Abstract
Celiac disease is a multisystem disorder. Celiac hepatitis characterized by gluten-responsive mild elevation of transaminases is the more common liver manifestation of celiac disease. Celiac disease may also be associated or coexist with other chronic liver disorders. Shared genetic risk and increased intestinal permeability has been suggested to be the most relevant events in the pathogenesis of liver injury in celiac disease. The aim of this article is to review the full spectrum of liver disorders in patients with celiac disease.
Keywords: Hepatitis, Cirrhosis, Alanine Aminotransferase
Introduction
Celiac disease (CD) is a multisystem disorder characterized by permanent intolerance to gluten (wheat, barley, and rye).1,2 Although the hallmark of CD is enteropathy; other organs including the liver may also be affected. Liver abnormalities in untreated CD are common.3 CD can cause direct liver damage (celiac hepatitis) but also may be associated with other liver conditions.4 Abnormal liver blood tests (especially hypertransaminasemia) may be the sole manifestations of hitherto unrecognized CD. The pathophysiology of liver injury in CD remains poorly understood. The aim of this study is to review the full spectrum of liver injury related to CD.
Initial Work-Up
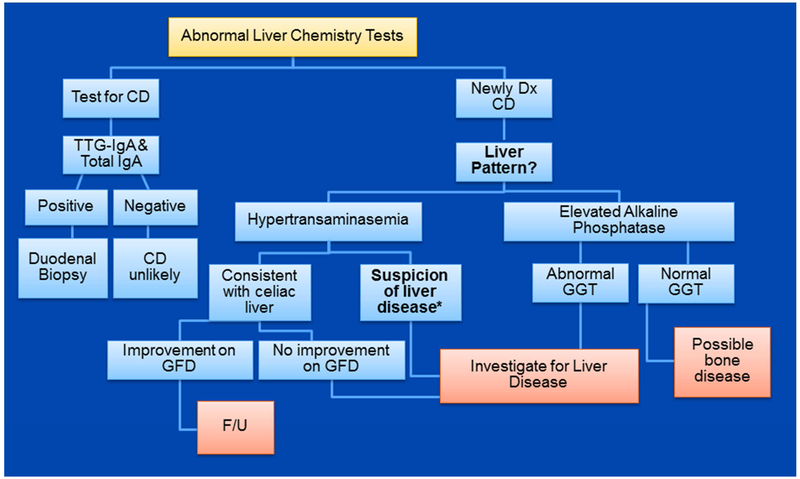
A complete liver test panel is strongly recommended in patients with newly diagnosed CD.3 Mild elevation of transaminases (3-5 times the upper limit of normal) in the absence of clinical manifestations of chronic liver disease is characteristic of celiac hepatitis. Resolution of the abnormal liver tests after strict adherence to a gluten-free diet (GFD) confirms the diagnosis.3 Thus, if liver tests were abnormal at the time of diagnosis, it should be re-checked after 6-12 months on a strict GFD.3,5 In patients with typical findings for celiac hepatitis, it is reasonable to treat with a GFD first and plan for further investigation in the subset of patients (10%-25%) with persistent liver test abnormalities after 1 year on strict adherence to GFD. However, an initial evaluation is strongly recommend for coexistent liver disorder in patients with symptoms or physical signs that suggest chronic liver disorder and/or transaminases levels greater than 5 times the upper limit of normal (see Fig. 1).6-8
Figure 1.
Suggested approach to abnormal liver test and celiac disease. *Clues for suspicion of concurrent liver disease includes: hyperbilirubinemia, hypertransaminasemia >5 times upper limit of normal, AST: ALT ratio >1.0, and abnormal physical exam.
