Abstract
Background:
Alcohol-related liver disease is one of the most prevalent chronic liver diseases worldwide. Mechanisms involved in the pathogenesis of alcohol-related liver disease are not well understood. Oxylipins play a crucial role in numerous biological processes and pathological conditions. Nevertheless, oxylipins are not well studied in alcohol-related liver disease.
Aims:
(1) To characterize the patterns of bioactive ω-3 and ω-6 polyunsaturated fatty acid metabolites in alcohol use disorder and alcoholic hepatitis patients, and (2) to identify associations of serum oxylipins with clinical parameters in patients with alcohol-related liver disease
Methods:
We performed a comprehensive liquid chromatography with tandem mass spectrometry (LC-MS/MS)analysis of serum and fecal oxylipins derived from ω-6 arachidonic acid, ω-3 eicosapentaenoic acid and docosahexaenoic acid in a patient cohort with alcohol-related liver disease.
Results:
Our results show profound alterations in the serum oxylipin profile of patients with alcohol use disorder and alcoholic hepatitis compared to non-alcoholic controls. Spearman correlation of the oxylipins with clinical parameters show a link between different serum oxylipins and intestinal permeability, aspartate aminotransferase, bilirubin, albumin, international normalized ratio, platelet count, steatosis, fibrosis and model for end-stage liver disease (MELD) score. Especially, higher level of serum 20- HETE was significantly associated with decreased albumin, increased hepatic steatosis, polymorphonuclear infiltration, and 90-day mortality.
Conclusions:
Patients with alcohol-related liver disease have different oxylipin profiles. Future studies are required to confirm oxylipins as disease biomarker or to connect oxylipins to disease pathogenesis.
Keywords: AA, EPA, DHA, PUFA, lipid mediator, metabolomics
Introduction
As one of the most prevalent types of chronic liver disease, alcohol-related liver disease is a major human health concern. It is a leading cause of morbidity and mortality worldwide and accounts for up to 48% of cirrhosis-associated deaths in the United States [1]. Many factors contribute to the pathogenesis of alcohol-related liver disease, including the amount of ethanol consumption, gut microbiota, genetic, epigenetic, and dietary factors [2]. However, to date, prevention and treatment strategies for alcohol-related liver disease are still limited.
Oxylipins are a group of bioactive fatty acid metabolites, which are endogenously produced via oxygenation of polyunsaturated fatty acids (PUFAs), such as linoleic acid (LA), arachidonic acid (AA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [3]. Oxylipins are potent inflammatory modulators and involved in biological processes such as inflammation, apoptosis, vascular tone, coagulation, blood vessel permeability, and blood pressure regulation [3,4]. These bioactive lipids act as autocrine and paracrine mediators by binding to G protein-coupled receptors (GPCRs) or peroxisome proliferator-activated receptors (PPARs) [5]. Oxylipins are associated with cardiovascular diseases, diabetes, and age-related degeneration, responses to host defense, tissue injury and surgical intervention [6,7].
Oxylipins are de novo synthesized in a tightly regulated manner [8]. Upon cell activation, polyunsaturated fatty acids are released from the cell membrane by phospholipase A2 (cPLA2) and converted to oxylipins by three major enzymatic pathways: cyclooxygenase, lipoxygenase, and cytochrome P-450 pathways [9]. Pharmaceuticals have been developed to target these enzymes such as cyclooxygenase inhibitors (e.g. acetaminophen) to reduce fever, pain and coagulation [10], and lipoxygenase inhibitor (e.g. zileuton) to reduce inflammatory conditions in asthma patients [11].
Not all PUFAs play an equal role in alcohol-related liver disease pathogenesis. A ω-6 PUFA metabolite LA and its metabolites are potential mediators of inflammatory responses and liver damage during the pathogenesis of alcohol-related liver disease [12,13]. Nevertheless, oxylipins derived from other ω-6 PUFAs, such as AA, and ω-3 PUFAs, such as EPA and DHA, are not well studied in alcohol-related liver disease patients. Here, we performed a comprehensive LC-MS/MS analysis of serum and fecal eicosanoids generated from ω-6 AA, ω-3 EPA, and decosanoids derived from ω-3 DHA in an alcohol-related liver disease patient cohort. The goal of the present study is to characterize the patterns of bioactive ω-3 and ω-6 PUFA metabolites in alcohol use disorder and alcoholic hepatitis patients, and to identify associations of serum oxylipins with clinical parameters in patients with alcohol-related liver disease.
Methods
Patients
Serum and fecal samples were collected from 16 non-alcoholic controls, 30 patients with alcohol use disorder and 13 patients with alcoholic hepatitis. Non-alcoholic controls (social drinkers) consumed less than 20g/day. Patients were diagnosed with alcohol use disorder (AUD) if they fulfill the DSM IV criteria [14]. They presented with various stages of liver disease and were recruited from an alcohol withdrawal unit where they followed a highly standardized and controlled 3-week detoxification and rehabilitation program. At admission, a complete medication and medical history is taken, and a complete physical examination is performed, including collection of basic demographic data, such as age, gender, weight, and height, and self-reported daily alcohol consumption. Patients reported long-term (>1 year) alcohol consumption >60 g/day and were actively drinking until the day of admission. They routinely and prospectively undergo a large panel of investigations including transient elastography (FibroScan), Doppler ultrasound and blood tests. FibroScan is performed on the day of admission and abdominal Doppler ultrasound within 72h. Non-alcoholic controls or patients with alcohol use disorder did not take antibiotics or immunosuppressive medication during the two months preceding enrollment. Other exclusion criteria were diabetes, inflammatory bowel disease, known liver disease of any other etiology, and clinically significant cardio-vascular, pulmonary or renal co-morbidities. Alcoholic hepatitis patients were enrolled as part of the InTeam Consortium. We analyzed a group of alcoholic hepatitis patients, who did not receive antibiotics or were not treated with corticosteroids. Inclusion criteria for alcoholic hepatitis were: 1. active alcohol use (> 50 g/day for men and > 40 g/day for women) in the last 3 months, 2. aspartate aminotransferase (AST) > alanine aminotransferase (ALT) and total bilirubin > 3 mg/dl in the past 3 months, and 3. liver biopsy and/or clinical picture consistent with alcoholic hepatitis. Exclusion criteria were: 1. Autoimmune liver disease (ANA > 1/320), 2. Chronic viral hepatitis, 3. Hepatocellular carcinoma, 4. Complete portal vein thrombosis, 5. Extrahepatic terminal disease, 6. Pregnancy, and 7. Lack of signed informed consent [15]. In all patients, the clinical picture was consistent with alcoholic hepatitis and in patients who underwent liver biopsy, the histology was in line with the diagnosis of alcoholic hepatitis. Liver biopsies were only done if clinically indicated as part of routine clinical care for diagnostic purposes of alcoholic hepatitis. Transient elastography using FibroScan® was performed for AUD patients. Model for end-stage liver disease (MELD) and sodium MELD score were calculated for alcoholic hepatitis patients. The baseline characteristics of all subjects are shown in Table 1A and 1B. The protocol was approved by the Ethics Committee of each participating center. Written informed consent was obtained from each subject.
