Abstract
Background: Although metabolic syndrome (MetS) was described in the late 80s, the molecular mechanisms underlying clustering of risk factors in certain individuals are not fully understood. The present study used targeted proteomics to establish cardiometabolic proteins related to all MetS components, thereby providing new hypotheses regarding pathways involved in the pathogenesis of MetS.
Methods: In the EpiHealth study, 249 cardiometabolic proteins were measured by proximity extension assay (PEA) and related to the five MetS components [consensus-modified National Cholesterol Education Program (NCEP) criteria] in 2,444 participants aged 45–75 years (50% women).
Results: Thirty-one proteins were associated with systolic blood pressure following adjustment for age and sex (P < 0.000040, taking multiple testing into account). The corresponding number of proteins significantly associated with fasting glucose, waist circumference, high-density lipoprotein cholesterol, and serum triglycerides were 58, 132, 127, and 148. Twenty-two proteins were significantly related to all 5 MetS components, and of those, 20 were with MetS as a binary outcome (n = 600, 24% of the sample) following adjustment for age, sex, fat mass, and lifestyle factors (alcohol intake, smoking, education, and exercise habits).
Conclusion: Using targeted proteomics, we identified 20 proteins reflecting a range of pathways, such as immunomodulation at different levels; regulation of adipocyte differentiation; lipid, carbohydrate, and amino acid metabolism; or insulin-like growth factor signaling, to be strongly associated with MetS independently of fat mass and lifestyle factors. Whether some of these proteins are causally involved in the pathogenesis of clustering of multiple risk factors in the same individual remains to be investigated.
Keywords: metabolic syndrome, proteomics, epidemiology
Introduction
Although clustering of cardiovascular risk factors was already described in the late 80s1,2 and metabolic syndrome (MetS) was first conceptualized in 1999–2001,3,4 the pathogenetic mechanisms underlying clustering of such risk factors in certain individuals are not fully understood. Insulin resistance, visceral adipose tissue accumulation, liver steatosis, and a poor skeletal muscle blood supply have all been suggested as unifying causes of MetS.1,5–9 Furthermore, specific proteins measured one by one using conventional techniques such as CRP, adiponectin, leptin, retinol-binding protein 4 (RBP-4), fetuin-A, and sex hormone-binding globulin (SHBG) have all been linked to MetS.10–14
In recent years, technical advances in mass spectrometry and/or antibody-based methods have provided an opportunity to measure multiple proteins in larger epidemiological studies. By use of the antibody-based proximity extension assay (PEA) technique, we and others have shown certain proteins to be linked to different components of MetS—systolic blood pressure, fasting glucose, waist circumference, high-density lipoprotein (HDL) cholesterol, and triglycerides15–17—suggesting that some of these proteins could be involved in the pathogenesis of the different components of MetS. However, no prior investigation has investigated whether these proteins are linked to all the five common components of MetS. If such proteins are found, it is possible that they could be involved in the clustering of risk factors.
Hence, the present study aimed to establish proteins associated with all five components of MetS, defined by the consensus-modified National Cholesterol Education Program (NCEP) criteria from 2009.18 To achieve this, we used data on 249 selected cardiometabolic proteins measured by the PEA technique in 2,444 individuals from the EpiHealth study.19 Our primary hypothesis was that we would discover several proteins being linked to all five MetS components. Identification of such proteins could reveal yet unknown pathways of MetS and identify novel targets for intervention.
Methods
Study sample
In the EpiHealth cohort study, males and females aged 45–75 years were invited to participate, as described previously in detail.19 Briefly, between 2011 and 2016, participants were randomly selected from the population registries of the Swedish cities, Malmö and Uppsala, and the response rate was ∼20%. Recruiting and sampling of participants have been completed in Uppsala, but are still ongoing in Malmö at the time of this study. The present study included a random subset of 2,467 individuals from the Uppsala site in whom we have performed proteomic measurements. The subset included the first 2,500 individuals recruited in Uppsala who underwent genotyping for a separate project, and 33 samples were excluded in proteomic quality control, leaving 2,467 participants for the present study. This study was approved by the regional ethics review board at Uppsala University, and all participants provided written informed consent.
