Abstract
Purpose of Review
Emerging research on the pediatric microbiome implicates the importance of the microbiome on the development of the immune system, nervous system, and growth. Changes to the microbiome during infancy are associated with the development of chronic illnesses such as asthma and inflammatory bowel disease. Additionally, the microbiome provides protection against certain pathogens, affects vaccine responses and alters drug metabolism. This review highlights what is known about the microbiome, the establishment of a healthy microbiome and the significance that changes to the microbiome composition have on growth and health of children and adolescents.
Recent Findings
Vaginal delivery, breastfeeding, maternal health and nutrition help shape a healthy microbiome. Caesarian delivery, formula feeding and antibiotic use perturb the microbiome and are associated with the development of type II diabetes, asthma, allergic diseases and obesity later in life. Specific interventions using pre- and probiotics in multiple settings are under investigation with limited success.
Summary
A better understanding of the microbiome and the interaction with the immune system may help guide interventions to alter the microbiome towards a state of lifelong health.
Keywords: Microbiome, Gastrointestinal, Microbiota, Humans, Breastmilk, Antibiotic use, Immune response
Introduction
The human microbiome, composed of bacteria, viruses, fungi and archaea, is an active and emerging field of study. These microbial communities are particularly relevant to pediatrics since animal and human data suggest a critical role in immune development and growth (1, 2). Although it is not yet known what organisms compose a healthy microbiome, the composition of the microbiome varies by age and by body site and it appears that health is associated with greater bacterial diversity in most anatomical sites. Early alterations in the microbiome composition have been linked to the development of diseases later in life such as asthma, atopy, inflammatory bowel disease, obesity and type I diabetes (3–9) and may also impact neurodevelopment (10–12). Likewise, certain bacteria in the microbiome may confer protection against various pathogens or may make a child more susceptible to specific infections including upper respiratory infections, malaria, and campylobacter infections (12–15). Lastly, the microbiome may influence both vaccine responses (16) and drug metabolism (17–19).
To date, the vast majority of microbiome research has focused on bacterial populations inhabiting the intestines. However, viruses and fungi also contribute to the microbiome, as a complex dynamic exists in the populations of bacteria, viruses and fungi that colonize various anatomical sites in the gastrointestinal tract. These populations and dynamics may influence the healthy intestinal microbiome as well as the development of other disease states in ways that we are only beginning to understand (20–22). In this review, we focus on the immune effects of the intestinal microbiome discussing when the microbiome is established, what constitutes a healthy microbiome, the timing of a critical window for the establishment of a healthy microbiome and the impact that changes such as antibiotics, delivery mode, breastfeeding and nutrition have on the microbiome and on the future health and growth of a child.
Are we sterile at birth?
Given the significance of the microbiome on the developing neonate and the potential for therapeutic interventions, it is important to establish when the infant first becomes colonized with microbes. It has been widely assumed that infants are sterile at birth. However, this notion has recently been challenged by the in utero hypothesis, which proposes that the fetus is exposed to bacteria in the placenta and amniotic fluid (23). Findings to support the in utero hypothesis include the detection of bacteria in the placenta and amniotic fluid at the time of delivery (24, 25); the presence of bacteria cultured from cord blood; and the amplification of bacterial sequences in meconium (25, 26). Arguments in favor of the sterile womb paradigm include the difficulty of proving the significance of the bacteria detected in the amniotic fluid and placenta as these results were largely obtained by detection of nucleic acid and culturing bacteria from these sites has not been successful (27, 28). A careful study in which contamination controls were utilized and compared to placental samples found that there was no significant difference in detection of unique bacterial populations between the two groups. This indicates that the reported detection of microbes likely represents background contamination of the reagents (28) which plagues the analysis of all low biomass studies (29). To date, most infants have no bacteria at birth, though the definitive answer to this question remains unknown.
