Abstract
Developmental exposures to bisphenol A (BPA), an estrogen receptor agonist, can disrupt development of the female reproductive tract in rodents and non-human primates. Due to an increased public knowledge of negative health effects associated with BPA exposure, BPA has begun to be phased out of many consumer products and in some cases it has been replaced with structurally similar compounds including bisphenol S (BPS). This study examined CD-1 mice exposed to a low dose of BPS during early development (200 µg/kg/day from gestational day 8 until postnatal day 19). BPS altered expression of estrogen-responsive genes in both the uterus and ovary, and induced increases in ovarian follicular development in pre-pubertal females evaluated at postnatal day 22. Prior studies have revealed that developmental exposures to environmental chemicals including BPA alter the response of animals to hormonal or carcinogen challenges experienced later in life. To evaluate whether early life exposures to BPS alter responses of females to an estrogen challenge, additional females were exposed to ethinyl estradiol from postnatal day 19 through postnatal day 21. BPS-treated females responded abnormally to this estrogen challenge, displaying heightened responses in the uterus and diminished responses in the ovary. Although additional studies are needed to characterize the mechanisms by which BPS alters the female reproductive tract, this pilot study provides evidence that a common BPA replacement chemical may have endocrine disrupting properties.
Keywords: apoptosis, endocrine disruptor, estrogen receptor, ethinyl estradiol, ovarian follicles, puberty, proliferation, uterine endometrium
1. Introduction
It is widely understood that early life exposures to synthetic estrogens can induce alterations to the female reproductive tract, some of which will manifest at puberty 1–3. One of the best known examples of this comes from human exposures to the pharmaceutical diethylstilbestrol (DES), which was prescribed to pregnant women to prevent spontaneous abortion 4. Girls exposed to DES during gestation, so-called DES daughters, often developed malformations of the reproductive tract and had an increased risk of developing clear cell adenocarcinoma of the vagina starting at puberty 5,6. Mice exposed to DES during early development are similarly affected, developing lesions of the reproductive tract that become obvious at puberty and in adulthood 7–9. Rodent models used in DES studies proved to be highly predictive of other adverse outcomes observed in DES daughters such as breast cancer 10.
A relatively large number of estrogenic endocrine disrupting chemicals (EDCs) have now been identified. Both controlled laboratory animal and epidemiological data suggest adverse effects on the female reproductive system as a result of early life exposures 11–15. In a recent review, Peretz and colleagues assessed all studies published from 2007–2013 that examined the impact of one xenoestrogen, bisphenol A (BPA), on the male and female reproductive systems 16. These authors concluded that BPA induced or was associated with disturbances of the ovary, oviduct and uterus in laboratory animals, and in human epidemiology studies. Another systematic review of all published studies reporting uterine effects in laboratory animals exposed to BPA during the period of uterine organogenesis and differentiation concluded that there is clear evidence for effects of early, developmental exposure to BPA leading to uterine abnormalities during adulthood in mice and rats 17. Further, consistent effects of BPA on a number of reproductive endpoints have been shown across studies conducted in multiple laboratories, and in different species 18.
Bisphenol S (BPS) is a chemical that is structurally similar to BPA 19. Because both scientific and public knowledge of adverse outcomes related to BPA exposure have increased 20,21, consumer demand for BPA-free products has encouraged the replacement of BPA with other related compounds including BPS 22–24. BPS is now used in a range of consumer products including thermal receipts, food-contact paper products, and canned foods 22,25–29. Biomonitoring studies reveal that human exposures to BPS are likely to be widespread 30; approximately 97% of individuals in the US have detectable levels of BPS metabolites in their urine 26. Back-calculations from these urinary concentrations suggest daily exposures in the range of 0.3 – 2 µg/day, however, these exposures will likely rise as the replacement of BPA in various consumer goods also increases. These back-calculations are based on limited toxicokinetic and exposure data and assumptions about clearance, and may therefore be an underestimate 31. Like BPA, human exposure to BPS appears to occur predominantly by two routes of exposure: absorption through the skin 32 and ingestion from plastic leachates, can linings, and dust 27,33.
In vitro studies show that BPS acts as an agonist for estrogen receptor (ER) α, ERβ, and membrane ER 34–37. Studies in zebrafish support the hypothesis that this chemical can disrupt both reproductive and developmental endpoints through endocrine pathways 38–41. Studies observing effects of BPS in mammals are limited, although there is evidence from our laboratory and others that it can disrupt maternal behaviors and induce uterotrophic responses, consistent with its suspected estrogenic properties 42–44. There is also evidence that BPS exposure can affect body weight and neurobehaviors in developmentally exposed male offspring 45.
Based on previous studies which observed changes in estrogen dependent endpoints due to early life BPA or BPS exposure, we hypothesized that developmental exposure to BPS would induce changes in estrogen dependent endpoints specifically of the female reproductive tract, with effects observed on a cellular and molecular level. We conducted a pilot study examining mice exposed to 200 µg BPS/kg/day from gestational day 8 – postnatal day [PND]19. This dose was selected because it is 1/50th of the toxicological NOAEL of 10 mg/kg/day 46. Developmental exposures to BPS resulted in modest alterations to gene expression in the female reproductive organs in the pre-pubertal mouse. Because many of the effects of DES on the female reproductive tract were not obvious until puberty, we also provided pre-pubertal females (treated with and without BPS during early development) with an estrogen challenge that was sufficient to induce an uterotrophic response. We noted that females perinatally exposed to BPS (referred to as BPS+EE females) responded to this estrogen challenge differently from controls (referred to as control+EE females), suggesting that BPS alters the responsiveness of the female reproductive tract to an estrogen challenge. These observations may be similar to how a female developmentally exposed to BPS will respond to natural pubertal hormones, and indicate that widespread exposures to BPS may have implications for public health.
2. Results
2.1 Effects of perinatal BPS exposures on the uterus and oviduct at PND22
2.1.1 Tissue Morphology
A number of characteristics of tissue organization in tissues of the female reproductive tract are known to be influenced by estrogen exposure including height of the uterine epithelium, e.g. the endometrium. To characterize the effects of developmental BPS exposure on the uterus, the height of the uterine epithelium was measured at multiple distinct locations along the endometrial layer. Perinatal exposure to BPS had no effect on tissue organization of the uterus including endometrial cell height (Figure 1A,B).
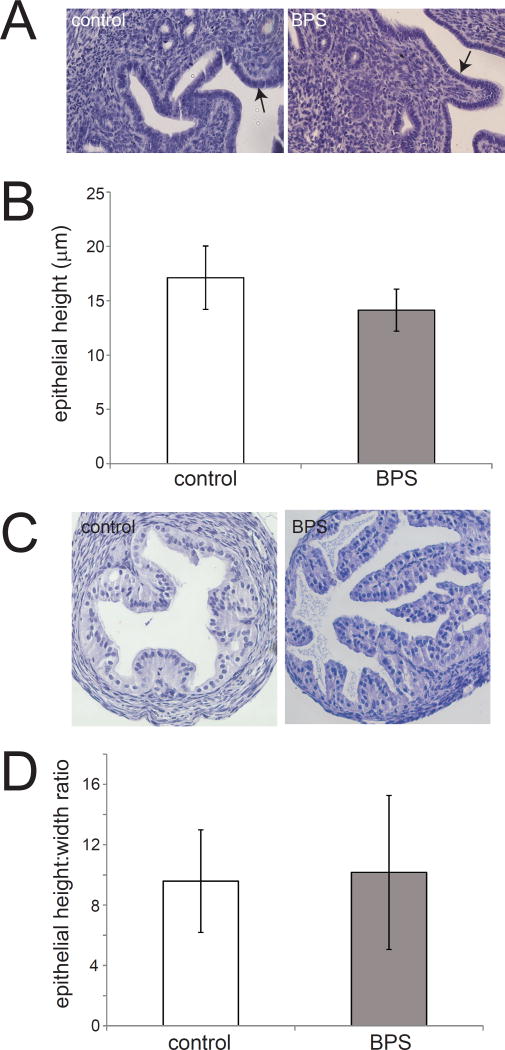
Figure 1. Uterine endometrial cell height and oviduct cell height are not affected by perinatal BPS exposure.