Physical examinations
Blood pressure was measured in the sitting position after 5 min of rest. Waist circumference was measured at the umbilical level. Body mass index was calculated from the measured height and weight as weight in kilograms divided by the square of body height in meters (kg/m2). Fat mass was measured using a bioimpedance scale (Tanita, Tokyo, Japan).
Questionnaire
A web-based questionnaire was completed by participants. They reported medication usage, leisure time, and physical activity in five levels from low (level 1) to strenuous physical activity (level 5). They also reported age, sex, alcohol intake given as drinks per week, education length (up to 9 years, 10–12 years, or >12 years), and current smoking habit. Fat mass was measured by a bioimpedance scale (Tanita).
MetS criteria
The harmonized NCEP criteria for MetS were used to define the five components of the syndrome and prevalent MetS (binary).18 Three of the following five criteria should be fulfilled: blood pressure ≥130/85 mmHg or antihypertensive treatment, fasting plasma glucose ≥5.6 mmol/L or antidiabetic treatment, waist circumference >102 cm in men and >88 cm in women, HDL cholesterol <1.0 mmol/L in men and <1.3 in women, and serum triglycerides ≥1.7 mmol/L.
Routine laboratory and proteomic analyses
Blood samples were collected by trained staff in the morning after a minimum of a 6-hr fast at the EpiHealth test center. The clinical chemistry laboratory at the University Hospital in Uppsala analyzed plasma glucose, HDL cholesterol, and serum triglycerides the same day by standard enzymatic methods on fresh samples. Plasma for proteins was frozen at −80°C for later analyses.
Analyses of proteins were performed with a high-throughput technique using the Olink Proseek® Multiplex Metabolism kit, cardiovascular disease (CVD) II and III arrays, measuring 275 preselected protein biomarkers of metabolism and cardiovascular disease. The kits are based on the PEA technology, and in each kit, 92 oligonucleotide-labeled antibody probe pairs can bind to their respective target in the sample.20 The panel assay characteristics, including a list of protein markers, detection limits, measurements of assay performance, and validations, are available from the manufacturer at www.olink.com. The 2,444 samples from the Uppsala part of the EpiHealth cohort were randomly chosen for protein analyses and stored at −80°C until analysis at the Clinical Biomarkers Facility, Science for Life Laboratory, Uppsala University. Twenty-six proteins were removed from further analysis since more than 25% of all samples were below the limit of detection (LOD). N-terminal prohormone brain natriuretic peptide (NT-proBNP) was measured with the metabolism chip, not the CVD III chip, to minimize values that were below LOD.
Statistical analysis
The protein levels were log2-transformed to promote normal distribution and thereafter transformed to a standard deviation scale to obtain comparable results for all proteins.
Fasting glucose and serum triglycerides were log-transformed to promote normal distributions.
First, linear regression analyses were performed for each of the 249 proteins (independent variables) to the 5 components of MetS: systolic blood pressure, fasting glucose, waist circumference, HDL cholesterol, and triglycerides (dependent variables) in separate models. First, we only adjusted for age and sex; additionally for lifestyle factors (alcohol intake, smoking, education, and exercise habits). Similarly, the limit for significance was set to P = 0.00040 (Bonferroni adjustment, 0.05/249).
Second, following the identification of 22 proteins related to all 5 components of MetS, logistic regression analyses with MetS as the binary outcome and each of the 22 proteins as independent variables in separate models were performed. These models were adjusted for age, sex, and lifestyle factors (alcohol intake, smoking, education, and exercise habits). In addition, the same models were reanalyzed with the addition of fat mass as an additional covariate. In this secondary analysis, the alpha threshold was P = 0.0023 (Bonferroni adjustment for 22 tests, 0.05/22).
Third, pairwise correlations between these 22 proteins were estimated and Pearson's correlation coefficients reported. In addition, a principal component analysis (PCA) was used to visualize the relationships between these proteins.
Fourth, ordinal or binary logistic regression analyses were performed to relate each of the 249 proteins (independent variables) to the number of MetS criteria (0–5, ordinal scale) or presence or absence of MetS (binary). The first set of models was adjusted only for age and sex. In another set of models, we additionally adjusted for lifestyle factors—alcohol intake, smoking, education, and exercise habits. The alpha threshold was set to P = 0.00040 (Bonferroni adjustment for 249 tests, 0.05/249).