Factors Influencing Early Microbial Colonization and their Long-term Health Consequences
Regardless of whether there is some exposure in utero, it is clear that after birth infants are rapidly colonized with a complex mixture of microbes primarily derived from initial environmental exposures. Infants delivered vaginally have a microbiome composition more similar to the mother’s vaginal flora containing Lactobacillus, Prevotella, Atopobium and/or Sneathia species (30). In contrast, infants born via Caesarean-section (31) have a microbiome that is more similar to the maternal skin flora (30) and is less diverse (32). The changes in the microbiome, depending on the delivery mode persist, remaining until approximately one year of age (31, 33, 34) but appear to be mitigated by breastfeeding (35). The extent to which the effects of C-sections are due to prophylactic antibiotics is unclear since most, but not all studies, report this confounder (35). These changes can have important life-long consequences as children born via C-section have a greater risk of developing asthma and other allergic diseases (36, 37). A single, small study swabbing infants with maternal vaginal fluid at the time of C-section appeared to alter the infant’s microbiome towards that of the vaginally-delivered infants, indicating that these changes in the microbiome may be reversible (38). Sample collection for this study ended at 30 days of life, so the persistence and full impact on future child and adolescent health is unknown.
Beyond delivery mode, breastfeeding is another important determinate in the establishment of the microbiome. Breast milk and the surrounding areolar skin are significant sources of bacteria for the infant’s intestinal microbiome, contributing an estimated 28% (breast milk) and 10% (areolar skin) during the first 30 days of life (39). The contribution of breast milk is dose-dependent. The microbiome of breastfed infants is a distinct microbial population compared to those who are formula fed and these differences persist into adulthood (31, 32, 40, 41). Breastfeeding reduces both upper and lower respiratory tract infections and gastrointestinal infections in infants. Early breastfeeding, within the first hour of life, compared to 2–23 hours of life, or greater than 24 hours of life significantly reduces infant mortality (42, 43). The benefits of breastfeeding also extend to increased survival in HIV-infected infants both in developed and developing countries (44–49). Breastfeeding also reduces the development of atopic dermatitis, asthma in children with a family history of asthma and possibly the development of obesity and type II diabetes later on in life (50–53).
How much of these benefits are due to microbial transfer versus other protective components of breast milk remains unknown. Breast milk contains multiple components that protect the infant against infection such as caseins, lactoferrin, lysozyme, immunoglobulin A (35), and human milk oligosaccharides (HMOs) (54). HMOs are the third largest constituent of breast milk and have many putative functions including some antibacterial activity (55). Interestingly these substances are not digested by humans and appear to serve as prebiotics, promoting the growth of commensal bacteria such as Bifidobacterium bifidum (56). Therefore, breastmilk contains both important prebiotics and probiotics for the establishment of the infant’s gut microbiome.
Maternal health is also important in the establishment of the infant microbiome. Perturbations of the infant’s microbiome have been noted in preterm infants whose mothers are obese, term infants whose mothers are on antibiotics during pregnancy and infants whose mothers are HIV-infected (57–59). HIV-exposed, uninfected infants experience considerable morbidity and mortality and decreased growth compared to infants in the same setting born to HIV negative women (60–65). HIV-exposed, uninfected infants have more abundant populations of Pseudomonadaceae and Thermaceae, decreased bacterial diversity, and a less mature microbiome in their stool than HIV-unexposed, uninfected infants (59). These perturbations may be one mechanism that accounts for the immunologic derangements and poor growth observed in these children.
The environment including race and ethnicity, geographical location, diet and exposure to pets has a significant effect on the microbiome (66). Genetic differences may also play an important role (67–72). Early exposure to pets correlates with an increase in Ruminoccocus and Oscillospira irrespective of delivery mode which may have implications towards decreased obesity and food allergies (73). Microbial differences are also observed in both infants and adults in specific ethnic groups and may explain the higher incidence of cardiovascular disease and diabetes amongst different ethnicities (74–76). This change begins early in life as differences are noted at one year of age in Canadian infants of South Asian descent and Caucasian descent (76). Race and ethnicity are significant determinants of the vaginal microbiome, and the microbial differences affect the risk of acquisition of sexually transmitted infections including HIV (77–79). Additionally, the vaginal microbiome alters the effectiveness of pre-exposure prophylaxis to prevent HIV transmission (17), so an understanding of racial and ethnic differences in microbiome composition has broad implications in health and disease.
The biogeography, or spatial organization, of bacteria along the gastrointestinal tract also plays a vital role in the establishment of the microbiome. The presence of naturally occurring antimicrobial peptides, secreted IgA, levels of oxygen, pH, and special anatomic constraints such as the appendix, colon crypts, and the mucous layer as well as other bacteria influence which bacteria inhabit specific areas of the gastrointestinal tract (80). Furthermore, certain bacteria present in the microbiome also modulate the immune system as Bifidobacteria and Clostridia species influence the development of T regulatory cells (81–83). Interactions with polysaccharides and short chain fatty acids also function as a stimulus for T regulatory cells (84–86).
