Abstract
Purpose: Aflibercept (Eylea™, Regeneron) is supplied in single-use glass vials along with 1 cc polycarbonate syringes. We sought to determine if storage of aflibercept for sustained periods within these syringes would result in loss of antivascular endothelial growth factor (anti-VEGF) activity.
Methods: Aflibercept samples were drawn from commercially available glass vials into manufacturer-supplied 1-mL syringes and stored at 4°C. Anti-VEGF activity was assessed using enzyme-linked immunosorbent assays at the following storage durations: 0, 4, 9, 14, and 28 days. Frozen samples stored at −20°C for 28 and 56 days were also assayed. Also, a subset of aflibercept samples was stored and then diluted to 1:10 and progressively smaller concentrations and the assays repeated. Aggregation of aflibercept was tested using a dynamic light scattering assay.
Results: There were no statistical differences in anti-VEGF activity among aflibercept samples of 1:1 or 1:10 dilution stored at either 4°C or −20°C at any of the storage intervals (P > 0.05). We also observed persistence of robust anti-VEGF activity for up to 14 days when diluted poststorage to 1:16,000, a concentration that would be expected after >7 vitreous half-lives within the eye (estimated at >50 days). No evidence of drug aggregation in specimens stored for 14 days was observed.
Conclusions: Our findings support feasibility of prefilling and storage of aflibercept within manufacturer-supplied polycarbonate syringes for as long as 14 days before use under pharmacy-based sterile conditions, facilitating greater safety and efficiency in many clinics delivering anti-VEGF therapy.
Keywords: aflibercept, anti-VEGF activity, syringe, storage, safety
Introduction
Aflibercept (Eylea™, Regeneron) is an antivascular endothelial growth factor (anti-VEGF) agent that is Food and Drug Administration approved for neovascular age-related macular degeneration, macular edema from diabetes or retinal vein occlusion, and diabetic retinopathy with diabetic macular edema. In the United States, it is provided in a single-use glass vial with manufacturer-supplied 1-mL polycarbonate syringes. Under the right conditions, prefilling of such syringes may provide greater efficiency and safety when large numbers of patients are administered intravitreal injections within a finite timeframe. Such a strategy would require sustained biologic activity of the medication when stored in such syringes, even if for short periods of time. Regarding aflibercept, the manufacturer provides evidence of its stability after storage in the supplied polycarbonate syringes for up to 6 h,1 with independent laboratories showing preservation of molecular integrity and drug activity for much longer in other types of plastic syringes.2,3 We evaluated persistence of in vitro anti-VEGF activity of aflibercept after storage in manufacturer-supplied syringes for various time intervals, including after frozen storage. In addition, we tested for aggregation of the drug molecule, a phenomenon that has been associated with sustained elevation of intraocular pressure following intravitreal injection of bevacizumab, another anti-VEGF agent.4,5
Methods
Aflibercept is administered clinically at a dose of 2 mg in 0.05 mL, provided in glass vials at a concentration of 40 mg/mL in 10 mM sodium phosphate, 40 mM sodium chloride, 0.03% polysorbate 20, and 5% sucrose, with pH of 6.2. In the United States, each package comes with one 1-mL syringe (BD Luer-lok, cat no. 309628; Becton Dickinson), one 19 gauge 1½-inch 5 μm filter needle for withdrawal of vial contents, and one 30 gauge ½-inch needle for intravitreal injection.