STATA 14 (Stata, Inc., College Station, TX) was used for calculations. R, version 3.4.4, and the Lattice package were used to create the figures.
Results
Basic characteristics of the sample are given in Table 1.
Table 1.
Basic Characteristics of the EpiHealth Sample
| Variable | Total sample (n = 2,444) | Women (n = 1,227) | Men (n = 1,217) |
|---|---|---|---|
| Mean (SD)/proportion | Mean (SD)/proportion | Mean (SD)/proportion | |
| MetS components | |||
| Systolic blood pressure (mmHg) | 134.7 (17.0) | 132.8 (17.4) | 136.5 (16.3) |
| Fasting plasma glucose (mmol/L) | 6.0 (0.99) | 5.8 (0.73) | 6.1 (1.1) |
| Waist circumference (cm) | 92.6 (11.7) | 87.5 (11.5) | 97.6 (9.7) |
| HDL cholesterol (mmol/L) | 1.51 (0.39) | 1.68 (0.39) | 1.34 (0.31) |
| Serum triglycerides (mmol/L) | 1.29 (0.75) | 1.17 (0.62) | 1.40 (0.85) |
| MetS (%) | 24 | 22 | 26 |
| No. of MetS components (%) | 0: 14 | 0: 17 | 0: 11 |
| 1: 34 | 1: 31 | 1: 35 | |
| 2: 28 | 2: 29 | 2: 27 | |
| 3: 16 | 3: 15 | 3: 18 | |
| 4: 6 | 4: 6 | 4: 7 | |
| 5: 2 | 5: 2 | 5: 2 | |
| Other clinical and lifestyle characteristics | |||
| Age | 60.6 (8.4) | 60.7 (8.3) | 60.6 (8.4) |
| Females (%) | 50 | 100 | 0 |
| Diastolic blood pressure (mmHg) | 83.4 (9.2) | 82.0 (8.9) | 84.8 (9.4) |
| BMI (kg/m2) | 26.5 (3.8) | 26.0 (4.2) | 26.9 (3.4) |
| Fat mass (%) | 24.0 (8.3) | 26.1 (8.8) | 21.8 (7.1) |
| Total cholesterol (mmol/L) | 5.97 (1.11) | 6.15 (1.08) | 5.8 (1.12) |
| LDL cholesterol (mmol/L) | 3.92 (0.98) | 3.98 (0.95) | 3.86 (1.00) |
| Education (%) | <10 Years: 21 | <10 Years: 21 | <10 Years: 19 |
| 10–12 Years: 28 | 10–12 Years: 30 | 10–12 Years: 28 | |
| >12 Years: 51 | >12 Years: 49 | >12 Years: 53 | |
| Years of smoking | 8.8 (9.2) | 8.7 (9.1) | 8.8 (9.2) |
| Drinks a week | 2.4 (2.9) | 1.8 (2.0) | 3.0 (3.4) |
| Physical activity, scale 1–5 (%) | 1: 3 | 1: 3 | 1: 4 |
| 2: 24 | 2: 24 | 2: 25 | |
| 3: 40 | 3: 43 | 3: 36 | |
| 4: 26 | 4: 24 | 4: 28 | |
| 5: 7 | 5: 6 | 5: 7 | |
SD, standard deviation; MetS, metabolic syndrome; HDL, high-density lipoprotein; BMI, body mass index; LDL, low-density lipoprotein.
First, 31 of the 249 proteins were significantly associated (P < 0.0004) with systolic blood pressure, following adjustment for age and sex. The corresponding number of proteins significantly related to fasting glucose, waist circumference, HDL cholesterol, and serum triglycerides were 58, 132, 127, and 148. Upon further adjustment for lifestyle factors (alcohol intake, smoking, education, and exercise habits), the P values for those abovementioned associations generally increased somewhat. However, in no case, a marked attenuation occurred, as can be seen in Supplementary Tables S1, S2, S3, S4, S5, and the numbers of significant associations were similar.
