Abstract
Bioresources are critical resources that support biomedical research because of their ability to appropriately collect, process, store, and distribute a wide range of high-quality biospecimens that meet the needs of specific investigators. Of note, some biorepositories are concerned by their growing inventories and their low rates of tissue utilization. This review discusses the technical characteristics of biospecimens that can cause morphological and molecular variability and/or limit the usefulness of biospecimens in research. This article also describes current challenges related to biospecimen characteristics that may affect biospecimen utilization. These include inadequate awareness of investigators about the availability of biospecimens with specific morphologic and molecular features, donor variability, preanalytical variables, technical problems inherent with an investigator's request for biospecimens, limited tissue availability from a biorepository based on requested sizes and/or numbers of available biospecimens, effects of times of warm and cold ischemia, damage of tissues during surgery, and molecular changes during storage. To ensure maximal biospecimen utilization of all types of biospecimens requires continual education of investigators from diverse fields, particularly on factors that cause variability in the morphological and molecular characteristics of tissues. The investigators' requests for biospecimens and associated data should be reviewed carefully, including by a bioresource-associated pathologist. Queries arising from the request/application form should be resolved by bioresource personnel directly with the investigator.
Keywords: bioresources, biodistributor, biospecimens, donors, preanalytical variables, utilization
Introduction
Biospecimen utilization is decreased when investigators cannot identify biospecimens to meet their research requirements. Meeting the biospecimen needs of investigators may be limited by some inherent aspects of the tissues. These include the lack of consistency of tissue (tissue heterogeneity) of morphologic and molecular features, potentially limiting the usefulness of such tissues in research.1–3 Improvements in medical care and cancer screening have reduced the sizes of some cancers such as breast carcinomas,4,5 Similarly, the increasing use of neoadjuvant therapy makes some tissues much less useful in research in that cancers may be effectively treated and cancer cells may be affected in unknown ways, restricting their usefulness in research. To ensure maximal utilization of biospecimens, the investigators' requests for biospecimens and associated data should be reviewed carefully, including by a pathologist, and all questions concerning the request should be resolved directly with the investigator.2–4
In studying tissue and medical aspects that may affect biospecimen utilization, problems can be subdivided into those involving (1) investigator requests, (2) sources of tissue, (3) inherent tissue characteristics, (4) donor variables, (5) presurgical changes in tissue, (6) surgical changes in tissue, (7) collection variables, (8) various approaches to processing, and (9) storage and distribution of tissues.
Investigator Requests
Investigators must realize that patients are at medical facilities for treatment and care—not to donate tissues for biomedical research; thus, even though patients may consent to the utilization of their tissues in research, medical care always takes precedence and biospecimens are only available when biospecimens and associated medical data do not interfere with current or future medical care. For example, it is our experience that all of a cancer that is ≤2 cm in its largest dimension frequently may be required for clinical care. Specifically, molecular biomarkers must be analyzed and some of the remaining tumor must be available for future clinically required molecular studies. In addition, some biospecimens, especially normal biospecimens, such as from the brain and heart, are usually not available for research from living patients, and some diseased tissues that are not removed at surgery will not be available from living patients. For example, diabetes mellitus is typically not a surgical disease and some tumors such as small cell carcinoma of the lung are not typically treated by surgery. Thus, such specimens must be obtained from autopsies, explicitly consented living donors, or deceased organ donors.
Some requests by investigators for biospecimens may be too vague, uninformed, and/or indicate a lack of understanding of tissues, medical care, and the collection, processing, storage, and distribution of biospecimens. Therefore, investigators' requests for biospecimens may be limited by the availability of tissues that can meet specific aspects of their requests. For example, there are several problems in meeting a request for 10 g of tissue from each of 100 HER2-positive breast tumors from African Americans.
First, the request for “breast tumors” is too vague. There are many subtypes of breast cancers as well as benign tumors and an investigator may not want all such biospecimens. Second, because most ductal and lobular carcinomas of the breast are small secondary to effective screening, a 10 g aliquot of most breast cancers is too large to be available for research. The molecular characteristics of HER2-positive breast adenocarcinomas (about 20% of breast cancers) and from the African American race (usually less than 20% of most academic medical center populations) will usually greatly restrict available specimens to less than 10% of breast adenocarcinomas. Thus, a request for 100 such biospecimens is too large to be provided even from multiple collaborating sites.
