Abstract
Background
Area-level indices are widely used to assess the impact of socio-environmental characteristics on cancer outcomes. While area-level measures of socioeconomic status (SES) have been previously used in cancer settings, fewer studies have focused on evaluating the impact of area-level health services supply (HSS) characteristics on cancer outcomes. Moreover, there is significant variation in the methods and constructs used to create area-level indices.
Methods
In this study, we introduced a psychometrically-induced, reproducible approach to develop area-level HSS and SES indices. We assessed the utility of these indices in detecting the effects of area-level characteristics on prostate, breast, and lung cancer incidence and stage at diagnosis in the US. The information on county-level SES and HSS characteristics were extracted from US Census, County Business Patterns data and Area Health Resource Files. The Surveillance, Epidemiology, and End Results database was used to identify individuals diagnosed with cancer from 2010 to 2012. SES and HSS indices were developed and linked to 3-year age-adjusted cancer incidence rates. SES and HSS indices empirically summarized the level of employment, education, poverty and income, and the availability of health care facilities and health professionals within counties.
Results
SES and HSS models demonstrated good fit (TLI = 0.98 and 0.96, respectively) and internal consistency (alpha = 0.85 and 0.95, respectively). Increasing SES and HSS were associated with increasing prostate and breast cancer and decreasing lung cancer incidence rates. The results varied by stage at diagnosis and race.
Conclusion
Composite county-level measures of SES and HSS were effective in ranking counties and detecting gradients in cancer incidence and stage at diagnosis. Thus, these measures provide valuable tools for monitoring geographic disparities in cancer outcomes.
Background
Research has found that a narrow focus on individuals outside of their social and physical contexts limits our understanding of disease etiology, outcomes, and interventions [1–3]. Socioeconomic forces that determine demand for health care and market forces that determine health services supply (HSS) may influence cancer outcomes directly or indirectly through individual characteristics [4]. These contextual effects lead to social patterning of cancer outcomes through area-level stratification processes that allow individuals to obtain health-enhancing knowledge, and take advantage of this through prevention, screening, and early detection.
Historically, researchers have used both single measures representing contextual attributes (e.g. percentage below poverty level), as well as composite indices consisting of several attributes to examine the impact of area-level characteristics on cancer outcomes [5–10]. A composite index of key indicators has the ability to reflect the multidimensional nature of a community’s socioeconomic status (SES) more accurately than single measures of area-level characteristics [5, 11, 12]. In addition, area-level indicators (e.g. income, poverty, and occupation) tend to be highly correlated, which may lead to multicollinearity in a multivariable analysis [13, 14]. Therefore, a composite index should have greater validity, robustness, and explanatory power than single area-level measures in documenting the impact of area-level characteristics on disease outcomes [5, 14]. However, research addressing area-level composite indices have paid limited attention to psychometric techniques that can be used to develop these measures. As a result, the rich tradition of psychometrics has not been fully exploited in the development and testing of area-level indices [2]. Limited focus on the application of standard psychometric techniques in the construction of area-level indices has contributed to a lack of consistency in variables used to create these measures, and comparability across measures [2, 12]. Composite measures of SES have included different combinations of occupation, employment, poverty, income, education, housing, ownership and living crowdedness variables, thus, limiting the ability to systematically compare and assess the impact of area-level SES on disease outcomes [12].
Prostate, breast, and lung and bronchus cancer are the three most frequently diagnosed, and the leading causes of cancer mortality in the US and worldwide [15, 16]. Globally, there is pronounced geographic variation in incidence rates across these three cancer sites [17–21]. The risk of these cancers is susceptible to human intervention through screening, early-detection, and prevention [22–24]. Thus, geographic variation in prostate, breast, and lung and bronchus cancer incidence rates has been attributed to differences in the use of screening tests and diagnostic practices, prevalence of risk factors such as smoking and obesity, as well as disparities in the distribution of socioeconomic characteristics and access to health care both in the US and worldwide [17, 21, 25, 26].
Area-based composite indices of SES have been used extensively in cancer settings in the US. However, fewer studies have explored the aggregate impact of HSS characteristics including a broad range of facilities, services, and physician/non-physician health care providers on the incidence of cancer. The availability of health care services has the potential to impact cancer incidence through health-seeking behavior, access to health-promoting resources, travel distance, crowding, waiting times, and patient-provider relationships. Measures capturing health care resource scarcity may provide useful tools for identifying areas to which resources could be allocated to improve delivery of health services and potentially reduce geographic disparities in cancer incidence [27].
In the current study, we focused on introducing a psychometrically-induced, reproducible approach to developing county-level indices to capture area-level SES and HSS characteristics. Using these composite measures, we examined the effects of county-level SES and HSS gradients on prostate, breast, and lung and bronchus cancer incidence and stage at diagnosis in the US.
Methods
Geographic unit
No general consensus exists regarding the geographic level at which area-based measures should be developed in the US [7]. SES indices have been developed at several levels using block, census tract, zip code, and county-level data [6–10, 14, 22, 28, 29]. Previous studies have used smaller areas, such as census tracts and blocks due to the likelihood of homogeneity in population characteristics, economic status, and living conditions [5, 6, 13]. While blocks and census tracts are more likely to be socioeconomically homogeneous than larger geographic units, they are more susceptible to change over time [5]. Zip codes, were established by the US Postal Service for efficient delivery of mail [7], and they do not provide a meaningful basis for economic or health services planning [30].
Counties are legislative areas with 100,000 persons on average, and are socio-politically and geographically more stable than census tracts and blocks [5, 13]. Counties provide an appropriate socioeconomic, political, and community context within which many social and public health policies are formulated and implemented in the US [5, 31, 32].
Data sources
We used the 2006–2010 American Community Survey (ACS) 5-Year estimates to extract county-level socioeconomic characteristics. Annual County Business Patterns (CBP) data for 2010 was used to obtain information on the availability of health care facilities and services [33]. Area Health Resource Files (AHRF) were used to obtain information on the availability of health care professionals in 2010. Data on cancer incidence and stage at diagnosis from 2010 to 2012 were collected using the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) Public Use Database [34]. Each of SEER’s 18 regional cancer registries collects data on patient demographic characteristics, primary tumor site, tumor morphology, stage at diagnosis, and first course of treatment on all diagnosed cancers within its region [34].