Table 1A:
Subject characteristics for oxylipin analysis
| Non-alcoholic Controls | Alcohol Use Disorder | Alcoholic Hepatitis | p-value | |
|---|---|---|---|---|
| Clinical parameter | ||||
| Total n | 16 | 30 | 13 | |
| Age, years, n=58 | 37 (27–71) | 41 (27–59) | 56 (40–75) | 0.0008* |
| Body Mass Index (BMI), kg/m2, n=58 | 21.9 (18.8–29.2) | 22.9 (18.3–31.2) | 26.9 (22.3–37.2) | 0.0067* |
| Gender (male), %, n=58 | 86.7 | 76.7 | 76.9 | 0.7203 |
| Laboratory parameter | ||||
| ALT (U/l), n=43 | 37 (11–184) | 63 (28–106) | 0.1154 | |
| AST (U/l), n=43 | 41 (15–283) | 167 (69–290) | 2.179E-05 | |
| Total bilirubin (mg/dl), n=40 | 0.4 (0.2–1.1) | 11.1 (3.1–36.2) | 3.786E-07 | |
| Alkaline phosphatase (U/l), n=39 | 69 (38–113) | 268 (81–1153) | 4.148E-06 | |
| GGT (U/l), n=36 | 41 (4–952) | 196 (7–2116) | 0.1439 | |
| Albumin (g/dl), n=37 | 4.7 (3.9–5.2) | 2.5 (1.8–3.5) | 1.176E-06 | |
| Platelet counts (×109/l), n=39 | 229 (80–434) | 115 (60–350) | 0.0001 | |
| Prothrombin time (s) n=12 | 22.6 (11.0–61.0) | |||
| Creatinine (mg/dl), n=40 | 0.8 (0.5–1.2) | 0.9 (0.4–2.0) | 0.3050 | |
| Sodium (mEq/L), n=13 | 132 (118–138) | |||
| INR, n=39 | 0.9 (0.8–1.2) | 1.6 (1.1–2.5) | 1.193E-06 | |
| Transient elastography (Kpa), n=30 | 5.5 (3.1–7.0) | |||
| ≤9.0 Kpa, n=30 | 5.5 (3.1–7.0) | |||
| ≥9.0 KPa, n=0 | ||||
| ≥11.7 Kpa, n=0 | ||||
| CAP (dB/m), n=30 | 279 (148–381) | |||
| ≤250 dB/m, n=7 | 208 (148–245) | |||
| ≥250 dB/m, n=10 | 268 (253–291) | |||
| ≥300 dB/m, n=13 | 325 (307–381) | |||
| Intestinal Permeability | ||||
| Small intestine permeability (%51Cr-EDTA/g creatinine), n=43 | 1.5 (0.3–4.2) | 1.9 (0.2–8.5) | 0.2595 | |
| Colon permeability (%51Cr-EDTA/g creatinine), n=42 | 1.0 (0.4–2.7) | 1.3 (0.7–5.5) | 0.1386 | |
| Total gut permeability (%51Cr-EDTA/g creatinine), n=43 | 1.0 (0.5–3.0) | 1.4 (0.4–5.5) | 0.03922 | |
| Medications | ||||
| Proton pump inhibitors, n=39 | 5 | 1 | ||
| NSAID, n=39 | 0 | 1 |
Values are presented as median and range in brackets. The number of patients for which the respective data was available is indicated in the first column. Kruskal- Wallis test (three groups) and Mann-Whitney-Wilcoxon test (two groups) were used to calculate p-values. Bold font indicates significance (raw p-value < 0.05).
Post hoc p-values (Dunn): Age: AUD vs. AH p=0.0003877; Ctrl vs. AH p=0.001359; Ctrl vs. AUD p=0.9104. BMI: AUD vs. AH p=0.009193; Ctrl vs. AH p=0.002597; Ctrl vs. AUD p=0.3821. INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl-transferase; AP, alkaline phosphatase; BMI, body mass index; CAP, controlled attenuation parameter. NSAID: nonsteroidal anti-inflammatory drugs.
Table 1B:
Characteristics of alcoholic hepatitis patients for oxylipin analysis
| Treatment at admission | Histology | ||||
|---|---|---|---|---|---|
| Steroids, n (%), n=13 | 0 (0) | 2 | 1 (14.3) | ||
| Antibiotics, n (%), n=13 | 0 (0) | Lobular fibrosis | 0 | 0 (0) | |
| Infection at admission, n (%), n=12 | 1 (8.3) | 1 | 1 (14.3) | ||
| Clinical scores and outcome | 2 | 2 (28.6) | |||
| Model for end-stage liver disease (MELD), n=12 | 21.9 (11.7–29.6) | 3 | 4 (57.1) | ||
| MELD>21, n (%), n=12 | 7 (58.3) | Pericellular fibrosis | 0 | 2 (28.6) | |
| 30 day mortality rate, n (%), n=12 | 0 (0) | 1 | 5 (71.4) | ||
| 90 day mortality rate, n (%), n=12 | 2 (16.7) | Grade of steatosis | 1 | 3 (42.85) | |
| Histology | 2 | 3 (42.85) | |||
| Liver biopsy available, n (%) | 7 (53.8) | 3 | 1 (14.3) | ||
| Stage of fibrosis | 0 | 0 (0) | Mallory bodies | 0 | 1 (14.3) |
| 1 | 0 (0) | 1 | 6 (85.7) | ||
| 2 | 1 (14.3) | Bilirubinostasis | 0 | 1 (16.7) | |
| 3 | 1 (14.3) | 1 | 4 (66.6) | ||
| 4 | 5 (71.4) | 2 | 0 (0) | ||
| PMN infiltration | 0 | 1 (14.3) | 3 | 1 (16.7) | |
| 1 | 4 (57.1) | Ballooning | 0 | 7 (100) | |
| 2 | 2 (28.6) | 1 | 0 (0) | ||
| Inflammatory grade | 0 | 0 (0) | Giant mitochondria | 0 | 5 (100) |
| 1 | 6 (85.7) | 1 | 0 (0) | ||
Values are presented as median and range in brackets. The number of patients for which the respective data was available is indicated in the first column. Fibrosis stage, 0 no fibrosis, 1 portal fibrosis, 2 expansive periportal fibrosis, 3 bridging fibrosis, 4 cirrhosis. Lobular fibrosis, 0 no fibrosis, 1 zone 3 (centrilobular) fibrosis, 2 zone 2+3 (midzonal) fibrosis, 3 panlobular fibrosis. Pericellular fibrosis, 0 absent, 1 present. Steatosis, 1 mild < 33%, 2 moderate < 33–66%, 3 marked > 66%. Mallory bodies, 0 absent, 1 present. Bilirubinostasis, 0 no, 1 hepato-canalicular, 2 cholangiolar, 3 both. Ballooning, 0 occasional hepatocellular, 1 marked hepatocellular, 2 none present. Megamitochondria, 0 absent, 1 present. PMN infiltration, 0 no, 1 mild, 2 severe. Inflammation, 0 no, 1 mild, 2 severe. PMN, polymorphonuclear infiltration.
Liver stiffness and controlled attenuation parameter (CAP) measurements
Liver fibrosis and steatosis were assessed non-invasively by determining liver stiffness using transient elastography and controlled attenuation parameter (CAP), respectively. The measurements were performed with the Fibroscan® device (Echosens, Paris, France) by an experienced examiner blinded to the patient’s data[16]. Liver stiffness was considered as valid if at least 10 validated measurements were obtained with an interquartile range <30%. Similarly, CAP was deemed valid if at least 10 validated measurements were obtained with an interquartile range ≤40 dB/m. The final result expressed in kPa or dB/m, respectively, was the median of all valid measurements obtained. The following validated fibrosis cut-offs for patients with alcohol use disorder were applied: 9.0 kPa for fibrosis ≥F1 (portal fibrosis) and 11.7 kPa for significant fibrosis ≥F2 (fibrosis bridges) [17]. Cut-offs for CAP values were derived from recent studies performed in NAFLD patients: ≥250 dB/m for significant and ≥300 dB/m for severe steatosis [18–21]. Written informed consent was obtained from all patients and controls. The study protocol was approved by the Ethics Committee of the Université Catholique de Louvain, in Brussels, Belgium.