Similarly, if there was a request for 20 lung adenocarcinomas with more than 90% malignant cells, it also would be problematic. If the proportion of malignant cells (tumor nuclei) is that high, there is likely to be few biospecimens that meet this requirement because there typically is dilution of the malignant cells of lung cancers by inflammatory, uninvolved, or other cells. The requirement for 90% malignant cells would reduce the number of available tissues. In addition, restricting requests to such an uncommon lung adenocarcinoma could severely bias experimental results with respect to the molecular features of typical lung cancers. Requests for other cancers with atypical features also might bias experimental results. Some investigators may request presence or absence of specific chronic diseases (e.g., Crohn's disease). This also may affect tissue availability and, hence, tissue utilization.
Optimizing requests from investigators for biospecimens is a key to increasing tissue utilization and is a very important function of biorepositories. Biospecimen requests should correspond to actual investigator needs,3 and all issues with biospecimen requests should be resolved. Improving requests may involve changes to investigators' experimental designs and educating investigators as to scientific results in the literature. When issues of the request are resolved, it is of great importance that extensive efforts are expended to find or to collect the biospecimens needed by investigators. This may include finding other biorepositories that can aid in providing necessary biospecimens for difficult to meet requests.
Sources of Biospecimens for Research
Biospecimens may be available from multiple sources such as surgery, autopsies, deceased organ donors, and from living tissue donors. The autopsy and/or organ donor is not a perfect source of nonsurgical tissues; however, recent studies have demonstrated that tissues obtained from autopsies can be used for many studies, including use of RNA in quantitative reverse transcriptase polymerase chain reaction analysis.6 Similarly, RNA from deceased organ donors also is a good source of RNA for a range of studies.7 Of note, not many hospital-based autopsies currently are performed and deceased organ donor cases are typically used for transplantation. Medical examiners cases, due to legal and consent requirements, also may not be available to support research. Some autopsies are performed on patients who have experienced vascular ischemia/shock for several days before death; ischemic and/or autolytic tissues from these cases are less useful to evaluate specific molecular characteristics.
Of great importance, if periods of shock do not precede death, the cells of most organs remain viable and in some cases can be cultured successfully 48 hours after death8; this includes most tissues such as normal bone marrow, pituitary, and liver.8,9 Tissues from autopsies should specify the time after death and should exclude cases with prolonged times of shock. Morphologic changes secondary to ischemia and shock usually can be recognized on microscopic examination by a pathologist during the quality control (QC) diagnosis.
Tissue Characteristics
The morphologic and molecular characteristics of tissues, even if they have the same diagnosis, may vary greatly and is one of the reasons that the QC of actual biospecimens provided to investigators is so important.2 This variation in morphologic and molecular features may prevent many tissues from meeting research needs and, hence, may reduce tissue utilization by biorepositories.
QC should be performed when biospecimens are collected. This permits a bioresource to know specifically what is in its inventory and to plan collections to match current or future needs for specific biospecimens.2,3
When a biospecimen is collected as a cancer or as uninvolved tissue matching to a cancer, the biospecimen might not match the original gross diagnosis. What appears as uninvolved tissue may be the lymphatic spread of cancer, and what appears to be cancer may be an area of fibrosis and/or an aliquot with only a small amount of cancer. The QC microscopic examination by a pathologist permits an evaluation of the collected research tissues so that an investigator's needs can be matched to the actual specimens that are available. This also applies to nonneoplastic tissues collected to support research and is especially important for tissues collected at autopsy or from deceased or living donors. Approaches to QC have been described2,3 and a thorough QC analysis is described in the website of the Cooperative Human Tissue Network.a
Even for tissues with the same diagnosis, all tissues have unique morphologic and molecular features. For example, breast tissue collected from a reduction mammoplasty and diagnosed as normal can range from areas that comprised only mature fat cells to areas with moderate numbers of normal appearing ductal-lobular units. There also may be areas of fibrocystic changes with extensive inflammation and with atypical hyperplasia. Dysplastic changes are more common when mastectomies are performed in patients who have BRCA1 and BRCA2 mutations. Thus, the surgical pathology, autopsy, and donor reported diagnoses do not necessarily represent the exact morphology of the tissues actually distributed to investigators.
