith et al., 1996; Yamaguchi et al., 2013). In mice, the thrombin‐cleaved form of OPN (Thr‐OPN) has been implicated in the pathogenesis of alcoholic hepatitis, ConA‐induced hepatitis, and RA (Diao et al., 2004; Morales‐Ibanez et al., 2013). Moreover, a recent study showed that Thr‐OPN enhanced the synthesis of type III collagen in myocardial fibrosis in a rat model of ischemic cardiomyopathy (Uchinaka et al., 2015).
However, there is limited information on the role of Thr‐OPN in human disease, and not much is still known about the function of Thr‐OPN in hepatic fibrosis progression. Therefore, in this study, we investigated the contribution of Thr‐OPN to liver fibrosis and assessed whether Thr‐OPN could be potentially used to stratify liver fibrosis severity.
2. MATERIALS AND METHODS
2.1. Human specimen collection
All patients involved in the study were admitted to the First Affiliated Hospital, School of Medicine, Zhejiang University (China) for diagnosis and treatment from January 2015 to August 2015. This study was approved by the Institutional Review Board of the First Affiliated Hospital, School of Medicine, Zhejiang University (reference number 2015‐311). Written consent was obtained from each patient before the procedure after full explanation of the purpose of the study. All patients were examined by ultrasound and/or computed tomography scans to detect and diagnose LC. Patients diagnosed with hepatocellular carcinoma were excluded in the study. Moreover, patients coinfected with human immunodeficiency virus, hepatitis A virus, hepatitis C virus or hepatitis D virus, or patients with resolved viral hepatitis were also excluded. In this study, 22 patients with LC group resulting from chronic hepatitis B (CHB) infection, and 36 patients with CHB without cirrhosis (CHB group) were screened. In addition, 30 healthy volunteers matched for age and sex were recruited as healthy controls (HC). The detailed information for all patients including age and sex are provided in Supporting Information Table S1. Plasma was collected from all patients in each group, and percutaneous liver biopsy using disposable needles (18 g × 16 cm, 22 mm penetration depth; Bard Max‐Core, Tempe, AZ) was performed on selected patients in each group.
2.2. Animals
All animal experimental procedures were approved by the Animal Care and Use Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (reference number 2015‐186). For the induction of Carbon tetrachloride (CCl4)‐mediated progressive liver fibrosis, 8 week–old male C57BL/6J mice received intraperitoneal injections of CCl4 (two injections per week of 25% CCl4 solution in olive oil, 2 ml/kg body weight). Progressive liver fibrosis models were divided into two groups: CCl4—six times group (two CCl4 injections per week for 3 weeks of, n = 6) and CCl4—eight times group (two CCl4 injections per week for 4 weeks, n = 6). Mice in both groups were anesthetized with ketamine hydrochloride (100 mg/kg intraperitoneal) and killed 48 hr after CCl4 injections. For the induction of liver inflammation, CCl4 was administered (once, n = 6) and mice were killed 24 hr later (CCl4—once group). Serum and liver tissue were stored at −80°C for further use. A piece of each liver was fixed with formaldehyde for histology.
2.3. Peptide preparation and CCl4‐mediated liver fibrosis in OPN‐deficient mice
Liver fibrosis was induced in age‐matched (8 week–old) male OPN‐deficient mice by intraperitoneal administration of CCl4 (two injections per week for 4 weeks, n = 6). For the indicated experiments, 400 μg of M5Ab or M1Ab (purchased from HuaAn Biotechnology, Hangzhou, China) were dissolved in 200 μl of phosphate‐buffered saline (PBS). These antibodies were administrated to wild‐type mice intravenously 3 hr before CCl4 challenge every time. Moreover, the cryptic epitope of OPN exposed by thrombin cleavage (VDVPNGRGDSLAYGLRS, M5 peptide; HuaAn Biotechnology), replacing Thr‐OPN, was administered (25 μg in 200 μl PBS per mouse, two injections per week for 4 weeks, n = 6) before intraperitoneal injections of CCl4. M1 peptide (LPVKVTDSGSSEEKL; HuaAn Biotechnology), the amino‐terminal portion of mouse OPN (amino acids 17–31), was used as a control. Mice were killed at 48 hr after the last injection.
2.4. Preparation of Thr‐OPN
Human Thr‐OPN was prepared by thrombin‐mediated cleavage of recombinant human FL‐OPN (R&D) at 10 μg FL‐OPN per 1 U of the enzyme at 37°C for 1 hr.
2.5. The details of the other methods including liver pathology
Immunohistochemical staining, cell culture, treatment, proliferation assay, migration assay, western blot analysis, analysis of messenger RNA expression, and the ELISA assays were described in the Supporting Information Materials and Methods
2.6. Statistical analysis
All data were expressed as the mean ± standard deviation. SPSS17.0 statistical software was used for statistical analysis. Statistical significance among different groups was analyzed by one‐way analysis of variance, and categorical variables were analyzed by Fisher's exact test or Rank sum test. Pearson's correlation analysis was performed for normally distributed variables. Statistical significance was set at p < 0.05.
3. RESULTS
3.1. Increased Thr‐OPN expression in patients with LC
Previous studies have shown that FL‐OPN expression is increased in patients with CHB (Ge et al., 2015). However, plasma Thr‐OPN levels have not been reported in patients with CHB and LC. We found that Thr‐OPN levels were significantly increased only in the patients with LC compared with those in the patients with CHB (p = 0.0027) and HCs (p = 0.0015). There was no obvious difference in Thr‐OPN levels between patients with CHB and HCs (p = 0.886; Figure 1a). Meanwhile, FL‐OPN levels were significantly higher in patients with LC than those in patients with CHB (p = 0.006). However, in contrast to the patterns of Thr‐OPN expression, FL‐OPN levels were increased in the CHB group compared with those in the HC group (p = 0.007). There was a sequential increase in plasma FL‐OPN expression across the HC group, patients CHB, and LC (Figure 1a). Moreover, in the liver of patients with CHB, inflammatory cell infiltration was observed (Figure 1b), and the Alanine transaminase levels were significantly increased (Supporting Information Table S1). Furthermore, the degree of fibrosis was exacerbated significantly in the liver of patients with LC (Figure 1b).