A) H&E staining of uterine sections from unexposed and BPS-exposed females. The endometrial layer is indicated by arrows. Photomicrographs were collected with a 20× EpiPlan Objective. B) Quantification of endometrial cell height in unexposed (control) and BPS-exposed females. C) H&E staining of oviduct sections from unexposed and BPS-exposed females. Photomicrographs were collected with a 20× EpiPlan Objective. D) Quantification of oviduct cell height in unexposed (control) and BPS-exposed females. In all groups, n=5 per treatment.
Tissue organization of the oviduct was analyzed similarly, although both cell height and width were measured. In the oviduct, no significant qualitative or quantitative changes were observed for epithelial height, epithelial width, or height:width ratios of the oviduct epithelium (Figure 1C,D and data not shown).
2.1.2 Apoptosis and proliferation
To determine whether developmental BPS exposures alter other cellular characteristics in the uterus, the number of cells expressing Ki67, a marker of proliferation, was quantified in both the epithelial and stromal compartments (the endometrium and lamina propria, respectively). No significant effects of BPS were noted in either tissue compartment (Figure 2A,B).
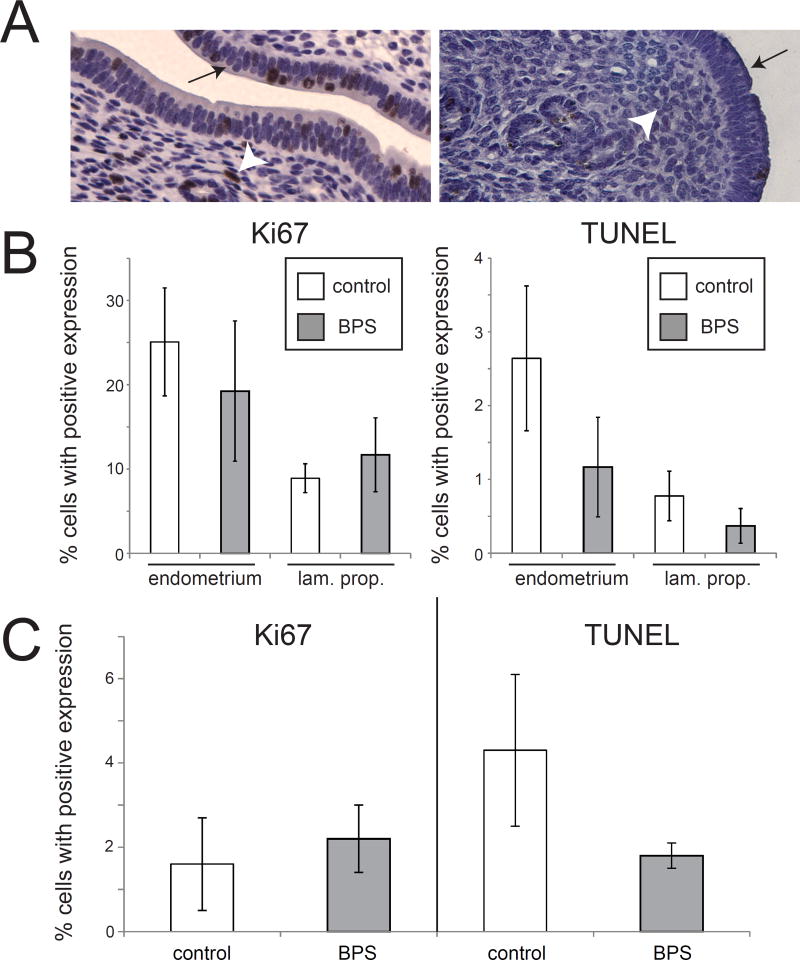
Figure 2. BPS does not alter expression of markers of proliferation or apoptosis in the uterus.
A) Expression of Ki67 and TUNEL labeling in the uterus. The endometrial layer is indicated by an arrow and the lamina propria is indicated by an arrowhead. B) Quantification of expression revealed no effect of BPS exposure on Ki67 in either the endometrium or the lamina propria of the uterus. Quantification of TUNEL also showed no effect of BPS on apoptosis in either tissue layer. C) Quantification of Ki67 and TUNEL in the oviduct also revealed no effect of BPS treatment. In all groups, n=5 per treatment.
Similarly, apoptotic cells were quantified using positive TUNEL (Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling) staining. No significant effects of BPS were noted (Figure 2A,B).
Ki67 and TUNEL expression were also analyzed in oviduct epithelial tissue. No effects of BPS treatment were observed for the number of epithelial cells in the oviduct expressing either the proliferation or apoptotic markers (Figure 2C).
2.1.3 Gene expression
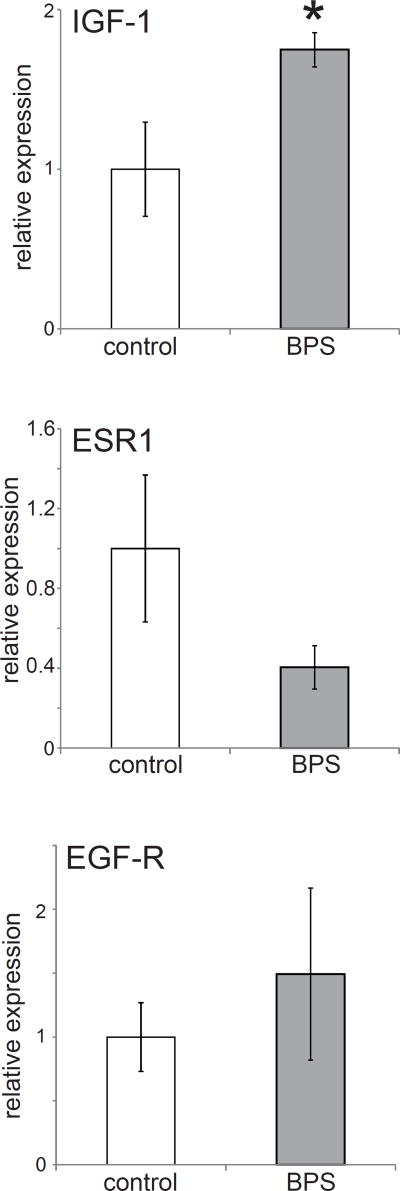
To quantify changes in uterine development influenced by early life BPS exposure, several genes involved in hormone responsiveness and known to be sensitive to estrogens were measured with the use of qPCR. These genes included: ESR-1 (estrogen receptor α), ESR-2 (estrogen receptor β), IGF-1 (insulin growth factor 1) and EGF-R (epidermal growth factor 1). Perinatal BPS exposure significantly increased the expression of IGF-1 (p<0.05) (Figure 3) but not EGF-R in the uterus. A non-significant decrease in ESR-1 was also observed (Figure 3). Expression levels of ESR-2 were very low, precluding accurate quantification (data not shown). Gene expression in the oviduct was not assessed due to a lack of available qPCR grade oviduct tissue.
Figure 3. Developmental exposure to BPS alters gene expression in the female reproductive tract.
Expression of three genes in the uterus at PND22. ESR2 expression levels were too low to be accurately quantified in these tissue samples (similar to (Couse et al., 1997)). In all panels, * indicates p<0.05, independent samples t-test. In all groups, n=5 per treatment.