Similarly, a colorectal cancer may have cancer cells intermixed with extensive inflammatory cells, including mature lymphocytes and plasma cells. This inflammation would be identified by QC examination and reported as a reduction in the proportion of malignant cells (cancer nuclei); of importance, analysis of the biospecimen would have molecular features of the inflammatory cells in combination with those of the malignant cells. This would vary with the proportion of inflammatory cells. Also, the matching uninvolved colon might have morphologic features of a range of conditions such as increased inflammation or diverticulitis.
The QC of the specific biospecimens provided to investigators indicates morphologic features and suggest molecular features that might be expected to vary from those of malignant cells. The QC examination also might identify an area with inflammation and reparative changes associated with a previous presurgical biopsy or changes caused by neoadjuvant therapy. All these features, especially neoadjuvant therapy, affect the morphologic and molecular features of the tissues available for research and may limit their usefulness in research. Some of the major differences among similar tissues may not be identified by QC and represent donor variability such as comorbidities, tobacco and drug abuse, inherited molecular characteristics, and therapeutic drug-induced changes.
Donors
Donor characteristics may affect biospecimen utilization in that researchers may need specific biospecimens from different sexes, racial, and/or ethnic or cultural groups. Because disease and incidence of disease may vary among donor categories, available specimens may be limited for specific patient categories. Some required biospecimens are rare such as breast carcinomas from males and may not be represented within the inventory of a biorepository. There are many other donor variables that may affect and decrease the optimal utilization of biospecimens; specifically, biospecimens from patients with a disease from only a specific racial or ethnic group may be needed to study the features of the disease in this group.
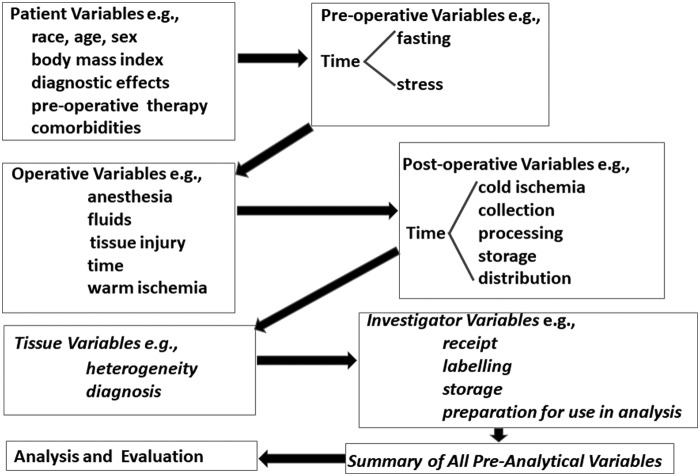
Comorbidities which include diseases such as neoplastic, rheumatologic, infectious, diabetic, or cardiovascular may affect the usefulness and, hence, availability of tissues in multiple ways; these may include the direct effects of the disease on the tissue as well as tissue effects of acute and/or chronic therapy for the specific diseases. In addition, there may be the presence or absence of inheritable genetic features, including specific mutations in tissues such as BRCA1 and BRCA2 as well as features that affect the risk that diseases may develop. Tissues with or without specific diseases/conditions, especially when accompanied by a racial restriction, can limit tissue availability. There are many donor characteristics such as those listed in Table 1. As indicated in Figure 1, some of the more important preanalytical variables may tend to build up in biospecimens. Specifications of donor characteristics may limit the availability of specimens. Of importance, investigators may not be aware of how donor characteristics may affect their research.
Table 1.