Second, 22 of the proteins were significantly associated with all 5 MetS criteria. As expected, all of these 22 proteins were significantly associated (P < 0.00227 adjusting for 22 tests) with MetS as a binary outcome when adjusting for age, sex, and lifestyle factors (Table 2). The associations with MetS were attenuated following further adjustment for fat mass for most of these proteins, but 20 of the proteins were still significantly related to MetS (P < 0.00227).
Table 2.
Associations of 22 Proteins (That Were Associated with All 5 Metabolic Syndrome Components) with Metabolic Syndrome (Binary)
| Adjusted for age, sex, and lifestyle factors | Additional adjustment for fat mass | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Abbreviation | OR | Lower 95% CI | Upper 95% CI | P | OR | Lower 95% CI | Upper 95% CI | P |
| Leptin | LEP | 5.58 | 4.45 | 7.01 | 1.10e-49 | 2.39 | 1.79 | 3.18 | 2.42e-09 |
| Low-density lipoprotein receptor | LDL receptor | 2.78 | 2.41 | 3.2 | 3.70e-45 | 2.51 | 2.15 | 2.93 | 4.00e-31 |
| Insulin-like growth factor-binding protein 2 | IGFBP-2 | 0.39 | 0.34 | 0.44 | 9.30e-43 | 0.53 | 0.45 | 0.61 | 4.20e-17 |
| Fatty acid-binding protein, adipocyte | FABP4 | 2.95 | 2.53 | 3.45 | 2.70e-42 | 1.61 | 1.34 | 1.93 | 3.41e-07 |
| Interleukin-1 receptor antagonist protein | IL-1ra | 2.66 | 2.31 | 3.06 | 4.20e-42 | 1.83 | 1.57 | 2.14 | 6.88e-15 |
| Paraoxonase (PON 3) | PON3 | 0.39 | 0.34 | 0.45 | 1.60e-41 | 0.52 | 0.45 | 0.60 | 4.00e-18 |
| Insulin-like growth factor-binding protein 1 | IGFBP-1 | 0.39 | 0.34 | 0.45 | 2.90e-40 | 0.58 | 0.50 | 0.68 | 3.63e-12 |
| Tissue-type plasminogen activator | t-PA | 2.57 | 2.20 | 2.99 | 4.10e-33 | 1.86 | 1.58 | 2.19 | 6.51e-14 |
| Retinoic acid receptor responder protein 2 | RARRES2 | 2.51 | 2.14 | 2.93 | 1.30e-30 | 1.65 | 1.39 | 1.95 | 8.13e-09 |
| Fibroblast growth factor 21 | FGF-21 | 2.06 | 1.8 | 2.36 | 4.00e-26 | 1.78 | 1.53 | 2.07 | 7.44e-14 |
| Plasminogen activator inhibitor 1 | PAI | 1.91 | 1.69 | 2.16 | 3.00e-25 | 1.57 | 1.37 | 1.8 | 1.06e-10 |
| Cathepsin D | CTSD | 1.89 | 1.68 | 2.14 | 1.00e-24 | 1.6 | 1.40 | 1.84 | 9.99e-12 |
| Retinal dehydrogenase 1 | ALDH1A1 | 1.7 | 1.52 | 1.91 | 4.60e-20 | 1.43 | 1.26 | 1.62 | 2.09e-08 |
| Nodal modulator 1 | NOMO1 | 1.80 | 1.59 | 2.05 | 7.70e-20 | 1.56 | 1.35 | 1.79 | 1.02e-09 |
| C-C motif chemokine 16 | CCL16 | 1.84 | 1.59 | 2.12 | 1.00e-16 | 1.41 | 1.21 | 1.65 | 0.000010 |
| Carbonic anhydrase 5A, mitochondrial | CA5A | 1.59 | 1.41 | 1.79 | 6.00e-15 | 1.31 | 1.14 | 1.49 | 0.000084 |
| Thimet oligopeptidase | THOP1 | 1.61 | 1.42 | 1.81 | 3.31e-14 | 1.51 | 1.32 | 1.73 | 3.56e-09 |
| Dihydropteridine reductase | QDPR | 1.53 | 1.37 | 1.72 | 2.85e-13 | 1.36 | 1.19 | 1.54 | 3.24e-06 |
| Leukocyte immunoglobulin-like receptor subfamily A member 5 | LILRA5 | 1.53 | 1.35 | 1.72 | 9.98e-12 | 1.31 | 1.14 | 1.5 | 0.00016 |
| NAD kinase | NADK | 1.48 | 1.32 | 1.66 | 3.40e-11 | 1.40 | 1.23 | 1.6 | 5.87e-07 |
| Amyloid-like protein 1 | APLP1 | 0.72 | 0.64 | 0.81 | 2.57e-08 | 0.87 | 0.76 | 0.99 | 0.046 |
| Matrix metalloproteinase-9 | MMP-9 | 1.36 | 1.21 | 1.53 | 1.15e-07 | 1.19 | 1.05 | 1.36 | 0.0077 |
OR, odds ratio; 95% CI, 95% confidence interval.