Measurement of intestinal permeability
Intestinal permeability was measured in 15 non-alcoholic controls and 28 patients with alcohol use disorder using 51Cr-EDTA as described previously [22]. Briefly, patients were fasted overnight. After the bladder was emptied, 200 mL Nutridrink (150 kcal/100 mL, Nutricia, Gaithersburg, MD) which contains 50 μCi 51Cr-EDTA was given to patients to drink. Urine from each patient was collected for 24 h to reflect the total intestinal permeability. Urine sample collected during 0–4h and 4–24h was expected to reflect small-bowel and colon permeability, respectively. Radioactivity was assessed with a gamma counter Cobra5003 (Canberra Packard, Schwadorf bei Wien, Austria). The percentage of the ingested dose of 51Cr-EDTA found in urine was calculated and normalized for creatinine. Written informed consent was obtained from all patients and controls. The study protocol was approved by the Ethics Committee of the Université Catholique de Louvain, in Brussels, Belgium.
Sample extraction
The extraction was performed as described previously with modifications [23]. For fecal sample extraction, 1mg fecal sample was treated with 10 μL antioxidants (0.2 mg/mL BHT/EDTA) and then spiked with 10 μL of internal standards (250 nM). The samples were extracted using 500 μL pre-chilled methanol with stainless steel grinding balls and homogenized using GenoGrinder for 60 s at 1,500 rpm and centrifuged at 14, 000 g for 2 min. The supernatant was collected and evaporated to dryness using Speed-vac. The dried extracts were reconstituted with 100 μL methanol/acetonitrile (v/v 1:1) containing 100 nM 1-cyclohexyluriedo-3-dodecanoic acid (CUDA) as a quality control marker, vortexed for 10s and sonicated for 5 min. The samples were set on ice for 15 min, centrifuged at 14,000 g for 3 min. The supernatant was filtered using Amicon Ultrafree- MC Durapore PVDF filter (pore-size 0 1 μM; Millipore, Bedford, MA) and centrifuged for 6 min. The filtrate was collected for LC-MS/MS analysis. For serum sample extraction, 25 μL antioxidant (0 2 mg/mL BHT/EDTA) and internal standards were added to 50 μL serum sample. Samples were extracted with pre-chilled acetonitrile and methanol (v/v, 1:1), vortexed for 30 s, centrifuged at 6°C for 5 min at 15,000 g. The supernatant was collected and filtered using Amicon Ultrafree-MC Durapore PVDF filter (pore-size 0.1 μM; Millipore, Bedford, MA). The filtrate was collected for LC-MS/MS analysis.
Liquid chromatography and mass spectrometry
Analytes were separated by reverse-phase high-performance liquid chromatography (HPLC) on a 1.7 μm Acquity BEH column (Waters, Milford, MA) coupled to 6500 QTrap (AB Sciex LLC. Framingham, MA). Oxylipins were quantified in negative electrospray ionization mode with methods reported previously [24]. 0.1% acetic acid in water was used as solvent A. 90:10 acetonitrile/isopropanol (v/v) with 0.1% acetic acid was used as solvent B. The flow rate was 0.250 mL/min with the following gradient: 0 min 25% B, 0–1 min 40% B, 2–2.5 min 42% B, 2.5–4.5 min 50% B, 4.5–10.5 min 65% B, 10.5–12.5 min 75% B, 12.5–14.0 min 85% B, 14.0–14.5 min 95% B, 14.5–16.0 min 25% B. Multiple reaction monitoring (MRM) transitions, ionization and fragmentation energies for all target analytes were reported in Supplemental Table S1.
Data processing
Peak detection and peak area integration were performed in Multi-Quant software Version 3.0 (AB Sciex LLC. Framingham, MA). Auto-integration was manually inspected and corrected if necessary. The obtained peak areas of targets were corrected by appropriate internal standards and calculated response ratios were used throughout the analysis.
Statistical analysis
Statistical analysis was performed using R (version 3.5.1). Kruskal-Wallis test was used to calculate the p-value in three groups and Mann-Whitney-Wilcoxon test was used for the comparison between two groups. Adjusted p-values were calculated using Benjamini-Hochberg procedure to control the false discovery rate. Spearman correlation was conducted to correlate serum and fecal oxylipin concentrations with clinical parameters. Partial least squares discriminant analysis was performed on serum and fecal oxylipins. Chemical similarity enrichment analysis was performed using ChemRICH [25]. Chemical similarity enrichment statistics was calculated by Kolmogorov-Smirnov test.
Results
Patient characteristics and laboratory parameters
Serum and fecal samples for oxylipin analysis were collected from 16 nonalcoholic controls, 30 patients with alcohol use disorder, and 13 patients with alcoholic hepatitis. Most of the alcoholic hepatitis patients were male (76.9%), with a median age of 56 years old and a body mass index (BMI) of 26.9 kg/m2. Alcohol use disorder patients, who underwent transient elastography, had liver stiffness measurements < 9 kPa indicating the absence of fibrosis. Based on the predefined CAP cutoffs, 23 out of 30 patients (76.7%) presented with steatosis among whom 13 patients (56.5%) had severe steatosis (CAP > 300 dB/m). Steatosis was confirmed by liver Doppler ultrasound (bright, hyperechoic livers) except in three patients who had CAP values close to the 250 dB/m cutoff. Eighty percent of patients with severe steatosis on CAP also showed increased AST and ALT levels at admission in favor of steatohepatitis. By contrast, elevated transaminases were seen in only 25% of patients with less severe steatosis on CAP suggesting the presence of simple steatosis in the majority of those patients. Among the remaining patients with normal liver Doppler ultrasound and normal CAP values, only one patient had slightly increased transaminase levels (1.5 × upper limit of normal) at admission. This group of alcohol use disorder patients likely has not developed significant liver disease. As expected, lower albumin values, platelet counts, and higher alkaline phosphatase (AP), aspartate aminotransferase (AST), total bilirubin, and international normalized ratio (INR) levels were found in patients with alcoholic hepatitis when compared to patients with alcohol use disorder (Table 1). BMI was higher in patients with alcoholic hepatitis, which might be due to the presence of ascites in 61.5% (8/13) of alcoholic hepatitis patients. The median MELD score in alcoholic hepatitis patients was 21.9 (Table 2). Liver biopsy was performed in 53.8% of the alcoholic hepatitis patients, among whom 71.4% had liver cirrhosis (Table 2). None of the subjects received antibiotics or steroids.
