Abstract
In this study, we investigated the effects of a newly synthesized α-galacto-oligosaccharide mixture (α-GOSg), 0.5% in drinking water, on high-fat/western-style diet (HFWD)-induced metabolic abnormality in mice in a study of 13 weeks. Raffinose family oligosaccharides (RFOs) were included as a comparison. Mice treated with α-GOSg had significantly lower body weight and body fat (p < 0.05), while RFOs were less effective. Both α-GOSg and RFOs significantly reduced serum levels of total cholesterol and low-density lipoprotein cholesterol, alanine aminotransferase and liver lipids. However, only α-GOS significantly decreased the histopathological score for liver steatosis and downregulated hepatic fatty acid synthesis gene acetyl CoA carboxylase-α. α-GOSg also significantly reduced the content of bile acids in the small intestine and significantly increased the abundance of gut Bifidobacterium and decreased the abundance of Clostridium leptum. These actions are proposed to be key mechanisms contributing to the beneficial health effects of α-GOSg.
Keywords: Metabolic syndrome, α-Galacto-oligosaccharides, Liver steatosis, Intestinal microflora
Graphic abstract
A newly synthesized α-galacto-oligosaccharide mixture (α-GOSg) alleviated HFWD-induced metabolic disorders mainly through the regulation of the intestinal environment.
1. Introduction
Galacto-oligosaccharides (GOS), one of the most commonly used prebiotics, contain a mixture of oligosaccharides formed by one to ten galactosyl moieties linked to a terminal glucose or galactose.1, 2 As non-digestible oligosaccharides, they can be utilized by gut microflora and modify the composition of gut microbiota.3 GOS have been reported to enhance the growth of beneficial bacteria (such as bifidobacterium and lactobacillum) and suppress the growth of pathogenic and putrefactive bacteria.4–6 In addition to being prebiotics, GOS exhibit other functions, such as preventing constipation, reducing the level of blood cholesterol, improving mineral absorption and alleviating certain acute or chronic diseases.7–10 In this study, a newly synthesized α-galacto-oligosaccharides mixture11 (designated as α-GOSg), derived from galactose (Gal), was investigated together with the naturally occurring raffinose family oligosaccharides (RFOs) as a comparison. RFOs usually exist in seeds from different plant families. Having a structure with 1–3 galactose units linked to a sucrose molecule, RFOs are typical α-GOS.12 RFOs have been shown to promote the growth of beneficial bacteria and immunomodulation and to alleviate metabolic abnormalities.5,13 Our previous study indicated that the α-GOSg was effective in preventing DSS-induced colitis than RFOs.14 In the present study, the effects of the newly synthesized α-GOSg on high-fat diet induced metabolic abnormities were investigated with RFOs included as a comparison. Our hypothesis is that α-GOSg alleviates metabolic abnormalities by altering gut microbiota, bile acid and lipid metabolism, or the expression of specific genes.
Metabolic syndrome is a set of disorders that include central obesity, hyperglycemia, insulin resistance, hyperlipidemia and hypertension.15 The development of metabolic syndrome is a complex process that involves genetic, environmental, and dietary factors. In the present study, metabolic syndrome was induced in mice with a high-fat western-style diet (HFWD). This diet was modified from the “new Western-style diet” developed by Newmark et al,16, 17 which mimics intake levels of nutrients that are major dietary risk factors for human colon cancer and other diseases in Western countries (higher fat combined with lower calcium, vitamin D3, fiber and one-carbon donors). The presently used HFWD was modified from the “new Western-style diet” by increasing the fat content from 40% to 60% of the total calories and was used previously in our laboratory to induce obesity and metabolic syndrome.16
Accumulating evidence suggests that dysbiosis of gut microbiota induced by a high fat/high calorie diet plays a key role in the development of obesity, insulin resistance and other hallmarks of metabolic syndrome.18,19 Reduction in beneficial bacteria and increases in pro-inflammatory/pathogenic bacteria are consistently associated with the development of obesity, adipose tissue and systemic inflammation, and metabolic comorbidities in both humans and rodents.20, 21 Currently, low-digestible carbohydrates have been widely studied and many are shown to be effective prebiotic factors. They are highly selective for the growth of Bifidobacterium and Lactobacillus, produce short-chain fatty acids and play a beneficial role in alleviating metabolic and inflammatory disorders such as obesity, diabetes and inflammatory bowel diseases.22–24 However, the effects of the newly synthesized α-GOSg in the prevention of metabolic syndrome were not known. This study aimed to investigate the effects of α-GOSg on HFWD-induced metabolic abnormalities.
2. Materials and Methods
2.1. Chemicals and diets
Low-fat diet (LF; 10% energy as fat) and HFWD (60% energy as fat, reduced levels of calcium, vitamin D3, choline, folate, and fiber)16 was prepared by Research Diets Inc. (New Brunswick, NJ). RFOs (containing 81.2% of raffinose, stachyose and verbascose, 16.5% of sucrose and 2.3% of ash) were purchased from Nanjing Duolong Bio-tech Co., Ltd (Nanjing, China). α-GOSg was enzymatically synthesized in our laboratory at Nanjing Agricultural University as described below.
2.2. Preparation of α-galactooligosaccharides
α-GOSg was synthesized from Galactose using α-galactosidase to catalyze the oligomerization. Galactose was dissolved in distilled water (96%, w/v) by autoclaving at 121°C for 20 min and then transferred to an incubator at a reaction temperature of 60°C. The reaction was started by the addition of α-galactosidase (35 U/g galactose). At interval, the reaction mixture was heated to 100°C for 10 min to inactivate the enzyme. The solution was loaded onto an activated carbon column (2.5 × 20 cm). The column was washed with 4% ethanol to elute galactose, and then eluted with 20% ethanol. The eluates containing the reaction products were collected, concentrated, and freeze-dried to yield a white powder. The α-GOSg contained 76.6% Gal2 (46.8% as Gal-α−1–6-Gal), 14.4% Gal3 (6.3% as Gal- α−1–6-Gal-α−1–6-Gal) and 10% free galactose.11
2.3. Animal studies
Male C57BL/6J mice (8 weeks old, 20–22 g) were obtained from Jackson Laboratories (Bar Harbor, ME). All animal experiments were carried out under protocol 91–024 approved by the Institutional Animal Care and Use Committee at Rutgers University (Piscataway, NJ). The mice were maintained in shoe-box cages in a controlled room (temperature 24 to 25°C, humidity 70%–75%, lighting regimen of 12-hour light-dark cycles), with free access to food and water. Mice were randomly divided into four groups LFD, HFWD, HFWD + α-GOSg, and HFWD + RFOs (n = 10 per group); they were maintained on the LFD or HFWD for 13 weeks. In α-GOSg and RFOs treated groups, mice were given α-GOSg or RFOs in drinking water (5 mg/ml). During the experiment, body weight, food consumption and water consumption were recorded weekly. Blood glucose levels were measured on weeks 0, 4, 8, and 12. Tail vein blood and fresh stool samples were collected on weeks 0, 4, 8, 10 and 12.