County-level characteristics
The literature is replete with different SES indices that are composed of various combinations of socioeconomic indicators [3]. Particularly important to this analysis were the area-based composite SES indices developed by Singh [5, 28], Yost [6], Krieger [7], Dayal [8], Saldana-Ruiz [10], and Rubin [22], as these indices have shown associations with cancer incidence and/or mortality at different geographic levels. The combinations of various area-level characteristics used by these SES indices belong to the broad domains of occupation, employment, education, poverty, income, housing, ownership, and living crowdedness. For example, the ‘poverty’ domain included percentage of persons below poverty, multiples of the poverty line, and percentage of families below poverty-level. We extracted thirty county-level socioeconomic characteristics belonging to the eight domains from the 2006–2010 ACS.
All health care personnel, facilities, and services that could potentially influence the uptake of screening services and early detection of cancer were considered for HSS index development. The number of facilities or providers available within the county was divided by the total land area of each county, and then multiplied by 1,000 to express each health care characteristic as the number available per 1,000 square miles to capture health care resource availability given the size of the county.
Statistical analysis
Index development
Structural equation modeling techniques were used to arrive at the number and nature of latent constructs needed to account for the correlations, and to capture the commonality among the variables. The analytic step was conducted using the SAS PROC Factor procedure with a maximum likelihood (ML) parameter estimator. We first tested the factor structures of the previously developed SES indices using county-level data extracted from the 2006–2010 ACS [5–8, 10, 22, 28]. After evaluating the psychometric properties of the existing indices, we created a new county-level SES index using a richer, psychometrically induced approach. Twenty-three indicators were selected for the new index based on the conceptual definitions of SES, and empirical evidence from the aforementioned studies highlighting the effects of area-level SES on cancer outcomes.
The creation of the new SES and HSS indices involved the following steps. All measures were normalized using rank transformations prior to being entered into ML factor analysis; tied values were assigned an average rank [13]. We used ML factor analysis with orthogonal rotations to simplify and clarify the data structure [35]. An orthogonal rotation was preferred to an oblique rotation as the factors were expected to be uncorrelated constructs [36]. The initial models were retained, based on scree plots and Kaiser criterion (i.e. Eigen value greater than 1.0) [37]. According to Costello [35], if an item has a factor loading of less than 0.40 it may either not be related to the other items, or it may suggest an additional factor that should be further explored. Cross-loaded items with values ≥0.40 on two or more factors were removed when other items had factor loadings of 0.50 or greater [35, 38]. We re-ran the model each time an item was deleted [38]. Factors which clearly indicated a theoretically and empirically meaningful clustering of the given indicators were retained. Factors with fewer than 3 items, with only a few substantial loadings which did not lend itself to any obvious theoretical interpretation were rejected [37]. The final models were chosen based on; 1) percentage of common variance explained (i.e. the proportion of the total variance accounted for by each factor), 2) achieving a “simple solution”, 3) interpretability of the factors, and 4) goodness-of-fit (TLI ≥0.95). Goodness-of-fit indices, such as the Tucker-Lewis Index (TLI), show how well a model fits data [39]. According to Hu and Bentler [40], the proposed TLI cut-off for an acceptable model fit in ML estimation is ≥0.95. Internal consistency, i.e. the degree to which responses are consistent across the items within a measure was tested using Cronbach’s alpha (α) [39]. In the final step, factor coefficients were used to construct weighted SES and HSS scores for each county.
Cancer incidence and stage at diagnosis
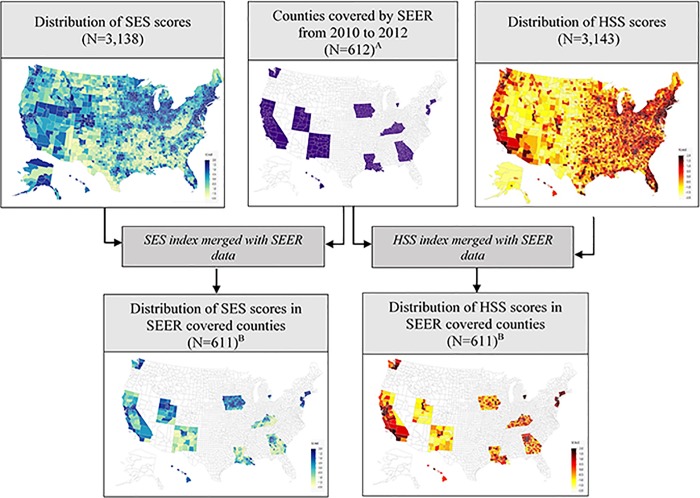
SEER data used to assess the gradients detected by SES and HSS composite measures included individuals at least 15 years of age, who lived in 611 counties covered by 17 SEER registries from 2010 to 2012. Unknown counties and Kalawao County in Hawaii were excluded due to lack of information on county-level characteristics. The county-level SES and HSS measures were merged with SEER data using the Federal Information and Processing Standards (FIPS) codes. FIPS codes recorded in SEER represent patient’s county of residence at the time of cancer diagnosis [13]. We examined the distribution of SES and HSS index scores across all counties (N = 3,138 and 3,143) and the counties covered by SEER (N = 611). SES and HSS scores were sorted from high to low, and divided as closely as possible into tertiles and quartiles with equal populations in each class based on their distribution across SEER county populations [13]. We examined the distribution of race/ethnicity and percentage living in urban/rural locations across the SES and HSS categories.