Once established, the microbiome itself is very resilient and not easily changed (87–89). It has been noted that 60% of the bacteria sequences comprising the microbiome remain stable over the course of five years in an adult (88). Administered antibiotics can change the diversity initially, but the microbiome becomes more similar to the pre-antibiotic state within weeks to months after last administration of the antibiotic (90, 91). The reason why the healthy microbiome remains impervious to change over time may be the result of the abundance of microbial species present and increased diversity as decreased bacterial diversity has been associated with diseases such as type II diabetes, obesity, and inflammatory bowel disease (92). Resilience in unhealthy microbiomes may even be associated with chronicity of certain diseases such as inflammatory bowel disease and obesity (92).
The Developmental Window
Given the significant health consequences associated with microbial perturbations, it has been proposed that the first 1–24 months of life represents a critical developmental window for the establishment of the microbiome (93). The first few years of life is also a key period in immune system development (94). As certain bacteria are required for parts of the immune system to develop or mature, these two processes are inextricably linked. For example, early infant colonization with Bacteroides fragilis is associated with B-cell maturation in the infant gut (95, 96).
Notable shift changes occur in the bacterial composition of the microbiome during the first few years of life. This microbial maturation can be assessed using an index developed by Subramanian et al (97). The index can be applied to compare communities, assess growth and assess severe acute malnutrition (97). Transition points have been recognized during the first month of life and with the introduction and expansion of solid foods. At two to three years of age, the microbiome becomes more similar to the diverse microbiome seen in adults (6, 40, 67) but this composition is still distinct even into adolescence (98).
While the microbiome changes more in the first few years of life, the composition in the first few months appears to be critical for allergic disease and imprinting. The Canadian Healthy Infant Longitudinal Development (35) study reported that the microbiome at three months of age, but not at one year, identified children who developed either atopy defined by skin testing or had clinical symptoms of wheezing (99). In this study, lower amounts of stool Veillonella, Lachnospira, Rothia, and Faecalibacterium was associated with atopy and wheezing (99). Moreover, in a murine model, administration of these bacteria attenuated airway inflammation (99). A US birth cohort also reported that the early stool microbiome was associated with atopy and asthma (3). In this study, stool profiles at one month but not six months of age were predictive (3). In this racially diverse group, risk was associated with lower bacterial levels of Bifidobacteria, Lactobacillus, Faecalibacterium, and Akkermansia and increased fungal levels of Saccharomyces, Rhodotorula, and Candida and decreased Malassezia (3). These studies are consistent with murine models in which early microbial colonization has life-long consequences on immune responsiveness (93).
At all ages, antibiotics can have a profound effect on the stool microbiome (40, 100, 101). Mouse models incorporating the use of a low dose antibiotic following weaning showed that changes in the microbiome correlated to more corpulence later on in life despite the microbiome normalizing (5, 102). However, the role of antibiotic exposure and current rates of childhood obesity is unclear as studies have been contradictory (103–107). Nevertheless, these concerns should prompt judicious use of antibiotics, especially as antibiotics are oftentimes overprescribed for treatment of viral illnesses.
Nutrition
The importance of proper nutrition in the developing child cannot be understated and an understanding of the complex interactions between the microbiome and nutritional status is starting to emerge. Undernutrition causes increased mortality in children under the age of five, has physical effects such as stunted growth, increased infections, decreased immunity, and impacts neurodevelopment (108, 109).
Malnourished children receiving ready-to-use therapeutic food (RUTF) do not gain the expected amount of weight compared to their healthy counterparts (97). Evidence suggests that microbes have an important role in the pathogenesis of malnutrition as highlighted by the efficacy of antibiotics in improving nutritional recovery and decreasing mortality in children with severe acute malnutrition (110). Transfer of IgA-targeted bacteria from the gut microbiota of undernourished children into germ-free mice disrupts the small intestinal and colonic epithelial barrier and induces weight loss and sepsis (111, 112). Characterization of the IgA-targeted bacteria demonstrated that Enterobacteriaceae in combination with ten other microbes was sufficient to produce enteropathy (111). Furthermore, the enteropathy could be prevented by administering two IgA-targeted bacteria from a healthy microbiota (Clostridium scindens and Akkermansia muciniphila) (111).