Examples of Presurgical Variables
| Race, age, ethnicity, sex |
| Neoadjuvant therapy |
| Failure of drug therapy |
| Biopsy site |
| Prior biopsy |
| Familial history (e.g., BRCA1, BRCA2) |
| Congenital abnormalities |
| Acquired conditions (e.g., immune deficiencies) |
| Comorbidities (e.g., diabetes) |
| Diet |
| Body mass index |
| Stress, acute and chronic (posttraumatic stress, stress of war, surgery) |
| Family support |
| Postinjuries |
| Drug abuse (alcohol, tobacco, illicit drug use) |
| Drug therapy |
| Environmental exposures (e.g., sun, radiation, chemical, biological exposures) |
| Sexual exposures (e.g., HIV, hepatitis C) |
| Employment (personnel and customer exposures) |
| Activity and exercise |
FIG. 1.
The buildup of some important preanalytical variables that can affect research.
In the last several decades, the molecular features of tissues, especially melanoma, breast, lung, and gastric cancers have become frequent interests of investigators because of molecular targeting in precision medicine. Molecular features may include inherited mutations, gene fusions, or mutations that develop during the progression of a disease/cancer. These include APC, MSH2, ALK, p53, and ERG. There are thousands of molecular features that may be studied at any one time; some are common and others are less common. Of special interest to some investigators are molecular features that are associated with clinical risk factors for a disease, clinical outcomes and/or clinical targets.
With the advent of precision medicine, more potential molecular targets are being evaluated, for example, PD-1 and/or PD-L1 in melanomas and non-small cell lung cancers; tissues expressing a wide range of such molecular targets are being studied and, hence, requested from biorepositories. Of note, unless the molecular features are analyzed as a component of standard medical care (e.g., HER2 expression in breast and gastric cancers), whether biospecimens contain specific molecular features is likely to be unknown. This complicates descriptions of biospecimens and may limit tissue utilization. Of note, it is important for biorepositories to add molecular characteristics to their informatics system so that tissues with specific molecular characteristics can be efficiently and readily provided to investigators.
Changes in tissues during and after surgery also may affect the morphology and molecular features of tissues used in research. In general, the exact effects of the various aspects of medical care during presurgery to postsurgery are unknown. Nevertheless, these effects may limit the availability of tissues to support specific research projects and, hence, may reduce tissue utilization, especially when there are multiple requirements that may be associated with clinical changes and medical care (e.g., failure of therapy by a specific drug).
Presurgical Changes
The presurgery period includes the initial period of diagnosis, including biopsy of the initial lesion as well as therapy before surgery (neoadjuvant therapy), which may include radiation and/or chemotherapy. Multiple changes in tissues may occur in the period before the surgical process begins, and these changes may prevent the use of tissues in specific types of research.
The diagnosis of a disease can begin by analysis of a sample of bodily fluids, a tissue biopsy, and/or molecular imaging by several approaches such as computerized tomography. In the case of cancer, the diagnosis frequently begins by evaluation of biological fluids, clinical imaging, and/or biopsy. Upon biopsy, the tissue is activated, inflamed, and repair is initiated. In some cases, the patient receives radiation and/or drug therapy before excisional surgery of cancer. The medical knowledge as to the effects of repair and/or neoadjuvant therapy on most tissues and types of cancer is limited. Morphological and molecular changes are likely to occur, especially in response to neoadjuvant therapy; however, the cancer target is changed cellularly and molecularly in unknown ways, and the treated cancer no longer morphologically or molecularly represents the untreated primary cancer. Also, a diagnosis of cancer puts the patient into a “high stress” state, which elevates stress hormones and changes the molecular characteristics of multiple tissues, which also can affect the remaining malignant tissues that will be removed at definitive excisional surgery.
Surgical Changes
The surgical process is initiated when a patient starts the immediate preparation for surgery by limiting food and water intake and in other preparations for the specific surgical process, for example, clearing the gastrointestinal system before the removal of colorectal polyps; such preparations are likely to increase stress. During surgery, changes in the targeted tissue may occur secondary to the administration of intravenous fluids and anesthesia. When the vascular system to the tissue that is to be removed is compromised, this begins a period designated as warm ischemia. During warm ischemia, the absence or reduced blood flow to the tissue that will be removed surgically creates ischemia and other stresses in the tissue and its cells. The cells of the tissue do not die, but the cells react to this stressful environment by molecular changes; typically ischemic, stress related, and chaperone genes and their associated proteins are upregulated.