To analyze disparities in cancer incidence and stage at diagnosis by SES and HSS, we obtained 3-year incidence rates that were age-standardized to the 2000 US standard population and expressed per 100,000 population using NCI’s SEER*Stat software (version 8.2.1 released on April 2015) [41]. Incidence rates were calculated by stage at diagnosis using SEER historic staging information. We also examined the relationships between the county-level composite measures and cancer incidence separately among White, African American, and Asian/Pacific Islander race groups. American Indian and Alaska Natives were excluded due to small sample size. The trends in the gradients detected by SES and HSS indices were verified using a nonparametric trend test [42] to detect trends across ordered groups.
Analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC, USA). SAS JMP software (11.2.0) was used to examine the distribution of the SES and HSS scores across the counties.
Results
County-level indices
The factor structures of the six indices tested [5–8, 10, 22, 28] varied with respect to the combinations of SES measures used, factor loadings, common variance explained, and goodness-of-fit (S1 Table). The new SES index developed using the candidate indicators retained three factors after rotation, with the first factor explaining 80% of the common variance (TLI = 0.79) (S2 Table). Fourteen of the indicators were clustered and had considerably larger loadings (≥0.40) on the first factor than on the second or third factor. Six SES indicators had cross loadings with values ≥0.40 on two factors. SES index development involved an iterative process of excluding cross-loaded items with values ≥0.40 on two or more factors, rejecting factors with fewer than 3 items, and retaining factors using multiple criteria including the Kaiser rule and scree plots [37]. The final single component model retained, employment rate, % of families below poverty level, median family income, and an education index [43] (weighted school years) (Table 1). The large absolute coefficients of poverty and income indicators suggest that they were dominant contributors to the SES index. The TLI value for the model created using the full sample (N = 3,138) was 0.98, indicating excellent model fit. The distribution of SES scores across the 3,138 counties ranged from -1.82 to 1.80, with lower values representing low SES, and higher values representing high SES (Mean: 0.00, SD: 0.96, Skewness tests: p-value = 0.92). The distributions of the SES scores across all 3,138 US counties and 611 SEER covered counties are shown in Fig 1. Majority of the relatively low SES counties were concentrated in the southwestern and southeastern regions of the US.
Table 1. Factor loadings and fit statistics of the new county-level composite SES and HSS indices.
| SES domain | County-level SES measures a | Factor Loadings |
|
SES index (N = 3,138) | ||
| Employed | Civilian labor force population aged ≥16 years employed, % (employment rate) | 0.794 |
| Poverty | Families below poverty level, % | - 0.872 |
| Income | Median family income | 0.926 |
| Education | Education index (weighted school years)b | 0.507 |
| TLI | 0.984 | |
| Cronbach’s alpha (Standardized) | 0.850 | |
| HSS domain | County-level HSS measures c |
HSS index (N = 3,143) |
| Facilities | Physician offices | 0.949 |
| Medical and Diagnostic Laboratories | 0.742 | |
| General Medical and Surgical Hospitals | 0.735 | |
| Providers | Primary Care Practitioners d | 0.952 |
| Physician Assistants | 0.800 | |
| Registered Nurses | 0.920 | |
| Public Health and General Preventive Medicine Practitioners e | 0.550 | |
| Pharmacists | 0.903 | |
| Diagnostic Radiology e | 0.776 | |
| TLI | 0.962 | |
| Cronbach’s alpha (Standardized) | 0.947 |
a: Extracted from the 2006–2010 American Community Survey 5-Year Estimates.
b: A weight of 16 was applied to the proportion of persons in the county with a college education (pc); 12 was applied to the proportion with a high school education (phs); and nine was applied to the proportion with less than a high school education (po). The average years of schooling in a given county, E = (16*pc) + (12*phs) + (9*po) [43]
c: Extracted from the 2010 Area Health Resource files and County Business Patterns data.
d: Primary Care Practitioners include non-Federal doctors of medicine and doctors of osteopathy providing direct patient care who practice principally in general or family practice and general internal medicine.
e: Subspecialty data extracted from the 2010 American Medical Association Physician Masterfiles.
SES Socioeconomic status, HSS Health services supply, N Number of counties, TLI Tucker-Lewis Index
Fig 1. Flow diagram illustrating data availability and the distribution of SES and HSS index scores.
In the maps showing the distribution of SES and HSS scores, the darker shades represent high SES and high HSS areas, and the lighter shades represent low SES and low HSS areas. Areas with no information are not shaded. A. SEER data extracted from 17 registries, excluding Alaska tumor registry. B. Kalawao County in Hawaii was excluded due to lack of SES data. N number of counties, SEER Surveillance, Epidemiology, and End Results program, SES Socioeconomic Status, HSS Health Services Supply.
Based on the Kaiser criterion and a scree plot, one component with a TLI of 0.96 was retained for the HSS index (Table 1). The composite measure combined 3 indicators of ‘facilities’ and 6 indicators of ‘providers’. The distribution of the HSS scores across 3,143 counties ranged from -1.70 to 1.86, with lower values representing low HSS, and higher values representing high HSS (Mean: 0.00, SD: 0.99, Skewness tests: p-value = 0.88). Majority of the relatively low HSS areas were located in rocky mountain and southwestern regions (Fig 1). Pearson’s correlation coefficient between SES and HSS measures was 0.29 (p<0.01), indicating a weak linear relationship between the two measures.
Effects of SES and HSS gradients on cancer incidence and stage at diagnosis
In the 611 counties covered by SEER from 2010 to 2012, a total of 167,959 men aged 15 years and above were diagnosed with prostate cancer, of which 151,163 (90%) were diagnosed with incident localized/regional prostate cancer, and 8,398 (5%) with incident distant prostate cancer. Similarly, among 180,748 women diagnosed with breast cancer, 63% were with localized, 27% were with regional, and 7% were with distant breast cancer at diagnosis. Among 151,740 individuals diagnosed with lung and bronchus cancer, 20% were with localized, 23% were with regional, and 51% were with distant cancer at diagnosis. Counties belonging to the high SES group consisted of a lower proportion of African Americans and individuals living in rural areas compared to the lowest SES category (Table 2). Counties belonging to the high HSS group consisted of a higher proportion of African Americans and individuals living in urban areas compared to the lowest HSS category (Table 2).