After treatment for 13 weeks, mice were sacrificed by CO2 asphyxiation. Blood was collected by cardiac puncture; plasma samples were prepared and stored at −80°C. Gallbladder was removed and stored at −80°C. Liver was quickly removed, one lobe of the liver was fixed in 10% formalin, one lobe was stored in RNA later solution (Ambion, NJ), and the remaining was frozen in dry ice and stored at −80°C. Small intestine was collected and stored in −80°C. Interscapular brown adipose tissue (BAT) and visceral white adipose tissues (WAT) (mesenteric, epididymal, and retroperitoneal depots) were collected and weighed.
2.4. Biochemical analyses of blood samples
Blood glucose levels were measured every 4 weeks using an Ascensia Contour Blood Glucose Meter (Bayer Healthcare LLC, Mishawaka, IN). Mice were fasted overnight (from 9:00 p.m. to 9:00 a.m.) before measurement; cage bedding was changed at 9:00 p.m. to avoid coprophagy. Blood samples were collected from the tail vein for serum preparation. ALT levels were determined using an ALT Discrete Pak Kit (Catachem Inc., Bridgeport, CT). Serum total cholesterol (TC) levels were determined using a Cholesterol Kit (Pointe Scientific, Inc., Canton, MI). Serum triglyceride (TG) levels were measured using a Triglycerides Liquid Kit (Pointe Scientific, Inc., Canton, MI). Serum HDL levels were measured using a Liquid autoHDL Cholesterol Kit (Pointe Scientific, Inc., Canton, MI). Serum LDL levels were calculated from the levels of cholesterol, triglyceride, and HDL using a formula: LDL=TC-HDL-TG/5.25 Serum insulin levels were measured using a Rat/Mouse Insulin ELISA Kit (Millipore Corporation, Billerica, MA), and insulin resistance was calculated according to the homeostasis assessment model (HOMA-IR).26 Total bile acid levels in serum samples were measured using a Total Bile Acid Assay Kit (Diazyme Laboratories, Poway, CA, USA)
2.5. Liver histology
Paraffin-embedded liver sample was sectioned at 4 μm thickness and stained with hematoxylin and eosin (H&E). Two H&E-stained sections (~30 μm apart) per liver were used for steatosis evaluation in a blind manner. Liver steatosis histopathological score was based on the sum of four parameters: macrovesicular steatosis, microvesicular steatosis, hepatocellular hypertrophy, and inflammation.27 Macrovesicular steatosis, microvesicular steatosis and hepatocellular hypertrophy were separately scored and the severity in each category was graded, based on the percentage of the total area affected, into the following categories: 0 (<5%), 1 (5–33%), 2 (34–66%) and 3 (>66%). Inflammation was evaluated by counting the number of inflammatory foci per field using a 100 × magnification. Five different fields were counted and the average was scored into the following categories: normal (<0.5 focus), slight (0.5–1.0 focus), moderate (1.0–2.0 foci), severe (>2.0 foci).
2.6. Lipid analysis
The analysis of total lipid levels was performed according to a reported method.28 In brief, 100–150 mg liver or stool samples were homogenized in 1 mL of buffer containing 18 mM Tris HCl, pH 7.5, 300 mM mannitol, 50 mM EGTA, and 0.1 mM phenylmethysulfonyl fluoride (PMSF). The homogenate (400 μl) was mixed with 2 mL of chloroform-methanol (2:1) and shaken overnight at room temperature. Then, 1 mL distilled water was added; the mixture was vortexed and centrifuged for 5 min at 3000 × g. The lower lipid phase was then collected and dried in a speedy vacuum concentrator. The lipid pellet was dissolved in a mixture of 60 μL of tert-butanol and 40 μL of Triton X-114: methanol (2:1) mix. Triglyceride and cholesterol levels were measured using the commercial kits as described above.
2.7. Measurement of bile acid pool size
The total bile acid pool size was determined as bile acid contents in the small intestine, gallbladder and liver. In brief, liver tissues (100–150 mg) were weighed, homogenized in 0.5 mL PBS, and mixed with 1 mL ethanol. The mixture was rotate mixed for 1 h and then centrifuged at 3500 rpm for 5 min. The supernatant was transferred to a new tube; the pellet was extracted for bile acid with 1.5 mL ethanol (rotating for 1 h) again. The supernatant was combined and stored at −20℃. The concentrations of bile acids were determined using a Total Bile Acid Assay Kit (Diazyme Laboratories, Poway, CA). The entire small intestine sample and gallbladder sample were each homogenized with 3 mL PBS and extracted with 3 mL ethanol for bile acid as described above. The pool size was expressed as micromoles of bile acid/100 g of body weight.
2.8. Analysis of liver gene expression
Total RNA was extracted from the liver using a Rneasy Mini Kit (Qiagen Inc., Valencia, CA). The content of RNA was estimated by measuring the absorbance at 260 nm. Purified total RNA was reverse-transcribed using the SuperScript First-Strand Synthesis System for RT-PCR Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s protocol. Primer sequences are shown as Table 1. Real-time PCR was performed by using an RT-PCR system (ABI ViiA™ 7 system) and Power SYBR® Green PCR Master Mix Kit (ABI Co., Ltd. Foster City, CA). In brief, the reaction was initiated by a 5 min activation at 95°C followed by 40 cycles of target cDNA amplification (15 s denaturing at 95°C and 35 s elongation at 60°C). All mRNA expression was normalized against GAPDH expression. The expression level of the target gene was calculated by the 2− ΔΔCT method.