Microbial diversity is decreased and microbiomes are immature in children with moderate and severe malnutrition (97, 113). Furthermore, lacking specific micronutrients can impact the microbiome by promoting growth of specific bacteria (114). Finally, environmental enteric dysfunction (EED) or tropical enteropathy may also play a role in malnutrition (115). EED is most commonly observed in developing countries and causes chronic inflammation in the small intestine and likely forms intestinal microerosions that impair the absorption of nutrients and promotes microbial translocation (115, 116). EED is thought to be caused by persistent exposure to microbes that promote chronic inflammation as a result of not having access to clean food and water, lack of access to toilets and lack of handwashing practices (115, 117). Improvement of child health from malnutrition will not only have to address macro and micronutrient deficiencies but may also need to develop strategies that also improve EED.
Therapeutic Potential
Given all the ways the microbiome impacts human health and disease, there is tremendous interest in manipulating the microbiome to improve health. However, data in humans is sparse. Fecal microbial transfers from healthy donors have been successful in manipulating the microbiome from a state of disease back to a state of health in the treatment of recurrent Clostridium difficile infections (118), but have not been as successful in other disease states (119, 120). It may be that treatment of C. difficile infection is uniquely successful because the disease has devastated the pre-existing communities creating an available niche for the microbiota from a healthy donor.
The use of prebiotics and probiotics has been another area of investigation with limited success. Prebiotics have shown some success in the desensitization of peanut allergies (121) as well as in reducing body weight and total body fat in obese children (122). Other studies have used a combination of pre-and probiotics, labelled symbiotics. One such study administered a symbiotic within the first 2–4 days after birth, for seven days, to healthy infants in a rural village in India and showed some success in reducing the combined primary outcome of death and sepsis as well as lowering the amount of lower respiratory tract infections (123). Partial success was also achieved with symbiotic administration in very low birth weight infants in reducing necrotizing enterocolitis (124). Other trials utilizing only a probiotic have had less success in reducing sepsis or necrotizing enterocolitis in pre-term infants (125–128). While therapeutic interventions to alter the microbiome show potential, more work needs to be done to understand the complex dynamics of the microbiome.
Finally, there is interest in using the knowledge gained about the microbiome and nutrition to improve health by improving available foods. This may be accomplished by finding foods that benefit microorganisms in the microbiome, by discovering nutritional components modified by microbes to benefit the human host, or by finding foods that benefit the human host (129). Once these components are identified, additional interventions may be done to alter crops in developing countries where specific nutrients essential for growth may be lacking in typical diets (129).
Conclusion
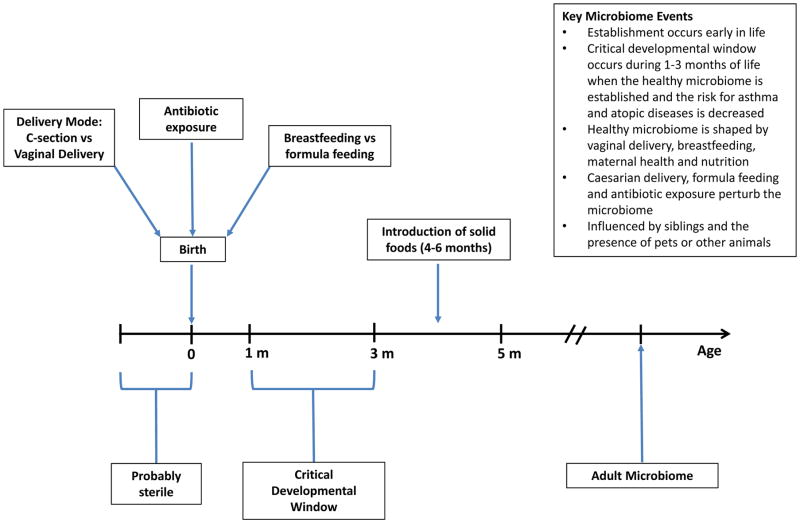
There is clear evidence that the microbiome has important long-term health implications for the growing child. The microbiome is established early and once established appears to be resilient to change (See Figure 1). A critical developmental window exists in the first one to three months of life, a window which decreases the risk of allergic diseases and establishes long-term health. Understanding all the factors that lead to a healthy microbiome is of critical importance and will likely include an understanding of the complex interactions between archea, viruses, bacteria and eukaryotes. In pediatrics, support of breastfeeding and good nutritional practices and avoiding procedures or interventions that alter the microbiome towards a state of dysbiosis are important for the health of a child. Avoiding medically unnecessary C-sections (36, 40, 130) and judicious use of antibiotics are critical, primum non nocere. More specific interventions, such as pre- and pro-biotics are as yet, of unproven benefit, but show potential and warrant further study.