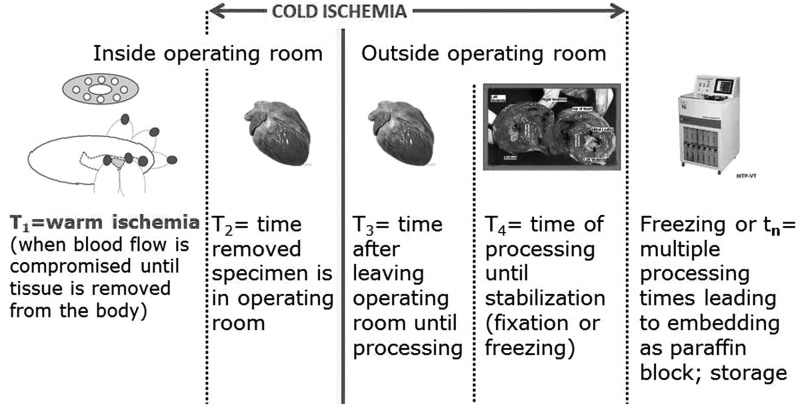
The tissue is removed from the body by cutting with a scalpel or cutting with a cautery, both of which can damage the margins or edges of the excised tissue. It is our observation that cautery results in wider molecular damage to the edges of the tissue than cutting with a scalpel. When a tissue is removed from the body, the surgical and warm ischemia periods for the tissue end and the period of cold ischemia begins (Fig. 2). There have been only a few studies of the effects of warm ischemia on the molecular and morphological characteristics of human tissues removed at surgery.
FIG. 2.
Warm and cold ischemia.
Although not studied, it is likely that some very labile molecules (e.g., phosphorylated proteins) may rapidly decay in the first few minutes of warm ischemia; however, such changes have not been studied adequately due to the infrastructure required for such human studies. A few studies of warm ischemia have found that, in general, most transcripts/genes do not change during warm ischemia, but of those that do change, most increase. These typically are genes associated with stress and ischemia.10 Of note, usually warm ischemia cannot be controlled because it represents a clinical phase of surgery.
However, with adequate resources, the time of cold ischemia can be controlled since it begins after the clinical period of surgery and is under control of the bioresource. Most studies of cold ischemia, which are large enough to be statistically sound, have reported that most transcripts/genes do not change for up to 4 to 6 hours of cold ischemia; however, of the transcripts/genes that do change, the great majority increase during the period of cold ischemia.10,11 This observation is supported somewhat by the Genotype-Tissue Expression Project study of deceased organ donors.7–13
The period of cold ischemia has been studied by multiple investigators; usually, the studies have been performed at 4°C, at room temperature of ∼25°C, 37°C (approximately equal to body temperature), or even 60°C. However, more studies have been performed at room temperature of about 25°C. These studies of cold ischemia have focused on changes in RNA Integrity Number (RIN), the 28S to 18S RNA ratio, and/or changes in transcripts/genes. Of note, RIN and the 28S to 18S ratios are measures of ribosomal RNA stability and may not be related directly to stability of mRNA. Most studies have found that RIN or transcripts do not change in up to 6 hours of cold ischemia12 and that the effects of warm ischemia are greater than the effects of cold ischemia.1,11–14 This is supported by the GTEx study of deceased organ donors who had an average RIN of 8.6 for a postmortem interval (PMI) of less than 4 hours and an average RIN of 6.7 for PMI's between 4 and 8 hours.7,13 This indicates that RIN does not extensively decrease, even in rapid autopsy tissues that are probably more stressed than surgical tissues.
Fewer studies have evaluated changes in proteins during cold ischemia. One of the largest of such studies found comparable results for selected proteins, including phosphoproteins; however, this is still an understudied area of tissue research.1,11–14 Although published results indicate that the measurements of transcripts and proteins do not require specimens to have cold ischemia times of less than 6 hours, many investigators want biospecimens be processed within 1 hour. This results in rejection of many acceptable biospecimens and decreased specimen utilization. Additional investigator education is needed in this area.