Table 2. Distribution of county characteristics by SES and HSS classes, 2010–2012 SEER 17 combined (N = 611).
| Index | Classification Scheme | Class | Population | Row Percent | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | White | African American | American Indian | Asian | Hispanic | Urban a | Rural b | |||
| SES | Tertile | High | 28,627,476 | 66.67 | 6.86 | 0.54 | 13.59 | 17.27 | 73.76 | 26.24 |
| Median | 25,161,164 | 67.31 | 12.92 | 0.91 | 5.95 | 20.77 | 54.65 | 45.35 | ||
| Low | 32,075,546 | 61.16 | 14.85 | 1.29 | 6.25 | 28.67 | 37.04 | 62.96 | ||
| Quartile | High | 24,432,212 | 67.19 | 6.41 | 0.52 | 13.82 | 16.84 | 76.40 | 23.60 | |
| 2 | 18,196,224 | 69.89 | 10.29 | 0.76 | 7.40 | 17.38 | 59.99 | 40.01 | ||
| 3 | 21,195,612 | 56.63 | 12.53 | 0.90 | 9.49 | 35.83 | 53.24 | 46.76 | ||
| Low | 22,040,140 | 65.80 | 17.61 | 1.53 | 2.97 | 20.39 | 36.48 | 63.52 | ||
| HSS | Tertile | High | 28,523,308 | 56.35 | 14.90 | 0.54 | 12.04 | 27.83 | 98.23 | 1.77 |
| Median | 28,635,348 | 64.54 | 11.51 | 0.70 | 10.47 | 18.02 | 89.50 | 10.50 | ||
| Low | 28,705,620 | 73.45 | 8.46 | 1.52 | 3.34 | 21.84 | 38.48 | 61.52 | ||
| Quartile | High | 24,544,348 | 55.25 | 15.63 | 0.54 | 11.89 | 29.47 | 98.93 | 1.07 | |
| 2 | 18,296,366 | 62.58 | 12.88 | 0.55 | 12.37 | 16.69 | 96.32 | 3.68 | ||
| 3 | 21,562,528 | 69.31 | 8.55 | 0.84 | 7.19 | 20.52 | 81.94 | 18.06 | ||
| Low | 21,461,036 | 73.08 | 9.03 | 1.77 | 3.07 | 21.69 | 36.19 | 63.81 | ||
a. Percentage living in urban areas consisting of urbanized areas of 50,000 or more people and urban clusters of at least 2,500 and less than 50,000 people.
b. Percentage living in rural areas. “Rural” encompasses all population, housing, and territory not included within an urban area.
SES Socioeconomic status, HSS Health services supply, N Total number of SEER covered counties
In terms of direction and statistical significance, the indices performed similarly across tertiles and quartiles (Table 3). Consistent with previous findings, decreasing SES was associated with decreasing prostate and breast cancer incidence rates [13], primarily due to decreasing early-stage (i.e. localized/regional) prostate and breast cancer incidence rates. However, decreasing SES was statistically significantly associated with increasing advanced (distant) breast cancer and all stages (i.e. localized, regional, and distant) of lung cancer incidence rates. Further, increasing HSS was associated with increasing overall prostate and breast cancer incidence rates. Specifically, increasing HSS was statistically significantly associated with increasing early-stage prostate and breast cancer incidence rates. The HSS gradients detected in distant prostate and breast cancer incidence rates were not statistically significant. However, decreasing HSS was statistically significantly associated with increasing regional and distant lung cancer incidence rates.
Table 3. Distributions of 3-year age-adjusted cancer incidence rates, by SES and HSS, in 611 SEERa counties.
| Index | Classification scheme | Class | 3-year age-adjusted incidence rates (per 100,000)b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostate cancer | Breast cancer | Lung and Bronchus cancer | |||||||||||
| All | Local/ Regional |
Distant | All | Local | Regional | Distant | All | Local | Regional | Distant | |||
| SES index |
Tertiles | High SES | 167.97 | 152.37 | 8.85 | 162.19 | 106.67 | 41.91 | 10.70 | 66.19 | 13.48 | 16.00 | 33.45 |
| SES 2 | 162.74 | 147.65 | 10.21 | 153.03 | 96.49 | 43.81 | 11.60 | 75.29 | 14.03 | 17.06 | 39.48 | ||
| Low SES | 154.55 | 137.72 | 10.49 | 142.06 | 86.14 | 41.18 | 13.50 | 99.11 | 18.35 | 23.48 | 50.47 | ||
| % Changec | 8.68 | 10.63 | - | 14.17 | 23.83 | 1.75 | -20.76 | -33.21 | -26.52 | -31.85 | -33.72 | ||
| p-value | <0.01 | <0.01 | 0.35 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | 0.02 | <0.01 | <0.01 | ||
| Quartiles | High SES | 169.05 | 153.55 | 8.37 | 166.32 | 109.38 | 43.25 | 10.63 | 67.96 | 14.51 | 15.82 | 33.93 | |
| SES 2 | 165.37 | 150.79 | 10.45 | 154.22 | 97.84 | 43.04 | 11.76 | 72.30 | 13.23 | 17.59 | 37.32 | ||
| SES 3 | 160.50 | 144.32 | 9.69 | 151.68 | 96.11 | 43.33 | 11.04 | 75.75 | 14.34 | 16.49 | 39.74 | ||
| Low SES | 154.40 | 137.60 | 10.53 | 141.53 | 85.78 | 41.03 | 13.55 | 99.35 | 18.33 | 23.46 | 50.35 | ||
| % Changec | 9.49 | 11.59 | - | 17.51 | 27.51 | 5.42 | -21.57 | -31.59 | -20.86 | -32.56 | -32.62 | ||
| p-value | <0.01 | <0.01 | 0.40 | <0.01 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||
| HSS index |
Tertiles | High HSS | 183.95 | 164.49 | 9.32 | 162.80 | 102.60 | 44.77 | 11.84 | 71.32 | 14.57 | 16.46 | 35.85 |
| HSS 2 | 176.34 | 159.27 | 9.13 | 165.35 | 105.17 | 45.43 | 11.69 | 79.63 | 16.51 | 18.65 | 40.08 | ||
| Low HSS | 154.96 | 138.91 | 10.39 | 144.09 | 88.82 | 41.28 | 11.39 | 91.26 | 16.83 | 21.49 | 46.66 | ||
| % Changec | 18.71 | 18.41 | - | 12.99 | 15.51 | 8.44 | - | -21.85 | - | -23.41 | -23.16 | ||
| p-value | <0.01 | <0.01 | 0.84 | <0.01 | <0.01 | <0.01 | 0.38 | <0.01 | 0.29 | <0.01 | <0.01 | ||
| Quartiles | High HSS | 185.80 | 164.92 | 9.40 | 163.21 | 102.56 | 44.34 | 12.26 | 73.02 | 14.94 | 16.94 | 36.21 | |