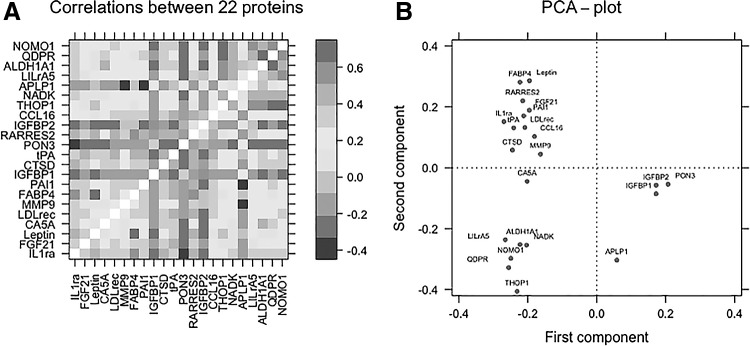
Third, a correlation matrix of the relationships between the 22 significantly associated proteins showed correlation coefficients ranging from −0.50 to 0.70. As can be seen in Fig. 1A, the majority of these relationships were positive. Exceptions to this included IGFBP1 and 2, PON3, and APLP1 that displayed inverse associations. The PCA showed that these 22 proteins form six components with an eigenvalue >1.0. As illustrated in Fig. 1B, the first component showed loadings ranging from 0.15 to 0.30 for all proteins, being negative for APLP1, PON3, IGFBP1, and IGFBP2—proteins showing inverse relationships to the other proteins (Fig. 1A), while the major proteins loading to the second component were the six proteins located in the upper right quadrant (THOP, QDPR, NOMO1, NADK, ALDH1A1, and LILRA5). The four proteins located in the upper left quadrant (APLP1, PON3, IGFBP1, and IGFBP2) were also the main proteins loading to the third component. APLP1, MMP9, and CA5A were the proteins mainly loading to the fourth, fifth, and sixth components.
FIG. 1.
Relationships between the 22 proteins associated with all components of metabolic syndrome. (A) Heat map showing Pearson's correlation coefficients of the 22 proteins of interest. A color-coded label of the correlation coefficients is given on the right side of the heat map. (B) PCA loading plot for these 22 proteins. See Table 2 for protein abbreviations. PCA, principal component analysis.
Fourth, 155 of the 249 proteins were significantly associated (P < 0.00040) with the number of components of MetS, following adjustment for age and sex. Of these, 107 were still significant following further adjustment for lifestyle factors (Supplementary Table S6). Similarly, 131 proteins were significantly associated with MetS (as a binary variable), following adjustment for age and sex. Of these, 101 were still significant following further adjustment for lifestyle factors (Supplementary Table S7).
Discussion
We studied 249 cardiometabolic proteins in 2,444 middle-aged to elderly individuals from the general population and identified 22 proteins that were related to all 5 components of MetS, and 20 of these were associated with MetS independently of fat mass.
Comparison with the literature
We and others have used the PEA technique to relate preselected proteins to different components of MetS.15–17 However, as far as we are aware, no prior study has aimed to identify specific proteins being related to all 5 components of MetS from a panel of more than 200 proteins. The rationale for this approach is to search for a common mechanism underlying the clustering of risk factors in MetS. It is plausible that proteins related to all components of the syndrome are more likely to be involved in a common pathophysiological pathway than a protein being related to just one or two MetS components.