Figure 1.
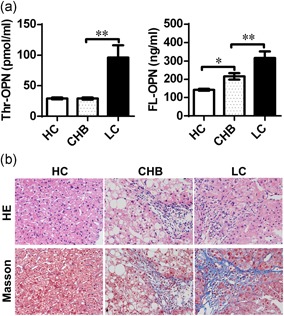
Marked increase of Thr‐OPN expression in patients with liver cirrhosis (LC). (a) Plasma levels of Thr‐OPN and FL‐OPN in patients with CHB and LC were determined. *p < 0.05; **p < 0.01. (b) Liver tissue from healthy controls (HC), and patients with chronic hepatitis B (CHB) and LC were stained with hematoxylin and eosin staining and Masson's trichrome staining (blue), original magnification, 400×. FL‐OPN: full‐length osteopontin; Thr‐OPN: thrombin‐cleaved osteopontin [Color figure can be viewed at wileyonlinelibrary.com]
Hyaluronic acid (HA), N‐terminal propeptide of procollagen III (PCIIINP), and collagen type IV (CIV) represent noninvasive markers of fibrosis, which are correlated to the degree of fibrosis in patients with chronic liver disease (El‐Shabrawi et al., 2012; Murawaki, Ikuta, Koda, & Kawasaki, 1994). Levels of HA, PCIIINP, and CIV were obviously elevated in patients with LC compared with those in patients with CHB (Supporting Information Figure S1a). Thr‐OPN levels showed a significantly positive correlation with plasma HA levels (r = 0.4389, p = 0.003). Further, there were significant positive correlations between Thr‐OPN and PCIIINP levels (r = 0.6878, p < 0.001) and between Thr‐OPN and CIV levels (r = 0.6383, p < 0.001; Supporting Information Figure S1b). Importantly, the correlation coefficients between Thr‐OPN levels and the three biomarkers were higher than those between FL‐OPN and the three biomarkers (Supporting Information Figure S1b and S1c).
TGF‐β stimulates the synthesis of ECM proteins, inhibits their degradation, and is considered to be the most potent fibrogenic cytokine in the liver (Friedman, 2008; Tsukamoto et al., 2011). Thr‐OPN levels also showed a significantly positive correlation with TGF‐β expression (r = 0.6080, p < 0.001; Supporting Information Figure S2a). In addition, expression of tumor necrosis factor (TNF)‐α, the important inflammatory cytokine, was increased in patients with both CHB and LC compared with those in HCs. However, there was no significant correlation between plasma Thr‐OPN and TNF‐α expression (r = 0.0716, p = 0.5766; Supporting Information Figure S2b).
3.2. Thr‐OPN expression was significantly increased with the progression of liver fibrosis in mice
To further validate the role of Thr‐OPN, we established the progressive model of liver fibrosis induced by intraperitoneal injection of CCl4 in mice. Liver histology studies showed obvious inflammatory cell infiltration and significantly increased serum ALT levels 24 hr after CCl4 administration compared with normal control mice (p < 0.001, Figure 2a,b; Supporting Information Figure S2c).
Figure 2.
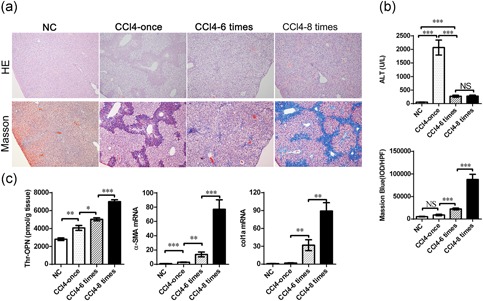
The Thr‐OPN expression level is significantly increased with the progression of liver fibrosis in mice. (a) Hematoxylin and eosin staining of liver tissue (above) and Masson's trichrome staining (below) were determined in CCl4‐induced hepatitis and progressive liver fibrosis in mice (original magnification, 100×). (b) Serum ALT (above) and the IOD value (below) were determined in CCl4‐induced hepatitis and progressive liver fibrosis in mice, data represented the average result of 10 random high‐power fields. (c) The level of Thr‐OPN, the expression of α‐SMA and collagen 1 mRNA in liver tissue was detected in CCl4‐induced hepatitis and progressive liver fibrosis in mice. *p < 0.05; **p < 0.01; ***p < 0.001. ALT: alanine transaminase; CCl4: carbon tetrachloride; IOD: integral optical density; mRNA: messenger RNA; Thr‐OPN: thrombin‐cleaved osteopontin; α‐SMA: alpha‐smooth muscle actin [Color figure can be viewed at wileyonlinelibrary.com]
Masson's trichrome staining (Figure 2a; Supporting Information Figure S2c) showed that there was a significant increase of the degree and distribution of liver fibrosis (blue) in CCl4 administration group, especially after eight times of CCl4 injection. To further quantify the degree of liver fibrosis, the integral optical density (IOD) per high‐power field was calculated and compared. Compared with normal control and CCl4—once the group, the IOD value was significantly increased in the CCl4—six times, and CCl4—eight times groups (all p < 0.001). Notably, the IOD value was significantly higher in the CCl4—eight times group than that in CCl4—six times group (p < 0.001; Figure 2b), which suggested a significant progression of liver fibrosis after six times and eight times injection.