2.2 Altered response in the uterus of BPS-exposed females to an estrogen challenge
Prior studies have revealed that developmental exposures to EDCs, including BPA, alter the response of animals to hormonal or carcinogen challenges experienced later in life 47–50. Here, one female from each litter was treated with 1 µg/kg/day ethinyl estradiol (EE) from PND19-PND21, prior to the onset of puberty (Supplemental Figure 1). This dosing paradigm is sufficient to induce an uterotrophic response in prepubertal mice, and thus can artificially induce some physiological responses consistent with puberty 51.
2.2.1 Tissue Morphology
In control+EE females, the EE challenge induced slight increases in uterine epithelial height, but these increases were not significantly different from unchallenged control animals (Figure 4A). Although the endometrium is known to be responsive to estrogen, prior studies suggest that higher doses are needed to induce statistically significant increases in cell height 51. Yet, surprisingly, in BPS+EE females, a significant increase in uterine epithelial height was observed following the pubertal estrogen challenge (Figure 4A). This result suggests that perinatal BPS exposure may act to enhance the uterine response to EE at puberty.
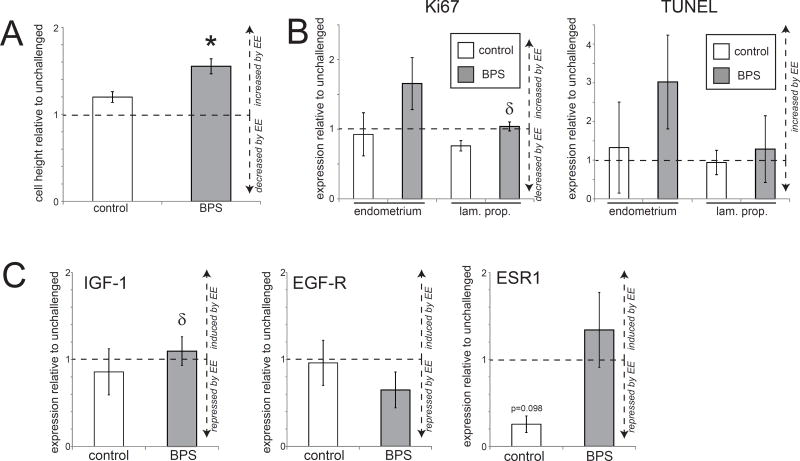
Figure 4. Tissue organization and gene expression in the uterus of BPS-treated females is disrupted by an EE challenge.
A) Height of the uterine epithelium was significantly increased in BPS+EE females after an estrogen challenge compared to unchallenged females. In contrast, an estrogen challenge did not significantly affect height of the uterine epithelium in control+EE females. B) Quantification of Ki67 and TUNEL expression in the uterus of control and BPS-treated females after an EE challenge. C) Expression of three genes in uteri is shown relative to unchallenged females from the same perinatal exposure group. Estrogen treatment induced a non-significant decrease in ESR1 in the uterus of control+EE females, but not in BPS+EE females. In all panels, * indicates p<0.05, independent samples t-test comparing EE-challenged with non-challenged females from the same perinatal treatment group (control or BPS). δ indicates significant differences, p<0.05, between control+EE females and BPS+EE females. In all groups, n=5 per treatment.
2.2.2 Apoptosis and proliferation
We again evaluated proliferation and apoptosis using immunohistochemistry for Ki67 and TUNEL labeling, respectively. In both control+EE and BPS+EE females, the EE challenge did not significantly affect the number of cells proliferating or undergoing apoptosis in the uterine stroma or epithelium, regardless of whether the animals were perinatally exposed to BPS (Figure 4B).
2.2.3 Gene expression
Finally, expression of IGF-1, EGF-R and ESR1 were evaluated in uterine samples following an EE challenge. In control+EE females, treatment with EE had no significant effects on uterine gene expression (Figure 4C), although a relative decrease of ESR-1 expression was observed. No effects on uterine gene expression were seen in BPS+EE females.
2.3 Effects of perinatal BPS exposures on the ovary at PND22
2.3.1 Tissue Morphology
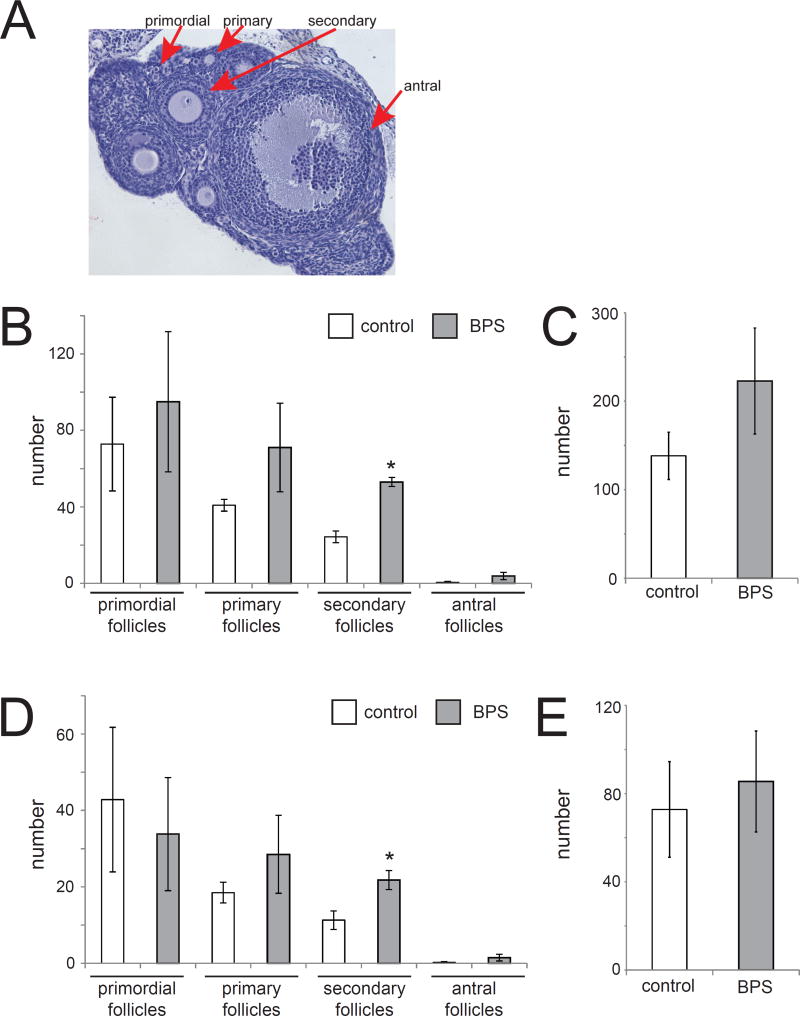
We next characterized the morphology of the ovary by counting the number of primordial, primary, secondary, and antral follicles in sections collected from animals exposed to BPS and unexposed controls (Figure 5A). Follicles were only counted if the nucleus of the oocyte was visible. Perinatal exposure to BPS significantly increased the number of secondary follicles compared to unexposed controls (Figure 5B). No significant effects were observed on other follicle stages or total follicles (Figure 5B,C).
Figure 5. BPS-induces significant increases in mature ovarian follicles.
A) H&E staining of an ovary collected at PND22. Examples of ovarian follicles at various stages of development are indicated. Photomicrographs were collected with a 10× EpiPlan Objective. B) BPS exposure induced significant increases in the number of secondary follicles in the entire ovary. C) The total number of follicles through the entire ovary was increased in BPS-treated females, although this increase was not statistically significant. D) To account for differences in ovarian size, four sections from the middle of the ovary were used to quantify follicle numbers in the various stages of development. BPS induced significant increases in the number of secondary follicles and non-significant increases in the number of primary and antral follicles. E) Non-significant increases were observed in total number of measured in four ovarian sections from BPS-treated ovaries. In all panels, * indicates p<0.05, independent samples t-test. In all groups, n=5 per treatment.
