Summary
Severe burn injuries are associated with systemic inflammation or even sepsis. A beneficial effect of probiotics on burn patients is reported by regulating the function of the intestinal barrier and reducing inflammation. Immunoglobulin A (IgA) acts as an anti-inflammation antibody, and interleukin 6 (IL-6) as a pro-inflammatory mediator, released extensively in burns. The aim of this study was to investigate the effect of single and mixed strain probiotics on the level of IgA and IL-6 in severe burn patients. A randomized double-blind trial was conducted in the burn centre of the Dr. Soetomo Hospital. Severe burn patients with more than 20% total body surface area burned were randomized into two groups. Group one received a single strain and the second group received mixed strain probiotics, once daily for fourteen days. Serum levels of IgA and IL-6 were measured on day 4 post burn injury (before treatment) and day 19 (after treatment). Seventeen burn patients were enrolled in this study. IgA increased significantly from 1.01±0.67 to 1.89±0.98 mg/mL (p<0.001) in the single strain group, and 0.96±0.48 to 2.10±1.09 mg/mL (p=0.025) in the mixed strain group by paired t-test. There was no significant decrease in IL-6 in either group. No significant differences between the two groups were observed for IgA or IL-6. Administration of single and mixed strain probiotics increased IgA level, while there was no decrease in IL-6 level.
Keywords: probiotics, burn injury, immunoglobulin A, Interleukin-6
Abstract
Les patients sévèrement brûlés développent SIRS et sepsis. Un effet positif des probiotiques chez ces patients a été évoqué. Il repose sur la régulation de la barrière intestinale et sur un effet anti inflammatoire. IgA est anti inflammatoire, IL-6, secrétée en très grande quantité chez le brûlé, comme pro inflammatoire. Le but de cette étude randomisée en double aveugle réalisée dans le CTB de l’hôpital Dr Soetomo était d’évaluer l’effet de probiotiques (1 ou plusieurs souches) sur les taux d’IgA et d’IL-6 de 17 patients gravement brûlés (> 20% SCT). Le groupe G1 recevait une seule souche de probiotique, le groupe G2 un mélange de souches, 1 fois par jour pendant 2 semaines. Les taux d’IgA et d’IL-6 étaient mesurés à J4 post-brûlure (avant traitement) et à J19 (après traitement). Les 2 groupes étaient statistiquement comparables. L’augmentation d’IgA était significative dans les 2 groupes : 1,01 +/- 0,67 puis 1,89 +/- 0,98 ; p< 0,001 dans le groupe G1 ; 0,98 +/- 0,48 puis 2,1 +/- 1,09 ; p=0,25 dans le groupe G2. Les variations d’IL6 n’étaient pas significatives. Donc l’administration de 1 ou plusieurs souches de probiotiques augmente les taux d’IgA et ne diminue pas ceux d’IL6.
Introduction
Severe burn injury is generally associated with systemic inflammation or systemic infection, leading to sepsis. It is characterized by extensive release of pro-inflammatory cytokines such as IL-6 and TNF-α and suppression of anti-inflammatory mediators. 1 When severe burn occurs, a process of inflammation begins, although the systemic response reaches peaks five to seven days after burn.2 When more than 20% of total body surface area (TBSA) is burned, a systemic inflammatory response is initiated with the release of many cytokines and other inflammatory mediators, leading to an immunosuppressed state and risk of sepsis.3
In severe burns, levels of all immunoglobulin serum usually decline within 2 to 3 days after trauma.4 Immunoglobulin A (IgA), one of the immunoglobulins in the human body, has anti-inflammatory properties and barely a systematic response. Under physiologic conditions, FcαRI (CD89) or IgA FC receptors bind to serum IgA and allow transmission of inhibitory signals, therefore playing an important role in the anti-inflammatory process.5 Interleukin 6 (IL-6) is an inflammatory mediator that has been widely used to predict mortality in patients with severe infection or sepsis.6 In severe burn patients with sepsis, IL-6 levels were significantly higher in some experimental and clinical studies and associated with increased mortality.7 Fortunately, when the level of IL-6 was blocked or reduced after burns or sepsis, there was an improvement in clinical outcome.8
Systemic infection due to pathogenic intestinal microbes is often seen after surgery or trauma.9 However, Bifidobacterium and Lactobacillus, probiotic bacteria of the gut, maintain the intestinal mucosal barrier and enhance the immune response.10 Antibiotics are the main therapy to treat infection or sepsis in burn patients. However, various antibiotic and pharmacological interventions have not been successful in reducing sepsis and have failed to increase the recovery rate in burn patients.11