Isolated elevation of alkaline phosphatase is not characteristic of celiac hepatitis. Metabolic bone disease may be the most common explanation for an isolated alkaline phosphatase in patients with CD.2,9 Check calcium, phosphate, 25 (OH) vitamin D, and parathyroid hormone for evaluation for osteomalacia.10 A very low 25-(0H) vitamin D, low calcium and phosphate, and elevated parathyroid hormone strongly support the diagnosis of malabsorption-related osteomalacia. Dual energy x-ray absorptiometry is suggested for all patients with newly-diagnosed CD.10 Thyroid stimulant hormone measurement is also useful.10 Chronic cholestatic liver disorders should be considered after exclusion of non-liver causes for isolated elevation of alkaline phosphatase.
Liver biopsy is not necessary in most patients with newly diagnosed CD who have isolated hypertransaminasemia.6,11 Liver biopsy may be useful in selected patients with suspicion of chronic cholestatic liver disease with negative non-invasive tests, unexplained persistent hypertransaminasemia (after 1-year on strict adherence to GFD), and coexistent liver disease in which the liver biopsy has therapeutic or prognostic significance.3
Celiac Hepatitis
Celiac hepatitis is a gluten-dependent injury and liver abnormalities resolved on a GFD, typically after 12 months of strict adherence.5-7,12,13 Histological changes also improve after a GFD.14
Hypertransaminasemia is frequent in untreated CD (13%-60%) (see Table 1).6,7,12 Conversely, CD is present in as many of the 9% of persons with unexplained hypertransaminasemia.15,16 Celiac patients have both an increased risk of subsequent liver disease and risk of death from liver cirrhosis than the general population. 3,15,17,18
Table 1.
Frequency of abnormal liver chemistry test and the effect of a GFD in patients with celiac disease
| Reference | Cases | Female (n, %) | Age (range) yrs |
Abnormal liver test (n, %) |
Response to GFD (n, %) |
Time on GFD |
|---|---|---|---|---|---|---|
| Bardella6 | 158 | 127, 80% | 18-68 | 67, 42% | 60/67, 90% | 6 months |
| Hagander7 | 74 | 43, 58% | 14-73 | 29/53, 55% | N/A* | N/A |
| Bonamico12 | 65 | 43, 66% | 0.5-18 | 37, 60% | N/A | N/A |
| Jacobsen13 | 132 | 64, 48% | 25-86 | 62, 47% | 24/32, 75% | 2 years |
| Dickey5 | 129 | 88, 68% | 17-88 | 17, 13% | 15/17, 88% | 6-12 months |
| Castillo, N57 | 463 | 328,71% | 44 (+/−14) | 190, 41% | 79% | 18 months |
| Lee, GJ58 | 388 | 235,61% | 10 | 185, 48% | 71% (only 21 repeat) | N/A |
| Aarela, L22 | 150 | 103, 69% | 7.3 (4.3-11.8) | 22, 15% | 80% | 12 months |
Transaminase levels fell significantly 2.5 - 8 weeks after starting a GFD
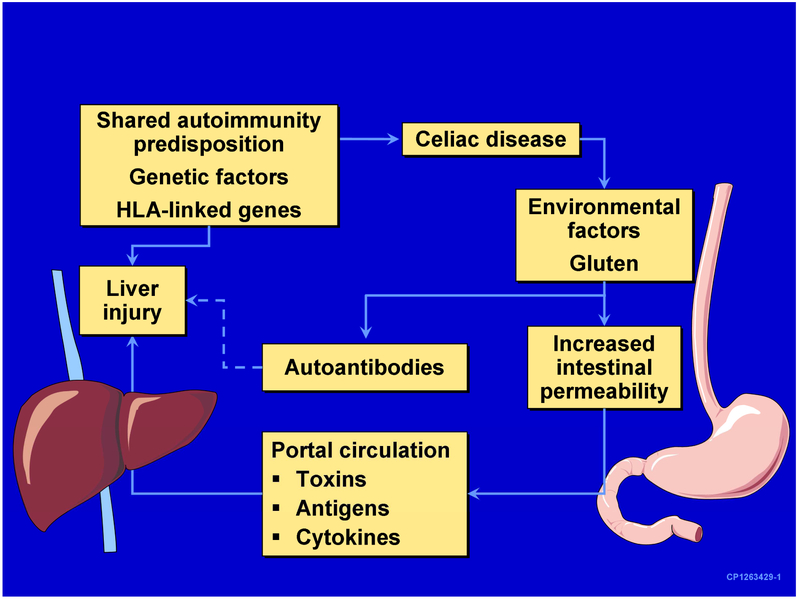
The mechanisms underlying celiac hepatitis are poorly understood.19 Intestinal permeability was quantitatively higher in patients with CD and hypertransaminasemia than in those with CD and normal liver tests.11 The phenomenon is gluten-dependent as demonstrated for normalization of both intestinal permeability and elevation of transaminases with a GFD.11 It has been speculated that increased intestinal permeability may facilitate the entry to the portal circulation (and then to the liver) of toxins, microbial and other antigens, cytokines, and/or other mediators of liver injury (see Fig.2).7,16,20,21 However, liver injury is not commonly seen in other intestinal disorders associated with increased intestinal permeability.