To investigate the effect of Thr‐OPN and FL‐OPN in liver fibrosis, we detected the levels of Thr‐OPN and FL‐OPN in liver tissue. As shown in Figure 2c, Thr‐OPN levels in liver tissues were increased in the CCl4—once group compared with the normal control mice (p < 0.01). Importantly, Thr‐OPN levels in liver tissues exhibited a significant sequential increase across the CCl4—once, CCl4—six times, and CCl4—eight times groups. This trend was consistent with the process of liver fibrosis. In addition, FL‐OPN levels were significantly elevated in the CCl4—once group compared with the normal control mice (p < 0.001) but were significantly lower in the fibrosis groups (p < 0.001; Supporting Information Figure S2d). This trend was consistent with the process of liver injury.
Alpha‐smooth muscle actin (α‐SMA) is an important marker of HSC activation. To further explore the role of Thr‐OPN in liver fibrosis, we identified the expression of α‐SMA and HSC marker desmin (Troeger et al., 2012) in liver tissues during the progression of liver fibrosis. Our experimental results indicated that the expressions of α‐SMA and desmin in liver tissue were gradually increased with the progression of liver fibrosis at both messenger RNA (mRNA) and protein levels (Figure 2c; Supporting Information Figures S3a–c). Moreover, as crucial factors in liver fibrosis, collagen 1a, TGF‐β, and tissue inhibitor of matrix metalloprotease‐1 exhibited a sustained increase in liver tissues with the progression of liver fibrosis (Supporting Information Figure S3d and S3e). Furthermore, Thr‐OPN expression was positively correlated to the degree of liver fibrosis (r = 0.8422, p < 0.001), as well as the mRNA levels of α‐SMA (r = 0.7651, p < 0.001) and collagen 1a (r = 0.8294, p < 0.001) in liver tissues (Supporting Information Figure S4a).
3.3. Thr‐OPN exacerbated CCl4‐induced liver fibrosis
Previous studies reported that a polyclonal antibody (M5Ab, raised against a synthetic peptide‐VDVPNGRGDSLAYGLRS) could block the functions of Thr‐OPN in mice (Asai et al., 2010; Diao et al., 2004). To provide direct evidence for the role of Thr‐OPN in liver fibrosis, M5Ab was used in CCl4‐induced liver fibrosis, and M1Ab was used as the control. As shown in Figure 3a and Supporting Information Figure S4b, Masson's trichrome staining showed that liver fibrosis was alleviated in M5Ab group versus M1Ab group. Moreover, a similar result was observed in the expression of a‐SMA in mice liver (Figure 3b; Supporting Information Figure S4b). In addition, a cryptic epitope of OPN exposed by thrombin cleavage (VDVPNGRGDSLAYGLRS, M5 peptide) was used to reflect the degree and progression of liver fibrosis in OPN‐deficient and wild‐type mice. We found that liver fibrosis was significantly decreased in OPN‐deficient mice compared with that in wild‐type mice after eight injections of CCl4 (Figure 3c; Supporting Information Figure S4c). Interestingly, administration of the M5 peptide significantly increased the degree of liver fibrosis in OPN‐deficient mice (Figure 3c; Supporting Information Figure S4c). In contrast, the administration of control peptide (M1 peptide) did not increase liver fibrosis in OPN‐deficient mice (Figure 3c; Supporting Information Figure S4c).
Figure 3.
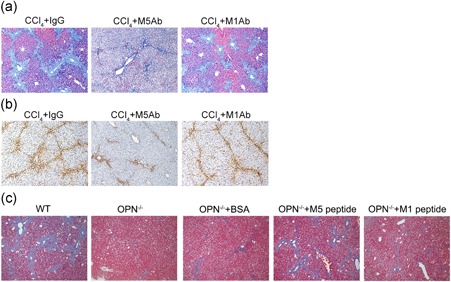
Thr‐OPN exacerbated CCl4‐induced liver fibrosis. (a) CCl4‐induced mice were treated with M5Ab or M1Ab before 4 hr CCl4 injection every time, and liver fibrosis (Masson's trichrome staining, blue) and (b) α‐SMA expression (yellow) were evaluated in wild‐type mice (original magnification, 100×). (c) Liver fibrosis (Masson's trichrome staining, blue) was evaluated in wild‐type mice and OPN‐deficient mice after administration of the exposed peptide of Thr‐OPN (M5 peptide) or control peptide (M1 peptide). CCl4: Carbon tetrachloride; OPN: osteopontin; Thr‐OPN: thrombin‐cleaved osteopontin; α‐SMA: alpha‐smooth muscle actin [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Thr‐OPN promoted the activation, proliferation, and migration of HSCs
Activation of HSCs has been identified as the central event in ECM deposition and the progression of liver fibrosis (Friedman, Roll, Boyles, & Bissell, 1985; Pellicoro et al., 2014). To further explore the role of Thr‐OPN in liver fibrosis, we analyzed the activation, proliferation, and migration of HSC line LX‐2 cells in vitro after the administration of Thr‐OPN and found that Thr‐OPN induced higher levels of α‐SMA and desmin in LX‐2 cells compared with those induced by FL‐OPN (p < 0.01; Figure 4a). Furthermore, Thr‐OPN stimulation also induced higher levels of TGF‐β and collagen 1a mRNA in LX‐2 cells (Figure 4b,c). Proliferation is a key change observed in HSCs in the sustained activation state (Paradis et al., 2002; Xiao et al., 2012). During a continuous 3‐day observation, the growth of LX‐2 cells was much more rapid in the Thr‐OPN stimulation group compared with that in the FL‐OPN stimulation and HC groups (Figure 4d). Moreover, based on migration assay of HSCs, Thr‐OPN stimulation exhibited powerful attraction ability for cell migration compared with that in response to FL‐OPN stimulation, from 0, 6, 18, 24, 36, to 48 hr after the consistent stimulation of Thr‐OPN or FL‐OPN, respectively (Supporting Information Figure S4d).
Figure 4.