Specimen Collection
The collection of tissue by a bioresource typically begins after the specimens are removed from the operating area. Depending on resources, bioresource personnel might be able to collect the specimens inside the operating room or in the operating area. During the collection period, the tissues experience cold ischemia (usually at 4°C or at room temperature). It is important to incorporate the times of warm and cold ischemia into the informatics system. Based on the literature, it is recommended that biospecimens be maintained at 4°C during collection, transport, and processing.
Changes During Processing of Biospecimens
Once biospecimens are collected, their processing should be relatively rapid. Processing may include aliquoting tissues to smaller biospecimens, uniquely labeling the specimens and entering these into the informatics system. When entered into the informatics system, the times of processing should be included as well as storage locations of the biospecimens. Of importance, samples for QC should be obtained during processing, and the results of QC should be incorporated into the informatics system. Performing QC confirms the diagnosis for each aliquot and identifies morphologic features of each aliquot that will be provided to investigators. This permits efficient and rapid matching of investigator needs with the computerized tissue inventory.3,15 The final step in processing is the initiation of stabilization by freezing and/or by fixation that usually leads to construction of formalin-fixed paraffin-embedded (FFPE) blocks for investigators. If specimens are frozen, FFPE blocks also are constructed for QC.2
Biospecimen Storage and Distribution
The storage of biospecimens depends upon whether the biospecimens are solid tissue or bodily fluids. Solid tissues may be stored at room temperature or colder (e.g., as FFPE blocks, frozen in optimal cutting temperature media or frozen neat). Biospecimens can also be preserved using heat and/or desiccation. Similarly, aliquots of bodily fluids may be frozen intact or separated into subcomponents (e.g., serum); storage is usually in cryovials. The typical temperatures at which most frozen biospecimens are stored include liquid nitrogen vapor (−190°C), dry ice (−109°C), or a range of mechanical freezers at −80°C and −130°C. To maintain cellular viability requires storage at −130°C or colder in fetal calf serum plus dimethyl sulfide (DMSO), so usually storage in liquid nitrogen vapor is chosen to store viable cells.
There should not be even short-term storage of frozen specimens in a self-defrost freezer; long-term storage at −20°C (or warmer) results in molecular changes after 5 to 8 months of storage compared to −80°C storage.1 Some studies of bodily fluids at −80°C have reported a change in molecules features after 1 to 2 years.1,16 During long-term storage (≥10 years) at −80°C versus the vapor phase of liquid nitrogen, no differences were reported based on mass spectrometry analysis, but better results at the RNA and mRNA levels were noted at −80°C storage. However, the RIN was low at both temperatures as was RNA recovery, suggesting changes at ultracold temperatures for both approaches of long-term storage.1,17
It is critical that biorepositories distribute biospecimens to support investigators' research; otherwise, biorepositories do not fulfill a role in biomedical research.15 To emphasize this point, we prefer the term “biodistributor” rather than biorepository or biobank. This issue is discussed in detail in other articles in this special issue.3,15 Many aspects of medical care and tissue variability may complicate identifying optimal biospecimens for distribution to meet the research needs of investigators. As described above, a very important role of the biorepository is to work closely with investigators to ensure that they receive the biospecimens they need as well as understand the potential limitations of tissues as to supporting their research.
Summary
Tissue-related factors and changes in tissues secondary to medical care and other factors influence tissue utilization. It is very important that QC be performed when the tissues are collected and processed so that tissue morphologic features and diagnoses are determined and are rapidly available to match with investigator requests. QC permits bioresources to have knowledge as to what specific biospecimens are in their inventories. Thus, when an investigator makes a request for specific tissues, the QC and other information (e.g., size, preparation) permit immediate matching of the request to the inventory of the bioresource or initiate the future collection of specific biospecimens by prospective collection. Medical research is shifting due to an increased focus on recognized molecular features of tissues, especially those that are targeted during standard or individualized medical therapy. With each new therapy such as targeting PD-1 or PD-L1 in lung adenocarcinomas, there are increased requests for research tissues with specific molecular characteristics. When such molecular characteristics are determined clinically, the biorepository should include them as data elements in describing their inventory to meet investigators' requirements or add them to prospective biospecimen collections. Diagnoses, molecular characteristics, and other tissue factors such as donor issues, neoadjuvant therapies, and biospecimen size, also may lead to problems meeting investigators' specific requirements and, hence, decrease tissue utilization.