Although there were no statistically significant differences in the number of sections evaluated between treatment groups, the total number of sections (and thus the size of the ovaries) was variable between individuals (Table 1). To account for possible differences in ovarian size, ovarian follicles were counted in four sections separated by at least 50 µm. The BPS-treated animals displayed modest increases in the number of primary, secondary and antral follicles (Figure 5D) and total follicles (Figure 5E), but only the increase in secondary follicles was statistically significant. Atretic follicles were not observed in any animal in any treatment group (data not shown).
Table 1.
Average number of sections containing ovary
| Treatment group (perinatal/EE) |
# Sections (n ± SEM) | Range (min, max) |
|---|---|---|
| Control + no challenge | 117.0 ± 12.6 | 89, 143 |
| BPS + no challenge | 115.4 ± 13.2 | 89, 151 |
| Control + EE | 125.0 ± 11.9 | 110, 160 |
| BPS + EE | 92.4 ± 18.0 | 59, 140 |
2.3.2 Apoptosis and proliferation
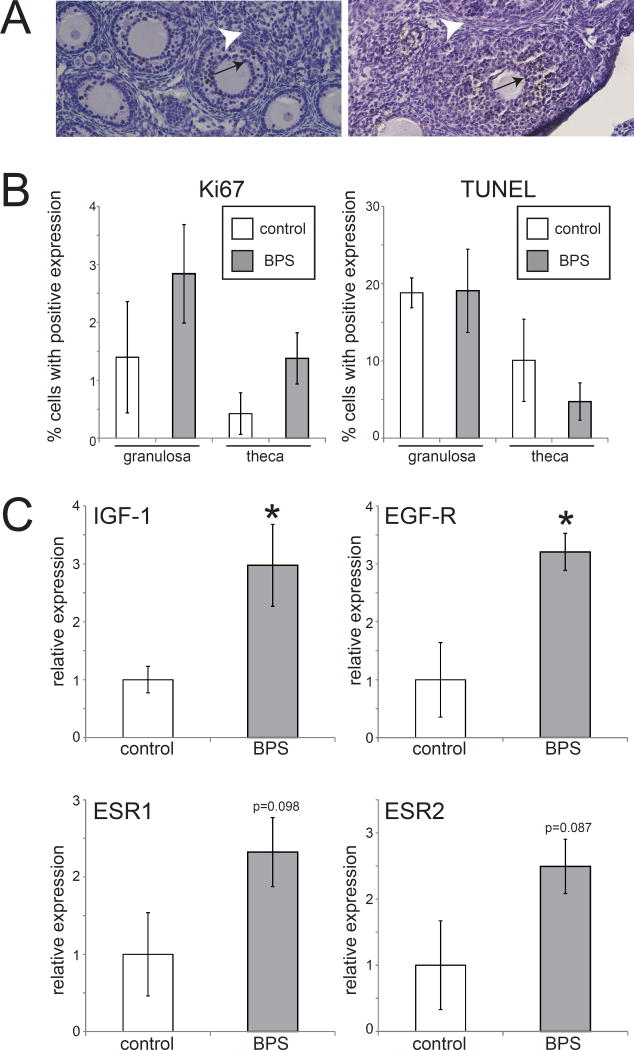
Ovarian tissue was assessed to characterize the effects of BPS on proliferation and apoptosis. BPS exposure was associated with modest increased expression of Ki67 in both granulosa cells and theca cells, although neither of these differences achieved statistical significance (Figure 6A,B). No effect of BPS was seen on TUNEL expression.
Figure 6. No significant effects of BPS on expression of markers of proliferation or apoptosis in the ovary.
A) Expression of Ki67 and TUNEL labeling in the ovary. Granulosa cells are indicated by an arrow and theca cells are indicated by an arrowhead. B) Quantification of expression revealed non-significant increases in Ki67 expression in both cell types in BPS-exposed ovaries. No differences were observed for TUNEL expression in BPS-treated ovaries. Photomicrographs were collected with a 20× EpiPlan Objective. C) Expression of four genes in the ovary at PND22. In all panels, * indicates p<0.05, independent samples t-test. In all groups, n=5 per treatment.
2.3.3 Gene expression
To quantify changes in ovarian development influenced by early life BPS exposure, the same genes that are known to be crucial for hormone regulation in the uterus were measured with the use of qPCR. Perinatal BPS exposure significantly increased the expression of IGF-1 (p<0.05) and EGF-R (p<0.05) in the ovary (Figure 6C). An increase in the expression of ESR-1 and ESR-2 was also observed in BPS-treated females, although this was not statistically significant compared to controls (Figure 6C). Overall, BPS induced increases in the expression of genes which are drivers of sexual maturity and regulate estrogen dependent pathways.
2.4 Altered response in the ovary of BPS-exposed females to an estrogen challenge
2.4.1 Tissue Morphology
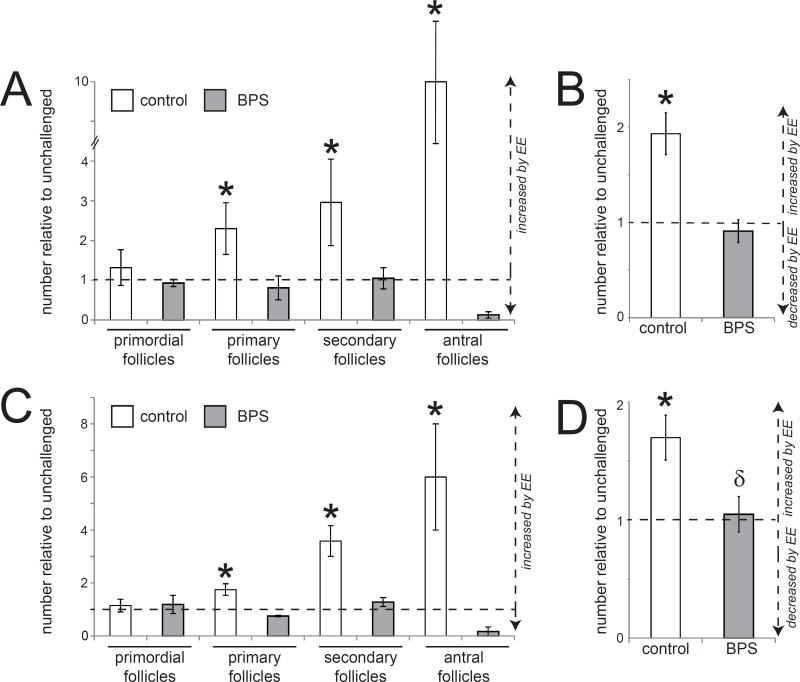
In control+EE females, the pubertal EE challenge significantly increased the number of primary, secondary and antral follicles, as well as the total number of follicles, when measured in the entire ovary (Figure 7A,B). When only four sections were evaluated in control+EE females, the EE challenge significantly increased the number of primary, secondary and antral follicles (Figure 7C) and total follicles (Figure 7D). This result is consistent with an estrogen-induced ovarian response, similar to what is seen in the maturation of follicles at the onset of puberty. In contrast, BPS+EE females saw no EE-induced increases in the number of total follicles, nor were any effects on specific follicle categories observed (Figure 7A,B). This was also true when only four sections were evaluated (Figure 7C,D). These results suggest that an estrogen challenge is ineffective at inducing an ovarian response in BPS-exposed females.
Figure 7. Tissue organization in the ovary of BPS-treated females is disrupted by an EE challenge.
A) An estrogen challenge induced significant ovarian development in control+EE females as measured by the increased number of primary, secondary and antral follicles. In contrast, the estrogen challenge had no effect on ovarian development in BPS+EE females. B) Total numbers of follicles counted throughout the entire ovary were significantly increased in control+EE females but unaffected in BPS+EE females. C) Similar patterns were observed when measures of ovarian development were limited to only four sections for specific types of follicles and D) total follicles in four sections of ovary. In all panels, * indicates p<0.05, independent samples t-test comparing EE-challenged with non-challenged females from the same perinatal treatment group (control or BPS). δ indicates significant differences, p<0.05, between EE-challenged controls and EE-challenged BPS-treated females. In all groups, n=5 per treatment.