Figure 1.
Timeline of key events in the development of the microbiome.
Key Points.
The microbiome has long-term ramifications for growing children.
The microbiome is established early in life and appears to be resilient to change.
A critical window for development exists during the first one to three months of life in which the healthy microbiome is established and the risk for asthma and allergies is decreased.
The healthy microbiome is shaped by vaginal delivery, breastfeeding, maternal health and nutrition.
Caesarian delivery, formula feeding and antibiotic use perturb the microbiome and are associated with the development of type II diabetes, asthma, allergic diseases and obesity later in life.
Acknowledgments
None
Financial support and sponsorship
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. TG is supported by a training grant from the NIH-NIAID [000064] under award number 5T32AI089398-08.
Footnotes
Conflicts of interest
None.
References
- 1.Koch MA, Reiner GL, Lugo KA, Kreuk LS, Stanbery AG, Ansaldo E, et al. Maternal IgG and IgA Antibodies Dampen Mucosal T Helper Cell Responses in Early Life. Cell. 2016;165(4):827–41. doi: 10.1016/j.cell.2016.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 3**.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–91. doi: 10.1038/nm.4176. This study is significant as it identifies a critical developmental window in infants at risk for atopy and asthma at one month of age using stool profiles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metsala J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Mother’s and offspring’s use of antibiotics and infant allergy to cow’s milk. Epidemiology. 2013;24(2):303–9. doi: 10.1097/EDE.0b013e31827f520f. [DOI] [PubMed] [Google Scholar]
- 5.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17(2):260–73. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–10. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–92. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell. 2016;167(4):915–32. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20(9):509–18. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao A, Ahmed AM, Subramanian S, Griffin NW, Drewry LL, Petri WA, Jr, et al. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature. 2014;515(7527):423–6. doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knecht H, Neulinger SC, Heinsen FA, Knecht C, Schilhabel A, Schmitz RA, et al. Effects of beta-lactam antibiotics and fluoroquinolones on human gut microbiota in relation to Clostridium difficile associated diarrhea. PLoS One. 2014;9(2):e89417. doi: 10.1371/journal.pone.0089417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159(6):1277–89. doi: 10.1016/j.cell.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–70. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris VC, Armah G, Fuentes S, Korpela KE, Parashar U, Victor JC, et al. Significant Correlation Between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J Infect Dis. 2017;215(1):34–41. doi: 10.1093/infdis/jiw518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356(6341):938–45. doi: 10.1126/science.aai9383. This paper shows the importance of the vaginal microbiome in altering drug metabolism in HIV negative women who received a vaginal microbicide for prophylaxis. [DOI] [PubMed] [Google Scholar]
- 18.Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013;341(6143):295–8. doi: 10.1126/science.1235872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmody RN, Turnbaugh PJ. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest. 2014;124(10):4173–81. doi: 10.1172/JCI72335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reyes A, Blanton LV, Cao S, Zhao G, Manary M, Trehan I, et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc Natl Acad Sci U S A. 2015;112(38):11941–6. doi: 10.1073/pnas.1514285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466(7304):334–8. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 2016;6:23129. doi: 10.1038/srep23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156(1):20–5. doi: 10.1016/j.jpeds.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornef M, Penders J. Does a prenatal bacterial microbiota exist? Mucosal Immunol. 2017;10(3):598–601. doi: 10.1038/mi.2016.141. [DOI] [PubMed] [Google Scholar]