Probiotics are ‘live micro-organisms’, usually used to treat gastrointestinal disorders such as irritable bowel syndrome, colitis ulcerative, etc.12 They also have some beneficial effects that improve the clinical outcome of burn patients.13 There are contradictory outcomes regarding the health effects of probiotics. Mixed strain probiotics can reduce or eliminate pathogens and toxins. Furthermore, mixed strain probiotics release nutrients, antioxidants and growth factors therefore they could be more effective than single strain probiotics. However, only a few studies have focused on the effects of probiotics in burn patients with a high risk of sepsis. Consequently, the purpose of this double-blind, randomized trial was to determine the effect of single and mixed strain probiotics containing Lactobacillus, Bifidobacterium and Streptococcus thermophilus on IgA and IL-6 serum level and clinical outcome in burn patients.
Materials and methods
This 14-day randomized, double-blind, parallel group, non-placebo-controlled, single centre trial was conducted at the intensive care unit and burn unit of Dr. Soetomo Hospital in Surabaya. The study protocol was approved by the Ethics Committee for Clinical Research of the Dr. Soetomo Hospital, with registration number 435/Panke.KKE/VI/2016 and 438/Panke.KKE/VI/2016.
Participant selection
Patients admitted between June and November 2016 to the intensive care unit (ICU) and burn unit of the Dr. Soetomo Hospital in Surabaya were included in this study. The researcher described the study (procedures, including risks and benefit) to patients. Patients or their relatives were given adequate time to consult with their doctors or families. They then signed informed consent forms before being included in the study. Inclusion criteria for patients to participate in this study were as follows: 1) age >18-years; 2) extensive burns ≥20% TBSA less than 24 hours after injury; 3) the patient could be fed orally or enterally during the study period; 4) they were willing to sign a letter agreeing to participate. Exclusion criteria included the following: 1) the patient could not be fed orally; 2) the patient had received probiotics before admission to hospital; 3) the patient had sepsis when admitted to the hospital; 4) the patient had a history of autoimmune disease. Anamnesis, physical examination and retrieval of materials for laboratory examination were performed in patients who met the inclusion criteria.
Intervention procedure
Patients were randomly assigned to one of two groups: a single strain probiotic group or a mixed strain group. A random scheme was prepared by one of the investigators, and the allocations were concealed in sealed envelopes, which were kept secret by the department of pharmacy in the burn unit of the Dr. Soetomo Hospital. These envelopes were opened once the study was completed. The probiotic capsules were identical in terms of appearance and texture, and were only differentiated by a code (“A” or “B”). The main researcher and the participants remained blinded to the contents of the capsule throughout the study procedure and the statistical analysis. An independent person who was not related to the study and the study product held the blinding codes. Code breaking was performed after analysis and study had been completed. All the participants received standard medical and nutrition therapy including antibiotics, analgesics, and enteral or parenteral nutrition.
Each capsule of single strain probiotics contained more than 107 CFU Lactobacillus acidophilus, Bifidobacterium longum, and Streptococcus thermophilus. Mixed strain probiotics contained more than 108 CFU Lactobacillus casei, Lactobacillus rhamnousus, Lactobacillus acidophilus, and Lactobacillus delbrueckii subsp. Bulgaricus, Bifidobacterium longum, Bifidobacterium breve, and Streptococcus salivarius subsp. Thermophilus. Probiotics were given once daily, the first capsule within 4 days after admission for 14 consecutive days. All study treatments consisted of one capsule of probiotics providing a total of >107 bacteria. Probiotics were administered to the patient orally or by a feeding tube. The patient was withdrawn from the study immediately if enteral nutrition was discontinued, the patient was discharged from hospital or passed away before study completion.