Table 1.
Primer Sequences Used in Real-Time PCR (RTqPCR) Assays
| Gene | Sequence (5’−3’) | Annealing temperature (°C) |
|---|---|---|
| ACC-α | F:AGGAGGGAAAGGGATCAGAAAAG | 60 |
| R:CAGAGCAGTCACGACCAAACAAA | ||
| FAS | F:CTGAGATCCCAGCACTTCTTGA | 60 |
| R:GCCTCCGAAGCCAAATGAG | ||
| CPT-1 | F:CATCCACGCCATACTGCT | 60 |
| R:GACCTTGAAGTAACGGCCTC | ||
| GAPDH | F:AGGTCGGTGTGAACGGATTTG | 60 |
| R:TGTAGACCATGTAGTTGAGGTCA |
2.9. Analysis of fecal microbial species
Total DNA was extracted from fresh fecal microbial samples according to the manufacturer’s instructions of a TIANamp Stool DNA Kit (Tiangen Biotechnology Co., Ltd., Beijing, China). The resulting DNA samples were assessed spectrophotometrically using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The specific primers for the 16S rRNA gene targeting group of different intestinal microbial species are shown in Table 2.6 The qPCR reaction was performed by using an RT-PCR system (ABI ViiA™ 7 system) according to the instructions of the Power SYBR® Green PCR Master Mix Kit (ABI Co., Ltd. Foster City, CA). The reaction was initiated by a 5 min activation at 95°C followed by 40 cycles of target cDNA amplification (15 s denaturing at 95°C and 35 s elongation at 60°C). The standard DNA template was used for quantification of target DNA copy number. In brief, a series of 10 times gradient dilutions of the standard products was used and at least six non-zero standard concentrations per assay were applied. The concentration of the target flora was expressed as log10 copy number. Each reaction was carried out in triplicate.
Table 2.
Group-specific Primers Based on 16S rRNA Sequences Used for qPCR
| Target bacterial group | Sequence (5’−3’) | PCR product size (bp) |
|---|---|---|
| All bacteria | F: ACTCCTACGGGAGGCAGCAG | 192 |
| R: ATTACCGCGGCTGCTGG | ||
| Bacteroides | F: GAGAGGAAGGTCCCCCAC | 108 |
| R: CGCTACTTGGCTGGTTCAG | ||
| Bifidobacterium | F: CGCGTCYGGTGTGAAAG | 244 |
| R: CCCCACATCCAGCATCCA | ||
| Lactobacillus | F: GAGGCAGCAGTAGGGAATCTTC | 126 |
| R: GGCCAGTTACTACCTCTATCCTTCTTC | ||
| Clostridium coccoides /Eubacterium rectale group | F: AAATGACGGTACCTGACTAA | 242 |
| R: CTTTGAGTTTCATTCTTGCGAA | ||
| Clostridium leptum group | F: GCACAAGCAGTGGAGT | 441 |
| R: CTTCCTCCGTTTTGTCAA |
2.10. Statistical Analysis
Statistical analysis was performed using SPSS software version 22.0. Data were presented as mean ± SD. Data were analyzed using one-way ANOVA and Student’s t-test. The level of statistical significance was set at p < 0.05.
3. Results
3.1. Body weight and body fat
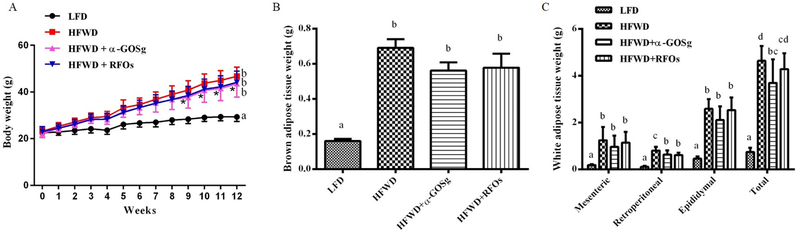
The average food consumption of LFD, HFWD, HFWD + α-GOSg, and HFWD + RFOs groups were 2.85 ± 0.22, 2.66 ± 0.13, 2.72 ± 0.20, and 2.57 ± 0.22 g/day per mouse, respectively. There was no significant difference in food consumption among the four groups throughout the experiment. The average water consumption of the four groups were respectively 3.51 ± 0.26, 3.15 ± 0.21, 3.25 ± 0.32, and 3.11 ± 0.32 ml/day per mouse. The water consumption was lower in mice fed the HFWD, and supplementation with α-GOSg or RFOs did not produce a significant effect. Mice in HFWD groups showed significantly higher body weight gain compared to the LFD group as shown in Figure 1A, but no significant differences were shown among the three HFWD-fed groups by ANOVA. However, the body weights of HFWD + α-GOSg group were lower than the HFWD group after week 9 based on Student’s t-test. Consistent with the effects on body weight, HFWD also significantly increased the weights of BAT and total WAT, including mesenteric, epididymal and retroperitoneal adipose tissues (p < 0.05, Figure 1B, 1C). Both α-GOSg and RFOs significantly decreased the weight of retroperitoneal adipose tissues (p < 0.05). Furthermore, α-GOSg significantly reduced the total WAT weight (p < 0.05).
fig. 1.
effects of α-gosg and rfos on body weight (a), brown adipose tissue weight (b) and white adipose tissue weight (c). white adipose tissue including mesenteric, epididymal, retroperitoneal, and total white adipose tissue. data are expressed as mean ± sd, n=10 for all groups. a-d represent significant differences among different groups (p < 0.05) by anova. for a, significant difference between the lfd group and the hfwd-fed groups was observed in all data points after week 1; * represents significant difference between hfwd group and hfwd + α-gosg group (p < 0.05) by student’s t-test observed after week 9.