2.4.2 Proliferation and Apoptosis
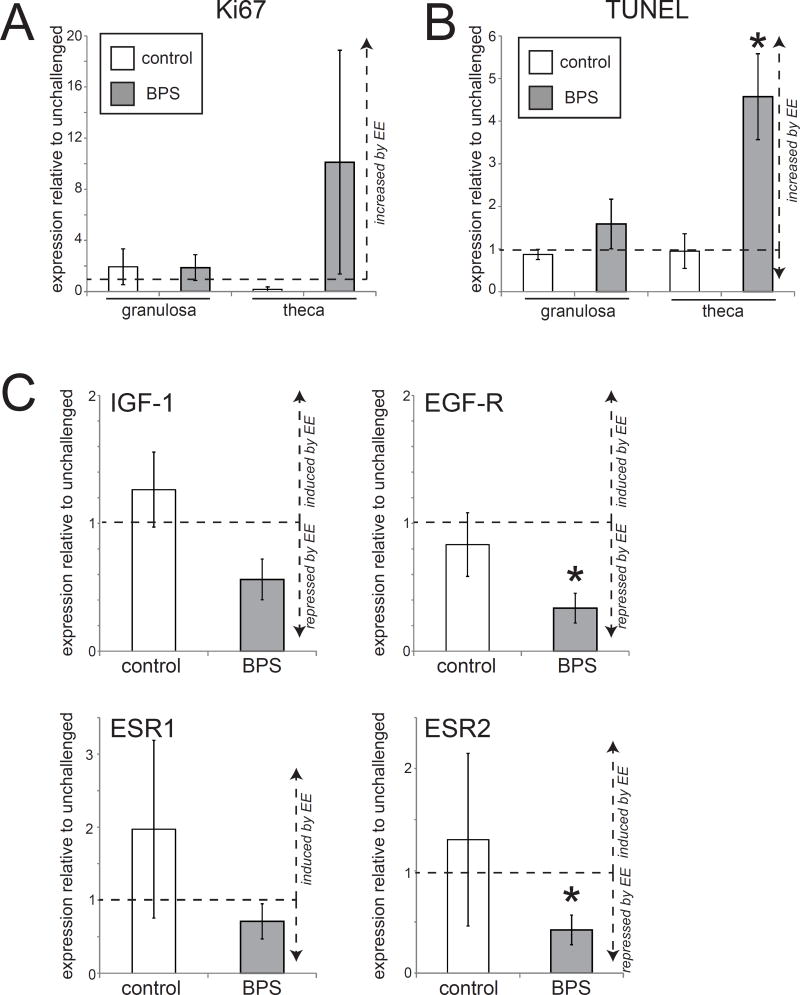
No changes in cell proliferation or apoptosis markers were observed in either the granulosa cells or the theca cells in the ovary of control+EE females following an estrogen challenge (Figure 8A,B). In contrast, BPS+EE females had increased numbers of theca cells that were positive for Ki67 and TUNEL, although only the apoptotic marker was statistically significant (Figure 8A,B).
Figure 8. Significant alterations in apoptosis and gene expression in the ovary of BPS-treated females after an estrogen challenge.
A) Quantification of Ki67 in the ovary of control+EE and BPS+EE females after an estrogen challenge. B) Quantification of TUNEL in the ovary of control+EE and BPS+EE females after an estrogen challenge. C) An estrogen challenge decreased the expression of estrogen-sensitive genes in the ovaries of BPS+EE females. In all panels, * indicates p<0.05, independent samples t-test comparing EE-challenged with non-challenged females from the same perinatal treatment group (control or BPS). δ indicates significant differences, p<0.05, between control+EE and BPS+EE females. In all groups, n=5 per treatment.
2.4.3 Gene expression
Control+EE females showed a relative (but non-significant) increase in ESR-1 expression in the ovary when compared to animals not provided with an EE challenge, suggesting that an estrogen challenge increases the expression of ERα in this organ (Figure 8C). In contrast, BPS+EE females experienced significant decreases in the expression of EGF-R and ESR-2 compared to BPS-treated females that were not given an estrogen challenge (Figure 8C).
3. Discussion
Here, we have shown that perinatal exposures to BPS, a common BPA replacement, can disrupt development of the mouse female reproductive tract. Relatively subtle effects were observed at PND22 in animals exposed to BPS alone, with statistically significant effects in the uterus limited to gene expression and effects in the ovary seen in both tissue morphology (e.g., number of secondary follicles) and gene expression. Further, when animals were provided with three days of estrogen treatment at a dose sufficient to induce an uterotrophic response, early life BPS exposure was associated with altered gene expression and abnormal tissue morphology in both the uterus and ovary.
In the pre-pubertal female mouse, the most striking effects of BPS were observed on gene expression endpoints, with significant effects on the expression of IGF-1 in the uterus and ovary and EGF-R in the ovary (Figure 3, Figure 6). IGF-1 was selected for analysis because prior studies have shown that estrogens can regulate expression of IGF-1 in uterine stromal cells and ovarian granulosa cells 52. EGF-R expression is also critically regulated by estrogen and expression of this gene increases late in folliculogenesis, consistent with our finding of increased numbers of mature follicles in BPS treated animals 53,54. Our results suggest that BPS may be able to act as an estrogen to stimulate increased expression of IGF-1 and EGF-R. Although the effects of BPS on ESR1 and ESR2 expression (encoding ERα and ERβ, respectively) were not statistically significant, likely due to the small sample size in this pilot study, their increased expression in BPS-treated ovaries is also consistent with BPS acting as an estrogen. One caveat that must be considered in the analysis of gene expression data is that these are evaluations of whole-organ cell lysates; because different gene expression is anticipated with different follicular stages, the changes in gene expression may be reflective of changes in follicular stage, including changes that we could not detect with our histological method (see further discussion below).
It has been reported previously that developmental exposure to estrogenic EDCs such as BPA and DES result in abnormal ovarian follicle formation 55–58 and tissue organization in the uterus 8,59–62. Here, we measured significant increases in the number of secondary follicles in ovaries of BPS-treated females (Figure 5), although no significant effects were observed on tissue organization, tissue morphology, cell proliferation or apoptosis in the uterus or oviduct (Figures 1, 2). This may be due to the limited size of this pilot study (n=5 per group) or the selection of a single BPS dose (200 µg/kg/day). Alternatively, these results could provide evidence that BPS induces alterations at the molecular level of biological organization in the uterus, but that more time is needed to observe effects at the level of the tissue. Additional studies are also needed to further explore the effects of BPS on the ovary, specifically to evaluate animals throughout adulthood. In particular, additional studies should examine not only the phenotype of the ovaries in BPS-exposed females (e.g. follicle number, follicle maturity, gross abnormalities etc.) they should also measure the quality and developmental competence of oocytes. If the number of mature follicles formed after puberty is significantly reduced in BPS-treated animals, this might indicate additional effects on measures of fecundity and fertility. Not only do these measures represent obvious adverse outcomes, they suggest possible endpoints with public health implications.