Serum and fecal oxylipin profiles in alcohol use disorder and alcoholic hepatitis patients
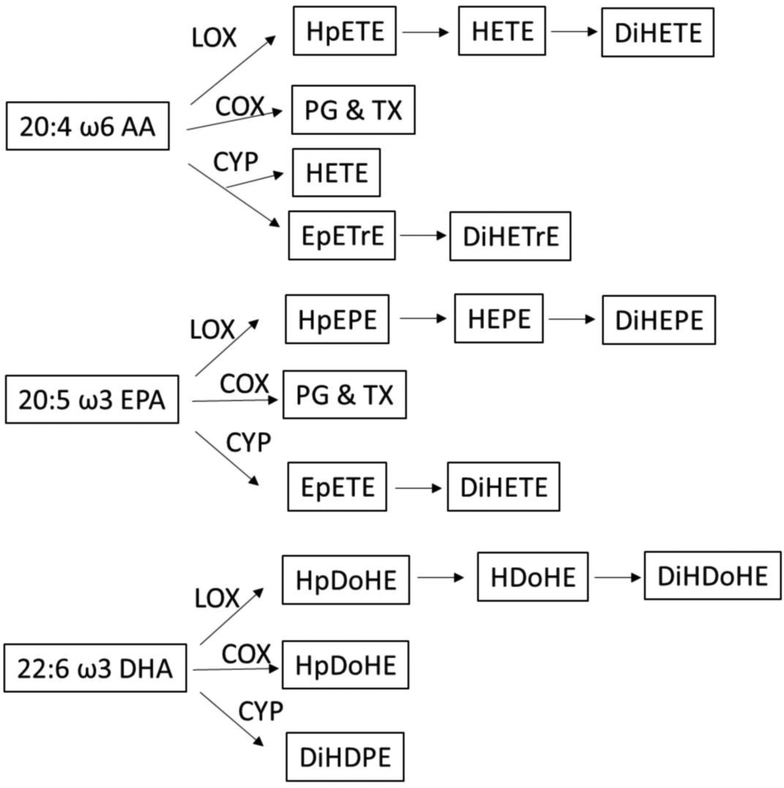
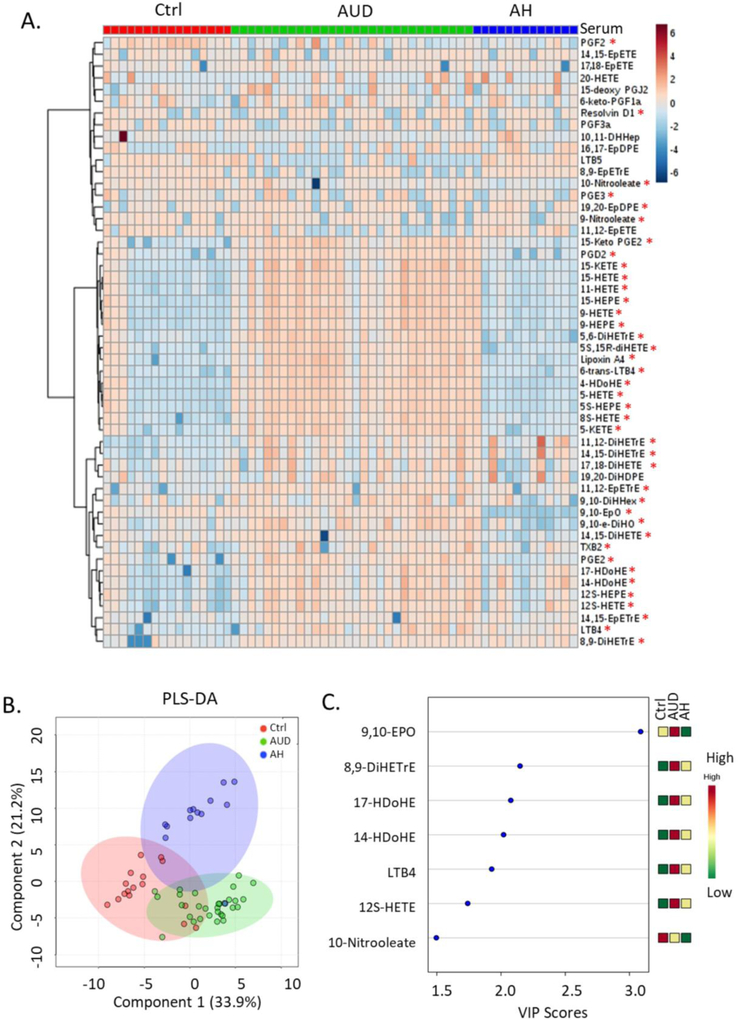
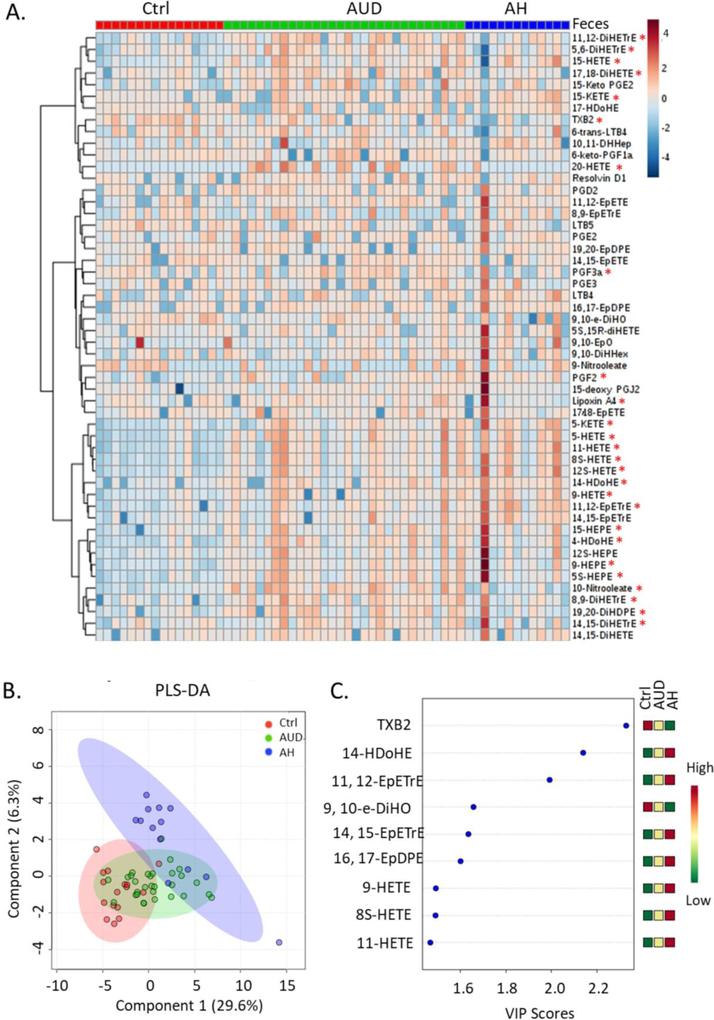
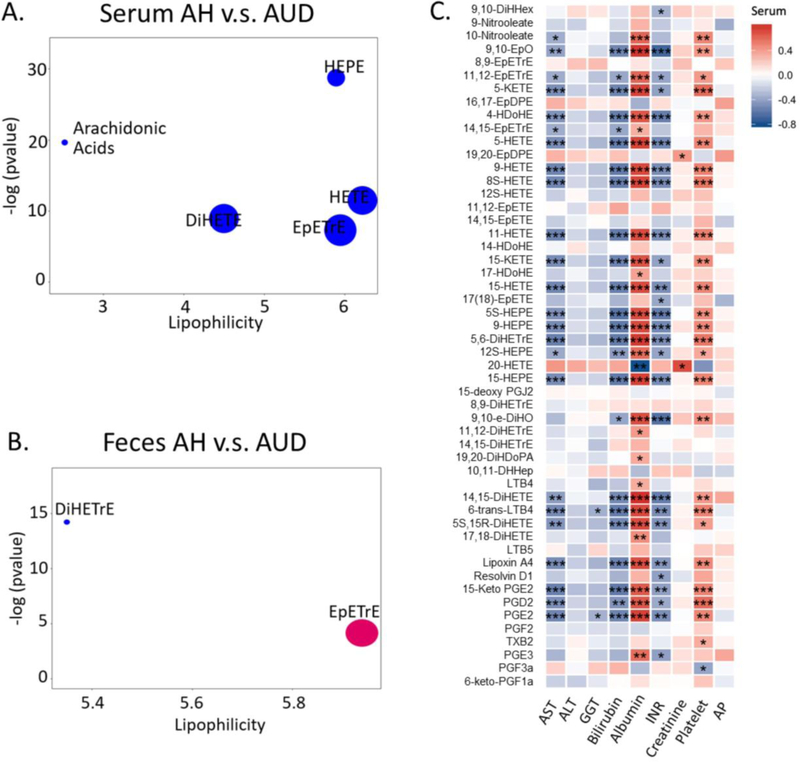
Most of the oxylipins analyzed in this study were generated from ω-6 AA, ω-3 EPA and DHA through lipoxygenase, cyclooxygenase and cytochrome P-450 pathways (Figure 1). Hierarchical clustering of serum oxylipins showed that the serum oxylipin profile was different in alcohol use disorder and alcoholic hepatitis patients compared to controls (Figure 2A). 40 out of the 52 were significantly different when comparing three groups (adjusted p-value < 0.05). Consistently, partial least squares discriminant analysis showed that alcohol use disorder, alcoholic hepatitis and controls were generally separated (Figure 2B). Most contributory variables in the serum partial least squares discriminant analysis model were shown in Figure 2C, including 9,10-EpO, 8,9-DiHETrE, 17-HDoHE, 14-HDoHE, LTB4, 12S-HETE and 10-Nitrooleate. Compared to serum samples, fewer fecal oxylipins showed significant difference (Figure 3A). Alcoholic hepatitis patients were better separated from controls compared to alcohol use disorder patients (Figure 3B). Most contributory variables in the fecal partial least squares discriminant analysis model were shown in Figure 3C. When comparing alcohol use disorder patients with controls, 26 oxylipins (adjusted p-value < 0.05) were found significantly different in both serum and feces (Supplemental Figure S1A). Four oxylipins were found significantly different between alcoholic hepatitis patients and the controls in both serum and feces (Supplemental Figure S1B). When comparing alcoholic hepatitis patients with alcohol use disorder patients, only 2 significantly altered oxylipins were found in both serum and feces (Supplemental Figure S1C).