3.2. Blood levels of glucose and insulin
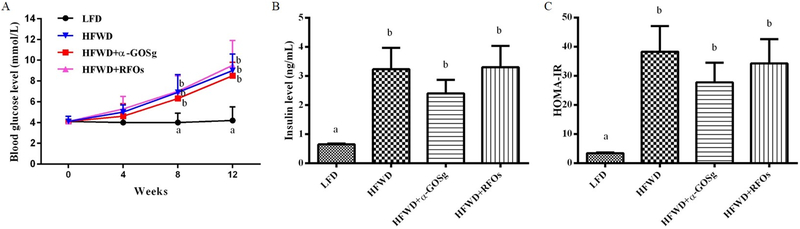
The changes in fasting blood glucose levels are shown in Figure 2A. HFWD fed mice significantly increased blood glucose levels after week 4 (p < 0.05) and continued to increase afterwards; while the blood glucose level of the LFD group stayed at the same value. At the end of the experiment, mice from the HFWD group showed blood glucose level 116% higher than that of the LFD group. Nevertheless, the blood glucose level was not significantly affected by the treatment with α-GOSg or RFOs. HFWD also significantly increased insulin level and insulin resistance (p < 0.05, Figure 2B, 2C). α-GOSg appeared to reduce insulin level and HOMA-IR (by 26–28%); however, the difference was not statistically significant.
fig. 2.
effects of α-gosg and rfos on fasting blood glucose level (a), insulin level (b) and homa-ir (c). data are expressed as mean ± sd, n=10 for all groups. a,b represents significant differences between different groups (p < 0.05) by anova.
3.3. Serum levels of alt, cholesterol and triglycerides
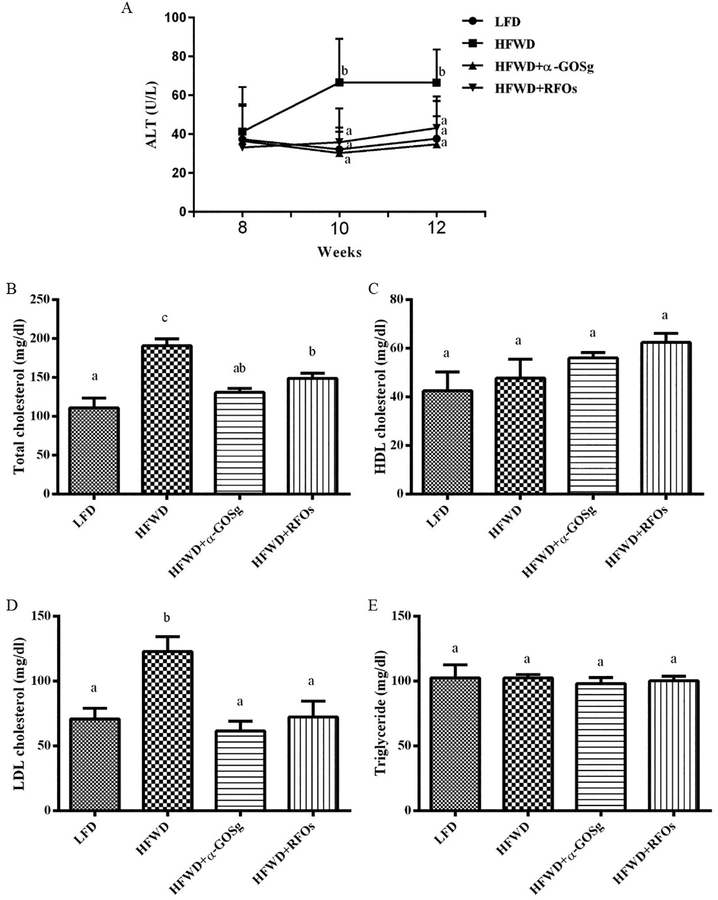
Serum ALT levels were measured every two weeks after week 8 (Figure 3A). The ALT levels were almost the same among the four groups on week 8. However, the ALT levels of mice in the HFWD group were significantly elevated by week 10 (p < 0.05). Both α-GOSg and RFOs significantly decreased ALT levels to the level of the LFD group on weeks 10 and 12.
fig. 3.
effects of α-gosg and rfos on serum alt levels (a), total cholesterol level (b), hdl cholesterol level (c), ldl cholesterol level (d), and triglyceride level (e). the alt levels were measured every 2 weeks after week 8. other parameters were measured at week 13 after sacrifice. data are expressed as mean ± sd, n=10 for all groups. a-c represents significant differences among different groups (p < 0.05) of the same week by anova.
The serum cholesterol and triglyceride concentrations were measured at the end of the experiment on week 13 (Figure 3B–E). The serum LDL cholesterol and total cholesterol levels of the HFWD fed mice were significantly higher than those fed the LFD (p < 0.05). Treatments with α-GOSg and RFOs significantly decreased serum total cholesterol and LDL cholesterol levels (p < 0.05). However, there was no significant difference in HDL cholesterol and triglyceride levels among the four groups.
3.4. Liver steatosis
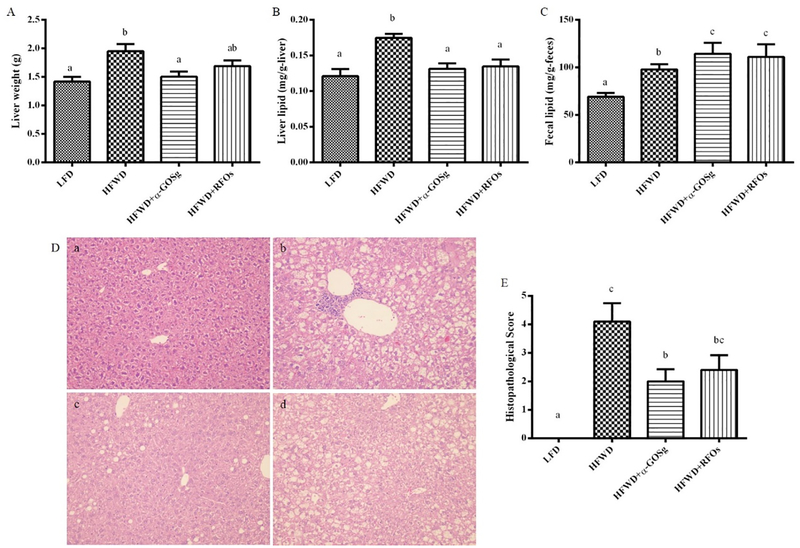
At the time of sacrifice, the liver was quickly removed and weighted. Mice fed the HFWD showed significant increased liver weight (p < 0.05) and α-GOSg treatment significantly reduced the liver weight (p < 0.05), while RFOs treatment did not significantly change the liver weight (Figure 4A). Biochemical analysis revealed that the liver lipid content of the HFWD group was significantly higher than the other three treatment groups (p < 0.05, Figure 4B).
fig. 4.
effects of α-gosg and rfos on hfwd-induced liver steatosis. (a) liver weight. (b) liver lipid content. (c) fecal lipid content. (d) histological sections of liver tissue stained with hematoxylin and eosin: (a) lfd group; (b) hfwd group; (c) hfwd + α-gosg group; (d) hfwd + rfos. (e) liver steatosis histopathological score of each group. data are expressed as mean ± sd, n=10 for all groups. a-c represents significant differences among different groups (p < 0.05) by anova.