| HSS 2 | 186.58 | 169.64 | 9.28 | 168.52 | 107.02 | 46.58 | 11.90 | 74.32 | 15.24 | 17.42 | 38.15 | ||
| HSS 3 | 171.49 | 155.58 | 8.78 | 159.51 | 102.01 | 43.72 | 11.03 | 83.25 | 17.17 | 19.50 | 41.76 | ||
| Low HSS | 153.85 | 137.72 | 10.54 | 143.72 | 88.34 | 41.31 | 13.00 | 91.76 | 16.82 | 21.61 | 46.95 | ||
| % Changec | 20.77 | 19.75 | - | 13.56 | 16.10 | 7.33 | - | -20.42 | - | -21.62 | -22.88 | ||
| p-value | <0.01 | <0.01 | 0.57 | <0.01 | <0.01 | <0.01 | 0.45 | <0.01 | 0.42 | <0.01 | <0.01 | ||
a. SEER data used to assess the gradients detected by SES and HSS measures included individuals at least 15 years of age, who lived in counties covered by 17 SEER registries at cancer diagnosis from 2010 to 2012. Alaska Natives, Kalawao County in Hawaii and unknown counties were excluded.
b. Rates are per 100,000 and age-adjusted to the 2000 US Standard Population (19 age groups).
c. % Change in incidence rates = ((High-Low category)/High category)X100
SEER Surveillance, Epidemiology, and End Results program, SES Socioeconomic status, HSS Health Services Supply.
The contribution of county-level SES and HSS to racial differences in cancer incidence varied by cancer site. Overall the prostate, breast, lung and bronchus cancer incidence rates among Whites and African Americans were higher than the rates among Asian/Pacific islanders. Decreasing SES and HSS were associated with decreasing prostate cancer incidence rates among Whites and increasing rates among Asian/Pacific islanders. Decreasing HSS was also statistically significantly associated with decreasing prostate cancer incidence rates among African Americans. Decreasing SES and HSS were both associated with increasing lung and bronchus cancer incidence rates among Whites, African Americans and Asian/Pacific islanders.
Discussion
Consistent with previous studies [6, 7, 13, 43], we found that increasing county-level SES was associated with increasing prostate and breast cancer, and decreasing lung cancer incidence rates [6, 7, 13, 43]. Although several studies have explored the impact of individual health services characteristics such as physician density or hospitals on cancer incidence [23, 24, 44–46], a wide range of health care services are needed to foster early detection and better health outcomes in the population. In order to address this gap in the literature, we developed a novel HSS index capturing the availability of a wide range of health care facilities (physician offices, medical and diagnostic laboratories, general medical and surgical hospitals), physicians (primary care practitioners, public health and general preventive medicine practitioners) and non-physician health care providers (physician assistants, nurses, pharmacists and diagnostic radiologists) to assess the effects of area-level health services on cancer outcomes. The novel county-level HSS index showed that increasing availability of health care resources within counties was associated with increasing prostate and breast cancer and decreasing lung cancer incidence rates.
These patterns may be explained by the unequal distribution of socioeconomic resources in the society that can cause differential knowledge diffusion regarding prevention. The high prostate and breast cancer incidence rates in increasing SES and HSS counties were driven by increasing incidence rates of early-stage diagnoses. These results indicate that, greater awareness about prevention and higher screening rates in high SES and HSS counties may have led to earlier diagnosis of these cancers [13]. High lung and bronchus cancer incidence rates may have stemmed from high prevalence of smoking in low SES counties [17, 47], and lower access to health care resources that could influence behaviors relating to smoking cessation and screening in low HSS counties [22]. Therefore, information provided by both HSS and SES indices may prove useful in identifying areas for dissemination of cancer site-specific preventive knowledge and health services.
In this study, we revisited the measurement of county-level characteristics considering a richer, psychometrically induced approach [2]. Drawing on a priori criteria outlined by Oakes et al.,[2] we required that the indices we developed, 1) were constructed from valid, reliable and easily accessible data, 2) were responsive for analysis at the county-level, 3) had sound psychometric properties, 4) employed terms/concepts that policy-makers understand; and perhaps most importantly, 5) were practical and useful in applied public health and epidemiological surveys.
Area-level indices can be developed using both factor analysis and principal component analysis. While both methods may produce similar results, there are conceptual distinctions between the two approaches [48]. Principal component analysis is more suitable for data reduction [35, 48]. In this study, we used factor analysis, as the goal of the analysis was to arrive at a parsimonious representation of the associations among the measured area-level characteristics by identifying latent constructs underlying the SES and HSS variables [48].