Importantly, a critical part of our experimental design included a pre-pubertal estrogen challenge. To our knowledge, no studies using developmental EDC exposures and a pre-pubertal estrogen challenge have been conducted using ovarian endpoints, making it difficult to make direct comparisons with our study. However, prior studies have revealed that developmental exposures to EDCs including BPA and DES alter the response of animals to hormonal or carcinogen challenges experienced later in life 47–50,63. In our study, one female from each litter was treated with 1 µg/kg/day EE for three days, from PND19-PND21; this dosing paradigm was selected because it is sufficient to induce an uterotrophic response in prepubertal mice, and thus can artificially induce some physiological responses consistent with puberty 51. We found that the estrogen challenge induced significant follicular maturation in control+EE females at PND22, with specific increases in the number of secondary and antral follicles, and the total number of follicles (Figure 7). These results are consistent with what is expected to occur in the ovary at the onset of puberty, when more advanced follicles are visible in the ovary 64,65. Although it is possible that EE prevents follicular loss, it is more likely that EE advances development of the ovary, increasing the number of mature follicles that are more likely to be counted with our method, which only evaluates sections every 50 µm and thus biases our counts toward larger follicles. Of course, these values are not counts of all follicles in the ovary; the method we use likely under-samples smaller follicles (primordial and primary), but the sampling methods are consistent between ovaries, allowing values to be compared between individuals. It is therefore not possible to determine whether BPS alters the complete pool of primordial follicles. This is potentially a sensitive endpoint that should be investigated in the future.
Strikingly, the increased number of mature follicles observed in control+EE females compared to controls that were not given an estrogen challenge were not observed in BPS+EE females; in these animals, the estrogen challenge did not appear to increase follicular maturation in the ovary. These results suggest that perinatal exposures to BPS may reduce responses of the ovary to estrogens at puberty (Figure 7), even when BPS exposure appears to heighten responses of the uterus when presented with an estrogen challenge (Figure 4A). Together, results observed in the ovary and uterus suggest that these organs respond in different ways to an estrogen challenge after perinatal BPS exposure. Additional analyses that examine additional aspects of the hypothalamic-pituitary-ovarian axis including gene expression in the hypothalamus and pituitary may help to elucidate the mechanisms by which BPS and similar compounds induce distinct alterations in estrogen-sensitive organs including the ovary and uterus.
BPA has a wide range of molecular behaviors including estrogen receptor agonist and antagonist behavior (both ERα and ERβ), an ability to bind to membrane ERα and the transmembrane estrogen receptor GPR30, actions as a thyroid hormone antagonist and an androgen receptor antagonist, and ability to bind to the estrogen related receptor (ERR)γ and the aryl hydrocarbon receptor (AhR) 66. In contrast, most studies of BPS point to its estrogen receptor agonist properties (via ERα, ERβ and membrane ERs) 34–37. Some of these studies suggest that BPS may be a more potent estrogen mimic than BPA, at least for some endpoints. Considering those molecular data, previous studies showing that BPS can disrupt a range of endpoints in zebrafish and rodents 39,40,43,45, and the data we present in this manuscript, it is clear there is a need for further studies to observe additional adverse outcomes associated with BPS exposure; it is equally clear that the data are not supportive of safety for this replacement chemical.
3.1 Conclusions
In the last year, a number of studies examining the effects of BPS have suggested that developmental exposures to this compound induce altered health outcomes through endocrine disrupting actions in rodents and zebrafish 23. The results we have acquired here are consistent with the hypothesis that developmental exposure to BPS disrupts cellular and molecular events in the developing mammalian female reproductive tract. The effects on the ovary that we observed were most pronounced after an estrogen challenge; we found that BPS induces premature ovarian development, but diminishes the response of the ovary from being further stimulated by estrogens. In contrast, the effects of BPS on uterine tissue organization are only apparent after an estrogen challenge meant to mimic the onset of puberty. Further studies are needed to extend this pilot study, both to examine additional endpoints of interest, to include older ages, and to examine apical endpoints related to fertility, fecundity, and reproductive health.
4. Materials and Methods
4.1 Animals
Adult outbred CD-1 mice of both sexes were obtained from Charles River Breeding Laboratories (Raleigh, NC). The CD-1 strain has previously been shown to be a sensitive model for the use of reproductive endocrine disruption studies16,60. Two adult females and one adult male were housed together in ventilated polysulfone cages until pregnancy was confirmed via the presence of a vaginal plug. Pregnant females were housed with one or two other females of the same dosing group until pregnancy day 17. On pregnancy day 18, each pregnant female was separated into her own cage in preparation for delivery. A 0600–1800 hours light cycle and a controlled temperature of 25–27 °C was followed for the entirety of the experiment. Standard rodent chow (Harlan Teklad 2018, which has been reported to have minimal estrogenic activity 67) and tap water (in glass water bottles) were provided ad libitum. All procedures with mice were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Massachusetts, Amherst.
4.2 Chemical administration
At the beginning of the dosing period, pregnant females were randomly assigned to exposure groups (n = 5 per group) using statistical methods to ensure similar body weights across treatment groups. Females were ear-tagged upon arrival to allow for their identification; identification tags were coded so that experimenters were blind to treatment throughout the experiment.
The day that a vaginal plug was observed was considered pregnancy day 1. Pregnant females were orally dosed with tocopherol-stripped corn oil (MP Biomedicals) alone (vehicle control, n=5) or 200µg BPS (Santa Cruz Biotechnology, 99% purity) /kg body weight/day in stripped corn oil (n=5). Mice were trained to drink these substances (1 µl per gram body weight) from the end of a pipette. Pregnant and lactating mice were treated from pregnancy day 8 until lactational day 19. This period of perinatal exposure was selected to include the period of organogenesis as well as critical periods of differentiation and development for multiple organs including the brain; evaluation of other organs, body weight, and neurobehaviors have been published elsewhere 42,45 or are forthcoming.
On postnatal day (PND) 19, two female offspring (F1 generation) were randomly selected from each litter, weaned from their mothers, and ear tagged. One female was fed tocopherol-stripped corn oil by pipette and the second was fed 1µg/kg/day ethinyl estradiol (EE) in stripped corn oil. Both females were fed their assigned solutions from PND 19 to PND21 and were killed by cervical dislocation on PND22, 24 hours after the last dose of oil or EE. During this pre-pubertal period, female offspring were housed only with other females receiving the same postnatal treatment (oil or EE).
This dosing procedure produced four treatment groups: perinatal vehicle + prepubertal oil; perinatal vehicle + prepubertal EE; perinatal BPS + prepubertal oil; perinatal BPS + prepubertal EE (see Supplemental Figure 1). In all four groups, n=5. This small sample size was selected for convenience rather than statistical power; this small sample size is a limitation of this pilot study.
4.3 Tissue and body weight collection
On PND22, female reproductive tissues including the ovary, oviduct and uterus were collected. All organs were examined for gross malformations including ovarian cysts. Uterine responsiveness to EE was confirmed visually via change in uterine size. One ovary and one uterine horn were snap-frozen in liquid nitrogen and stored at −80C for qPCR analysis. One ovary, one oviduct, and one horn of the uterus were fixed in neutral buffered formalin for histological analyses. Body weight was also measured at time of sacrifice (data not shown).
4.4 Analysis of tissue morphology
Fixed tissues were processed through a series of alcohols and embedded in paraffin. Paraffin blocks were sectioned at 5µm on a Fisher rotary microtome and mounted on positively-charged glass slides. Sections were processed through a series of xylene and ethanol, stained with hematoxylin and eosin, dehydrated, and coverslipped with permanent mounting medium. Images were collected on a Zeiss AxioImager Inverted Microscope with ZEN imaging software and a Zeiss high resolution color camera with a 20× EpiPlan Objective.
To analyze morphology of the oviduct and uterus, epithelial cell height was measured with ZEN imaging software. To score ovarian follicles, every tenth section throughout the entire ovary was analyzed, corresponding to one section per 50µm of ovary. This method is not intended to count every follicle in the ovary; smaller follicles (primordial and primary) will be under-represented because of their small size. However, counting sections at this distance will prevent accidental counting of larger follicles more than once.
Follicles were scored using methods modified from those described previously 55 and categorized depending on the stage of follicle development. Only follicles containing oocytes with visibly stained nuclei were counted. As expected, corpora lutea were not observed in any animal at PND22. To minimize statistical error due to variation between ovarian sizes, four sections (50µm apart) from the medullary region were analyzed separately. For all tissues, sections were coded allowing for scoring by a observer blind to treatment group.