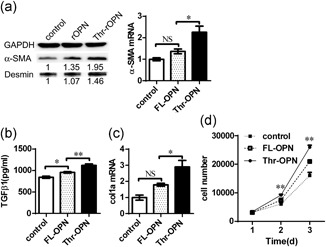
Thr‐OPN promotes the activation, proliferation, and migration of HSCs in vitro. The expression of α‐SMA protein and desmin mRNA (a), the level of TGF‐β (b), and the expression of col1a (c) mRNA by LX‐2 cells after stimulation with Thr‐OPN stimulation were compared with those after FL‐OPN stimulation. (d) The effects of Thr‐OPN and FL‐OPN on LX‐2 cell proliferation were evaluated by Microfluidic System. *p < 0.05; **p < 0.01. FL‐OPN: full‐length osteopontin; HSC: hepatic stellate cell; mRNA: messenger RNA; TGF‐β: transforming growth factor‐β; Thr‐OPN: thrombin‐cleaved osteopontin; α‐SMA: alpha‐smooth muscle actin
3.5. Thr‐OPN activated HSCs by α9 and α4 integrins that presented high expressions in liver fibrosis of both patients and the mice model
Cleavage of OPN by thrombin exposes an additional integrin‐binding motif (SVVYGLR sequence) that is SLAYGLR in mice, thereby promoting the adherence of cells expressing α4 and α9 integrins (Senger et al., 1995). Expression of both α9 and α4 in liver tissues was increased in patients with LC compared with that in patients with CHB (Figure 5a). The mRNA and proteins levels of α9 and α4 integrins exhibited a sustained increase with the progression of liver fibrosis across the CCl4—once, CCl4—six times, and CCl4—eight times groups (Figure 5b,c).
Figure 5.
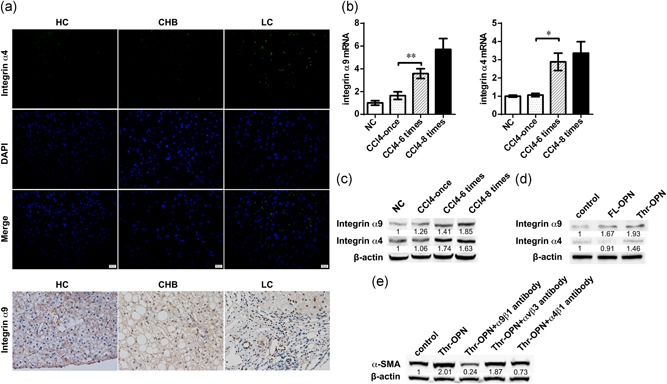
Thr‐OPN activated HSCs by α9 and α4 integrins that presented high expressions in liver fibrosis. (a) α4 and α9 integrin expression in liver from healthy control (HC) individuals, patients with chronic hepatitis B (CHB), and liver cirrhosis (LC, original magnification, 400×). (b) α9 and α4 integrin mRNA expressions in the liver in a CCl4‐induced progressive liver fibrosis mouse model. (c) α9 and α4 integrin protein expressions in the liver in the mouse model. (d) The expression of α9 and α4 integrin protein in LX‐2 cells after stimulation with Thr‐OPN or FL‐OPN. (e) The expression of α‐SMA protein in Thr‐OPN‐stimulated LX‐2 cells after administration of blocking antibodies to integrin α9β1, ανβ3, and α4β1. *p < 0.05; **p < 0.01. CCl4: carbon tetrachloride; FL‐OPN: full‐length osteopontin; HSC: hepatic stellate cell; Thr‐OPN: thrombin‐cleaved osteopontin; α‐SMA: alpha‐smooth muscle actin [Color figure can be viewed at wileyonlinelibrary.com]
To provide further evidence for the contribution of α9 and α4 integrins to liver fibrosis, we analyzed the relationships between Thr‐OPN expression and integrin α9 and α4 in LX‐2 cells after stimulation with FL‐OPN or Thr‐OPN. HSCs activated by Thr‐OPN stimulation expressed higher levels of α4 and α9 integrins compared with those expressed after FL‐OPN stimulation (Figure 5d). Moreover, blocking antibodies to integrin α9β1, α4β1, and ανβ3 were applied to search for the receptors of Thr‐OPN on HSCs activation. Expressions of both α‐SMA and col1a were inhibited by α9β1 and α4β1 antibodies after Thr‐OPN stimulation, but not ανβ3 antibody (Figure 5e; Supporting Information Figure S5a).
3.6. Thr‐OPN activated HSCs by integrins α9 and α4 via mitogen‐activated protein kinase and NF‐κB signaling pathway
Previous studies indicated that OPN exerted its function by mitogen‐activated protein kinase (MAPK) and NF‐κb pathway in cell survival and activation (Diao et al., 2012; Urtasun et al., 2012; Zhang et al., 2015). Thus, we analyzed the expression of crucial proteins involved in HSCs activation after Thr‐OPN stimulation, such as p38, extracellular signal‐regulated kinase (Erk) 1/2, c‐Jun N‐terminal kinase (JNK), and NF‐κB p65. First, our laboratory data revealed that the changes in p38, Erk1/2, JNK, and NF‐κb p65 of HSCs were increased after Thr‐OPN stimulation. After the blockage by integrins α9 and α4 antibodies, the expressions of the total protein or the phosphorylation of p38, Erk1/2, JNK, and NF‐κb p65 were inhibited (Figure 6a; Supporting Information Figure S5b). To further confirm the signaling pathway of HSCs activation after Thr‐OPN stimulation, protein inhibitors of MAPK and NF‐κb signaling pathways were used. In genome and protein expression level, we found that α‐SMA and col1a expressions of HSC after Thr‐OPN stimulation were higher than the controls. However, the elevated α‐SMA and col1a expressions of HSC were inhibited with the inhibitor of p38, Erk1/2, and NF‐κb, but not the inhibitor of JNK (Figure 6b–d).
Figure 6.