Figure 2.
Potential mechanisms of liver injury in celiac disease (From Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007;46(5):1650-1658, with permission.)
Most patients with celiac hepatitis have no symptoms or signs of liver disease.6,7,15 Thus, the presence of palmar erythema, jaundice, ascites, splenomegaly, encephalopathy, coagulopathy, or portal hypertension suggests advanced liver disease or the coexistence with other chronic liver disease.3,6 Mild to moderate (less than 5 times the upper limit of normal) levels of aspartate aminotransferase and/or alanine aminotransferase are typical.6,7,12,16 The ratio aspartate to alanine aminotransferase is usually less than 1.3 Elevated alanine aminotransferase is associated with poor growth and severe villous atrophy in children.22
Conjugated hyperbilirubinemia is not expected in the absence of advance cirrhosis.6,10,23 Abdominal ultrasound is not necessary during the initial work-up, and findings on the liver vary according to the degree of liver injury; from normal (most common) to coarse echo texture.23 Other non-specific abdominal ultrasound findings suggestive of CD include dilated small bowel loops, enlarged mesenteric lymph nodes, non-occlusive intussusception, abnormal jejunum folds, and increased fasting gallbladder volume.24,25
Liver biopsy is rarely needed for celiac hepatitis. Mild and/or nonspecific histological changes are seen.13,26 Extensive fibrosis and cirrhosis are rare (see Table 2).23
Table 2.
Liver pathology of patients with celiac disease
most common findings
Finally, there is considerable evidence and expert opinion support for testing for CD in patients with unexplained abnormal liver tests.27 Advanced liver disease is associated with false positive results of the tissue transglutaminase antibody (especially if titer is less than 3 times upper limit of normal).3 Endomysial antibodies are more specific in this context and may be helpful in the diagnostic evaluation of patients with advanced liver disease. Biopsy confirmation of CD is strongly recommended.3
Celiac Disease and Selected Liver Disorders
Autoimmune Liver Disorders
Primary biliary cholangitis and autoimmune hepatitis may be associated with CD.3,28,29 The frequency of CD in patients with primary biliary cholangitis (1% - 7%) and primary biliary cholangitis in patients with CD (0.1% - 3%) is variable between studies (see Table 3 and Table 4).8,30-37 CD is present in 4% -6% of patients with both type 1 and type 2 autoimmune hepatitis.3,28-30 There are also case reports of primary sclerosing cholangitis and CD.38
Table 3.
Selected studies on screening of celiac disease with biopsy confirmation in primary biliary cirrhosis
Table 4.
