The heart is composed of a heterogeneous population of cells including cardiomyocytes, fibroblasts, smooth muscle cells, endothelial cells, and immune cells. As in most tissues, the primary immune cells that reside in the healthy heart are macrophages1. Cardiac tissue injury, such as myocardial infarction (MI), triggers a dynamic cellular cascade that initially activates resident stromal and immune cells, and over time evolves in a coordinated manner that leads to the recruitment of diverse leukocyte populations into the inflamed tissue2. An initial wave of polymorphonuclear leukocytes arrives within minutes after onset of ischemia. Monocytes also begin to accumulate within the first hours of ischemia and give rise to inflammatory macrophages, which participate in clearing of dead and dying cells in the infarct in order to prepare for tissue rebuilding and regeneration. Initial inflammation is followed by a reparative phase during which new matrix is generated by fibroblasts to provide mechanical stability to the left ventricle. Another hallmark of the reparatory phase is angiogenesis, regulated by macrophage-derived vascular endothelial growth factor (VEGF). Macrophages active during the reparative phase are less inflammatory and express genes associated with tissue repair, guiding angiogenesis and myofibroblast activation2. Intercellular signaling and cross talk between macrophages, other leukocytes, cardiomyocytes, endothelial cells and fibroblasts are critical in the biphasic macrophage response after MI, and dictate the balance between myocardial health and pathological left ventricular remodeling that may lead to post-MI heart failure3, 4.
In this issue of Circulation Research, Chen et al5 describe for the first time a critical in vivo role of macrophage Smad3 in the biphasic macrophage response and cardiac repair after ischemic injury (Figure). Smad3 is a member of the ubiquitously expressed intracellular effector family of Smads, which regulate gene expression in the canonical transforming growth factor (TGF)-β signaling cascade6. TGF-β itself is a pleiotropic cytokine with an essential role in remodeling of the infarcted heart through modulation of the inflammatory and reparative response post-MI7. However, a detailed understanding of macrophage Smad3 in post-MI remodeling is lacking. The authors demonstrate in vitro that macrophage Smad3 is not only activated after stimulation with TGF-β but also shorty after phosphatidylserine-dependent phagocytosis of apoptotic cells. The latter stimulus is independent of TGF-β activity as shown by the authors in cultured bone marrow-derived macrophages.
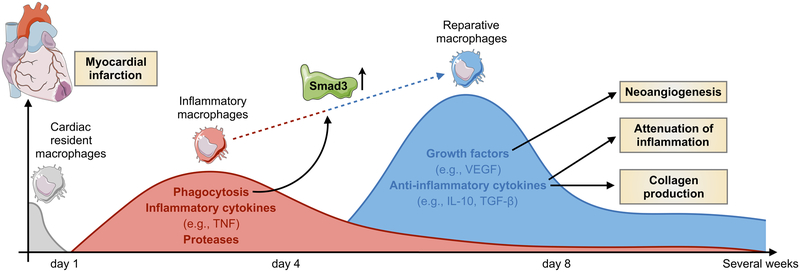
Figure. Smad3 in the biphasic macrophage response after myocardial infarction.
The figure summarizes macrophage activity in the heart at different time points after ischemia in the area of myocardial infarction. Resident cardiac macrophages are lost shortly after onset of ischemia, and replaced by inflammatory monocytes from the circulation15. Recruited monocytes and macrophages participate in the phagocytosis of dead and dying cells and secrete proteases and inflammatory cytokines, such as tumor necrosis factor (TNF), that further fuel inflammation. Within 3–7 days, monocytes continue to accumulate and give rise to tissue macrophages with a reparative phenotype that produce vascular endothelial growth factor (VEGF), IL-10 and transforming growth factor-β (TGF-β). These lead to neoangiogenesis, attenuation of inflammation and collagen production by fibroblasts, respectively. Chen et al5 describe for the first time a critical role of macrophage Smad3 signaling in phagocytosis, mediating the transition from inflammatory to reparative phase and protection from adverse remodeling.
Using cell-specific loss-of-function strategies, the authors report no active role of macrophage Smad3 in steady-state cardiac function with exception of a modest but significant PR prolongation, a change that indicates impaired atrioventricular conduction. This finding is consistent with a recently described role of cardiac resident macrophages in electrical conduction8. After permanent coronary occlusion, macrophage-specific Smad3 knockout (LysM-Smad3−/−) mice showed infarct expansion, increased inflammation, more adverse remodeling, worse systolic dysfunction and higher late post-infarction mortality. Poor survival in infarcted LysM-Smad3−/− mice is not caused by cardiac rupture but relates to impaired activation of macrophage’s phagocytic program and a delayed transition to their anti-inflammatory and reparative phenotype.