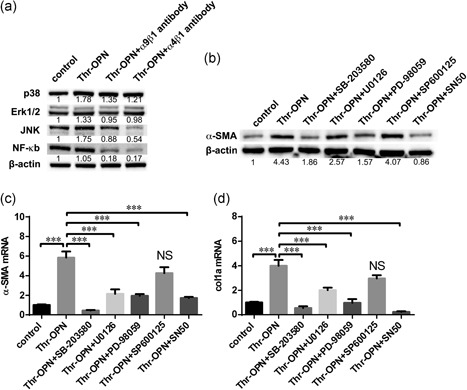
Thr‐OPN activated HSCs by integrins α9 and α4 via MAP kinase and NF‐κB signaling pathway. (a) The expression of p38, Erk1/2, JNK, and NF‐κb p65 protein in Thr‐OPN‐stimulated LX‐2 cells with or without blocking antibodies to integrin α9β1 and α4β1. (b) The expression of α‐SMA protein in Thr‐OPN‐stimulated LX‐2 cells with or without inhibitors of p38, Erk1/2, JNK, and NF‐κb p65. And the expression of α‐SMA (c) and col1a (d) mRNA expression by Thr‐OPN‐stimulated LX‐2 cells were analyzed (qPCR) with or without inhibitors of p38, Erk1/2, JNK, and NF‐κb p65. ***p < 0.001. Erk: extracellular signal‐regulated kinase; HSC: hepatic stellate cell; JNK: c‐Jun N‐terminal kinase; MAP: mitogen‐activated protein kinase; mRNA: messenger RNA; NF: nuclear factor; Thr‐OPN: thrombin‐cleaved osteopontin; qPCR: quantitative polymerase chain reaction; α‐SMA: alpha‐smooth muscle actin
4. DISCUSSION
In the current study, we examined whether Thr‐OPN played a pivotal role in the pathogenesis of liver fibrosis. We found higher expression of Thr‐OPN in patients with LC compared with that in patients with CHB, whereas there was no difference between the levels detected in patients with CHB and healthy volunteers. Importantly, Thr‐OPN level was positively correlated to the degree of liver fibrosis. Therefore, we investigated the contribution of Thr‐OPN to LC. We found that Thr‐OPN promoted the activation, function, and proliferation of HSC cells, and played a pivotal role in the progression of liver fibrosis both in vitro and in vivo.
Histopathological examination of percutaneous biopsies is the “gold standard” method for the diagnosis of LC; however, this approach is limited by the invasive nature of the method. Previous reports have indicated that human plasma cytokines, such as TGF‐β and IL‐17, are gradually elevated during the progression from hepatitis to LC (Matsue et al., 2015; Yu et al., 2014), whereas few cytokines are elevated only in liver fibrosis but not by the process of inflammation. To date, noninvasive markers (plasma cytokines) of fibrosis have been validated for clinical use, and potentially promising biomarkers are only rarely tested in prediagnostic samples. We found that plasma Thr‐OPN was significantly increased in patients with LC, whereas there was no difference in Thr‐OPN levels in patients with CHB and healthy volunteers. Relevant to this investigation, a recent study reported that Thr‐OPN enhances the synthesis of collagen type III in myocardial fibrosis in rats (Uchinaka et al., 2015). We also found that Thr‐OPN levels were remarkably elevated in the liver tissue in the CCl4‐induced liver fibrosis mouse model. In our preliminary experiment, we found that ALT level was sharply increased without liver fibrosis in acute liver injury induced by CCl4 or ConA in mice, However, multiple and repeated stimulations induced a low and more adapted response of liver injury. Therefore, mice could recover from liver injury once they lived through the attack. And also, it showed an attenuated elevated ALT level after CCl4—six times and CCl4—eight times. Interestingly, our in vivo experiments also indicated that the changes in Thr‐OPN levels in liver tissues were consistent with the degree of liver fibrosis. These observations further confirmed the crucial role of Thr‐OPN in the progression of liver fibrosis. Thus, Thr‐OPN was implicated as a predictive factor for liver fibrosis.
A polyclonal antibody (M5Ab) raised against a synthetic peptide (a cryptic epitope of OPN exposed by thrombin cleavage, VDVPNGRGDSLAYGLRS, M5 peptide) has been shown to block the functions of Thr‐OPN in mice (Asai et al., 2010; Diao et al., 2004; Diao et al., 2008). In ConA‐induced hepatitis, OPN‐deficient mice and M5Ab were used to identify the function of Thr‐OPN because OPN‐deficient mice were refractory to Con A‐induced hepatitis, and Thr‐OPN blockage by M5Ab also could ameliorate liver injury (Diao et al., 2008). In contrast, the M1Ab, which was a polyclonal antibody raised against the M1 peptide (LPVKVTDSGSSEEKL) that formed the amino‐terminal portion of murine OPN, did not block the functions of Thr‐OPN (Diao et al., 2004; Kon et al., 2002), and the M1Ab was used as control antibody of M5Ab in a series of studies (Asai et al., 2010; Diao et al., 2004). In our study, we found that only M5Ab, not M1Ab, alleviated CCl4‐induced liver fibrosis in wild‐type mice. Furthermore, previous studies had shown that this synthetic M5 peptide functions in the same way as Thr‐OPN in cell culture (Cui et al., 2015; Yamamoto et al., 2003). Thus, the M5 peptide was used to verify the function of Thr‐OPN in the progression of liver fibrosis, and the M1 peptide was used as the control. We found that liver fibrosis was significantly alleviated in OPN‐deficient mice compared with that in wild‐type mice. Interestingly, when the M5 peptide was administered, liver fibrosis was significantly exacerbated in OPN‐deficient mice, whereas the control M1 peptides had no significant effect. These data directly indicated that Thr‐OPN was critically involved in the pathogenesis of liver fibrosis.