We found 22 proteins to be related to all 5 components of MetS. These were, as expected, all highly associated with MetS as such (when analyzed as a binary variable) when adjusting for age, sex, and lifestyle factors. Furthermore, for 20 of these proteins, associations were significant even after taking fat mass measured by bioimpedance into account. There is a possibility that these proteins could be involved in the pathogenesis of clustering of MetS risk factors independently of being associated with general obesity. However, additional studies with alternative designs would be required to establish this prospect.
Correlation analyses and PCAs of the 22 identified proteins showed that many of the proteins are highly correlated with other proteins. For example, the four proteins showing inverse relationships with MetS (PON3, IGFBP1, IGFBP2, and APLP1) were strongly related to each other; and it is therefore hard to tell which (if any) of these proteins are linked to MetS and which show an association just by being correlated with another protein.
Not all obese subjects fulfill the criteria for having MetS; an observation that has been named metabolically healthy obesity (MHO).21,22 Conversely, not all subjects with MetS are obese (metabolically obese, but normal weight, MONW). However, fat mass is closely related to MetS and the number of MetS components.23 For this reason, we adjusted for fat mass to not report proteins that are only linked to obesity. This further adjustment for fat mass weakened all relationships [except the one for the low-density lipoprotein (LDL) receptor], but most were still strongly related to MetS. We believe that the possibility to find a common pathophysiological pathway for clustering of risk factors is higher among these proteins being related to MetS also following adjustment for fat mass.
Toward this goal, we studied Reactome pathways represented by the 22 strongly associated proteins (Table 3). Several of the pathways described in Table 3 have been previously linked to MetS, such as immunomodulation,10 regulation of adipocyte differentiation,24 lipid, carbohydrate, and amino acid metabolism,25,26 insulin-like growth factor (IGF) signaling pathways,27 signaling by PDGF,28 and dissolution of fibrin clot.29 However, many pathways not yet well known to be associated with the pathogenesis of clustering of multiple risk factors were noted, such as signaling by receptor tyrosine kinases, collagen degradation, nodal signaling, heart development, trophoblast cell proliferation, neuronal differentiation, and synaptogenesis. Several of those pathways are likely to be a cause of, rather than causing, MetS, but it cannot be excluded that such pathways are involved in the pathogenesis of MetS.
Table 3.
Physiological Pathways Related to the 22 Proteins Being Related to All 5 Components of Metabolic Syndrome According to www.reactome.org
| Protein | Abbreviation | Pathways |
|---|---|---|
| Leptin | LEP | Transcriptional regulation of white adipocyte differentiation |
| Low-density lipoprotein receptor | LDL receptor | Plasma lipoprotein assembly, remodeling, and clearance, metabolism of fat-soluble vitamins, clathrin-mediated endocytosis, and vesicle-mediated transport |
| Insulin-like growth factor-binding protein 2 | IGFBP-2 | Regulation of IGF transport and uptake |
| Fatty acid-binding protein, adipocyte | FABP4 | Transcriptional regulation of white adipocyte differentiation and triglyceride metabolism |
| Interleukin-1 receptor antagonist protein | IL-1ra | MAPK family signaling cascades and cytokine signaling, |
| Paraoxonase (PON 3) | PON3 | Synthesis of 5-eicosatetraenoic acids |
| Insulin-like growth factor-binding protein 1 | IGFBP-1 | Regulation of IGF transport and uptake |
| Tissue-type plasminogen activator | t-PA | dissolution of fibrin clot, signaling by PDGF, and signaling by receptor tyrosine kinases |
| Retinoic acid receptor responder protein 2 | RARRES2 | Platelet activation, signaling, and aggregation |
| Fibroblast growth factor 21 | FGF-21 | Assembly of active LPL and LIPC lipase complexes, cellular hexose transport, and SLC-mediated transmembrane transport |