The authors further identify, in cultured macrophages, the “eat me” signal milk fat globule-EGF factor-8 and nuclear peroxisome proliferator-activated receptors (PPAR)-γ and -δ as players partially involved in the Smad3-mediated phagocytic and anti-inflammatory macrophage phenotype, respectively. Smad3 loss in cultured macrophages showed lower levels of the angiogenic growth factor Vegfa and fibrogenic mediator TGF-β1 suggesting a role for Smad3 in angiogenesis and myofibroblast activation, both hallmarks of the reparative phase after ischemic injury. However, infarcted LysM-Smad3−/− mice had no significant defects in infarct angiogenesis, vascular maturation, myofibroblast density and collagen deposition. These at first glance conflicting findings highlight the caution one should undertake while translating in vitro results to a more complex in vivo situation. Indeed, organ function in living organisms relies on close, highly dynamic interactions between parenchymal and stromal cells; such interactions cannot be easily mimicked in cell culture experiments. Another example of oversimplification often used in macrophage research is the concept of M1/M2 macrophage polarization, which is derived from in vitro studies and does not reflect the more subtle phenotypes observed in vivo. Tissue macrophages do not form stable subsets but respond to a combination of factors resulting in complex, even mixed, phenotypes. By combining single-cell RNA sequencing with fate-mapping strategies, Dick et al9 recently identified 13 infarct macrophage clusters, 6 of which precisely overlapped with clusters present in noninfarcted hearts, and 7 clusters which are unique to infarcted tissue and distinctly relate to functional processes such as inflammation or repair mechanisms. These findings reveal the functional heterogeneity of the macrophage cell compartment post-MI beyond the M1 versus M2 polarization model and raise the question to what extend Smad3 critically regulates function of each macrophage subpopulation, and what mechanisms compensate for the loss of Smad3 in vivo. Macrophage density also chronically expands in the nonischemic remote myocardium post-MI, which may play a critical role in adverse cardiac remodeling and heart failure10. The role of Smad3 in macrophage function in noninfarcted remote myocardium still remains to be determined.
The importance of TGF-β/Smad3 signaling in infarcted tissue is not limited to macrophages. In previous work11, the same authors studied the role of cardiomyocyte- and fibroblast-specific Smad3 in regulating cardiac repair and remodeling post-MI. They showed an opposing action of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium: inhibition of Smad3 in fibroblasts impairs scar remodeling and accentuates adverse remodeling while Smad3 deletion in cardiomyocytes exerts protective actions by attenuating apoptosis and reducing protease-driven adverse remodeling11. These opposing findings highlight the challenges in designing therapeutic approaches targeting the TGF-β/Smad3 cascade in cardiovascular disease. The completion of CANTOS, the clinical trial that targeted the inflammatory cytokine IL-1β to treat cardiovascular disease provided the first convincing proof that modulating inflammation improves patients’ cardiovascular health12. Macrophages are likely excellent therapeutic targets because of their functional plasticity and ease of delivering drug-loaded nanomaterials to these phagocytes13. Therefore, one could for example consider to specifically stimulate macrophage Smad3 signaling using macrophage-targeting nanoparticles in which a potent agonist of PPAR-γ is encapsulated. A similar therapeutic strategy to modulate macrophages towards a less inflammatory phenotype has previously been used to prevent plaque destabilization and rupture in atherosclerotic mice14, further emphasizing the importance and feasibility of targeting inflammatory pathways in cardiovascular disease.
Acknowledgments
SOURCE OF FUNDING
This work was funded in part by federal funds from the NIH HL139598, HL125428 and HL131495. M.H. was supported by an American Heart Association Career Development Award (19CDA34490005). M.N. was supported by the Massachusetts General Hospital Research Scholar Program.
Footnotes
DISCLOSURES
The authors declare that they have no competing interests.
REFERENCES
- 1.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nature Reviews Immunology. 2018;18:733–744. [DOI] [PubMed] [Google Scholar]
- 3.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forte E, Furtado MB, Rosenthal N. The interstitium in cardiac repair: role of the immune–stromal cell interplay. Nature Reviews Cardiology. 2018;15:601–616. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Huang S, Su Y, Wu Y-J, Hanna A, Brickshawana A, Graff J, Frangogiannis NG. Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circ Res. 2019;doi: 10.1161/CIRCRESAHA.119.315069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmierer B, Hill CS. TGFβ–SMAD signal transduction: molecular specificity and functional flexibility. Nature reviews Molecular cell biology. 2007;8:970–982. [DOI] [PubMed] [Google Scholar]
- 7.Frangogiannis NG. The role of transforming growth factor (TGF)-β in the infarcted myocardium. Journal of thoracic disease. 2017;9:S52–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulsmans M, Clauss S, Xiao L, et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell. 2017;169:510–522.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick SA, Macklin JA, Nejat S, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sager HB, Hulsmans M, Lavine KJ, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong P, Shinde AV, Su Y, Russo I, Chen B, Saxena A, Conway SJ, Graff JM, Frangogiannis NG. Opposing actions of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium. Circulation. 2018;137:707–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 13.Duivenvoorden R, Senders ML, van Leent MMT, Pérez-Medina C, Nahrendorf M, Fayad ZA, Mulder WJM. Nanoimmunotherapy to treat ischaemic heart disease. Nature Reviews Cardiology. 2019;16:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashiro S, Matoba T, Umezu R, Koga J-i, Tokutome M, Katsuki S, Nakano K, Sunagawa K, Egashira K. Pioglitazone-incorporated nanoparticles prevent plaque destabilization and rupture by regulating monocyte/macrophage differentiation in ApoE−/− mice. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:491–500. [DOI] [PubMed] [Google Scholar]
- 15.Leuschner F, Rauch PJ, Ueno T, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]