Outcomes
Primary outcome was the serum level of IgA and IL-6. Secondary outcomes were clinical outcome, including infection related-sepsis, GI disturbance or incidence of diarrhea and duration of antibiotic use. Three millilitres of blood were obtained from each patient to evaluate IgA and IL-6 levels before initiation of the treatment (day 4) and on day 19. Serum was separated from whole blood by centrifugation, and then kept at -800 C. Pre- and post-treatment serums were collected from all study subjects, and levels of IgA and IL-6 were determined by ELISA in the laboratory of clinical pathology, a diagnostics centre of the Dr. Soetomo Hospital.
Statistical analysis
The total sample size of subjects was calculated based on published levels of secretory IgA (sIgA) differences in burn patients. The Kolmogorov-Smirnov test was used to assess the normality of distribution of the variables. The independent t-test was performed on all baseline data and to assess differences between groups. The paired t-test was performed to evaluate differences before and after treatment for each group. The data were analyzed by statistical software (SPSS 22.0; SPSS Inc, Chicago, Illinois, USA). Statistical significance was accepted when p<0.05.
Results
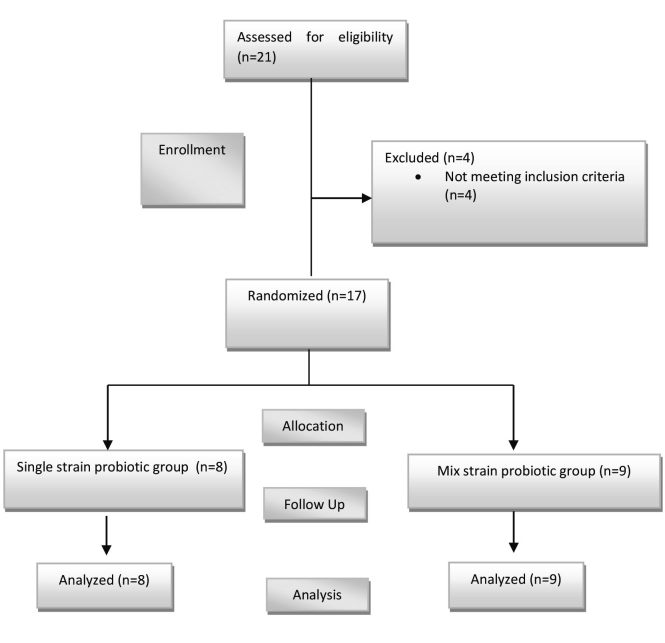
Between June and November 2016, 17 burn patients fulfilled the inclusion criteria. There were no significant differences between the groups regarding demographic data, as presented in Table I. Eight of the seventeen patients enrolled in the study were enrolled in a single strain probiotics group and nine in the mixed strain probiotics group, as shown in Fig. 1. The causes of burn in this study are shown in Fig. 2. All collected data, including demographic characteristics, IgA and IL-6 levels were normally distributed by Kolmogorov-Smirnov.
Table I. Demographic data of this study.
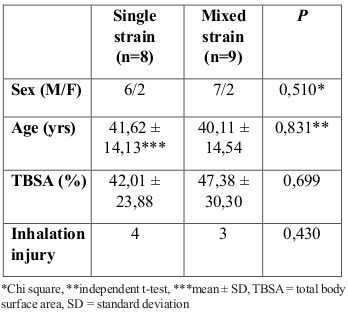
Fig. 1. Overview of the study.
Fig. 2. Causes of burns.