Figure 1.
Oxylipins derived from arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid. AA: arachidonic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; LOX: lipoxygenase; COX: cyclooxygenase; CYP: cytochrome p450; HpETE: hydroperoxyeicosatetraenoic acid; HETE: hydroxyeicosatetraenoic acid; DiHETE: dihydroxyeicosatetraenoic acid; PG: prostaglandin; TX: thromboxane X; EpETrE: epoxyeicosatrienoic acid; DiHETrE: dihydroxyeicosatrienoic acid; HpEPE: hydroperoxy-eicosapentaenoic acid; HEPE: hydroxyeicosapentaenoic acid; DiHEPE: dihydroxy-eicosapentaenoic acid; EpETE: epoxyeicosatetraenoic acid; HpDoHE: hydroperoxy-docosahexaenoic acid; HDoHE: hydroxydocosahexaenoic acid; DiHDoHE: dihydroxy-docosahexaenoic acid; DiHDPE: dihydroxy-docosapentaenoic acid
Figure 2.
Hierarchical clustering of serum oxylipins in control, alcohol use disorder and alcoholic hepatitis patients (A). *: adjusted p-value < 0.05. Partial least squares discriminant analysis of serum oxylipins (B). Important serum oxylipins identified by variable importance in projection scores (C). Ctrl: non-alcoholic control; AUD: alcohol use disorder; AH: alcoholic hepatitis.
Figure 3.
Hierarchical clustering of fecal oxylipins in controls, alcohol use disorder and alcoholic hepatitis patients (A). *: adjusted p-value < 0.05. Partial least squares discriminant analysis of fecal oxylipins (B). Important fecal oxylipins identified by variable importance in projection scores (C). Ctrl: non-alcoholic control; AUD: alcohol use disorder; AH: alcoholic hepatitis.
Oxylipins and intestinal permeability in alcohol use disorder patients
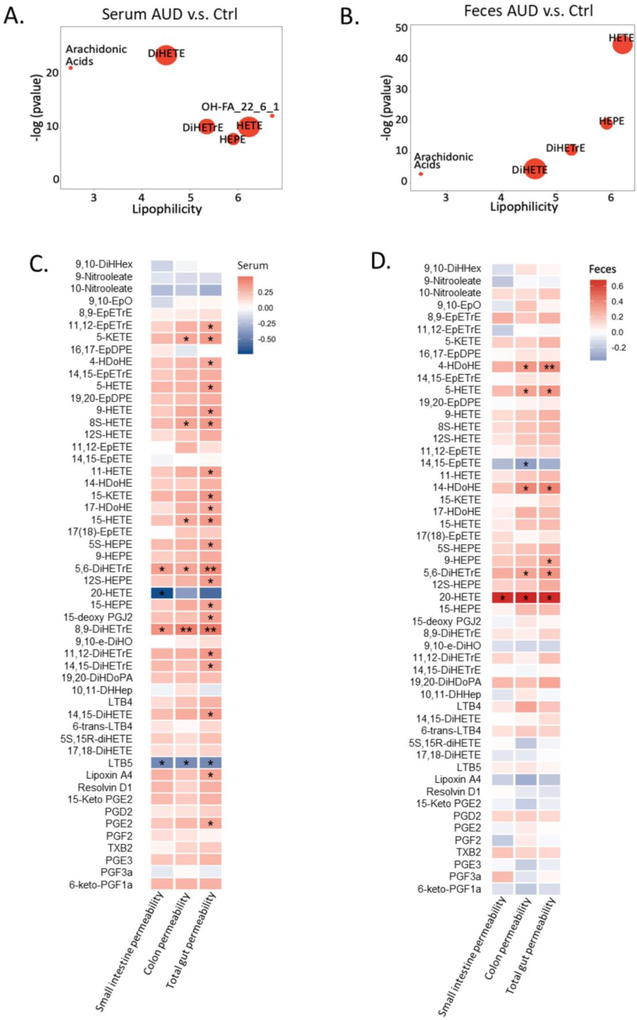
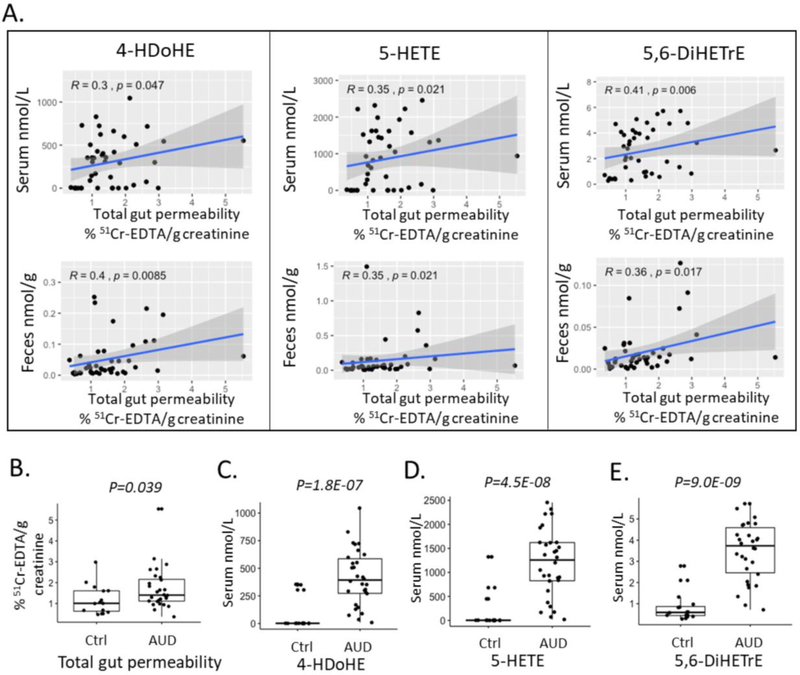
Five compound clusters were significantly increased in both serum and fecal samples in alcohol use disorder patients compared to controls, including AA, AA-derived DiHETrE, HETE, and EPA-derived HEPE and DiHETE (Figure 4A, B). Intestinal permeability was measured in 15 non-alcoholic controls and 28 patients with alcohol use disorder using 51Cr-EDTA. Spearman association showed that 4, 6 and 22 serum oxylipins were correlated with the gut permeability in small intestine, colon and the total gut permeability, respectively (p-value < 0.05, Figure 4C). In addition, 1, 6, and 6 fecal oxylipins showed significant correlation with intestinal permeability in small intestine, colon and the total intestinal permeability, respectively (p value < 0.05, Fig. 4d). Notably, elevated level of three oxylipins in both serum and fecal samples was associated with increased total gut permeability, including AA-derived 5-HETE, 5,6-DiHETrE, and DHA-derived 4-HDoHE (Figure 5A). Compared with control subjects, alcohol use disorder patients showed elevated total gut permeability (Figure 5B). Serum levels of 5-HETE, 5,6-DiHETrE, and 4-HDoHE were also significantly increased in alcohol use disorder patients (Figure 5C, D, E).
Figure 4.