The development of fatty liver was also examined after sacrifice. As compared to mice fed the LFD, all 10 mice fed the HFWD had enlarged liver, pink in color with white spots on the surface. Interestingly, only 2 of 10 mice in the α-GOSg group, and 4 of 10 mice in the RFOs group showed features of fatty liver. Histopathological analysis showed increased lipid deposition in the livers of HFWD fed mice (Figure 4D). In all livers, the cytoplasm of the centrilobular hepatocytes showed microvesicular steatosis (the presence of numerous small lipid droplets) as well as macrovesicular steatosis (the presence of large lipid droplets). Clusters of inflammatory cells were also observed. In the α-GOSg-treated group, the liver showed slight fatty degeneration, with the average percentage of microvesicular steatosis and macrovesicular steatosis less than 33%. Livers of several mice from this group showed a markedly attenuated degree of liver steatosis (compared to those of the HFWD group), with the fatty change primarily consisting of the accumulation of widely scattered large lipid droplets in the centrilobular zone, with little evidence of microvesicular involvement. Thus, α-GOSg treatment significantly reduced the severity of fatty liver (p < 0.05) (Figure 4E). RFOs appeared to be less effective; mice showed moderate fatty degeneration, with appearance of microvesicular steatosis in the liver.
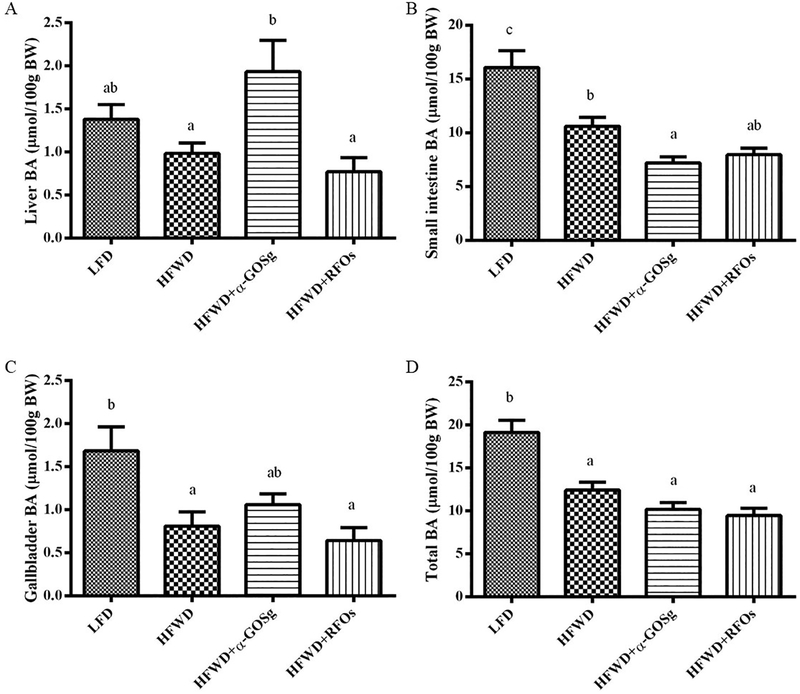
3.5. Bile acid pool size
In HFWD-fed mice, the bile acid levels in gallbladder and small intestine were significantly lower (p < 0.05) than those of the LFD group (Figure 5). Both α-GOSg and RFOs did not significantly change the total bile acid pool size, and RFOs did not significantly change the tissue distribution of bile acids. However, α-GOSg significantly increased the hepatic bile acid level and decreased the small intestinal bile acid level (p < 0.05), which may be caused by obstruction of bile flow. However, we do not have evidence for this possibility.
fig. 5.
effects of α-gosg and rfos on bile acid pool size. data are expressed as mean ± sd, n=10 for all groups. the bile acid pool size was expressed as micromoles of bile acid/100 g of body weight. a-c represents significant differences among different groups (p < 0.05) by anova.
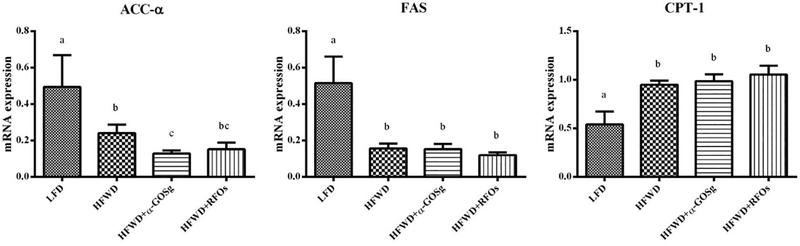
3.6. Expression of metabolic genes
In the liver samples of the HFWD group, the mRNA levels of acetyl CoA carboxylase (ACC)-α and fatty acid synthase (FAS) genes were significantly down-regulated, while the expression of carnitine palmitoyl transferase (CPT)-1 was significantly up-regulated (p < 0.05, Figure 6). α-GOSg further significantly attenuated the expression of ACC-α (p < 0.05). The two oligosaccharides, however, had no effects on the expression of FAS and CPT-1.
fig. 6.
effects of α-gosg and rfos on expression of hepatic lipid metabolism genes. the relative expression levels of genes in the liver tissue are expressed as mean ± sd, n=10 for all groups. gapdh was used as an internal control for normalizing the mrna level. the results were repeated in at least 3 independent experiments. a-c represents significant differences among different groups (p < 0.05) by anova.