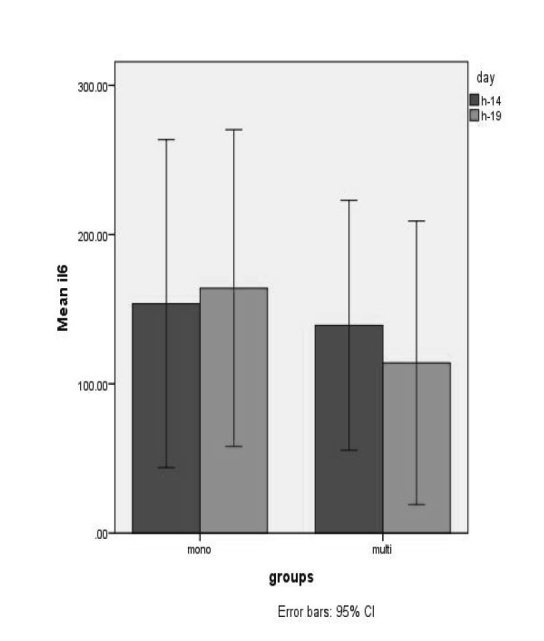
No serious adverse effects of probiotic administration were observed during this study. In patients with sepsis, no Lactobacillus were observed in blood or wound culture. For IgA, a significant increase was seen for both single strain (P<0,001) and mixed strain (p=0,025) probiotics after 14 days of treatment, as shown in Fig. 3. There were no significant differences between the groups on day 4 or day 19, as presented in Table II. Clinical outcome data are presented in Table III For IL-6, no significant changes were observed in the single strain probiotics group (p=0.804) or the mixed strain probiotics group (p=0.683), as shown in Fig. 4. Between the two groups, no significant differences were assessed on day 4 or after 14 days of treatment, as shown in Table II.
Fig. 3. Mean (±SD) IgA levels in the two groups of patients *Patients in the single strain probiotic group (P=0,000) and mixed strain probiotic group (P=0,025) had significantly higher IgA levels by day 19.
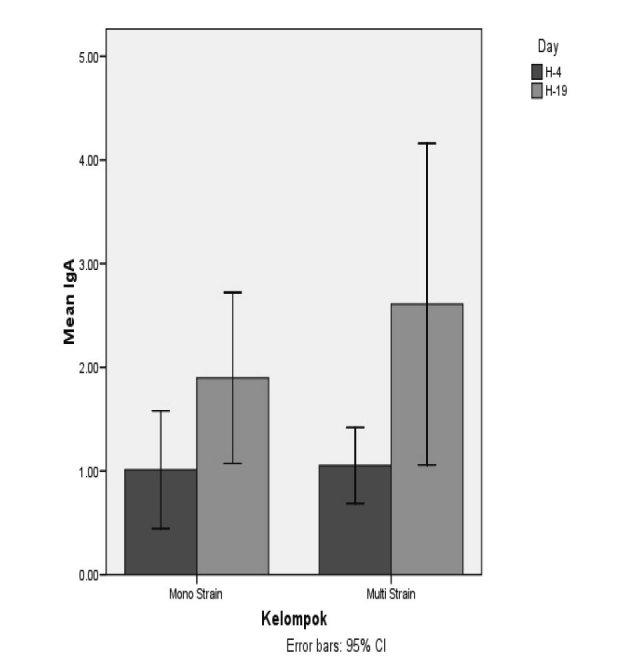
Table II. Serum levels of IgA and IL-6 at day 4 and day 19.
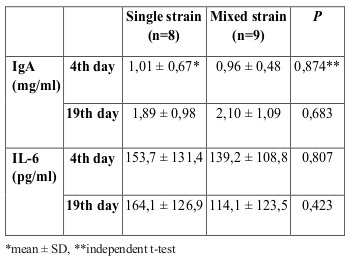
Table III. Outcome data of this study.
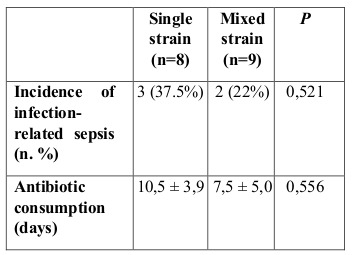
Fig. 4. Mean (±SD) IL-6 levels in the two groups of patients *Patients in the single strain probiotic group (P=0,804) and mixed strain probiotic group (P=0,683) had no significantly lower IL-6 levels by day 19.