ChemRICH analysis of serum oxylipins in alcoholic use disorder patients and controls (A). Ctrl: non-alcoholic control; AUD: alcohol use disorder; AH: alcoholic hepatitis. Significantly impacted metabolite clusters are shown in the plot (p < 0.05). The y-axis shows the most significantly altered clusters on the top. Red = increased in AUD patients. ChemRICH analysis of fecal oxylipins in alcoholic use disorder patients and controls (b). Ctrl nonalcoholic control; AUD alcohol use disorder; AH alcoholic hepatitis. Significantly impacted metabolite clusters are shown in the plot (p < 0.05). The y-axis shows the most significantly altered clusters on the top. Red=increased in AUD patients. Spearman correlation of serum oxylipins with intestinal permeability (C). Color intensity represents the correlation coefficient (R); Red: positive correlation; Blue: negative correlation. * p<0.05; ** p<0.01; *** p<0.001. Number of controls N=15; Number of AUD patients N=28. Spearman correlation of fecal oxylipins with intestinal permeability (D). Color intensity represents the correlation coefficient (R); Red: positive correlation; Blue: negative correlation. * p<0.05; ** p<0.01; *** p<0.001. Number of controls N=15; Number of AUD patients N=28.
Figure 5.
Spearman correlation of serum or fecal 4-HDoHE, 5-HETE, 5,6-DiHETrE with total gut permeability (A). Number of controls N=15; Number of alcoholic use disorder patients N=28. Total gut permeability was elevated in alcohol use disorder patients compared with control subjects (B). AUD: alcohol use disorder. Number of controls N=15; Number of alcoholic use disorder patients N=28. Serum levels of 4-HDoHE (C), 5-HETE (D), 5,6-DiHETrE (E) were increased in alcohol use disorder patients compared with controls. AUD: alcohol use disorder. Number of controls N=15; Number of alcoholic use disorder patients N=28.
Association between oxylipins and clinical parameters in alcohol use disorder and alcoholic hepatitis patients
Compared to alcohol use disorder patients, serum oxylipins in alcoholic hepatitis patients showed a decrease in five compound clusters, including AA, AA-derived DiHETE, EpETrE, HETE and EPA-derived HEPE (Figure 6A). In contrast, only two AA- derived compound clusters were significantly different between the fecal oxylipins in alcoholic hepatitis patients and the alcohol use disorder patients, among which DiHETrE cluster was significantly decreased, while EpETrE cluster showed an increase in alcoholic hepatitis patients compared to alcohol use disorder patients (Figure 6B). We further examined the correlation of oxylipin levels with laboratory parameters in alcohol use disorder and alcoholic hepatitis patients including AST, alanine aminotransferase (ALT), gamma-glutamyl-transferase (GGT), bilirubin, albumin, INR, creatinine, platelet count and AP. We found the majority of serum oxylipins showed strong negative correlation with AST, GGT, bilirubin and INR, and positive correlation with albumin, creatinine and platelet count (p-value < 0.001, Figure 6C). Interestingly, increased level of serum 20-HETE was associated with decreased serum albumin. We also found some correlations between fecal oxylipins and clinical parameters, however, they were not as strong as for serum metabolites (Supplemental Figure S2).
Figure 6.
ChemRICH analysis of serum oxylipins in alcoholic hepatitis and alcohol use disorder patients (A). AUD: alcohol use disorder; AH: alcoholic hepatitis. Significantly impacted metabolite clusters are shown in the plot (p<0.05). The y-axis shows the most significantly altered clusters on the top. Red=increased in AH patients. Blue=decreased in AH patients. ChemRICH analysis of fecal oxylipins in alcoholic hepatitis and alcoholic use disorder (B). AUD: alcohol use disorder; AH: alcoholic hepatitis. Significantly impacted metabolite clusters were shown in the plot (p<0.05). The y-axis shows the most significantly altered clusters on the top. Red=increased in AH patients. Blue=decreased in AH patients. Purple=some increased compounds, others decreased in AH patients. Spearman correlation of serum oxylipins with laboratory parameters in alcohol use disorder and alcoholic hepatitis patients (c). Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05, ** p<0.01, *** p<0.001. INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl-transferase; AP, alkaline phosphatase.
Oxylipins and liver disease stage in alcohol use disorder and alcoholic hepatitis patients
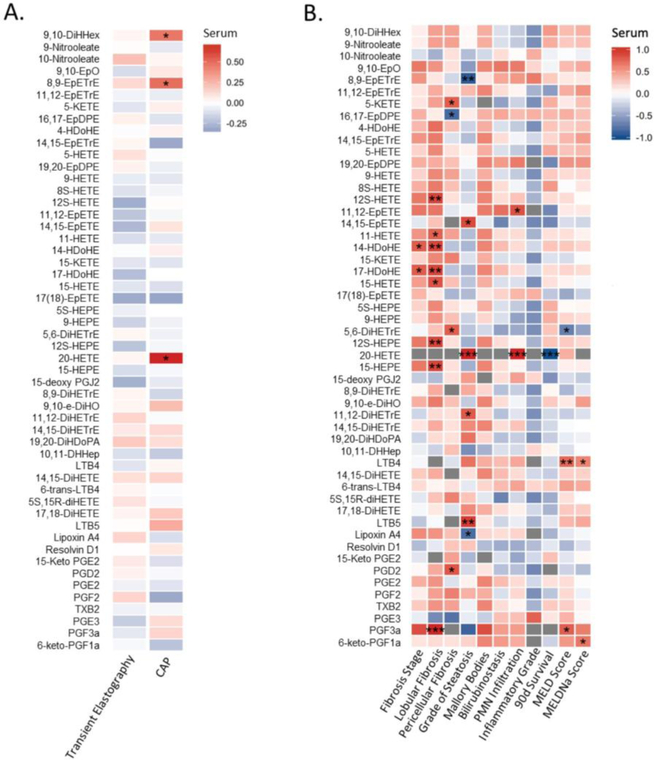
We further examined the correlation of serum oxylipin levels with stage of liver disease in alcohol-related liver disease patients. In alcohol use disorder patients, only three serum oxylipins were positively correlated with steatosis as non-invasively measured by controlled attenuation parameter (CAP), including 9,10-DiHHex, 8,9-EpETrE and 20-HETE (Figure 7A). In alcoholic hepatitis patients, serum 20-HETE was also positively correlated with the grade of steatosis and PMN infiltration on liver biopsy, and negatively correlated with the 90-day survival (Figure 7B). In addition to 20-HETE, the serum level of two other AA metabolites, 11,12-DiHETrE and LTB5, and an EPA metabolite, 14(15)-EpETE, were also positively correlated with the grade of steatosis on liver biopsy in alcohol hepatitis patients, while two AA-derived 8(9)-EpETrE and Lipoxin A4, were negatively correlated with the grade of steatosis (Figure 7B). Serum level of an EPA metabolite 11(12)-EpETE was positively correlated with PMN infiltration on liver biopsy in alcohol hepatitis patients (Figure 7B).
Figure 7.
Spearman correlation of serum oxylipins with parameters of liver disease stage in alcohol use disorder patients (A). CAP: controlled attenuation parameter. Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05. Number of alcohol use disorder patients N=30. Spearman correlation of serum oxylipins with liver histology and clinical scores in alcoholic hepatitis patients (B). Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05, ** p<0.01, *** p<0.001. MELD: model for end-stage liver disease; MELDNa: sodium model for end-stage liver disease. Number of alcoholic hepatitis patients N=7.
An association between serum oxylipins with fibrosis status on liver biopsy, 90 day mortality and clinical predictors of outcome was also found in alcoholic hepatitis patients (Figure 7B). For example, serum level of DHA-derived 14-HDoHE and 17-HDoHE was positively correlated with fibrosis stage. AA-derived 12-HETE, 11-HETE, 15-HETE, EPA-derived 12-HEPE, 15-HEPE, PGF3α, and DHA-derived 14-HDoHE and 17-HDoHE were positively correlated with lobular fibrosis. Serum level of AA-derived 5-KETE, 5,6-DiHETrE and PGD2 was positively correlated with pericellular fibrosis, while DHA- derived 16(17)-EpDPE was negatively correlated with pericellular fibrosis. Serum LTB4 (AA metabolite) was positively correlated with model for end-stage liver disease (MELD) and sodium model for end-stage liver disease (MELDNa) scores. Some fecal oxylipins were also significantly correlated with the liver histology (Supplemental Figure S3).