Selected studies on the prevalence of primary biliary cirrhosis in patients with celiac disease
The reasons for the association between CD and autoimmune liver disorders are unknown. Shared genetic susceptibility to autoimmunity and perhaps vulnerability of both biliary and small intestine epithelium to immune-mediated damage may play a role.32 CD and primary sclerosing cholangitis share the at risk gene risk HLA-DQ2. The presence of HLA-DQ2 is associated with an increased rapid progression of the liver disease in primary sclerosing cholangitis.39 Likewise, homozygosity for DQ2 increase CD risk and perhaps severity.40,41 A GFD appears to have little effect on coexistent liver disease outcome as it may not improve liver tests or symptoms.29,31
Viral Hepatitis and Vaccines
There is no association between CD and chronic hepatitis C.42 Most patients with concurrent CD and hepatitis C have a well-defined route of transmission for hepatitis C.43 Hepatitis C treatment with interferon-α and/or ribavirin may activate silent or latent CD.3,44,45 The clinical relevance of this observation has decreased with the newly available direct active antiviral drugs. Non-response to hepatitis B vaccine given prior to diagnosis of CD is frequent (54%- 68%).46,47 Rate of seroconversion correlated with the amount of gluten ingestion and greater than 95% of CD patients vaccinated after treatment with a GFD may respond.48 HLA-DQ2 may play a role in vaccine non-response.46,49
Nonalcoholic Fatty Liver
The frequency of CD in patients with nonalcoholic fatty liver disease is 3% - 7%.50,51 Screening all patients with nonalcoholic fatty liver disease for CD is controversial. However, a high index of suspicion may result in early diagnosis. Active screening is reasonable in patients with nonalcoholic fatty liver disease with unexplained anemia, nutritional deficiencies, and recurrent abdominal symptoms.51 The GFD may improve liver tests in patients with nonalcoholic fatty liver disease and CD, but it is unclear if this effect is independent of nutritional factors.50 Moreover, there is an increased risk of nonalcoholic fatty liver disease (hazard ratio = 2.8) following a GFD and close monitoring of weight is recommended after GFD.52 Increased risk was higher in children and non-overweight CD patients.52,53 Nonalcoholic fatty liver disease risk remains elevated even beyond 15 years after the diagnosis of CD.52
Liver Transplantation
The prevalence of CD in liver transplant patients with end-stage liver disease of multiple causes varied from 3% - 4.3%.23,54 Strict adherence for 6 months to a GFD in a small group of enlisted patients with CD and end-staged liver disease improved liver function to the point that made liver transplant unnecessary. 23,54,55 A large Swedish study showed no increased risk of liver transplantation in diagnosed CD despite increased risk of acute hepatitis, chronic hepatitis, primary sclerosing cholangitis, fatty liver disease, liver failure, liver cirrhosis/fibrosis, and primary biliary cholangitis.17
Mortality of Liver Cause in Celiac Disease
Mortality of liver cause is increased in patients with CD (standardized mortality ratio 3.10) although the absolute risk of liver-related mortality is modest.56
Conclusion
Liver blood tests abnormalities are common in patients with CD. Conversely, abnormal liver blood tests (especially hypertransaminasemia) may be the sole manifestations of unrecognized CD. Celiac hepatitis is the most common liver manifestation of CD and responsive to GFD. Finally, CD may be associated with selected liver conditions especially immune-mediated and the effect of GFD on the progression of coexistent liver disease is unclear.