In our study, the expression of Thr‐OPN was positively correlated with plasma TGF‐β levels, suggesting that Thr‐OPN was also important in the progression of liver fibrosis. Furthermore, Thr‐OPN administration induced higher expression of α‐SMA, desmin, TGF‐β, and collagen 1a, as well as a faster growth rate in HSCs compared with the effects of FL‐OPN administration. Not only did these data suggest that Thr‐OPN promoted HSC activation, proliferation and contributed to liver fibrosis, but also that Thr‐OPN possessed a more powerful capacity in this regard than FL‐OPN. Recent reports indicated that plasma FL‐OPN levels were elevated in LC (Matsue et al., 2015; Syn et al., 2011; Zhao et al., 2008) via the activation of HSCs (Urtasun et al., 2012; Xiao et al., 2012). In our study, we also found a sequential increase in plasma FL‐OPN expression across the HC group, patients with CHB, and LC; however, in contrast to the patterns of FL‐OPN expression, Thr‐OPN levels were significantly increased only in the patients with LC compared with those in the patients with CHB and HCs. There was no obvious difference in Thr‐OPN levels between patients with CHB and HCs. OPN interacted with a variety of cell surface receptors, including αvβ3, αvβ5, α4β1, and α8β1 integrins, as well as CD44 to stimulate cell adhesion, migration, chemotaxis, and tissue remodeling, as well as cell survival and inflammatory cell chemoattraction and tumor metastasis (Bataller & Brenner, 2005; Senger et al., 1995; Uchinaka et al., 2015; Uede, 2011; Yamamoto et al., 2003). The previous study indicated that αvβ3 integrin antibody blocked the increase of collagen‐I expression after OPN stimulation, but neutralization of other integrins (i.e. β1, β5, and β6) failed to prevent the increase of collagen‐I (Uchinaka et al., 2015). Both FL‐OPN and Thr‐OPN bound to α4 integrin, whereas only Thr‐OPN bound to α9 integrin via SVVYGLR sequence that was exposed by thrombin‐mediated cleavage (Green, Ludbrook, Miller, Horgan, & Barry, 2001; Smith & Giachelli, 1998). Thr‐OPN performed functions such as promoting cell adhesion, migration, and the production of α‐SMA and collagen through its interactions with α4β1 and α9β1 integrins (Senger et al., 1995; Uchinaka et al., 2015; Uede, 2011; Yamamoto et al., 2003). In this study, we found higher expression of both α4 and α9 integrins in liver tissues of patients with LC compared with that in patients with CHB. The expression of α4 and α9 integrins in the mouse livers also exhibited a sustained increase in the progression of liver fibrosis at both the mRNA and protein levels. Furthermore, we also found that the activated HSCs expressed higher levels of α4 and α9 integrins after Thr‐OPN stimulation compared with the levels detected after FL‐OPN stimulation in vitro. Moreover, α9β1 and α4β1 antibodies blocked both α‐SMA and col1a expressions of HSCs after Thr‐OPN stimulation. In addition, we found that α‐SMA and col1a expressions of HSC after Thr‐OPN stimulation were inhibited by the inhibitor of p38, Erk1/2, and NF‐κb. However, inhibition is slight with JNK inhibitor, which could be due to the crosstalk with other pathways. These observations indicated that Thr‐OPN was involved in promoting the progression of liver fibrosis via a positive feedback mechanism (Supporting Information Figure S5c).
The activation, function, and proliferation of HSCs are promoted by Thr‐OPN via MAP kinase and NF‐kB signal pathways when interacted with α9 and α4 integrin receptors. Besides, Thr‐OPN is implicated in many other diseases. It was reported that Thr‐OPN could regulate hemopoietic stem and progenitor cell functions through interactions with integrins (Grassinger et al., 2009). Also, administration of Thr‐OPN did not affect preterm brain injury, but OPN‐derived peptides increased the severity of brain injury in the preterm brain injury model (Albertsson et al., 2014). Shio et al. (2010) found that thrombin‐cleaved OPN was also found increased in the urine of patients with RA, which may reflect the severity of active inflammatory arthritis.
In conclusion, compared with CHB patients, Thr‐OPN was expressed at higher levels in patients with LC, whereas there was no difference in the levels detected in patients with CHB and healthy volunteers. Importantly, Thr‐OPN level was positively correlated with liver fibrosis. Moreover, Thr‐OPN was involved in liver fibrosis progression both in vitro and in vivo. In addition, Thr‐OPN induced HSCs activation by higher expression of α4 and α9 integrins via MAP kinase and NF‐κB signaling pathway. These results suggested that Thr‐OPN, not FL‐OPN, was critically involved in the pathogenesis of liver fibrosis by α9 and α4 integrins via MAP kinase and NF‐κB signaling pathway, thus representing a novel diagnostic biomarker and treatment target for LC.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interests.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This study was supported by The National Natural Science Foundation of China (No. 81571953, 81702757), The National Basic Research Program (2013CB531405), Zhejiang Provincial Natural Science Foundation of China (LY16H190002), and The National Hygiene Bureau and Zhejiang Province Co‐construction Project (2015118507).