Aflibercept samples were drawn directly from commercially available glass vials into manufacturer-supplied syringes using the provided filter needle, and stored at 4°C in the provided syringes. Anti-VEGF activity was assessed at the following storage durations: 0, 4, 9, 14, and 28 days. Frozen samples stored at −20°C for 28 and 56 days were also assessed. Three samples at each time interval were tested and the experiment repeated. Also, a subset of aflibercept samples was stored and then diluted 1:10 in phosphate-buffered saline (PBS) to increase sensitivity of the experiment. Recombinant human VEGF (rhVEGF, cat no. PHC9391; Fisher Scientific) was diluted to 100 ng/mL with PBS containing 0.1% bovine serum albumin (BSA) and was co-incubated with all aflibercept samples for 30 min at room temperature, and then assayed for residual VEGF. A Human VEGF ELISA Kit (cat no. KHG0111; Fisher Scientific) was used for the assay. The rhVEGF-aflibercept complex was added to the enzyme-linked immunosorbent assay (ELISA) wells and allowed to bind for 2 h at room temperature. The wells were rinsed with wash buffer to remove any unbound complex. An antibody to VEGF (biotin conjugated) was added to the wells and incubated for 1 h. The wells were washed to remove unbound antibody and a detection reagent (streptavidin-horseradish peroxidase) was added to bind to the biotin-labeled detection antibody. After a 30-min incubation, the wells were washed and a chromogen reagent was added to bind to the antibody complex. After a 30-min incubation, a stop solution was added and the optical densities (absorbance at 450 nm) were read using a spectrophotometric plate reader. For positive controls, dilutions of rhVEGF alone (100, 50, and 25 ng/mL) were run in the ELISA along with the syringe-stored aflibercept samples. The above methodology has been implemented in related studies.6
To increase sensitivity of the anti-VEGF activity assay to drug concentration effects, ELISA of poststorage dilutions of aflibercept at 40 mg/mL (stock concentration), 100, 10, 5, 2.5, 1, and 0 μg/mL (control) were performed in triplicate after storage intervals of 0, 9, and 14 days at 4°C. In addition, assays of protein aggregation of aflibercept at stock concentration in a variety of syringe types used in our clinics for intravitreal injection (BD Luer Lok cat no. 309628, Becton Dickinson; NORM-JECT Tuberkulin 1 mL Luer, cat no. 410-200V0, Henke Sass Wolf, silicone-free; BD 1 mL Tuberculin Slip Tip cat no. 309659, Becton Dickinson) were performed in triplicate using dynamic light scattering (Zetasizer uV; Malvern Panalytical, Malvern, United Kingdom) after storage intervals of 0, 9, and 14 days at 4°C.
Results
Our assays showed persistence of anti-VEGF activity of aflibercept samples stored at either 4°C for up to 28 days or −20°C for up to 56 days. No statistical difference among storage durations was detected (P > 0.05, analysis of variance, Tukey's multiple comparison, Fig. 1). Samples diluted poststorage to 1:10 also showed persistence of activity and no difference among these same intervals.
FIG. 1.
Anti-VEGF activity of aflibercept samples by storage temperature, and duration, as shown by ELISA of unbound VEGF after 30 min of incubation with poststorage aflibercept samples. Lower unbound levels of VEGF indicate higher levels of anti-VEGF activity. Error bars are standard deviation. ELISA, enzyme-linked immunosorbent assay; VEGF, vascular endothelial growth factor.
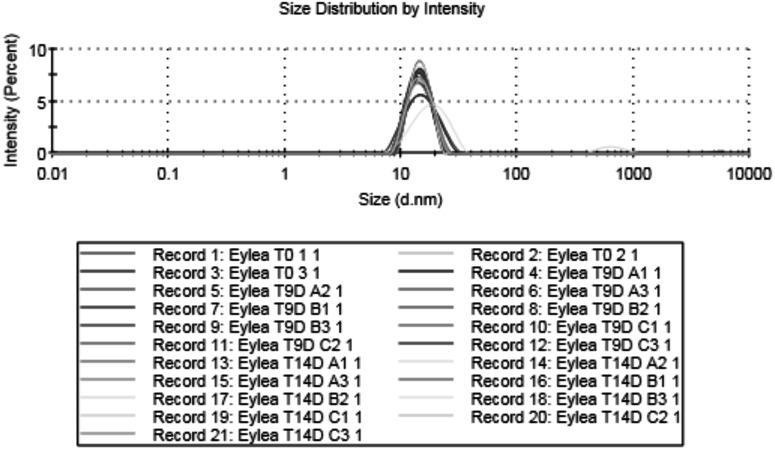
Aflibercept stored for 0, 9, and 14 days in manufacturer-supplied polycarbonate syringes showed persistence of anti-VEGF activity even when diluted poststorage to very low concentrations (Fig. 2). Notably, we observed persistence of robust anti-VEGF activity for up to 14 days when diluted poststorage to 1:16,000, a concentration that would be expected after >7 vitreous half-lives within the eye. There were no signs of protein aggregation within any of the above-specified syringes when stored for these intervals (Figs. 3 and 4).