Discussion
A profound change in the profile of serum and fecal oxylipins was found in patients with alcohol-related liver disease (Figure 2, 3). For example, the chemical cluster of HETE (hydroxyl-FA), primarily lipoxygenase-derived oxidized products of AA, was significantly increased in alcohol use disorder patients compared with controls (Figure 4A). Consistently, in a C57BL/6 male mice model of alcohol-related liver disease, ethanol and dietary unsaturated fat (enriched in corn oil) caused elevated plasma ALT levels, hepatic steatosis and inflammation. These pathologies were associated with increased levels of inflammatory lipid metabolites, including HETEs and DiHETrEs, in parallel with increased pro-resolving mediators [26]. Increased plasma HETEs concentrations were also documented from alcohol-related cirrhosis patients in line with the increase of 5- and 15-lipoxygenase-2 mRNA expression in liver samples [13].
In addition to alcohol-related liver disease, oxylipins have also been implicated to play a key role in non-alcoholic fatty liver disease (NAFLD). Reduced dietary ω-6 to ω-3 fatty acid ratio is protective against chronic high-fat diet-induced steatohepatitis in the 12/15 lipoxygenases knockout mouse model [27]. Increases in inflammatory HODEs and 11-HETE were found in obese mice compared with lean mice, with a higher magnitude of change due to the interaction of obesity and alcohol [28]. An increase of lipoxygenase metabolites 5-HETE, 8-HETE and 15-HETE in patients has been reported to characterize the progression of NAFLD [29]. In a randomized placebo-controlled trial of nonalcoholic steatohepatitis (NASH), therapy with pentoxifylline, a methylxanthine derivative which increases red blood cell flexibility, reduces blood viscosity, and decreases platelet aggregation, was associated with decreases in 8-HETE, 9-HETE and 11-HETE compared to placebo [30]. Statistically significant correlations were demonstrated between the decrease in HETEs and improved lobular inflammation [30]. Consistently, decreased serum level of HETEs (11, 12S and 15-HETE) were significantly correlated with less lobular fibrosis in our study (Figure 7).
Oxylipins derived from the ω-6 fatty acid arachidonic acid generally increase inflammation, hypertension and platelet aggregation. Alternatively, most oxylipins derived from ω-3 fatty acids have anti-inflammatory, anti-aggregatory, and vasodilatory effects [6]. In the present study, serum levels of ω-6 AA-derived HETEs (5-, 8-, 9-, 11-, 12-, and 15-HETE), and 5,6-DiHETrE were negatively correlated with AST, bilirubin, INR and positively correlated with albumin and platelet count (Figure 6C). In line with ω- 6 fatty acid AA-derived oxylipins, several ω-3 fatty acid derived oxylipins were also negatively correlated with AST, bilirubin, INR and positively correlated with albumin and platelet count, including HEPEs (5-, 9-, 12- 15-HEPE, hydroxyl-metabolites of EPA via lipoxygenase pathway) and HDoHEs (DHA hydroxyl-metabolites generated via lipoxygenase pathway) (Figure 6C). Our results suggest that inflammation or platelet aggregation and resolution of inflammation or platelet aggregation are tightly connected processes in alcohol use disorder and alcoholic hepatitis patients, and the enhanced resolution are initiated as an adaptive response to inflammation or platelet aggregation. If the deleterious effects of inflammatory ω-6 PUFAs metabolites outweigh the benefits of anti-inflammatory and pro-resolving ω-3 bioactive lipid mediators, it may suggest severe liver pathology and tip balance toward liver damage.
Unsaturated fat diet (corn oil/linoleic acid) itself induced the dysregulation of tight junction integrity and increased the gut permeability, which was exacerbated by alcohol in a C56BL/6N mouse model [31]. In our study, we found that increased total gut permeability was significantly correlated with the increase of AA-derived 5-HETE, 5,6-DiHETrE, and DHA-derived 4-HDoHE in both serum and feces, which suggested the oxylipins might play a role in the regulation of the gut permeability. However, the impact of different PUFA-derived oxylipins on the intestinal permeability and inflammation warrants future investigation.
Oxylipins are generally formed in situ and they are found in all tissues of the body [6]. Serum oxylipins are measured to characterize the levels of circulating oxylipins in the body. In addition to the host, another source of oxylipins is eukaryotic microbes such as fungi, protozoa and helminths [32]. Oxylipins play a role in both metabolism or maturation of the microbial organism and its communication with the host [33]. For instance, the fungal oxylipins function as signaling molecules to modulate the spore development, toxin production and pathogenesis [33]. Fecal oxylipins could better characterize the oxylipins co-metabolism by host and the gut microbes. The analysis of both serum and fecal oxylipins provides us a comprehensive understanding of the oxylipin profiles in patients with alcohol-related liver disease.
One limitation of our study is the control population. As 71.4% of alcoholic hepatitis patients have underlying cirrhosis, the ideal way to capture the contribution of the inflammatory process would have been to include patients with cirrhosis as controls. The age of controls and alcoholic hepatitis patients is significantly different (control vs. alcoholic hepatitis p-value = 0.001359; control vs. alcohol use disorder p-value = 0.9104; alcohol use disorder vs. alcohol hepatitis p-value = 0.0003877) (Table 1A). Pearson correlation results showed that six serum oxylipins correlate with age (p-value < 0.05), including 10-Nitrooleate, 14,15-DiHETE, 9,10-EpO, 9-Nitrooleate, PGE3 and 6- trans-LTB4 (Supplemental Table S2). We found one fecal oxylipin, Lipoxin A4 correlates with age (p-value < 0.05) (Supplemental Table S2). The remaining serum and fecal oxylipins didn’t show any significant correlation with age. In addition, there is significant (p-value < 0.05) correlation between oxylipins detected in feces and serum (Supplemental Table S3).
In summary, our findings reveal alterations of serum and fecal oxylipin profiles in alcohol use disorder and alcoholic hepatitis patients and suggest potential links between oxylipins and the pathogenesis of alcohol-related liver disease. Further research is required to elucidate the molecular mechanisms by which oxylipins exert their effects during the development and progression of alcohol-related liver disease, and identify novel therapeutic targets and intervention strategies for disease prevention and treatments. Alternatively, oxylipins might be developed as biomarkers for patients with alcohol-related liver disease.
Supplementary Material
Supplemental Figure S1 Venn diagram of significantly altered oxylipins found in both serum and fecal samples (adjusted p-value < 0.05). Ctrl: controls; AUD: alcoholic use disorder; AH: alcoholic hepatitis.
Supplemental Figure S2 Spearman correlation of fecal oxylipins with laboratory parameters in alcoholic hepatitis and alcohol use disorder patients. Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05, ** p<0.01, *** p<0.001. INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl-transferase; AP, alkaline phosphatase.
Supplemental Figure S3 Spearman correlation of fecal oxylipins with parameters of liver disease stage in alcohol use disorder patients (A). CAP: controlled attenuation parameter. Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05. Number of alcohol use disorder patients N=30. Spearman correlation of fecal oxylipins with liver histology and clinical scores in alcoholic hepatitis patients (B). Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05, ** p<0.01, *** p<0.001. MELD, Model for End-stage Liver Disease; MELDNa, Sodium Model for End-stage Liver Disease. Number of alcoholic hepatitis patients N=7.
Acknowldegements:
This study was supported in part by NIH grants R01 AA020703, R01 AA24726, U01 AA021856, U01 AA026939 and by Award Number BX004594 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to B.S.), and by Fond National de Recherche Scientifique (FNRS) Belgium grants CDR J.0146.17 and PDR T.0217.18 (to P.S.).