Discussion
A number of studies have reported the positive benefits of probiotics for humans and concluded that probiotics can be used to treat and prevent a number of diseases.14 However, other benefits of probiotics in burn patients are still very limited.15 In this study, we aimed to evaluate the effect of probiotics on inflammation marker IL-6 and humoral immunity, IgA levels and on burn patients. Seventeen acutely burned patients were randomly allocated into two equal groups. Regarding demographic data, we observed no significant differences between the two groups. The immune system is regulated by probiotics depending on dose and strain.16 Especially, some strains of Lactobacilli and Bifidobacteria can activate the production of secretory IgA and IgG.17,18 A large amount of secretory IgA in the gut can strengthen the mucous layer and prevent the translocation of pathogenic bacteria into the systemic circulation.19 In our study, administration of both single and mixed strain probiotics significantly increased IgA production on day 19 post admission.
A similar finding in a mouse model was reported by Indrayanto et al., who showed that viable L. acidophilus, B. longum and B. Bifidum significantly increased IgA levels compared to a sepsis group.20 Our data are consistent with a study by Alberda et al., who evaluated the effect of probiotics on ICU patients and showed a significant increase in IgA levels in the treatment group.21 Another study by El-Ghazeli et al. showed that probiotic administration of Lactobacillus fermentum and Lactobacillus debulrecki to pediatric thermal burns had a significant effect on serum IgA levels compared to a placebo group at day 14 post admission (P=0,033).22 However, in this study there was no significant difference in serum IgA levels between the two groups after 14 days of treatment with probiotics (p=0.683). This suggests that in the process of modulating the immune system, probiotics are largely determined by differences in strains.17
IL-6 can be used as a predictor for cytokine cascade activity and organ dysfunction and mortality in clinical studies.6 In our study, administration of both single and mixed strain probiotics did not significantly decrease IL-6 levels. This result is in contrast with the findings of Sanaie et al., who reported that administration of probiotics to critically ill patients resulted in significantly lower levels of IL-6 in the probiotic group compared with the controls.23
In our study, the level of IL-6 on day 4 post burns was similar to that in a study by Yeh et al., who observed circulating levels of interleukin 6 in burned patients. It showed that the peak level of IL-6 of the five burn patient survivors was 78 to 540 pg/ml within 4 days post burns.24 In our study, in the mixed strain probiotic group, there was a decrease in IL-6 level on day 19 after administration of probiotics. The decrease of IL-6 is lower with mixed strain probiotics than single strain probiotics, which showed that proinflammatory capacity decreases with the use of mixed strain probiotics, although it is not significant.
A study by Babu et al. showed that on day 4 post burns the level of IL-6 was 13,23 ± 0,11 ng/ml and on day 14 it was 13,42 ± 0,38 ng/ml in burn patient survivors. In non survivors, on day 14 the level of IL-6 was 19,96 ± 0,26 ng/ml. It showed that the decrease of IL-6 level on day 14 post burns was not significant, even though conservative therapy had been given.25
A study by Nijsten et al. showed that the level of IL-6 was elevated within several hours of burns and remained elevated for several weeks afterwards. There is a positive correlation that the elevated CRP level is associated with elevated levels of IL-6 (P < 0,0001).26
In our study diarrhea, especially antibiotic-associated diarrhea, was not found in either group. This result is similar to that of the study by El-Ghazali et al., which showed that the administration of probiotics in pediatric burn patients significantly decreased the frequency of diarrhea compared to the control group (p=0,038).22 The beneficial benefits of probiotics have also been reported in a meta-analysis study evaluating 25 randomized controlled trial studies that found that probiotic administration was able to reduce the frequency of diarrhea and incidence of antibiotic associated-diarrhea due to Clostridium difficile infection.27
In our study, there were no significant differences in the duration of antibiotic use. The most widely administered antibiotic in our patients was ceftazidime (70%). Our result is consistent with a study by Mayes et al., in which the duration of antibiotics was 8,5 ± 1,8 days for the probiotic group, and 8,7 ± 1,8 days for the placebo group (p=0,94).28 In this study there was a decrease in the number of patients with sepsis, and there was no significant difference between the two groups. This is similar to a study by Kotzampassi et al., who observed the effects of probiotic supplementation on critically ill patients. The result was a decrease in infection rates, ventilator-associated pneumonia and mortality rates.29
One of our limitations is the small sample size. These results may not represent the entire population because of a limited number of samples. Moreover, the daily dose of probiotics is probably not the maximum dose that can be given, since one capsule daily was administered for all degrees of burns. In another study, a larger dose was used.23 Another limitation of our study was that there was no placebo group to clearly show that the changes observed in cytokine levels are due to probiotic administration and are not merely changes that could be expected normally over time in burn patients.