FIG. 2.
Anti-VEGF activity of aflibercept samples by concentration and duration, as shown by ELISA of unbound VEGF after 30 min of incubation with aflibercept samples stored at 4°C. A 2.5 μg concentration (1:16,000 dilution of stock concentration) is estimated to be equivalent to >7 half-lives within the eye. Error bars are standard deviation.
FIG. 3.
Distribution of particle size of aflibercept stored in various syringes for up to 14 days, as tested by dynamic light scattering, shows no evidence of obvious protein aggregation as demonstrated by coincident distribution curves for the various time points and syringe types A, B, and C. Curves for all syringe types, storage durations of 0, 9, and 14 days, and triplicate data are shown simultaneously on the graph.
FIG. 4.
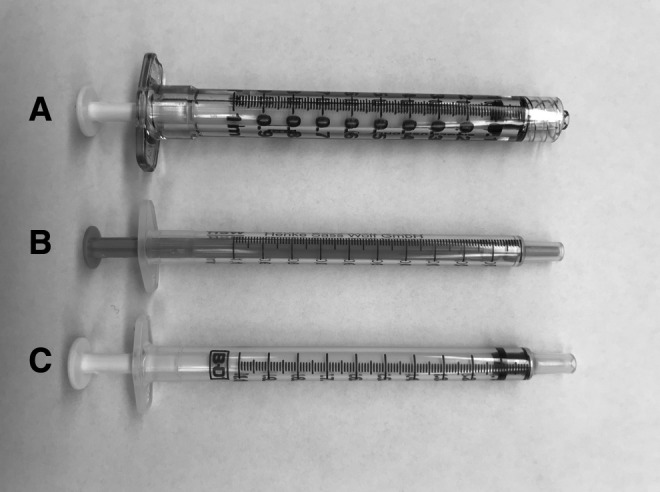
Syringe types in which drug aggregation studies were performed. Syringe A: BD Luer Lok, cat no. 309628, Becton Dickinson. Syringe B: NORM-JECT Tuberkulin 1 mL Luer, cat no. 410-200V0, Henke Sass Wolf, silicone-free. Syringe C: BD 1 mL Tuberculin Slip Tip, cat no. 309659, Becton Dickinson.
Discussion
Because of demand for efficient delivery of anti-VEGF therapy, many clinics prefill and store anti-VEGF medication in syringes for injection, for short periods and often in large quantities. In such instances, any variations in environmental conditions, personnel, and adherence to sterile technique create opportunity for contamination and endophthalmitis risk. A qualified pharmacy could prepare syringes and markedly reduce such variation. However, sustained biological activity and sterility during the period of transit from pharmacy to clinic would be important, both to assure efficacy and reduce drug wastage.
Our findings corroborate with those of Cao et al.2 and Sivertsen et al.3 in demonstrating persistence of anti-VEGF activity of aflibercept stored for 4 weeks. In addition, we showed absence of aggregation of aflibercept kept unfrozen for 14 days when stored in plastic syringes. As a complement to the aforementioned studies, we evaluated storage within the specific polycarbonate syringes distributed by the manufacturer for clinical use in the United States. Stability of bevacizumab, another commonly used anti-VEGF agent, has been observed after sustained storage in syringes made of polycarbonate.7
We observed persistence of anti-VEGF activity of aflibercept (Eylea) stored in the above syringes for up to 14 days, then diluted 1:16,000 to a concentration that would be expected after >7 vitreous half-lives, estimated to be more than 50 days by mathematical modeling.8 Our findings thus appear to support a robust anti-VEGF effect of aflibercept when stored under appropriate conditions for up to at least 14 days. Aggregation effects of storage in syringes were also analyzed and no detrimental effect was noted. We also observed evidence of persistent anti-VEGF activity of aflibercept for up to 56 days after being stored frozen at −20°C in the manufacturer-distributed syringes.
Our study has clinical implications. It suggests that a good therapeutic response might be expected from aflibercept stored at 4°C for as long as 14 days in the manufacturer-provided syringe. Also, we found no evidence of aggregation of aflibercept under these conditions in any of three plastic syringe types used in our clinics. Notably, sustained elevation of intraocular pressure has been attributed to injection of compounded bevacizumab in which drug protein has aggregated.4,5 Our findings suggest that sustained intraocular pressure elevations from such a mechanism would be unlikely if aflibercept were stored in the specified syringes under the above conditions.