3.7. Fecal lipids and gut microbiota
The lipid contents in the fecal samples of mice treated with α-GOSg and RFOs were significantly higher than those of the HFWD group (p < 0.05, Figure 4C), suggesting that α-GOSg and RFOs treatment decreased the absorption of lipid.
The shift of specific gut microflora is shown in Table 3. The total amount of intestinal bacteria did not change with the dietary treatment for 13 weeks, but the structure of intestinal microflora shifted. The mice fed the HFWD showed significant less amount of Bifidobacterium (p < 0.05). α-GOSg treatment significantly increased the quantity of Bifidobacterium and decreased the quantity of Clostridium leptum, while RFOs only decreased the quantity of Clostridium leptum (p < 0.05). No significant differences were observed on the amount of Bacteroides and Eubacterium rectale among the different groups.
Table 3.
Effects of different treatments on colonic microbiota composition
| Bacterial group | Treatments | |||
|---|---|---|---|---|
| LFD | HFWD | HFWD + α-GOSg | HFWD + RFOs | |
| All bacteria | 11.79 ± 0.21 a | 11.64 ± 0.38 a | 11.50 ± 0.08 a | 11.24 ± 0.68 a |
| Bacteroides | 11.03 ± 0.13 a | 10.95 ± 0.26 a | 10.77 ± 0.08 a | 10.57 ± 0.56 a |
| Bifidobacterium | 9.34 ± 0.25 c | 7.78 ± 0.37 a | 8.46 ± 0.23 b | 8.31 ± 0.51 ab |
| Lactobacillus | 8.23 ± 0.22 a | 7.93 ± 0.21 a | 8.29 ± 0.19 a | 8.32 ± 0.28 a |
| Clostridium coccoides/ Eubacterium rectale group | 9.88 ± 0.40 a | 9.61 ± 0.34 a | 9.57 ± 0.28 a | 9.45 ± 0.61 a |
| Clostridium leptum group | 9.51 ± 0.35 ab | 10.02 ± 0.30 b | 9.18 ± 0.29 a | 9.04 ± 0.66 a |
Data are expressed as log10 copy number/g of freeze-dried fecal sample.
represent significant differences among different groups of certain bacterial group (p < 0.05).
4. Discussion
In this study, mice fed the HFWD had excessive weight gain and higher levels of serum total cholesterol, glucose and ALT, as well as increased insulin resistance, as compared to the LFD group. It also led to hepatic steatosis with the cytoplasm of the centrilobular hepatocytes showed microvesicular and macrovesicular steatosis. The HFWD had a higher fat content but insufficient amount of calcium, vitamin D3, and methyl-donor nutrients. Methyl-donor nutrients such as choline are essential for lipid transport and metabolism; deficiency of methyl donor nutrients is known to promote the development of hepatic steatosis and result in elevated ALT levels in the blood.29 Our results are consistent with previous reports16 and demonstrate that the HFWD affords a robust model of metabolic syndrome and hepatic steatosis.
The present results provide direct evidence showing that α-GOSg significantly decreased body weight, total adipose tissue weight and liver steatosis, as well as down regulated the hepatic expression of ACC-α. ACC-α catalyzes the first committed step in fatty acid biosynthesis.30 Our result suggests that α-GOSg may inhibit de novo fatty acid biosynthesis. RFOs also significantly decreased weight of the retroperitoneal fat and the levels of liver lipid, serum cholesterol and serum ALT. At the dose used, α-GOSg was more effective than RFOs in preventing many of the deleterious effects induced by HFWD. In structure, α-GOSg is only composed of galactose and more than 50% is composed with α−1–6-galactoside linkages, while RFOs end with the structure of Gul-β−1–2-Fru. Structural differences of oligosaccharides could influence their biological activities.11,31–32 Sanz et al.33 compared the influence of different glycosidic linkages and monosaccharide compositions of disaccharides on the selectivity of microbial fermentation, and found that disaccharides with 1–2, 1–4, and 1–6 linkages were more effective than those with 1–1, 1–3 and 1–5 linkages in promoting the proliferation of bifidobacteria and lactobacilli. Fructose-containing disaccharides usually had lower prebiotic indexes than galactobiose.33 Another study showed that glycosidic with 1–6 and 1–2 linkages between galactose and glucose monomers were significantly more resistant to gastrointestinal digestion than 1–4 linkage.34 Our previous work also indicated that α-GOS linked with α-(1→6)-galactosidic linkage had better immunomodulatory effect than other linkage types.11 However, the structural basis for the higher activities of α-GOSg (than RFOs) remains to be elucidated. Another issue is that the preparation of RFOs contained 16.5% free sucrose and the preparation of GOSg contained 10% free galactose. It is unclear whether the presence of these disaccharide and monosaccharide would affect the relative strength of the α-GOSg and RFOs.
Many reports suggested that diet-induced obesity is associated with alterations in gut microbiota with decreased lactobacilli (beneficial microbial genera), leading to increased metabolic syndrome.17,35 Animal studies and human trials have shown that some probiotics effectively lowered hepatic steatosis, colon inflammation, blood glucose level and insulin concentrations.15,36 Oligosaccharides are prebiotics, which have been reported to increase the abundance of health-promoting bacteria such as bifidobacteria and lactobacilli.3,5 In this study, species that are more responsive to oligosaccharides and related to metabolic abnormities were characterized. We found that α-GOSg significantly increased the quantity of Bifidobacterium and decreased that of Clostridium leptum. While RFOs only significantly reduced the amount of Clostridium leptum. Bifidobacterium has been shown to ameliorate visceral fat accumulation and insulin sensitivity of the mice fed high fat diet.37 Reduction of Clostridium leptum has been shown to be associated with the alleviation of metabolic abnormalities.38 The shift of these microbial species by α-GOSg may help alleviate some metabolic abnormities. However, there were some inconsistencies between our results and previous reports. Bifidobacterium and Lactobacillus have shown to be enriched by RFOs.5, 39–40 In our study, in comparison to the HFWD group, RFOs group had higher quantities in Bifidobacterum (8.31 ± 0.51 vs. 7.78 ± 0.37) and Lactobacillus (8.32 ± 0.28 vs. 7.93 ± 0.21). However, they were not statistically different. One possibility is that these bacteria were increased by RFOs, but our data did not reach statistical significance. Another possibility is that in our experimental system, RFOs did not significantly increase these bacteria. The previously reported increase of these bacteria by RFOs were mainly obtained from in vitro fermentation experiment and one study in mice with a commercial oligosaccharide mixture “Deshipue stachyose granules”.5, 39–40
Bile acids are produced in hepatocytes, stored in the gallbladder, and released into the duodenum upon ingestion of a meal to facilitate absorption of triglycerides, cholesterol and fat-soluble vitamins.41 Gut microbiota may influence bile acid metabolism by promoting deconjugation, dehydrogenation, and hydroxylation of primary bile acids in the distal small intestine and colon42 and affect the level of bile acids in the small intestine. The lower levels of bile acids in the small intestine of α-GOSg-treated mice, as we observed, could affect the formation of micelles and decrease the adsorption of lipids, resulting in more lipid excretion in feces.42, 43 Decreased lipid absorption could also lead to the lower levels of blood lipids and hepatic steatosis. Therefore, modulating the composition of intestinal microbes and their metabolism of bile acids may play a central role in the alleviation of metabolic syndrome by α-GOSg. α-GOSg may also directly affect the metabolism of cholesterol, bile acids and lipids. Further studies on these mechanisms are needed.