- 28**.Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4(1):29. doi: 10.1186/s40168-016-0172-3. This is an important study which highlights the importance of the use of contamination controls in low biomass samples such as the placenta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D, Hofstaedter CE, Zhao C, Mattei L, Tanes C, Clarke E, et al. Optimizing methods and dodging pitfalls in microbiome research. Microbiome. 2017;5(1):52. doi: 10.1186/s40168-017-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185(5):385–94. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stokholm J, Thorsen J, Chawes BL, Schjorring S, Krogfelt KA, Bonnelykke K, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138(3):881–9. e2. doi: 10.1016/j.jaci.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. 2016;16(1):86. doi: 10.1186/s12876-016-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–93. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 36.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38(4):634–42. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 37.Negele K, Heinrich J, Borte M, Berg A, Schaaf B, Lehmann I, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatric Allergy and Immunology. 2004;15(1):48–54. doi: 10.1046/j.0905-6157.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–3. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017 doi: 10.1001/jamapediatrics.2017.0378. This article demonstrates the significant contribution of bacteria in breast milk and the surrounding areolar skin to the infant microbiome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509(7500):357–60. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neovita Study G. Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. The Lancet Global Health. 2016;4(4):e266–e75. doi: 10.1016/S2214-109X(16)00040-1. [DOI] [PubMed] [Google Scholar]
- 43.Smith ER, Hurt L, Chowdhury R, Sinha B, Fawzi W, Edmond KM, et al. Delayed breastfeeding initiation and infant survival: A systematic review and meta-analysis. PLoS One. 2017;12(7):e0180722. doi: 10.1371/journal.pone.0180722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobin NH, Aldrovandi GM. Immunology of pediatric HIV infection. Immunol Rev. 2013;254(1):143–69. doi: 10.1111/imr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19(7):699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 46.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. The Lancet. 2007;369(9567):1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 47.Piwoz EG, Humphrey JH, Tavengwa NV, Iliff PJ, Marinda ET, Zunguza CD, et al. The impact of safer breastfeeding practices on postnatal HIV-1 transmission in Zimbabwe. Am J Public Health. 2007;97(7):1249–54. doi: 10.2105/AJPH.2006.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359(2):130–41. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tozzi AE, Pezzotti P, Greco D. Does breast-feeding delay progression to AIDS in HIV-infected children? AIDS. 1990;4(12):1293–4. doi: 10.1097/00002030-199012000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Blaymore Bier JA, Oliver T, Ferguson A, Vohr BR. Human milk reduces outpatient upper respiratory symptoms in premature infants during their first year of life. J Perinatol. 2002;22(5):354–9. doi: 10.1038/sj.jp.7210742. [DOI] [PubMed] [Google Scholar]
- 51.Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–25. doi: 10.1542/peds.2008-3256. [DOI] [PubMed] [Google Scholar]
- 52.Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007;(153):1–186. [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer MS, Guo T, Platt RW, Sevkovskaya Z, Dzikovich I, Collet JP, et al. Infant growth and health outcomes associated with 3 compared with 6 mo of exclusive breastfeeding. Am J Clin Nutr. 2003;78(2):291–5. doi: 10.1093/ajcn/78.2.291. [DOI] [PubMed] [Google Scholar]
- 54.Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol. 2004;4(7):565–72. doi: 10.1038/nri1393. [DOI] [PubMed] [Google Scholar]
- 55.Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22(9):1147–62. doi: 10.1093/glycob/cws074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stepans MB, Wilhelm SL, Hertzog M, Rodehorst TK, Blaney S, Clemens B, et al. Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med. 2006;1(4):207–15. doi: 10.1089/bfm.2006.1.207. [DOI] [PubMed] [Google Scholar]
- 57.Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, Aagaard K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol. 2015;212(5):653e1–16. doi: 10.1016/j.ajog.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blaser MJ, Dominguez-Bello MG. The Human Microbiome before Birth. Cell Host Microbe. 2016;20(5):558–60. doi: 10.1016/j.chom.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Bender JM, Li F, Martelly S, Byrt E, Rouzier V, Leo M, et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci Transl Med. 2016;8(349):349ra100. doi: 10.1126/scitranslmed.aaf5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chatterjee A, Bosch RJ, Hunter DJ, Fataki MR, Msamanga GI, Fawzi WW. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-Infected women in Tanzania. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2007;46(5):599–606. doi: 10.1097/QAI.0b013e31815a5703. [DOI] [PubMed] [Google Scholar]