This study has a few limitations that deserve mention. First, we combined different datasets to obtain information pertaining to health care facility and provider characteristics within counties, each of which probably has different sources of random error. Second, limited information on psychometric and distributional properties of previously developed indices restricted our ability to select an existing index to detect socioeconomic gradients at the county-level [5–8, 10, 22, 28]. Although there is no consensus definition of SES, the new index captures the main concepts of SES including employment, poverty, income and education. Housing, ownership and living crowdedness domains did not converge on to the same component during the modeling process. [13] Housing, ownership and living crowdedness tend to have different interpretations across rural and urban areas. For example, people in rural areas are more likely to own a house, own a car, and live in less crowded conditions. Therefore, exclusion of these measures and including only those measures that have the same relative meaning across geographic regions allowed the new index to have relatively simpler and uniform interpretations across the counties. Third, due to limited sample size we only created tertiles and quartiles for our analysis. However, previous studies have noted that tertiles may be more appropriate when the sample size is limited [13]. Fourth, county-level SES may not accurately represent individual socioeconomic characteristics, and equating differentials observed at the county-level to individual-level SES may lead to ecologic bias.[5, 49, 50] Therefore, caution should be exercised when comparing county-level vs. individual-level effects of SES on cancer outcomes. Finally, there may be other population-level social and behavioral factors contributing to these observed trends which were not explored in the current study [5].
Conclusions
County-level SES and HSS indices are useful to assess geographic disparities in cancer incidence and stage at diagnosis. Further, these indices can be used in multi-level modeling studies to explain county-level variation in cancer outcomes.[51, 52] The gradients detected by county-level indices quantify cancer disparities attributable to county-level SES and HSS characteristics. Therefore, these measures may have the potential to provide useful tools to identify areas where resources could be allocated to reduce disparities in cancer outcomes. Future research could use these indices to examine the effects of county-level SES and HSS on population distributions of screening rates, smoking, crime, and environmental pollution to further explain geographic disparities in cancer outcomes.
Supporting information
a. White-collar occupations include management, professional, and related occupations.
b. Working class includes sales and office occupations, construction, extraction, and maintenance occupations, and production, transportation, and material moving occupations.
c. A weight of 16 was applied to the proportion of persons in the county with a college education (pc); 12 was applied to the proportion with a high school education (phs); and nine was applied to the proportion with less than a high school education (po). The average years of schooling in a given county, (E) = (16*pc) + (12*phs) + (9*po) [7].
d. Income disparity in year 2010 was defined as the 100×ratio of number of households with < $15,000 income to number of households with ≥$75,000 income.
e. Geographic level and US census years used to extract data in the original study to develop the index.
N Number of counties in 2000 US census, SES Socioeconomic status, HH Household, N/A Not Applicable.
(PDF)
a. White-collar occupations include management, professional, and related occupations.
b. A weight of 16 was applied to the proportion of persons in the county with a college education (pc); 12 was applied to the proportion with a high school education (phs); and nine was applied to the proportion with less than a high school education (po). The average years of schooling in a given county, E, is thus E = (16*pc) + (12*phs) + (9*po). [1]
c. Income disparity in year 2010 was defined as the 100×ratio of number of households with < $15,000 income to number of households with ≥$75,000 income.
SES Socioeconomic Status, N Number of counties, HH Household, TLI Tucker-Lewis Index.
(PDF)
Acknowledgments
This work is based on the dissertation submitted by Dr. Jinani Jayasekera in partial fulfillment of the Ph.D. requirements at the University of Maryland, Baltimore.
Abbreviations
- ACS
American Community Survey
- AHRF
Area Health Resource Files
- CBP
County Business Patterns
- FIPS
Federal Information and Processing Standards
- HSS
Health Services Supply
- ML
Maximum Likelihood
- NCI
National Cancer Institute
- SEER
Surveillance, Epidemiology, and End Results
- SES
Socioeconomic Status
- TLI
Tucker-Lewis Index
Data Availability
The data underlying the results in this study belong to the following third party sources and are freely available to interested researchers by following the provided links: 2006-2010 American Community Survey (ACS) 5-Year estimates data belongs to the US Census Bureau and can be found at https://factfinder.census.gov/bkmk/table/1.0/en/ACS/10_5YR/DP03/0600000US3401351000+; the Annual County Business Patterns (CBP) data for 2010 data belongs to US Census Bureau County Business Patterns and can be found at https://www.census.gov/data/datasets/2010/econ/cbp/2010-cbp.html); the Area Health Resource File 2010 release belongs to the Health Resources and Services Administration and can be found at https://data.hrsa.gov/data/download. Additionally, data from 2010-2012 for each of the cancer sites (lung and bronchus, breast and prostate cancer) were obtained from Surveillance Epidemiology, and End Results (SEER) Public Use Database via SEER*stat software owned by the National Cancer Institute. This data can be found at https://seer.cancer.gov/data/options.html. Interested researchers who choose to download SEER data (Public Use Database) via the SEER*stat software can also do so by using the link provided. The authors of this study did not receive special access privileges to the data. Interested researchers will be able to obtain the data in the same manner as the authors, and replicate the results of this study using the data sources listed above in SAS software version 9.2 (SAS Institute, Inc., Cary, NC 196 USA), SAS JMP software (11.2.0), and by following the detailed protocol described in the methods section.