Cui G, Chen J, Wu Z, et al. Thrombin cleavage of osteopontin controls activation of hepatic stellate cells and is essential for liver fibrogenesis. J Cell Physiol. 2019;234:8988–8997. 10.1002/jcp.27571
References
REFERENCES
- Albertsson, A. M. , Zhang, X. , Leavenworth, J. , Bi, D. , Nair, S. , Qiao, L. , … Wang, X. (2014). The effect of osteopontin and osteopontin‐derived peptides on preterm brain injury. Journal of Neuroinflammation, 11, 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, S. , Kimbara, N. , Tada, T. , Imai, M. , Campbell, W. , Okada, H. , & Okada, N. (2010). Procarboxypeptidase R deficiency causes increased lethality in concanavalin A‐induced hepatitis in female mice. Biological and Pharmaceutical Bulletin, 33(7), 1256–1259. [DOI] [PubMed] [Google Scholar]
- Bataller, R. , & Brenner, D. A. (2005). Liver fibrosis. The Journal of Clinical Investigation, 115(2), 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruemmer, D. , Collins, A. R. , Noh, G. , Wang, W. , Territo, M. , Arias‐Magallona, S. , … Hsueh, W. A. (2003). Angiotensin II‐accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin‐deficient mice. Journal of Clinical Investigation, 112(9), 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabas, D. , Baranzini, S. E. , Mitchell, D. , Bernard, C. C. , Rittling, S. R. , Denhardt, D. T. , … Steinman, L. (2001). The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science, 294(5547), 1731–1735. [DOI] [PubMed] [Google Scholar]
- Cui, G. Y. , Chen, J. N. , He, J. Q. , Lu, C. , Wei, Y. F. , Wang, L. , … Diao, H. Y. (2015). Osteopontin promotes dendritic cell maturation and function in response to HBV antigens. Drug Design, Development and Therapy, 9, 3003–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, H. , Kon, S. , Iwabuchi, K. , Kimura, C. , Morimoto, J. , Ito, D. , … Uede, T. (2004). Osteopontin as a mediator of NKT cell function in T cell‐mediated liver diseases. Immunity, 21(4), 539–550. [DOI] [PubMed] [Google Scholar]
- Diao, H. , Iwabuchi, K. , Li, L. , Onoe, K. , Van Kaer, L. , Kon, S. , … Uede, T. (2008). Osteopontin regulates development and function of invariant natural killer T cells. Proceedings of the National Academy of Sciences of the United States of America, 105(41), 15884–15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, H. , Liu, X. , Wu, Z. , Kang, L. , Cui, G. , Morimoto, J. , … Li, L. (2012). Osteopontin regulates interleukin‐17 production in hepatitis. Cytokine, 60(1), 129–137. [DOI] [PubMed] [Google Scholar]
- El‐Shabrawi, M. H. F. , Zein El Abedin, M. Y. , Omar, N. , Kamal, N. M. , Elmakarem, S. A. , Khattab, S. , … Ali, A. S. M. (2012). Predictive accuracy of serum hyaluronic acid as a non‐invasive marker of fibrosis in a cohort of multi‐transfused Egyptian children with beta‐thalassaemia major. Arab Journal of Gastroenterology, 13(2), 45–48. [DOI] [PubMed] [Google Scholar]
- Friedman, S. L. (2008). Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews, 88(1), 125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, S. L. , Roll, F. J. , Boyles, J. , & Bissell, D. M. (1985). Hepatic lipocytes: The principal collagen‐producing cells of normal rat liver. Proceedings of the National Academy of Sciences of the United States of America, 82(24), 8681–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, T. , Shen, Q. , Wang, N. , Zhang, Y. , Ge, Z. , Chu, W. , … Qin, W. (2015). Diagnostic values of alpha‐fetoprotein, dickkopf‐1, and osteopontin for hepatocellular carcinoma. Medical Oncology, 32(3), 59. [DOI] [PubMed] [Google Scholar]
- Grassinger, J. , Haylock, D. N. , Storan, M. J. , Haines, G. O. , Williams, B. , Whitty, G. A. , … Nilsson, S. K. (2009). Thrombin‐cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood, 114(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Green, P. M. , Ludbrook, S. B. , Miller, D. D. , Horgan, C. M. T. , & Barry, S. T. (2001). Structural elements of the osteopontin SVVYGLR motif important for the interaction with alpha(4) integrins. FEBS Letters, 503(1), 75–79. [DOI] [PubMed] [Google Scholar]
- Kon, S. , Yokosaki, Y. , Maeda, M. , Segawa, T. , Horikoshi, Y. , Tsukagoshi, H. , … Uede, T. (2002). Mapping of functional epitopes of osteopontin by monoclonal antibodies raised against defined internal sequences. Journal of Cellular Biochemistry, 84(2), 420–432. [DOI] [PubMed] [Google Scholar]
- Lozano, R. , Naghavi, M. , Foreman, K. , Lim, S. , Shibuya, K. , Aboyans, V. , … Memish, Z. A. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet, 380(9859), 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsue, Y. , Tsutsumi, M. , Hayashi, N. , Saito, T. , Tsuchishima, M. , Toshikuni, N. , … George, J. (2015). Serum osteopontin predicts degree of hepatic fibrosis and serves as a biomarker in patients with hepatitis C virus infection. PLoS One, 10, e0118744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui, Y. , Rittling, S. R. , Okamoto, H. , Inobe, M. , Jia, N. , Shimizu, T. , … Uede, T. (2003). Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E‐deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 23(6), 1029–1034. [DOI] [PubMed] [Google Scholar]
- Morales‐Ibanez, O. , Domínguez, M. , Ki, S. H. , Marcos, M. , Chaves, J. F. , Nguyen‐Khac, E. , … Bataller, R. (2013). Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology, 58(5), 1742–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawaki, Y. , Ikuta, Y. , Koda, M. , & Kawasaki, H. (1994). Serum type‐Iii procollagen peptide, type‐Iv collagen 7s domain, central Triple‐Helix of Type‐Iv collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver‐disease ‐ relationship to liver histology. Hepatology, 20(4), 780–787. [DOI] [PubMed] [Google Scholar]