- 61.Omoni AO, Ntozini R, Evans C, Prendergast AJ, Moulton LH, Christian PS, et al. Child Growth According to Maternal and Child HIV Status in Zimbabwe. Pediatr Infect Dis J. 2017;36(9):869–76. doi: 10.1097/INF.0000000000001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adler C, Haelterman E, Barlow P, Marchant A, Levy J, Goetghebuer T. Severe Infections in HIV-Exposed Uninfected Infants Born in a European Country. PLoS One. 2015;10(8):e0135375. doi: 10.1371/journal.pone.0135375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slogrove AL, Cotton MF, Esser MM. Severe Infections in HIV-Exposed Uninfected Infants: Clinical Evidence of Immunodeficiency. Journal of Tropical Pediatrics. 2009;56(2):75–81. doi: 10.1093/tropej/fmp057. [DOI] [PubMed] [Google Scholar]
- 64.Epalza C, Goetghebuer T, Hainaut M, Prayez F, Barlow P, Dediste A, et al. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics. 2010;126(3):e631–8. doi: 10.1542/peds.2010-0183. [DOI] [PubMed] [Google Scholar]
- 65.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. The Lancet. 2007;369(9571):1440–51. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 66.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takeshita T, Matsuo K, Furuta M, Shibata Y, Fukami K, Shimazaki Y, et al. Distinct composition of the oral indigenous microbiota in South Korean and Japanese adults. Sci Rep. 2014;4:6990. doi: 10.1038/srep06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyakht AV, Kostryukova ES, Popenko AS, Belenikin MS, Pavlenko AV, Larin AK, et al. Human gut microbiota community structures in urban and rural populations in Russia. Nat Commun. 2013;4:2469. doi: 10.1038/ncomms3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Guo Z, Xue Z, Sun Z, Zhang M, Wang L, et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 2015;9(9):1979–90. doi: 10.1038/ismej.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tun HM, Konya T, Takaro TK, Brook JR, Chari R, Field CJ, et al. Exposure to household furry pets influences the gut microbiota of infant at 3–4 months following various birth scenarios. Microbiome. 2017;5(1):40. doi: 10.1186/s40168-017-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Luo X, Mao X, Tao Y, Ran X, Zhao H, et al. Gut microbiome analysis of type 2 diabetic patients from the Chinese minority ethnic groups the Uygurs and Kazaks. PLoS One. 2017;12(3):e0172774. doi: 10.1371/journal.pone.0172774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu W, Zhang J, Wu C, Cai S, Huang W, Chen J, et al. Unique Features of Ethnic Mongolian Gut Microbiome revealed by metagenomic analysis. Sci Rep. 2016;6:34826. doi: 10.1038/srep34826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stearns JC, Zulyniak MA, de Souza RJ, Campbell NC, Fontes M, Shaikh M, et al. Ethnic and diet-related differences in the healthy infant microbiome. Genome Med. 2017;9(1):32. doi: 10.1186/s13073-017-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity. 2017;46(1):29–37. doi: 10.1016/j.immuni.2016.12.013. This paper demonstrates that differences in the vaginal microbiome may place women at increased risk for HIV infection as South African women with higher vaginal microbiome diversity had quadruple the number of HIV infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen J. INFECTIOUS DISEASE. Vaginal microbiome affects HIV risk. Science. 2016;353(6297):331. doi: 10.1126/science.353.6297.331. [DOI] [PubMed] [Google Scholar]
- 79.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160(Pt 10):2272–82. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 84.Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci U S A. 2012;109(6):2108–13. doi: 10.1073/pnas.1115621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–7. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jakobsson HE, Jernberg C, Andersson AF, Sjolund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017 doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 93.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–44. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol. 2017;13:3. doi: 10.1186/s13223-016-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gronlund MM, Arvilommi H, Kero P, Lehtonen OP, Isolauri E. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0–6 months. Arch Dis Child Fetal Neonatal Ed. 2000;83(3):F186–92. doi: 10.1136/fn.83.3.F186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39(4):518–26. doi: 10.1111/j.1365-2222.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- 97**.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. doi: 10.1038/nature13421. This study is important as it provides a key model for microbial diversity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta TA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 100.Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. doi: 10.1186/s40168-017-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Localio AR, et al. Antibiotic Exposure During the First 6 Months of Life and Weight Gain During Childhood. JAMA. 2016;315(12):1258–65. doi: 10.1001/jama.2016.2395. [DOI] [PubMed] [Google Scholar]