4.5 Immunohistochemistry
For immunohistochemical analysis of uterine, oviduct and ovarian tissues, standard protocols were used 60. Briefly, slides were processed through a series of xylene and ethanol washes followed by antigen retrieval using microwave-heated citric acid buffer. Endogenous peroxidase activity was quenched with hydrogen peroxide treatment and non-specific antibody sites were blocked using normal goat serum 1:20 in 1.5% milk. Ki67 antibodies were obtained from Vector Labs (rabbit polyclonal, Cat# VP-RM04) and used at concentrations of 1:1000. After overnight incubation with the primary antibody at 4°C, the tissue was incubated with the biotin labeled anti-rabbit secondary antibody (Abcam Cat# ab64256) for one hour at room temperature, and the reaction was developed with streptavidin peroxidase complex (Abcam Cat# ab64269). Finally, a color-change reaction was obtained using 3,3-diaminobenzidine (DAB, Abcam Cat# ab64238), tissue was counterstained with hemotoxylin and cover-slipped with permanent mounting media.
To quantify Ki67 expression, one section of the uterus and the medullary region of the ovary were analyzed per female. Each sample was imaged with a Zeiss AxioImager Inverted Microscope with a 20× EpiPlan Objective with ZEN imaging software and a Zeiss high-resolution color camera. All analyses were conducted by an observer blind to treatment. In the ovary, follicles were analyzed by counting the number of positive and negative cells (both granulosa and theca cells were counted). The total percentage of granulosa and theca cells that were positive for the specific antibody being tested was then calculated (# positive / total number counted × 100). Whenever possible, at least two follicles of the different stages were counted. In uterine and oviduct tissues, positive and negative cells were counted in the epithelial and stromal compartments, as appropriate.
4.6 TUNEL apoptosis assay
The Trevigen TACS 2 TdT-DAB in situ apoptosis detection kit was used for detection of apoptotic cells in ovarian and uterine tissue sections. Briefly, one section of the uterus and the medullary region of the ovary were analyzed per female. Slides were processed through a series of xylene and ethanol washes followed by 1X PBS. Samples incubated with Proteinase K solution for 15 minutes at room temperature, immersed in a hydrogen peroxide quenching solution and incubated with the reaction mix containing TdT enzyme and incubated for one hour at 37°C. The reaction was developed using a Strep-HRP and DAB solution. Samples were counterstained with hematoxylin, dehydrated, and cover-slipped with permanent mounting media.
Each sample was imaged with a Zeiss AxioImager Inverted Microscope with a 20× EpiPlan Objective with ZEN imaging software and a Zeiss high-resolution color camera. Samples were assessed using the same methods stated above for immunohistochemical analyses.
4.7 RNA isolation, cDNA synthesis and qPCR
Total RNA extracted from ovaries or uterine horns of individual mice using Trizol reagent (Ambion) was reverse transcribed using an iScript Reverse Transcription Supermix for RT-qPCR kit (Cat 170–884, BioRad). Primers were designed using free online software Primer3Plus and are listed in Table 2. The expression of four genes related to estrogen response was analyzed: Esr1 (ER α). Esr2 (ER β), IGF1 (insulin growth factor 1), and EGF-R (epidermal growth factor receptor). beta-2-microglobulin (B2M) was used as a housekeeping gene; although this gene can respond to estradiol 68, we saw no changes in B2M expression associated with BPS or EE treatment (data not shown). Triplicate 5-µl real-time PCR mixtures, each containing iTaq Universal SYBR Green Supermix (Cat 172–5124, BioRad), qPCR primers, and cDNA template were loaded onto a 384-well plate and run through 40 cycles on a CFX384 real time cycler (Bio-Rad Laboratories, Inc). Data were analyzed using the manufacturer's CFX manager software, version 3.1. Relative quantification was determined using the ΔΔCq method to correct for differences in the reference gene 69.
Table 2.
Primer sequences used for qPCR analysis
| Gene | Forward primer sequence |
Reverse primer sequence |
|---|---|---|
| Housekeeping gene: beta-2-microglobulin/B2M | CCG GCC TGT ATG CTA TCC AG | TGT TCG GCT TCC CAT TCT CC |
| Epidermal growth factor receptor/EGF-R | TCT TCA AGG ATG TGA AGT GTG | TGT ACG CTT TCG AAC AAT GT |
| Estrogen Receptorα/ESR1 | TGC AAT GAC TAT GCC TCT GG | CTC CGG TTC TTG TCA ATG GT |
| Estrogen Receptor®/ESR2 | ACT GCC AAT CAT CGC TTC TC | AGT AAC AGG GCT GGC ACA AC |
| Insulin like Growth Factor 1/IGF1 | GGA CCA GAG ACC CTT TGC GGG G | GGC TGC TTT TGT AGG CTT CAG TGG |
4.8 Statistical analysis
All statistical analysis was carried out with the use of SPSS statistical software version 22. Effect of chemical treatment was assessed for using independent sample t-tests to compare control and BPS groups, or oil and EE groups, as appropriate. Graphs indicate mean ± SEM. Results were considered significant at p<0.05.
Supplementary Material
Supplemental Figure 1: A schematic overview of the experimental design.
Acknowledgments
The authors gratefully acknowledge helpful feedback from Alicia Timme-Laragy, Mary Catanese, and members of the Vandenberg laboratory. The experiments described in this study were supported by start-up funds to AS and LNV and Award Number K22ES025811 from the National Institute of Environmental Health Sciences of the National Institutes of Health to LNV. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Massachusetts.
Abbreviations
- BPA
bisphenol A
- BPS
bisphenol S
- DAB
3,3-diaminobenzidine
- DES
diethylstilbestrol
- EDCs
endocrine disrupting chemicals
- EE
ethinyl estradiol
- ER
estrogen receptor
- NOAEL
no observed adverse effect level
- PBS
phosphate buffered saline
- PND
postnatal day
Footnotes
Disclosure Statement: LNV has received travel reimbursement from Universities, Governments, NGOs and Industry, to speak about endocrine-disrupting chemicals. CEH, SS and AS have nothing to disclose.