| Plasminogen activator inhibitor 1 | PAI | Dissolution of fibrin clot, signaling by PDGF, BMAL1:CLOCK, and NPAS2 activates circadian gene expression, ECM proteoglycans, and RNA polymerase II transcription |
| Cathepsin D | CTSD | Peptide hormone metabolism, ESR-mediated signaling, metabolism of angiotensinogen to angiotensins, collagen degradation, MHC class II antigen presentation, and neutrophil degranulation |
| Retinal dehydrogenase 1 | ALDH1A1 | Fructose metabolism, ethanol oxidation, retinoic acid biosynthesis pathway, and phase I—functionalization of compounds |
| Nodal modulator 1 | NOMO1 | Nodal signaling, heart development, and trophoblast cell proliferation |
| C-C motif chemokine 16 | CCL16 | Chemokine receptors bind chemokines |
| Carbonic anhydrase 5A, mitochondrial | CA5A | Reversible hydration of carbon dioxide |
| Thimet oligopeptidase | THOP1 | Class I MHC-mediated antigen processing and presentation |
| Dihydropteridine reductase | QDPR | Metabolism of amino acids and derivatives |
| Leukocyte immunoglobulin-like receptor subfamily A member 5 | LILRA5 | Immunoregulatory interactions between a lymphoid cell and a nonlymphoid cell |
| NAD kinase | NADK | Metabolism of water-soluble vitamins and cofactors |
| Amyloid-like protein 1 | APLP1 | Neuronal differentiation, synaptogenesis, neurite outgrowth, and synaptic plasticity |
| Matrix metalloproteinase-9 | MMP-9 | Degradation of the ECM, neutrophil degranulation, EPH-ephrin-mediated repulsion of cells, cytokine signaling in the immune system, and signaling by receptor tyrosine kinases |
IGF, insulin-like growth factor; ECM, extracellular matrix.
Limitations of the present study include lack of an independent replication sample, its cross-sectional design, and unknown generalizability of other populations. We were unaware of any independent sample with measurements of the same proteins that we could use as a replication sample, so we used a strict Bonferroni adjustment for 249 tests in the main analysis, the relationships between the proteins, and the 5 components of MetS. Since our study is cross sectional, we cannot distinguish causality from correlation. One approach to study directionality and causality of these associations would be by Mendelian randomization (MR) studies that use genetic variants linked to proteins and to MetS components to investigate potential causal directions of the relationships between proteins and MetS. The present sample size of about 2,400 individuals is, however, too small to perform an MR study with reasonable power, but it is an important direction for future studies. The generalizability of our results is unknown, especially to populations with other ethnic and geographical characteristics. The major strengths of our study include the assessment of a large number of proteins in parallel and the large study sample of individuals from the general population.
In conclusion, using targeted proteomics assessing 249 cardiometabolic proteins in 2,444 middle-aged to elderly individuals from the general population, we identified 22 cardiometabolic proteins as being related to all 5 MetS criteria and 20 to MetS independently of fat mass. These proteins represent a range of physiological pathways related to cardiometabolic traits, such as immunomodulation at different levels, regulation of adipocyte differentiation, lipid, carbohydrate, and amino acid metabolism, or IGF signaling pathways. Whether some of these proteins are causally involved in clustering of multiple risk factors in the same individual remains to be investigated in MR studies and in experimental investigations. In addition to addressing causality, future studies should also investigate the role of these protein biomarkers in risk prediction, diagnostics, therapeutic choices, and other aspects of clinical treatment of MetS and its components.
Supplementary Material
Acknowledgments
The authors would like to thank the participants of the EpiHealth cohort for their generous contribution. This study was conducted with support from the National Institutes of Health (R01DK106236).