In conclusion, our study demonstrates for the first time that α-GOSg administration attenuated HFWD-induced metabolic disorders, including decreased body fat weight and levels of LDL cholesterol and TC. It also affected bile acid metabolism and decreased lipid absorption, hepatic expression of ACC-α, liver lipid content and the severity of liver steatosis. On the other hand, RFOs were less or not effective in affecting these parameters. Administration of α-GOSg also increased the abundance of the beneficial bacteria Bifidobacterium and decreased that of the deleterious bacteria Clostridium leptum; whereas the RFOs decreased Clostridium leptum but not significantly increased Bifidobacterium. The beneficial effects of α-GOSg on metabolic syndrome may be due to that 1) α-GOSg increase the abundance of beneficial gut bacteria and suppress deleterious gut bacteria, 2) α-GOSg decreases lipid absorption by decreasing intestinal BA content, and 3) α-GOSg inhibits the de novo fatty acid biosynthesis process by downregulating ACC-α gene. The possible beneficial health effects of α-GOSg in humans remain to be investigated.
Acknowledgements
This work was supported by grants from the U.S. National Institutes of Health (CA120915 and shared facilities funded by CA72720 and ES05022), the John L. Colaizzi Chair Endowment fund, Grants-in-Aid for scientific research from the National Natural Science Foundation of China (31171750 and 31801541), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Abbreviations
- ACC
acetyl CoA carboxylase
- α-GOSg
α-galacto-oligosaccharide mixture
- BAT
brown adipose tissue
- CPT
carnitine palmitoyl transferase
- FAS
fatty acid synthase
- GOS
galacto-oligosaccharides
- Gal
galactose
- Fru
fructose
- H&E
hematoxylin and eosin
- HFWD
high-fat/western-style diet
- HOMA-IR
homeostasis assessment model
- LF
low-fat diet
- PMSF
phenylmethysulfonyl fluoride
- RFOs
raffinose family oligosaccharides
- TC
total cholesterol
- TG
triglyceride
- WAT
white adipose tissues
Footnotes
Conflict of interest
The authors have declared no conflict of interest.
References
- 1.Moreno FJ and Sanz ML, Food oligosaccharides: production, analysis and bioactivity, John Wiley & Sons, 2014. [Google Scholar]
- 2.Park AR and Oh DK, Galacto-oligosaccharide production using microbial beta-galactosidase: current state and perspectives, Appl Microbiol Biot, 2010, 85, 1279–1286. [DOI] [PubMed] [Google Scholar]
- 3.Torres DPM, Gonçalves M. d. P. F., Teixeira JA and Rodrigues LR, Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics, Compr Rev Food Sci Food Saf, 2010, 9, 438–454. [DOI] [PubMed] [Google Scholar]
- 4.Fernando WM, Hill J, Zello G, Tyler R, Dahl W and Van Kessel A, Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults, Benef microbes, 2010, 1, 197–207. [DOI] [PubMed] [Google Scholar]
- 5.Ehara T, Izumi H, Tsuda M, Nakazato Y, Iwamoto H, Namba K and Takeda Y, Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice, Br J Nutr 2016, 116, 270–278. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Manzano MC, Abecia L, Hernandez-Hernandez O, Sanz ML, Montilla A, Olano A, Rubio LA, Moreno FJ and Clemente A, Galacto-oligosaccharides derived from lactulose exert a selective stimulation on the growth of Bifidobacterium animalis in the large intestine of growing rats, J Agric Food Chem, 2013, 61, 7560–7567. [DOI] [PubMed] [Google Scholar]
- 7.Gosling A, Stevens GW, Barber AR, Kentish SE and Gras SL, Recent advances refining galactooligosaccharide production from lactose, Food Chem, 2010, 121, 307–318. [Google Scholar]
- 8.Vulevic J, Juric A, Tzortzis G and Gibson GR, A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults, J Nutr, 2013, 143, 324–331. [DOI] [PubMed] [Google Scholar]
- 9.Whisner CM, Martin BR, Schoterman MH, Nakatsu CH, McCabe LD, McCabe GP, Wastney ME, van den Heuvel EG and Weaver CM, Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial, Br J Nutr, 2013, 110, 1292–1303. [DOI] [PubMed] [Google Scholar]
- 10.Dai Z, Su D, Zhang Y, Sun Y, Hu B, Ye H, Jabbar S and Zeng X, Immunomodulatory activity in vitro and in vivo of verbascose from mung beans (Phaseolus aureus), J Agric Food Chem, 2014, 62, 10727–10735. [DOI] [PubMed] [Google Scholar]
- 11.Dai Z, Lyu W, Xiang X, Tang Y, Hu B, Ou S and Zeng X, Immunomodulatory effects of enzymatic-synthesized alpha-galactooligosaccharides and evaluation of the structure-activity relationship, J Agric Food Chem, 2018, 66, 9070–9079. [DOI] [PubMed] [Google Scholar]
- 12.Kuo TM, VanMiddlesworth JF and Wolf WJ, Content of raffinose oligosaccharides and sucrose in various plant seeds, J Agric Food Chem, 1988, 36, 5. [Google Scholar]
- 13.Yang X, Zhao Y, He N and Croft KD, Isolation, characterization, and immunological effects of α-galacto-oligosaccharides from a new source, the herb Lycopus lucidus Turcz, J Agric Food Chem, 2010, 58, 8253–8258. [DOI] [PubMed] [Google Scholar]
- 14.Dai Z, Feng S, Liu A, Wang H, Zeng X and Yang CS, Anti-inflammatory effects of newly synthesized α-galacto-oligosaccharides on dextran sulfate sodium-induced colitis in C57BL/6J mice, Food Res Int, 2018, 109, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JE, Strissel K, Zhao L, Obin M and Shen J, Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice, ISME J, 2015, 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP and Yang CS, Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice, J Agric Food Chem, 2011, 59, 11862–11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh DP, Khare P, Zhu J, Kondepudi KK, Singh J, Baboota RK, Boparai RK, Khardori R, Chopra K and Bishnoi M, A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice, Int J Obesity, 2016, 40, 487–496.. [DOI] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh P, Klein S and Gordon JI, Microbial ecology: human gut microbes associated with obesity, Nature, 2006, 444, 1022–1023. [DOI] [PubMed] [Google Scholar]