Abbreviations:
- CD
celiac disease
- GFD
gluten-free diet
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Catassi C The world map of celiac disease. Acta Gastroenterol Latinoam. 2005;35(1):37–55. [PubMed] [Google Scholar]
- 2.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357(17):1731–1743. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007;46(5):1650–1658. [DOI] [PubMed] [Google Scholar]
- 4.Sood A, Khurana MS, Mahajan R, et al. Prevalence and clinical significance of IgA anti-tissue transglutaminase antibodies in patients with chronic liver disease. J Gastroenterol Hepatol. 2017;32(2):446–450. [DOI] [PubMed] [Google Scholar]
- 5.Dickey W, McMillan SA, Collins JS, Watson RG, McLoughlin JC, Love AH. Liver abnormalities associated with celiac sprue. How common are they, what is their significance, and what do we do about them? J Clin Gastroenterol. 1995;20(4): 290–292. [PubMed] [Google Scholar]
- 6.Bardella MT, Fraquelli M, Quatrini M, Molteni N, Bianchi P, Conte D. Prevalence of hypertransaminasemia in adult celiac patients and effect of gluten-free diet. Hepatology. 1995;22(3):833–836. [PubMed] [Google Scholar]
- 7.Hagander B, Berg NO, Brandt L, Norden A, Sjolund K, Stenstam M. Hepatic injury in adult coeliac disease. Lancet. 1977;2(8032):270–272. [DOI] [PubMed] [Google Scholar]
- 8.Lawson A, West J, Aithal GP, Logan RF. Autoimmune cholestatic liver disease in people with coeliac disease: a population-based study of their association. Aliment Pharmacol Ther. 2005;21(4):401–405. [DOI] [PubMed] [Google Scholar]
- 9.Buess M, Steuerwald M, Wegmann W, Rothen M. Obstructive jaundice caused by enteropathy-associated T-cell lymphoma in a patient with celiac sprue. J Gastroenterol. 2004;39(11): 1110–1113. [DOI] [PubMed] [Google Scholar]
- 10.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006; 131 (6): 1981–2002. [DOI] [PubMed] [Google Scholar]
- 11.Novacek G, Miehsler W, Wrba F, Ferenci P, Penner E, Vogelsang H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol. 1999;11(3):283–288. [DOI] [PubMed] [Google Scholar]
- 12.Bonamico M, Pitzalis G, Culasso F, et al. [Hepatic damage in celiac disease in children]. Minerva Pediatr. 1986;38(21):959–962. [PubMed] [Google Scholar]
- 13.Jacobsen MB, Fausa O, Elgjo K, Schrumpf E. Hepatic lesions in adult coeliac disease. Scand J Gastroenterol. 1990;25(7):656–662. [DOI] [PubMed] [Google Scholar]
- 14.Majumdar K, Sakhuja P, Puri AS, Gaur K, Haider A, Gondal R. Coeliac disease and the liver: spectrum of liver histology, serology and treatment response at a tertiary referral centre. J Clin Pathol. 2018;71(5):412–419. [DOI] [PubMed] [Google Scholar]
- 15.Bardella MT, Vecchi M, Conte D, et al. Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology. 1999;29(3):654–657. [DOI] [PubMed] [Google Scholar]
- 16.Volta U, De Franceschi L, Lari F, Molinaro N, Zoli M, Bianchi FB. Coeliac disease hidden by cryptogenic hypertransaminasaemia. Lancet. 1998;352(9121):26–29. [DOI] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Elfstrom P, Broome U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol. 2007;5(1):63–69 e61. [DOI] [PubMed] [Google Scholar]
- 18.Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003. ;163(13): 1566–1572. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmanova I, Sanchez D, Tuckova L, Tlaskalova-Hogenova H. Celiac Disease and Liver Disorders: From Putative Pathogenesis to Clinical Implications. Nutrients. 2018;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelaez-Luna M, Schmulson M, Robles-Diaz G. Intestinal involvement is not sufficient to explain hypertransaminasemia in celiac disease? Med Hypotheses. 2005;65(5):937–941. [DOI] [PubMed] [Google Scholar]