- 104.Principi N, Esposito S. Antibiotic administration and the development of obesity in children. Int J Antimicrob Agents. 2016;47(3):171–7. doi: 10.1016/j.ijantimicag.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 105.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37(1):16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology. 2016;151(1):120–9. e5. doi: 10.1053/j.gastro.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–26. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 108.Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science. 2016;352(6293):1533. doi: 10.1126/science.aad9359. [DOI] [PubMed] [Google Scholar]
- 109.Krebs NF, Lozoff B, Georgieff MK. Neurodevelopment: The Impact of Nutrition and Inflammation During Infancy in Low-Resource Settings. Pediatrics. 2017;139(Suppl 1):S50–S8. doi: 10.1542/peds.2016-2828G. [DOI] [PubMed] [Google Scholar]
- 110.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013;368(5):425–35. doi: 10.1056/NEJMoa1202851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111**.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7(276):276ra24. doi: 10.1126/scitranslmed.aaa4877. This is a significant study that shows specific bacteria bound to IgA from malnourished children when transferred to germ-free mice causes enteropathy which can be prevented when IgA-bound bacteria are administered from the stools of healthy children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339(6119):548–54. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275) doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW, et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med. 2017;9(390) doi: 10.1126/scitranslmed.aal4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trehan I, Kelly P, Shaikh N, Manary MJ. New insights into environmental enteric dysfunction. Arch Dis Child. 2016;101(8):741–4. doi: 10.1136/archdischild-2015-309534. [DOI] [PubMed] [Google Scholar]
- 116.Kelly P, Besa E, Zyambo K, Louis-Auguste J, Lees J, Banda T, et al. Endomicroscopic and Transcriptomic Analysis of Impaired Barrier Function and Malabsorption in Environmental Enteropathy. PLoS Negl Trop Dis. 2016;10(4):e0004600. doi: 10.1371/journal.pntd.0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374(9694):1032–5. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 118.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 119.Jung Lee W, Lattimer LD, Stephen S, Borum ML, Doman DB. Fecal Microbiota Transplantation: A Review of Emerging Indications Beyond Relapsing Clostridium difficile Toxin Colitis. Gastroenterol Hepatol (N Y) 2015;11(1):24–32. [PMC free article] [PubMed] [Google Scholar]
- 120.Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol. 2012;5(6):403–20. doi: 10.1177/1756283X12453637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsiao K-C, Ponsonby A-L, Axelrad C, Pitkin S, Tang MLK, Burks W, et al. Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomised, double-blind, placebo-controlled trial. The Lancet Child & Adolescent Health. 2017 doi: 10.1016/S2352-4642(17)30041-X. [DOI] [PubMed] [Google Scholar]
- 122.Nicolucci AC, Hume MP, Martinez I, Mayengbam S, Walter J, Reimer RA. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology. 2017;153(3):711–22. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 123.Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548(7668):407–12. doi: 10.1038/nature23480. [DOI] [PubMed] [Google Scholar]
- 124.Dilli D, Aydin B, Fettah ND, Ozyazici E, Beken S, Zenciroglu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr. 2015;166(3):545–51. e1. doi: 10.1016/j.jpeds.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 125.Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic Effects on Late-onset Sepsis in Very Preterm Infants: A Randomized Controlled Trial. Pediatrics. 2013;132(6):1055–62. doi: 10.1542/peds.2013-1339. [DOI] [PubMed] [Google Scholar]
- 126.Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011;(3):CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 127.Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. The Lancet. 2016;387(10019):649–60. doi: 10.1016/S0140-6736(15)01027-2. [DOI] [PubMed] [Google Scholar]
- 128.Sinha A, Gupta SS, Chellani H, Maliye C, Kumari V, Arya S, et al. Role of probiotics VSL#3 in prevention of suspected sepsis in low birthweight infants in India: a randomised controlled trial. BMJ Open. 2015;5(7):e006564. doi: 10.1136/bmjopen-2014-006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barratt MJ, Lebrilla C, Shapiro HY, Gordon JI. The Gut Microbiota, Food Science, and Human Nutrition: A Timely Marriage. Cell Host Microbe. 2017;22(2):134–41. doi: 10.1016/j.chom.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.World Health Organization Human Reproduction Programme A. WHO Statement on caesarean section rates. Reprod Health Matters. 2015;23(45):149–50. doi: 10.1016/j.rhm.2015.07.007. [DOI] [PubMed] [Google Scholar]