- 19.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER and Gordon JI, An obesity-associated gut microbiome with increased capacity for energy harvest, Nature, 2006, 444, 1027–1031. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y and Zhao L, Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice, ISME J, 2010, 4, 232–241. [DOI] [PubMed] [Google Scholar]
- 21.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K and Wang J, A metagenome-wide association study of gut microbiota in type 2 diabetes, Nature, 2012, 490, 55–60. [DOI] [PubMed] [Google Scholar]
- 22.Rastall RA , Gluco and galacto-oligosaccharides in food: update on health effects and relevance in healthy nutrition, Curr Opin Clin Nutr, 2013, 16, 675–678. [DOI] [PubMed] [Google Scholar]
- 23.Ye EQ, Chacko SA, Chou EL, Kugizaki M and Liu SM, Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain, J Nutr, 2012, 142, 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM and Mackay CR, Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43, Nature, 2009, 461, 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI and Fredrickson DS, Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin Chem, 1972, 18,499–502. [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF and Turner RC, Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man, Diabetologia, 1985, 28, 412–419. [DOI] [PubMed] [Google Scholar]
- 27.Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, Havekes LM, Kleemann R and van den Hoek AM, Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology, PloS one, 2014, 9, e115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folch J, Lees M and Sloane-Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues, J biol Chem, 1957, 226, 497–509. [PubMed] [Google Scholar]
- 29.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J and Ament ME, Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation, Hepatol, 1995, 22, 1399–1403. [PubMed] [Google Scholar]
- 30.Lee J, Walsh MC, Hoehn KL, James DE, Wherry EJ and Choi Y, Regulator of fatty acid metabolism, acetyl coenzyme a carboxylase 1, controls T cell immunity, J Immunol, 2014, 192, 3190–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laparra JM, Díez-Municio M, Herrero M and Moreno FJ. Structural differences of prebiotic oligosaccharides influence their capability to enhance iron absorption in deficient rats, Food Funct, 2014, 5, 2430. [DOI] [PubMed] [Google Scholar]
- 32.Damen B, Verspreet J, Pollet A, Broekaert WF, Delcour JA and Courtin CM, Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence, Mol Nutr Food Res, 2011, 55, 1862–1874. [DOI] [PubMed] [Google Scholar]
- 33.Sanz ML, Gibson GR and Rastall RA. Influence of disaccharide structure on prebiotic selectivity in vitro, J Agri Food Chem, 2005, 53, 5192–5199. [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Hernández O, Marín-Manzano MC, Rubio LA, Moreno FJ, Sanz ML and Clemente A, Monomer and linkage type of galacto-oligosaccharides affect their resistance to ileal digestion and prebiotic properties in rats, J Nutr, 2012, 142, 1232–1239. [DOI] [PubMed] [Google Scholar]
- 35.Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A, Bohm C, Wenning M, Wagner M, Blaut M, Schmitt-Kopplin P, Kuster B, Haller D and Clavel T, High-fat diet alters gut microbiota physiology in mice, ISME J, 2014, 8, 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M and Colecchia A, Gut microbiota and metabolic syndrome, World J Gastroentero, 2014, 20, 16079–16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Wang R, Li XF and Wang RL, Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome, Brit J Nutr, 2012, 107, 1429–1434. [DOI] [PubMed] [Google Scholar]
- 38.Eslinger AJ, Eller LK and Reimer RA, Yellow pea fiber improves glycemia and reduces Clostridium leptum in diet-induced obese rats, Nutr Res, 2014, 34, 714–722. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Hernandez O, Côté GL, Kolida S, Rastall RA and Sanz ML, In vitro fermentation of alternansucrase raffinose-derived oligosaccharides by human gut bacteria, J Agric Food Chem, 2011, 59, 10901–10906. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Lu X and Yang X, Stachyose-enriched α-galacto-oligosaccharides regulate gut microbiota and relieve constipation in mice, J Agric Food Chem, 2013, 61, 11825–11831 [DOI] [PubMed] [Google Scholar]
- 41.Mataki C, Magnier BC, Houten SM, Annicotte JS, Argmann C, Thomas C, Overmars H, Kulik W, Metzger D, Auwerx J and Schoonjans K, Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1, Mol Cell Biol, 2007, 27, 8330–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayin SI, Wahlstrom A, Felin J, Jantti S, Marschall HU, Bamberg K, Angelin B, Hyotylainen T, Oresic M and Backhed F, Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist, Cell metab, 2013, 17, 225–235. [DOI] [PubMed] [Google Scholar]
- 43.Yokota A, Fukiya S, Islam KS, Ooka T, Ogura Y, Hayashi T, Hagio M and Ishizuka S, Is bile acid a determinant of the gut microbiota on a high fat diet?, Gut Microbes, 2012, 3, 455–459. [DOI] [PubMed] [Google Scholar]