- 21.Korponay-Szabo IR, Halttunen T, Szalai Z, et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53(5):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aarela L, Nurminen S, Kivela L, et al. Prevalence and associated factors of abnormal liver values in children with celiac disease. Dig Liver Dis. 2016;48(9):1023–1029. [DOI] [PubMed] [Google Scholar]
- 23.Kaukinen K, Halme L, Collin P, et al. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122(4): 881–888. [DOI] [PubMed] [Google Scholar]
- 24.Fraquelli M, Colli A, Colucci A, et al. Accuracy of ultrasonography in predicting celiac disease. Arch Intern Med. 2004;164(2):169–174. [DOI] [PubMed] [Google Scholar]
- 25.Rettenbacher T, Hollerweger A, Macheiner P, Huber S, Gritzmann N. Adult celiac disease: US signs. Radiology. 1999;211(2):389–394. [DOI] [PubMed] [Google Scholar]
- 26.Pollock DJ. The liver in coeliac disease. Histopathology. 1977;1(6): 421–430. [DOI] [PubMed] [Google Scholar]
- 27.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112(1): 18–35. [DOI] [PubMed] [Google Scholar]
- 28.Volta U, De Franceschi L, Molinaro N, et al. Frequency and significance of anti-gliadin and anti-endomysial antibodies in autoimmune hepatitis. Dig Dis Sci. 1998;43(10):2190–2195. [DOI] [PubMed] [Google Scholar]
- 29.Villalta D, Girolami D, Bidoli E, et al. High prevalence of celiac disease in autoimmune hepatitis detected by anti-tissue tranglutaminase autoantibodies. J Clin Lab Anal. 2005;19(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volta U, Rodrigo L, Granito A, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol. 2002;97(10):2609–2613. [DOI] [PubMed] [Google Scholar]
- 31.Dickey W, McMillan SA, Callender ME. High prevalence of celiac sprue among patients with primary biliary cirrhosis. J Clin Gastroenterol. 1997;25(1):328–329. [DOI] [PubMed] [Google Scholar]
- 32.Kingham JG, Parker DR. The association between primary biliary cirrhosis and coeliac disease: a study of relative prevalences. Gut. 1998;42(1):120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niveloni S, Dezi R, Pedreira S, et al. Gluten sensitivity in patients with primary biliary cirrhosis. Am J Gastroenterol. 1998;93(3):404–408. [DOI] [PubMed] [Google Scholar]
- 34.Floreani A, Betterle C, Baragiotta A, et al. Prevalence of coeliac disease in primary biliary cirrhosis and of antimitochondrial antibodies in adult coeliac disease patients in Italy. Dig Liver Dis. 2002;34(4):258–261. [DOI] [PubMed] [Google Scholar]
- 35.Gillett HR, Cauch-Dudek K, Jenny E, Heathcote EJ, Freeman HJ. Prevalence of IgA antibodies to endomysium and tissue transglutaminase in primary biliary cirrhosis. Can J Gastroenterol. 2000;14(8):672–675. [DOI] [PubMed] [Google Scholar]
- 36.Bardella MT, Quatrini M, Zuin M, et al. Screening patients with celiac disease for primary biliary cirrhosis and vice versa. Am J Gastroenterol. 1997;92(9):1524–1526. [PubMed] [Google Scholar]
- 37.Sorensen HT, Thulstrup AM, Blomqvist P, Norgaard B, Fonager K, Ekbom A. Risk of primary biliary liver cirrhosis in patients with coeliac disease: Danish and Swedish cohort data. Gut. 1999;44(5):736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hay JE, Wiesner RH, Shorter RG, LaRusso NF, Baldus WP. Primary sclerosing cholangitis and celiac disease. A novel association. Ann Intern Med. 1988;109(9):713–717. [DOI] [PubMed] [Google Scholar]
- 39.Boberg KM, Spurkland A, Rocca G, et al. The HLA-DR3,DQ2 heterozygous genotype is associated with an accelerated progression of primary sclerosing cholangitis. Scand J Gastroenterol. 2001;36(8):886–890. [DOI] [PubMed] [Google Scholar]