Although our study showed persistence of anti-VEGF activity of aflibercept after storage of frozen specimens at −20°C, further study is needed before clinical use of stored, frozen aflibercept can be recommended. Our evaluation of frozen specimens was a preliminary exploration. For our purposes, we did not perform as stringent an analysis on such specimens, since freezing would not be necessary for the maximal storage limit of 14 days imposed by our pharmacy for purposes of sterility assurance. However, such information may still be of relevance because of the potential for unintended freezing that might occur when medications in transit are kept with frozen ice packs or the equivalent, even for short durations.
It should be noted that our study did not address the issue of sterility, a foremost concern when contemplating long-term storage of intravitreally injected agents. In a similar scenario in which compounded sterile preparations of bevacizumab are provided in batch quantities for later injection, it has been considered mandatory that the drug preparation adheres to standards provided by the United States Pharmacopeial Convention (USP Chapter 797 Compounding Guide) as a means to reduce contamination risk.9 These standards far exceed that of a typical clinic facility with respect to environmental controls, sterile technique, and training and certification of personnel. As a countermeasure against contamination risk, our clinics utilize an attached hospital inpatient pharmacy that adheres to these standards. Furthermore, considerable attention to details such as the proper capping of prepared syringes for transport, labeling, inventory, and temperature control is required. This reduces the risk of subsequent contamination, medication error, usage of an expired drug, and drug wastage, any of which can jeopardize the mission of achieving maximal safety and efficient care. At our institution, we have imposed an expiration period of 14 days postpreparation on manufacturer-supplied syringes prefilled with aflibercept, and have experienced no adverse events attributable to this endeavor after 1 year of implementation and several thousand injections. Our investigation of persistence of anti-VEGF activity after syringe storage was just the first step in the implementation of this practice.
Acknowledgments
This study was supported, in part, by the Jack A. and Elaine D. Klieger Professorship (D. Han); the Thomas M. Aaberg Retina Research Fund; and NEI grant K08EY024645, Iris Kassem Laboratory, Medical College of Wisconsin, Milwaukee, Wisconsin.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.“EYLEA® (aflibercept) Injection and Syringe Stability.” Written communication from Regeneron regarding Eylea stability, received August 25, 2016 [Google Scholar]
- 2. Cao S., Cui J., Matsubara J., Forooghian F. Long-term in vitro functional stability of compounded ranibizumab and aflibercept. Can. J. Ophthalmol. 52: 273–276, 2017 [DOI] [PubMed] [Google Scholar]
- 3. Sivertsen M.S., Jørstad, Ø.K., Grevys A., Foss S., and Moe M.C., and Andersen J.T. Pharmaceutical compounding of aflibercept in prefilled syringes does not affect structural integrity, stability or VEGF and Fc binding properties. Sci. Rep. 8:1–9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahook M.Y., Liu L., Ruzycki P., et al. High-molecular-weight aggregates in repackaged bevacizumab. Retina. 30:887–892, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Gal-Or O., Dotan A., Dachbash M., et al. Bevacizumab clearance through the iridocorneal angle following intravitreal injection in a rat model. Exp. Eye Res. 145:412–416, 2016 [DOI] [PubMed] [Google Scholar]
- 6. Mansour A.M., Al-Ghadban S.I., Yunis M.H., El-Sabban Me. Ziv-aflibercept in macular disease. Br. J. Ophthalmol. 99:1055–1059, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalili H., Sharma G., Froome A., Khaw P.T., and Brocchini S. Storage stability of bevacizumab in polycarbonate and polypropylene syringes. Eye. 29: 820–827, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stewart M.W. What are the half-lives of ranibizumab and aflibercept (VEGF Trap-eye) in human eyes? Calculations with a mathematical model. Eye Reports. 1:2011 [Google Scholar]
- 9. Shienbaum G., and Flynn H.W., Jr. Compounding bevacizumab for intravitreal injection: does USP <797> always apply? Retina. 33:1733–1734, 2013 [DOI] [PubMed] [Google Scholar]