Literature Cited
- 1.Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90(4):911–40. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandrup KR, Jacobsen PR, Isling LK, et al. Effects of perinatal ethinyl estradiol exposure in male and female Wistar rats. Reprod Toxicol. 2013;42:180–91. doi: 10.1016/j.reprotox.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Soto AM, Rubin BS, Sonnenschein C. Endocrine disruption and the female. In: Gore A, editor. Endocrine-Disrupting Chemicals. Totowa, N.J.: Humana Press; 2007. [Google Scholar]
- 4.McLachlan JA. Commentary: prenatal exposure to diethylstilbestrol (DES): a continuing story. Int J Epidemiol. 2006;35(4):868–70. doi: 10.1093/ije/dyl140. [DOI] [PubMed] [Google Scholar]
- 5.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971;284(15):878–81. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 6.Hoover RN, Hyer M, Pfeiffer RM, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365(14):1304–14. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 7.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50(23):7677–81. [PubMed] [Google Scholar]
- 8.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24(2):253–8. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki A, Sugihara A, Uchida K, et al. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16(2):107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 10.Soto AM, Vandenberg LN, Maffini MV, et al. Does breast cancer start in the womb? Basic and Clinical Pharmacology and Toxicology. 2008;102(2):125–33. doi: 10.1111/j.1742-7843.2007.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman A, Heindel JJ, Kasten T, et al. The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect. 2013;121(4):A104–6. doi: 10.1289/ehp.1205448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36(6):E1–150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunt PA, Susiarjo M, Rubio C, et al. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–13. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maffini MV, Rubin BS, Sonnenschein C, et al. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–86. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Rochester JR. Bisphenol A and human health: A review of the literature. Reprod Toxicol. 2013;42C:132–55. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Peretz J, Vrooman L, Ricke WA, et al. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect. 2014;122(8):775–86. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suvorov A, Waxman DJ. Early programing of uterine tissue by bisphenol A: Critical evaluation of evidence from animal exposure studies. Reprod Toxicol. 2015 doi: 10.1016/j.reprotox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandenberg LN, Ehrlich S, Belcher SM, et al. Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies. Endocrine Disruptors. 2013;1(1):e25078. [Google Scholar]
- 19.Rosenmai AK, Dybdahl M, Pedersen M, et al. Are structural analogues to bisphenol a safe alternatives? Toxicol Sci. 2014;139(1):35–47. doi: 10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- 20.Chapin RE, Adams J, Boekelheide K, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83(3):157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 21.Vandenberg LN, Hunt PA, Myers JP, et al. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28(1):37–58. doi: 10.1515/reveh-2012-0034. [DOI] [PubMed] [Google Scholar]
- 22.Mathew M, Sreedhanya S, Manoj P, et al. Exploring the interaction of bisphenol-s with serum albumins: a better or worse alternative for bisphenol a? J Phys Chem B. 2014;118(14):3832–43. doi: 10.1021/jp500404u. [DOI] [PubMed] [Google Scholar]
- 23.Rochester JR, Bolden AL, Bisphenol SF. A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015;123(7):643–50. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenberg LN, Luthi D, Quinerly D. Plastic bodies in a plastic world: multi-disciplinary approaches to study endocrine disrupting chemicals. J Cleaner Production. 2017;140(1):373–85. [Google Scholar]
- 25.Liao C, Kannan K. Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655–62. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- 26.Liao C, Liu F, Alomirah H, et al. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol. 2012;46(12):6860–6. doi: 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- 27.Liao C, Liu F, Guo Y, et al. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol. 2012;46(16):9138–45. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- 28.Liao C, Liu F, Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol. 2012;46(12):6515–22. doi: 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- 29.Liao C, Liu F, Moon HB, et al. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol. 46(21):11558–65. doi: 10.1021/es303191g. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, Wong LY, Kramer J, et al. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ Sci Technol. 2015;49(19):11834–9. doi: 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lv Y, Lu S, Dai Y, et al. Higher dermal exposure of cashiers to BPA and its association with DNA oxidative damage. Environ Int. 2017;98:69–74. doi: 10.1016/j.envint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Porras SP, Heinala M, Santonen T. Bisphenol A exposure via thermal paper receipts. Toxicol Lett. 2014;230(3):413–20. doi: 10.1016/j.toxlet.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Bittner GD, Yang CZ, Stoner MA. Estrogenic chemicals often leach from BPA-free plastic products that are replacements for BPA-containing polycarbonate products. Environ Health. 2014;13(1):41. doi: 10.1186/1476-069X-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grignard E, Lapenna S, Bremer S. Weak estrogenic transcriptional activities of Bisphenol A and Bisphenol S. Toxicol in Vitro. 2012;26(5):727–31. doi: 10.1016/j.tiv.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Kuruto-Niwa R, Nozawa R, Miyakoshi T, et al. Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol. 2005;19(1):121–30. doi: 10.1016/j.etap.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Molina-Molina JM, Amaya E, Grimaldi M, et al. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol. 2013 doi: 10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Vinas P, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121(3):352–8. doi: 10.1289/ehp.1205826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji K, Hong S, Kho Y, et al. Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol. 2013;47(15):8793–800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- 39.Kinch CD, Ibhazehiebo K, Jeong JH, et al. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proc Natl Acad Sci U S A. 2015;112(5):1475–80. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naderi M, Wong MY, Gholami F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Qiu W, Zhao Y, Yang M, et al. Actions of Bisphenol A and Bisphenol S on the Reproductive Neuroendocrine System During Early Development in Zebrafish. Endocrinology. 2016;157(2):636–47. doi: 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- 42.Catanese MC, Suvorov A, Vandenberg LN. Beyond a means of exposure: a new view of the mother in toxicology research. Toxicol Res. 2015;4:592–612. [Google Scholar]
- 43.Catanese MC, Vandenberg LN, Bisphenol S. (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology. 2016 doi: 10.1210/en.2016-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki K, Noda S, Imatanaka N, et al. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol Lett. 2004;146(2):111–20. doi: 10.1016/j.toxlet.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Kim B, Colon E, Chawla S, et al. Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ Health. 2015;14:64. doi: 10.1186/s12940-015-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.EPA U. [Accessed 14 November 2016];Bisphenol A alternatives in thermal paper, Final Report. 2014 https://www.epa.gov/sites/production/files/2015-08/documents/bpa_final.pdf af, ed.
- 47.Betancourt AM, Eltoum IA, Desmond RA, et al. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ Health Perspect. 2010;118(11):1614–9. doi: 10.1289/ehp.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durando M, Kass L, Piva J, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prins GS, Birch L, Tang WY, et al. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol. 2007;23(3):374–82. doi: 10.1016/j.reprotox.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadia PR, Vandenberg LN, Schaeberle CM, et al. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect. 2007;115(4):592–8. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandenberg LN, Wadia PR, Schaeberle CM, et al. The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization? Journal of Steroid Biochemistry and Molecular Biology. 2006;101(4–5):263–74. doi: 10.1016/j.jsbmb.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 52.Ogo Y, Taniuchi S, Ojima F, et al. IGF-1 gene expression is differentially regulated by estrogen receptors alpha and beta in mouse endometrial stromal cells and ovarian granulosa cells. J Reprod Dev. 2014;60(3):216–23. doi: 10.1262/jrd.2013-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Hayek S, Demeestere I, Clarke HJ. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proc Natl Acad Sci U S A. 2014;111(47):16778–83. doi: 10.1073/pnas.1414648111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garnett K, Wang J, Roy SK. Spatiotemporal expression of epidermal growth factor receptor messenger RNA and protein in the hamster ovary: follicle stage-specific differential modulation by follicle-stimulating hormone, luteinizing hormone, estradiol, and progesterone. Biol Reprod. 2002;67(5):1593–604. doi: 10.1095/biolreprod.102.005470. [DOI] [PubMed] [Google Scholar]
- 55.Hunt PA, Lawson C, Gieske M, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109(43):17525–30. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peretz J, Gupta RK, Singh J, et al. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119(1):209–17. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera OE, Varayoud J, Rodriguez HA, et al. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol. 2011 doi: 10.1016/j.reprotox.2011.06.118. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez HA, Santambrosio N, Santamaria CG, et al. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol. 2010;30(4):550–7. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Markey CM, Michaelson CL, Veson EC, et al. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109(1):55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markey CM, Wadia PR, Rubin BS, et al. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biology of Reproduction. 2005;72(6):1344–51. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 61.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199(2):142–50. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 62.Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117(6):879–85. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newbold RR, Jefferson WN, Padilla-Banks E, et al. Developemental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol. 2004;18(3):399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Monget P, Bobe J, Gougeon A, et al. The ovarian reserve in mammals: a functional and evolutionary perspective. Mol Cell Endocrinol. 2012;356(1–2):2–12. doi: 10.1016/j.mce.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 65.Omoto Y, Lathe R, Warner M, et al. Early onset of puberty and early ovarian failure in CYP7B1 knockout mice. Proc Natl Acad Sci U S A. 2005;102(8):2814–9. doi: 10.1073/pnas.0500198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandenberg LN, Maffini MV, Sonnenschein C, et al. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocrine Reviews. 2009;30(1):75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vandenberg LN, Maffini MV, Schaeberle CM, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–9. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schroder AL, Pelch KE, Nagel SC. Estrogen modulates expression of putative housekeeping genes in the mouse uterus. Endocrine. 2009;35(2):211–9. doi: 10.1007/s12020-009-9154-6. [DOI] [PubMed] [Google Scholar]
- 69.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: A schematic overview of the experimental design.