- 40.Murray JA, Moore SB, Van Dyke CT, et al. HLA DQ gene dosage and risk and severity of celiac disease. Clin Gastroenterol Hepatol. 2007;5(12):1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Toma A, Goerres MS, Meijer JW, Pena AS, Crusius JB, Mulder CJ. Human leukocyte antigen-DQ2 homozygosity and the development of refractory celiac disease and enteropathy-associated T-cell lymphoma. Clin Gastroenterol Hepatol. 2006;4(3):315–319. [DOI] [PubMed] [Google Scholar]
- 42.Fine KD, Ogunji F, Saloum Y, Beharry S, Crippin J, Weinstein J. Celiac sprue: another autoimmune syndrome associated with hepatitis C. Am J Gastroenterol. 2001;96(1): 138–145. [DOI] [PubMed] [Google Scholar]
- 43.Thevenot T, Boruchowicz A, Henrion J, Nalet B, Moindrot H, Angh. Celiac disease is not associated with chronic hepatitis C. Dig Dis Sci. 2007;52(5):1310–1312. [DOI] [PubMed] [Google Scholar]
- 44.Bardella MT, Marino R, Meroni PL. Celiac disease during interferon treatment. Ann Intern Med. 1999;131(2):157–158. [DOI] [PubMed] [Google Scholar]
- 45.Adinolfi LE, Durante Mangoni E, Andreana A. Interferon and ribavirin treatment for chronic hepatitis C may activate celiac disease. Am J Gastroenterol. 2001. ;96(2): 607–608. [DOI] [PubMed] [Google Scholar]
- 46.Noh KW, Poland GA, Murray JA. Hepatitis B vaccine nonresponse and celiac disease. Am J Gastroenterol. 2003;98(10):2289–2292. [DOI] [PubMed] [Google Scholar]
- 47.Park SD, Markowitz J, Pettei M, et al. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr. 2007;44(4):431–435. [DOI] [PubMed] [Google Scholar]
- 48.Nemes E, Lefler E, Szegedi L, et al. Gluten intake interferes with the humoral immune response to recombinant hepatitis B vaccine in patients with celiac disease. Pediatrics. 2008;121(6):e1570–1576. [DOI] [PubMed] [Google Scholar]
- 49.Craven DE, Awdeh ZL, Kunches LM, et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med. 1986;105(3):356–360. [DOI] [PubMed] [Google Scholar]
- 50.Bardella MT, Valenti L, Pagliari C, et al. Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36(5):333–336. [DOI] [PubMed] [Google Scholar]
- 51.Kamal S, Aldossari KK, Ghoraba D, et al. Clinicopathological and immunological characteristics and outcome of concomitant coeliac disease and non-alcoholic fatty liver disease in adults: a large prospective longitudinal study. BMJ Open Gastroenterol. 2018;5(1): e000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reilly NR, Lebwohl B, Hultcrantz R, Green PH, Ludvigsson JF. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol. 2015;62(6):1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tovoli F, Negrini G, Fari R, et al. Increased risk of nonalcoholic fatty liver disease in patients with coeliac disease on a gluten-free diet: beyond traditional metabolic factors. Aliment Pharmacol Ther. 2018;48(5):538–546. [DOI] [PubMed] [Google Scholar]
- 54.Rubio-Tapia A, Abdulkarim AS, Wiesner RH, Moore SB, Krause PK, Murray JA. Celiac disease autoantibodies in severe autoimmune liver disease and the effect of liver transplantation. Liver Int. 2008;28(4):467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalekos GN, Bogdanos DP, Neuberger J. Celiac disease-related autoantibodies in end-stage autoimmune liver diseases: what is the message? Liver Int. 2008;28(4):426–428. [DOI] [PubMed] [Google Scholar]
- 56.Holmes GKT, Muirhead A. Mortality in coeliac disease: a population-based cohort study from a single centre in Southern Derbyshire, UK. BMJ Open Gastroenterol. 2018;5(1):e000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castillo NE, Vanga RR, Theethira TG, et al. Prevalence of abnormal liver function tests in celiac disease and the effect of a gluten-free diet in the US population. Am J Gastroenterol. 2015;110(8):1216–1222. [DOI] [PubMed] [Google Scholar]
- 58.Lee GJ, Boyle B, Ediger T, Hill I. Hypertransaminasemia in Newly Diagnosed Pediatric Patients With Celiac Disease. J Pediatr Gastroenterol Nutr. 2016;63(3):340–343. [DOI] [PubMed] [Google Scholar]