Acknowledgments
Supported by CHTN grants 1UM1CA183728 to UAB; the Tissue Procurement Shared Facility of the UAB Comprehensive Cancer Center P30CA13148-41; the Pulmonary Hypertension Breakthrough Initiative 1R24HL123767-01; the Hepato/Renal Fibrocystic Diseases Core Center P30DK074038; and the NCI Institutional National Research T32 Award 5T32CA183926-02.
Footnotes
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1. Atherton DS, Sexton KC, Otali D, et al. Factors affecting the use of human tissues in biomedical research: Implications in the design and operation of a biorepository. In: Grützmann R, Pilarsky C. (eds). Cancer Gene Profiling Methods and Protocols. New York: Springer Science+Business Media; 2016: 1–38 [DOI] [PubMed] [Google Scholar]
- 2. Grizzle WE, Gunter EW, Sexton KC, et al. Quality management of biorepositories. Biopreserv Biobank 2015;13:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grizzle WE, Sexton KC, McGarvey D, et al. Lessons learned during three decades of operations of two prospective bioresources. Biopreserv Biobank 2018. 16:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coburn NG, Chung MA, Fulton J, et al. Decreased breast cancer tumor size, stage, and mortality in Rhode Island: An example of a well-screened population. Cancer Control 2004;11:222–230 [DOI] [PubMed] [Google Scholar]
- 5. Welch HG, Prorok PC, O'Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016;375:1438–1447 [DOI] [PubMed] [Google Scholar]
- 6. van der Linden A, Blokker BM, Kap M, et al. Post-mortem tissue biopsies obtained at minimally invasive autopsy: An RNA-quality analysis. PLoS One 2014;9:e115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carithers LJ, Ardlie K, Barcus M, et al. A novel approach to high-quality postmortem tissue procurement: The GTEx Project. Biopreserv Biobank 2015;13:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aupet S, Simone G, Heyd B, et al. Isolation of viable human hepatic progenitors from adult livers is possible even after 48 hours of cold ischemia. Tissue Eng Part C Methods 2013;19:497–506 [DOI] [PubMed] [Google Scholar]
- 9. D'Ippolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (Miami) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 2004;117:2971–2981 [DOI] [PubMed] [Google Scholar]
- 10. Schlomm T, Nakel E, Lubke A, et al. Marked gene transcript level alterations occur early during radical prostatectomy. Eur Urol 2008;53:333–344 [DOI] [PubMed] [Google Scholar]
- 11. Cacciatore S, Hu X, Viertler C, et al. Effects of intra- and post-operative ischemia on the metabolic profile of clinical liver tissue specimens monitored by NMR. J Proteome Res 2013;12:5723–5729 [DOI] [PubMed] [Google Scholar]
- 12. Grizzle WE, Otali D, Sexton KC, et al. Effects of cold ischemia on gene expression: A review and commentary. Biopreserv Biobank 2016;14:548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Consortium GT. Human genomics. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science; 2015;348:648–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gundisch S, Hauck S, Sarioglu H, et al. Variability of protein and phosphoprotein levels in clinical tissue specimens during the preanalytical phase. J Proteome Res 2012;11:5748–5762 [DOI] [PubMed] [Google Scholar]
- 15. Grizzle WE, Sexton KC. Commentary on improving biospecimen utilization by classic biobanks: Identifying past and minimizing future mistakes. Biopreserv Biobank 2019;17:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Potter DM, Butterfield LH, Divito SJ, et al. Pitfalls in retrospective analyses of biomarkers: A case study with metastatic melanoma patients. J Immunol Methods 2012;376:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Auer H, Mobley JA, Ayers LW, et al. The effects of frozen tissue storage conditions on the integrity of RNA and protein. Biotech Histochem 2014;89:518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]