Funding Statement
This work received support from the Georgetown-Lombardi American Cancer Society Young Investigator Award (ACS IRG 92-152-20) (JJ) and the Cancer Prevention Research Fellowship sponsored by the American Society of Preventive Oncology and Breast Cancer Research Foundation (ASPO-17-001) (JJ). URL: https://www.cancer.org/; https://www.bcrf.org/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klassen AC, Platz EA. What Can Geography Tell Us About Prostate Cancer? American Journal of Preventive Medicine. 2006;30(2, Supplement):S7–S15. 10.1016/j.amepre.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Social science & medicine (1982). 2003;56(4):769–84. Epub 2003/02/01. . [DOI] [PubMed] [Google Scholar]
- 3.MacKinnon JA, Duncan RC, Huang Y, Lee DJ, Fleming LE, Voti L, et al. Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(4):756–62. Epub 2007/04/10. 10.1158/1055-9965.epi-06-0392 . [DOI] [PubMed] [Google Scholar]
- 4.Mobley LR, Kuo T-M, Watson L, Gordon Brown G. Geographic disparities in late-stage cancer diagnosis: Multilevel factors and spatial interactions. Health & Place. 2012;18(5):978–90. 10.1016/j.healthplace.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. American journal of public health. 2003;93(7):1137–43. Epub 2003/07/02. 10.2105/ajph.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer causes & control: CCC. 2001;12(8):703–11. Epub 2001/09/20. . [DOI] [PubMed] [Google Scholar]
- 7.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. American journal of epidemiology. 2002;156(5):471–82. Epub 2002/08/28. 10.1093/aje/kwf068 . [DOI] [PubMed] [Google Scholar]
- 8.Dayal HH, Power RN, Chiu C. Race and socio-economic status in survival from breast cancer. Journal of chronic diseases. 1982;35(8):675–83. [DOI] [PubMed] [Google Scholar]
- 9.Robert SA, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, Remington PL. Socioeconomic risk factors for breast cancer: distinguishing individual-and community-level effects. Epidemiology. 2004;15(4):442–50. [DOI] [PubMed] [Google Scholar]
- 10.Saldana-Ruiz N, Clouston SA, Rubin MS, Colen CG, Link BG. Fundamental causes of colorectal cancer mortality in the United States: understanding the importance of socioeconomic status in creating inequality in mortality. American journal of public health. 2013;103(1):99–104. 10.2105/AJPH.2012.300743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. International Journal of Epidemiology. 2002;31(3):600–13. 10.1093/ije/31.3.600 [DOI] [PubMed] [Google Scholar]
- 12.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. The development of a standardized neighborhood deprivation index. Journal of urban health: bulletin of the New York Academy of Medicine. 2006;83(6):1041–62. Epub 2006/10/13. 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu M, Tatalovich Z, Gibson J, Cronin K. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes & Control. 2014;25(1):81–92. 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 14.Pruitt SL, Shim MJ, Mullen PD, Vernon SW, Amick BC. Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiology Biomarkers & Prevention. 2009;18(10):2579–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2011;378(9804):1707–16. Epub 2011/10/25. 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. Epub 2018/09/13. 10.3322/caac.21492 . [DOI] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 18.Crabbe JC, Gregorio DI, Samociuk H, Swede H. Secular trends, race, and geographic disparity of early-stage breast cancer incidence: 25 years of surveillance in connecticut. American journal of public health. 2015;105 Suppl 3:e64–70. Epub 2015/04/24. 10.2105/ajph.2015.302640 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng I, Witte JS, McClure LA, Shema SJ, Cockburn MG, John EM, et al. Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer causes & control: CCC. 2009;20(8):1431–40. Epub 2009/06/16. 10.1007/s10552-009-9369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. Global Health Equity: Cancer Care Outcome Disparities in High-, Middle-, and Low-Income Countries. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(1):6–13. Epub 2015/11/17. 10.1200/JCO.2015.62.2860 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamangar F, Dores GM, Anderson WF. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. Journal of Clinical Oncology. 2006;24(14):2137–50. 10.1200/JCO.2005.05.2308 . [DOI] [PubMed] [Google Scholar]
- 22.Rubin MS, Clouston S, Link BG. A fundamental cause approach to the study of disparities in lung cancer and pancreatic cancer mortality in the United States. Social science & medicine (1982). 2014;100:54–61. Epub 2014/01/22. 10.1016/j.socscimed.2013.10.026 . [DOI] [PubMed] [Google Scholar]
- 23.Major JM, Norman Oliver M, Doubeni CA, Hollenbeck AR, Graubard BI, Sinha R. Socioeconomic status, healthcare density, and risk of prostate cancer among African American and Caucasian men in a large prospective study. Cancer causes & control: CCC. 2012;23(7):1185–91. Epub 2012/06/08. 10.1007/s10552-012-9988-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coughlin SS, Richardson LC, Orelien J, Thompson T, Richards TB, Sabatino SA, et al. Contextual Analysis of Breast Cancer Stage at Diagnosis Among Women in the United States, 2004. The open health services and policy journal. 2009;2:45–6. Epub 2009/01/01. [PMC free article] [PubMed] [Google Scholar]
- 25.Ezzati M, Friedman AB, Kulkarni SC, Murray CJ. The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. PLoS medicine. 2008;5(4):e66 Epub 2008/04/25. 10.1371/journal.pmed.0050066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grauman DJ, Tarone RE, Devesa SS, Fraumeni JF Jr., Alternate ranging methods for cancer mortality maps. Journal of the National Cancer Institute. 2000;92(7):534–43. Epub 2000/04/06. 10.1093/jnci/92.7.534 . [DOI] [PubMed] [Google Scholar]
- 27.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health services research. 2013;48(2 Pt 1):539–59. Epub 2012/07/24. 10.1111/j.1475-6773.2012.01449.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. Journal of cancer epidemiology. 2011;2011:107497 Epub 2011/01/01. 10.1155/2011/107497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC cancer. 2010;10:603 Epub 2010/11/06. 10.1186/1471-2407-10-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis AR, Konrad TR, Thomas KC, Morrissey JP. County-level estimates of mental health professional supply in the United States. Psychiatric services (Washington, DC). 2009;60(10):1315–22. Epub 2009/10/03. 10.1176/appi.ps.60.10.1315 . [DOI] [PubMed] [Google Scholar]
- 31.Statistics NCfH. Health, United States: Socioeconomic status and health chartbook: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 1998.
- 32.DeFranco EA, Lian M, Muglia LJ, Schootman M. Area-level poverty and preterm birth risk: a population-based multilevel analysis. BMC Public Health. 2008;8(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y, Yang Y, Inoue LY, Munsell MF, Miller AB, Berry DA. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. Journal of the National Cancer Institute. 2005;97(16):1195–203. Epub 2005/08/18. 10.1093/jnci/dji239 . [DOI] [PubMed] [Google Scholar]
- 34.Surveillance, Epidemiology, and End Results (SEER) Program Populations (1973–2012) (www.seer.cancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program, released November 2014.