Abbreviations
- AP
alkaline phosphatase
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- BMI
body mass index
- CAP
controlled attenuation parameter
- INR
international normalized ratio
- MELD
model for end-stage liver disease
- MELDNa
sodium model for end-stage liver disease
- PMN
polymorphonuclear infiltration
- AA
arachidonic acid
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- HpETE
hydroperoxyeicosatetraenoic acid
- HETE
hydroxyeicosatetraenoic acid
- DiHETE
dihydroxyeicosatetraenoic acid
- PG
prostaglandin
- TX
thromboxane X
- EpETrE
epoxyeicosatrienoic acid
- DiHETrE
dihydroxyeicosatrienoic acid
- HpEPE
hydroperoxy-eicosapentaenoic acid
- HEPE
hydroxyeicosapentaenoic acid
- DiHEPE
dihydroxy-eicosapentaenoic acid
- EpETE
epoxyeicosatetraenoic acid
- HpDoHE
hydroperoxy-docosahexaenoic acid
- HDoHE
hydroxydocosahexaenoic acid
- DiHDoHE
dihydroxy-docosahexaenoic acid
- DiHDPE
dihydroxy-docosapentaenoic acid;
- PLS-DA
partial least squares discriminant analysis
- VIP
variable importance in projection
- MRM
multiple reaction monitoring
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: B.S. is consulting for Ferring Research Institute.
References
- 1.Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries American Journal of Gastroenterology. 2017;112. [DOI] [PubMed] [Google Scholar]
- 2.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets Gastroenterology. 2011;141:1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs Advances in nutrition (Bethesda, Md.). 2015;6:513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology Journal of lipid research. 2009;50:1015–1038; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barquissau V, Ghandour RA, Ailhaud G et al. Control of adipogenesis by oxylipins, GPCRs and PPARs Biochimie. 2017;136:3–11. [DOI] [PubMed] [Google Scholar]
- 6.Caligiuri SPB, Parikh M, Stamenkovic A, Pierce GN, Aukema HM. Dietary modulation of oxylipins in cardiovascular disease and aging American Journal of Physiology-Heart and Circulatory Physiology. 2017;313:H903–H918. [DOI] [PubMed] [Google Scholar]
- 7.Tourdot BE, Ahmed I, Holinstat M. The emerging role of oxylipins in thrombosis and diabetes Frontiers in pharmacology. 2014;4:176–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk CD. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology Science. 2001;294:1871. [DOI] [PubMed] [Google Scholar]
- 9.Yeung J, Hawley M, Holinstat M. The expansive role of oxylipins on platelet biology Journal of molecular medicine (Berlin, Germany). 2017;95:575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner D, Westover K, Simmons DL. Nonsteroidal Anti-Inflammatory Drugs, Acetaminophen, Cyclooxygenase 2, and Fever Clinical Infectious Diseases. 2000;31:S211–S218. [DOI] [PubMed] [Google Scholar]
- 11.Liu MC, Dubé LM, Lancaster J. Acute and chronic effects of a 5-lipoxygenase inhibitor in asthma: A 6-month randomized multicenter trial Journal of Allergy and Clinical Immunology. 1996;98:859–871. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Zhong W, Sun Q, Sun X, Zhou Z. Hepatic overproduction of 13-HODE due to ALOX15 upregulation contributes to alcohol-induced liver injury in mice Scientific reports. 2017;7:8976–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldstein AE, Lopez R, Tamimi TA-R et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis Journal of lipid research. 2010;51:3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball SA, Tennen H, Poling JC, Kranzler HR, Rounsaville BJ. Personality, temperament, and character dimensions and the DSM-IV personality disorders in substance abusers J Abnorm Psychol. 1997;106:545–553. [DOI] [PubMed] [Google Scholar]
- 15.Brandl K, Hartmann P, Jih LJ et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis Journal of Hepatology. 2018;69:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen-Khac E, Thiele M, Voican C et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis The Lancet Gastroenterology & Hepatology. 2018;3:614–625. [DOI] [PubMed] [Google Scholar]
- 17.Salavrakos M, Piessevaux H, Komuta M, Lanthier N, Starkel P. Fibroscan Reliably Rules Out Advanced Liver Fibrosis and Significant Portal Hypertension in Alcoholic Patients J Clin Gastroenterol. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Buzzetti E, Lombardi R, De Luca L, Tsochatzis EA. Noninvasive Assessment of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease International journal of endocrinology. 2015;2015:343828–343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddowes PJ, Sasso M, Allison M et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Non-alcoholic Fatty Liver Disease Gastroenterology. 2019. [DOI] [PubMed] [Google Scholar]
- 20.Caussy C, Alquiraish MH, Nguyen P et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis Hepatology. 2018;67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlas T, Petroff D, Sasso M et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis Journal of Hepatology. 2017;66:1022–1030. [DOI] [PubMed] [Google Scholar]
- 22.Leclercq S, Matamoros S, Cani PD et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters Journal of lipid research. 2010;51:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luria A, Weldon SM, Kabcenell AK et al. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice The Journal of biological chemistry. 2007;282:2891–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barupal DK, Fiehn O. Chemical Similarity Enrichment Analysis (ChemRICH) as alternative to biochemical pathway mapping for metabolomic datasets Scientific reports. 2017;7:14567–14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warner DR, Liu H, Ghosh Dastidar S et al. Ethanol and unsaturated dietary fat induce unique patterns of hepatic ω-6 and ω-3 PUFA oxylipins in a mouse model of alcoholic liver disease PloS one. 2018;13:e0204119–e0204119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazic M, Inzaugarat ME, Povero D et al. Reduced dietary omega-6 to omega-3 fatty acid ratio and 12/15-lipoxygenase deficiency are protective against chronic high fat diet-induced steatohepatitis PloS one. 2014;9:e107658–e107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri P, Xu J, Vihervaara T et al. Alcohol produces distinct hepatic lipidome and eicosanoid signature in lean and obese Journal of lipid research. 2016;57:1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puri P, Wiest MM, Cheung O et al. The plasma lipidomic signature of nonalcoholic steatohepatitis Hepatology (Baltimore, Md.). 2009;50:1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zein CO, Lopez R, Fu X et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism Hepatology (Baltimore, Md.). 2012;56:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirpich IA, Feng W, Wang Y et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease Alcoholism, clinical and experimental research. 2012;36:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes Clinical microbiology reviews. 2003;16:517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host–fungal communication signals Trends in Microbiology. 2007;15:109–118. [DOI] [PubMed] [Google Scholar]
- 34.Arnold C, Konkel A, Fischer R, Schunck W-H. Cytochrome P450–dependent metabolism of ω-6 and ω-3 long-chain polyunsaturated fatty acids Pharmacological Reports. 2010;62:536–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 Venn diagram of significantly altered oxylipins found in both serum and fecal samples (adjusted p-value < 0.05). Ctrl: controls; AUD: alcoholic use disorder; AH: alcoholic hepatitis.
Supplemental Figure S2 Spearman correlation of fecal oxylipins with laboratory parameters in alcoholic hepatitis and alcohol use disorder patients. Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05, ** p<0.01, *** p<0.001. INR, international normalized ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl-transferase; AP, alkaline phosphatase.
Supplemental Figure S3 Spearman correlation of fecal oxylipins with parameters of liver disease stage in alcohol use disorder patients (A). CAP: controlled attenuation parameter. Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05. Number of alcohol use disorder patients N=30. Spearman correlation of fecal oxylipins with liver histology and clinical scores in alcoholic hepatitis patients (B). Color intensity represents the correlation coefficient (R); Red: positive correlation. Blue: negative correlation. * p<0.05, ** p<0.01, *** p<0.001. MELD, Model for End-stage Liver Disease; MELDNa, Sodium Model for End-stage Liver Disease. Number of alcoholic hepatitis patients N=7.