- Ohshima, S. , Yamaguchi, N. , Nishioka, K. , Mima, T. , Ishii, T. , Umeshita‐Sasai, M. , … Saeki, Y. (2002). Enhanced local production of osteopontin in rheumatoid joints. The Journal of Rheumatology, 29(10), 2061–2067. [PubMed] [Google Scholar]
- Paradis, V. , Dargere, D. , Bonvoust, F. , Vidaud, M. , Segarini, P. , & Bedossa, P. (2002). Effects and regulation of connective tissue growth factor on hepatic stellate cells. Laboratory Investigation, 82(6), 767–774. [DOI] [PubMed] [Google Scholar]
- Pellicoro, A. , Ramachandran, P. , Iredale, J. P. , & Fallowfield, J. A. (2014). Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nature Reviews Immunology, 14(3), 181–194. [DOI] [PubMed] [Google Scholar]
- Senger, D. R. , Brown, L. F. , Perruzzi, C. A. , Papadopoulossergiou, A. , & Vandewater, L. (1995). Osteopontin at the tumor host interface—Functional regulation by thrombin‐cleavage and consequences for cell‐adhesion. Osteopontin: Role in Cell Signalling and Adhesion, 760, 83–100. [DOI] [PubMed] [Google Scholar]
- Shio, K. , Kobayashi, H. , Asano, T. , Saito, R. , Iwadate, H. , Watanabe, H. , … Ohira, H. (2010). Thrombin‐cleaved osteopontin is increased in urine of patients with rheumatoid arthritis. The Journal of Rheumatology, 37(4), 704–710. [DOI] [PubMed] [Google Scholar]
- Smith, L. L. , & Giachelli, C. M. (1998). Structural requirements for alpha(9)beta(1)‐mediated adhesion and migration to thrombin‐cleaved osteopontin. Experimental Cell Research, 242(1), 351–360. [DOI] [PubMed] [Google Scholar]
- Smith, L. L. , Cheung, H. K. , Ling, L. E. , Chen, J. , Sheppard, D. , Pytela, R. , & Giachelli, C. M. (1996). Osteopontin N‐terminal domain contains a cryptic adhesive sequence recognized by alpha(9)beta(1) integrin. Journal of Biological Chemistry, 271(45), 28485–28491. [PubMed] [Google Scholar]
- Steinman, L. , & Zamvil, S. (2003). Transcriptional analysis of targets in multiple sclerosis. Nature Reviews Immunology, 3(6), 483–492. [DOI] [PubMed] [Google Scholar]
- Syn, W. K. , Choi, S. S. , Liaskou, E. , Karaca, G. F. , Agboola, K. M. , Oo, Y. H. , … Diehl, A. M. (2011). Osteopontin is induced by Hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology, 53(1), 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeger, J. S. , Mederacke, I. , Gwak, G. Y. , Dapito, D. H. , Mu, X. , Hsu, C. C. , … Schwabe, R. F. (2012). Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology, 143(4), 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto, H. , Zhu, N. L. , Asahina, K. , Mann, D. A. , & Mann, J. (2011). Epigenetic cell fate regulation of hepatic stellate cells. Hepatology Research, 41(7), 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchinaka, A. , Hamada, Y. , Mori, S. , Miyagawa, S. , Saito, A. , Sawa, Y. , … Kawaguchi, N. (2015). SVVYGLR motif of the thrombin‐cleaved N‐terminal osteopontin fragment enhances the synthesis of collagen type III in myocardial fibrosis. Molecular and Cellular Biochemistry, 408(1‐2), 191–203. [DOI] [PubMed] [Google Scholar]
- Uede, T. (2011). Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathology International, 61(5), 265–280. [DOI] [PubMed] [Google Scholar]
- Urtasun, R. , Lopategi, A. , George, J. , Leung, T. M. , Lu, Y. , Wang, X. , … Nieto, N. (2012). Osteopontin, an oxidant stress sensitive cytokine, upregulates collagen‐I via integrin aV ss 3 engagement and PI3K/pAkt/NF?B signaling. Hepatology, 55(2), 594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X. , Gang, Y. , Gu, Y. , Zhao, L. , Chu, J. , Zhou, J. , … Fan, D. (2012). Osteopontin contributes to TGF‐beta 1 mediated hepatic stellate cell activation. Digestive Diseases and Sciences, 57(11), 2883–2891. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, Y. , Shao, Z. , Sharif, S. , Du, X. Y. , Myles, T. , Merchant, M. , … Leung, L. L. K. (2013). Thrombin‐cleaved fragments of osteopontin are overexpressed in malignant Glial tumors and provide a molecular niche with survival advantage. Journal of Biological Chemistry, 288(5), 3097–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, N. , Sakai, F. , Kon, S. , Morimoto, J. , Kimura, C. , Yamazaki, H. , … Uede, T. (2003). Essential role of the cryptic epitope SLAYGLR within osteopontin in a murine model of rheumatoid arthritis. Journal of Clinical Investigation, 112(2), 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, C. , Evason, K. J. , Asahina, K. , & Stainier, D. Y. R. (2013). Hepatic stellate cells in liver development, regeneration, and cancer. The Journal of Clinical Investigation, 123(5), 1902–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Guo, R. , Ming, D. , Su, M. , Lin, C. , Deng, Y. , … Su, Z. (2014). Ratios of regulatory T cells/T‐helper 17 cells and transforming growth factor‐beta 1/interleukin‐17 to be associated with the development of hepatitis B virus‐ associated liver cirrhosis. Journal of Gastroenterology and Hepatology, 29(5), 1065–1072. [DOI] [PubMed] [Google Scholar]
- Yumoto, K. , Ishijima, M. , Rittling, S. R. , Tsuji, K. , Tsuchiya, Y. , Kon, S. , … Noda, M. (2002). Osteopontin deficiency protects joints against destruction in anti‐type II collagen antibody‐induced arthritis in mice. Proceedings of the National Academy of Sciences of the United States of America, 99(7), 4556–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhou, C. , Zha, X. , Xu, Z. , Li, L. , Liu, Y. , … Zhu, B. (2015). Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Molecular and Cellular Biochemistry, 407(1‐2), 41–50. [DOI] [PubMed] [Google Scholar]
- Zhao, L. , Li, T. , Wang, Y. , Pan, Y. , Ning, H. , Hui, X. , … Fan, D. (2008). Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. International Journal of Clinical Practice, 62(7), 1056–1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