- 35.Costello A, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval 2005; 10. pareonline net/getvn asp. 2011;10:7. [Google Scholar]
- 36.Byrne BM. Structural equation modeling with Mplus: Basic concepts, applications, and programming: Routledge Academic; New York; 2011. [Google Scholar]
- 37.Cabrera-Nguyen P. Author guidelines for reporting scale development and validation results in the Journal of the Society for Social Work and Research. Journal of the Society for Social Work and Research. 2010;1(2):99–103. [Google Scholar]
- 38.Salkind NJ. Encyclopedia of research design,Volume 1 Thousand Oaks, CA: Sage; 2010. [Google Scholar]
- 39.Kline RB. Principles and Practice of Structural Equation Modeling. Third ed: Guilford Press; 2011. [Google Scholar]
- 40.Lt Hu, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- 41.Surveillance Research Program, National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.2.1. [cited December 2, 2015]
- 42.Cuzick J. A wilcoxon-type test for trend. Statistics in Medicine. 1985;4(1):87–90. 10.1002/sim.4780040112 [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Deapen D, Bernstein L. Socioeconomic status and cancers of the female breast and reproductive organs: a comparison across racial/ethnic populations in Los Angeles County, California (United States). Cancer Causes & Control. 1998;9(4):369–80. [DOI] [PubMed] [Google Scholar]
- 44.Campbell RJ, Ramirez AM, Perez K, Roetzheim RG. Cervical cancer rates and the supply of primary care physicians in Florida. Family medicine. 2003;35(1):60–4. Epub 2003/02/05. . [PubMed] [Google Scholar]
- 45.Roetzheim RG, Pal N, van Durme DJ, Wathington D, Ferrante JM, Gonzalez EC, et al. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. Journal of the American Academy of Dermatology. 2000;43(2 Pt 1):211–8. Epub 2000/07/25. 10.1067/mjd.2000.106242 . [DOI] [PubMed] [Google Scholar]
- 46.Ananthakrishnan AN, Hoffmann RG, Saeian K. Higher physician density is associated with lower incidence of late-stage colorectal cancer. Journal of general internal medicine. 2010;25(11):1164–71. Epub 2010/07/27. 10.1007/s11606-010-1457-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. Epub 2004/04/06. . [DOI] [PubMed] [Google Scholar]
- 48.Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychological methods. 1999;4(3):272. [Google Scholar]
- 49.Yabroff KR, Gordis L. Does stage at diagnosis influence the observed relationship between socioeconomic status and breast cancer incidence, case-fatality, and mortality? Social science & medicine (1982). 2003;57(12):2265–79. Epub 2003/10/24. . [DOI] [PubMed] [Google Scholar]
- 50.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health. 1992;82(5):703–10. Epub 1992/05/01. 10.2105/ajph.82.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jayasekera J, Onukwugha E, Tom S, Harrington D, Pradel FG, Naslund M. Contextual Analysis Of The Effect Of Prostate-Specific Antigen Testing On Prostate Cancer Outcomes Among Elderly Men. Value in Health. 2016;19(3):A98 10.1016/j.jval.2016.03.1709 [DOI] [Google Scholar]
- 52.Onukwugha E, Jayasekera J, Gandhi AB, Vapiwala N, Mair C, Lehning A, et al. Geographic Variation in Utilization of Physician Services Among Older Adults Diagnosed with Non-Hodgkin Lymphoma. Value in Health. 2018;21:S136 10.1016/j.jval.2018.04.912 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a. White-collar occupations include management, professional, and related occupations.
b. Working class includes sales and office occupations, construction, extraction, and maintenance occupations, and production, transportation, and material moving occupations.
c. A weight of 16 was applied to the proportion of persons in the county with a college education (pc); 12 was applied to the proportion with a high school education (phs); and nine was applied to the proportion with less than a high school education (po). The average years of schooling in a given county, (E) = (16*pc) + (12*phs) + (9*po) [7].
d. Income disparity in year 2010 was defined as the 100×ratio of number of households with < $15,000 income to number of households with ≥$75,000 income.
e. Geographic level and US census years used to extract data in the original study to develop the index.
N Number of counties in 2000 US census, SES Socioeconomic status, HH Household, N/A Not Applicable.
(PDF)
a. White-collar occupations include management, professional, and related occupations.
b. A weight of 16 was applied to the proportion of persons in the county with a college education (pc); 12 was applied to the proportion with a high school education (phs); and nine was applied to the proportion with less than a high school education (po). The average years of schooling in a given county, E, is thus E = (16*pc) + (12*phs) + (9*po). [1]
c. Income disparity in year 2010 was defined as the 100×ratio of number of households with < $15,000 income to number of households with ≥$75,000 income.
SES Socioeconomic Status, N Number of counties, HH Household, TLI Tucker-Lewis Index.
(PDF)
Data Availability Statement
The data underlying the results in this study belong to the following third party sources and are freely available to interested researchers by following the provided links: 2006-2010 American Community Survey (ACS) 5-Year estimates data belongs to the US Census Bureau and can be found at https://factfinder.census.gov/bkmk/table/1.0/en/ACS/10_5YR/DP03/0600000US3401351000+; the Annual County Business Patterns (CBP) data for 2010 data belongs to US Census Bureau County Business Patterns and can be found at https://www.census.gov/data/datasets/2010/econ/cbp/2010-cbp.html); the Area Health Resource File 2010 release belongs to the Health Resources and Services Administration and can be found at https://data.hrsa.gov/data/download. Additionally, data from 2010-2012 for each of the cancer sites (lung and bronchus, breast and prostate cancer) were obtained from Surveillance Epidemiology, and End Results (SEER) Public Use Database via SEER*stat software owned by the National Cancer Institute. This data can be found at https://seer.cancer.gov/data/options.html. Interested researchers who choose to download SEER data (Public Use Database) via the SEER*stat software can also do so by using the link provided. The authors of this study did not receive special access privileges to the data. Interested researchers will be able to obtain the data in the same manner as the authors, and replicate the results of this study using the data sources listed above in SAS software version 9.2 (SAS Institute, Inc., Cary, NC 196 USA), SAS JMP software (11.2.0), and by following the detailed protocol described in the methods section.