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607 [DOI] [PubMed] [Google Scholar]
- 2. Lind L, Jakobsson S, Lithell H, et al. Relation of serum calcium concentration to metabolic risk factors for cardiovascular disease. BMJ 1988;297:960–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 4. Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 1999;16:442–443 [DOI] [PubMed] [Google Scholar]
- 5. Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006;38:52–63 [DOI] [PubMed] [Google Scholar]
- 6. Wolff L, Bos D, Murad SD, et al. Liver fat is related to cardiovascular risk factors and subclinical vascular disease: The Rotterdam Study. Eur Heart J Cardiovasc Imaging 2016;17:1361–1367 [DOI] [PubMed] [Google Scholar]
- 7. Wu WC, Wang CY. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: Case-control retrospective study. Cardiovasc Diabetol 2013;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H, Ma Z, Pan L, et al. Hepatic fat content is a determinant of metabolic phenotypes and increased carotid intima-media thickness in obese adults. Sci Rep 2016;6:21894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J 1993;125:1494–1497 [DOI] [PubMed] [Google Scholar]
- 10. Han TS, Sattar N, Williams K, et al. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 2002;25:2016–2021 [DOI] [PubMed] [Google Scholar]
- 11. Liu Z, Liang S, Que S, et al. Meta-analysis of adiponectin as a biomarker for the detection of metabolic syndrome. Front Physiol 2018;9:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee SW, Jo HH, Kim MR, et al. Association between metabolic syndrome and serum leptin levels in postmenopausal women. J Obstet Gynaecol 2012;32:73–77 [DOI] [PubMed] [Google Scholar]
- 13. Zachariah JP, Quiroz R, Nelson KP, et al. Prospective relation of circulating adipokines to incident metabolic syndrome: The Framingham heart study. J Am Heart Assoc 2017;6 pii: . doi: 10.1161/JAHA.116.004974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akin F, Bastemir M, Alkis E, et al. SHBG levels correlate with insulin resistance in postmenopausal women. Eur J Intern Med 2009;20:162–167 [DOI] [PubMed] [Google Scholar]
- 15. Nowak C, Sundstrom J, Gustafsson S, et al. Protein biomarkers for insulin resistance and type 2 diabetes risk in two large community cohorts. Diabetes 2016;65:276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enroth S, Johansson A, Enroth SB, et al. Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat Commun 2014;5:4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Figarska SM, Gustafsson S, Sundstrom J, et al. Associations of circulating protein levels with lipid fractions in the general population. Arterioscler Thromb Vasc Biol 2018;38:2505–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 19. Lind L, Elmstahl S, Bergman E, et al. EpiHealth: A large population-based cohort study for investigation of gene-lifestyle interactions in the pathogenesis of common diseases. Eur J Epidemiol 2013;28:189–197 [DOI] [PubMed] [Google Scholar]
- 20. Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab 2004;30:569–572 [DOI] [PubMed] [Google Scholar]
- 22. Ärnlov J, Ingelsson E, Sundström J, et al. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010;121:230–236 [DOI] [PubMed] [Google Scholar]
- 23. Lind L, Arnlov J, Lampa E. The interplay between fat mass and fat distribution as determinants of the metabolic syndrome is sex-dependent. Metab Syndr Relat Disord 2017;15:337–343 [DOI] [PubMed] [Google Scholar]
- 24. Oliva-Olivera W, Coin-Araguez L, Lhamyani S, et al. Adipogenic impairment of adipose tissue-derived mesenchymal stem cells in subjects with metabolic syndrome: Possible protective role of FGF2. J Clin Endocrinol Metab 2017;102:478–487 [DOI] [PubMed] [Google Scholar]
- 25. Pujos-Guillot E, Brandolini M, Petera M, et al. Systems metabolomics for prediction of metabolic syndrome. J Proteome Res 2017;16:2262–2272 [DOI] [PubMed] [Google Scholar]
- 26. Zhong F, Xu M, Bruno RS, et al. Targeted high performance liquid chromatography tandem mass spectrometry-based metabolomics differentiates metabolic syndrome from obesity. Exp Biol Med (Maywood) 2017;242:773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguirre GA, De Ita JR, de la Garza RG, et al. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tisato V, Toffoli B, Monasta L, et al. Patients affected by metabolic syndrome show decreased levels of circulating platelet derived growth factor (PDGF)-BB. Clin Nutr 2013;32:259–264 [DOI] [PubMed] [Google Scholar]
- 29. Mosimah CI, Murray PJ, Simpkins JW. Not all clots are created equal: A review of deficient thrombolysis with tissue plasminogen activator (tPA) in patients with metabolic syndrome. Int J Neurosci 2018;1–18. DOI: 10.1080/00207454.2018.1550400 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.