Conclusion
The results of this randomized trial suggest that administration of single and mixed strain probiotics to burn patients significantly increases the level of IgA. However, there is no significant difference between the two groups. This study also showed that mixed strain probiotics reduce the level of IL-6 as a marker of the pro-inflammatory mediator. Further studies with larger sample sizes and placebo controls are needed to clarify their usefulness for the clinical outcome of burn patients.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Belcher HJR. Churchill Livingstone. New York: 1996. Principles and practice of burns management; pp. 163–176. [Google Scholar]
- 3.Jeschke MG, Kamolz LP, Shahrokhi S. Burn Care and Treatment. Springer-Verlag; Wien: 2013. Pathophysiology of burn injury; pp. 415–440. [Google Scholar]
- 4.Olas K, Butterweck H, Teschner W, Schwarz HP. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human singlecytes and peripheral blood singlenuclear cells. Clin Exp Immunol. 2005;140(3):478–490. doi: 10.1111/j.1365-2249.2005.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteiro RC. Immunoglobulin A as an anti-inflammatory agent. Clin Exp Immunol. 2014;178 (Suppl 1):108–110. doi: 10.1111/cei.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth. 1996;77(1):110–117. doi: 10.1093/bja/77.1.110. [DOI] [PubMed] [Google Scholar]
- 7.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 8.Haasper C, Kalmbach M, Dikos GD, Meller R. Prognostic value of procalcitonin (PCT) and/or interleukin-6 (IL-6) plasma levels after multiple trauma for the development of mix organ dysfunction syndrome (MODS) or sepsis. Technol Health Care. 2010;18(2):89–100. doi: 10.3233/THC-2010-0571. [DOI] [PubMed] [Google Scholar]
- 9.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45(2):223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu K, Ogura H, Goto M, Asahara T. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60(1):126–133. doi: 10.1097/01.ta.0000197374.99755.fe. [DOI] [PubMed] [Google Scholar]
- 11.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman HM, Niers LE, Ridwan BU, Koning CJ. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr Edinb Scotl. 2007;26(4):450–459. doi: 10.1016/j.clnu.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. . Curr Pharm. 2009;15(13):1428–1518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 14.Borchers AT, Selmi C, Meyers FJ, Keen CL. Probiotics and immunity. J Gastroenterol. 2009;44(1):26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 15.Koren L, Gurfinkel R, Glezinger R, Perry ZH. The effect of Lactobacillus bacteria supplement on sepsis and its complications in patients with acute burns. Burns. 2007;33(5):594–598. doi: 10.1016/j.burns.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Kemgang TS, Kapila S, Shanmugam VP, Kapila R. Cross-talk between probiotic lactobacilli and host immune system. J Appl Microbiol. 2014;117(2):303–319. doi: 10.1111/jam.12521. [DOI] [PubMed] [Google Scholar]
- 17.Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet MEB. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007;14(5):485–492. doi: 10.1128/CVI.00406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinne M, Kalliomaki M, Arvilommi H, Salminen S. Effect of probiotics and breastfeeding on the bifidobacterium and lactobacillus/enterococcus microbiota and humoral immune responses. . J Pediatr. 2005;147(2):186–191. doi: 10.1016/j.jpeds.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Bengmark S. Synbiotics and the mucosal barrier in critically ill patients. Curr Opin Gastroenterol. 2005;21(69:712–716. doi: 10.1097/01.mog.0000182858.65927.81. [DOI] [PubMed] [Google Scholar]
- 20.Indrayanto Y, Prasetyo DH. Probiotic effect of mortality, degree of intestinal inflammation, and IgA levels in sepsis mice model. MKB. 2006;45(1):10–15. [Google Scholar]
- 21.Alberda C, Gramlich L, Meddings J, Field C. Effects of probiotic therapy in critically ill patients: a randomized, doubleblind, placebo-controlled trial. Am J Clin Nutr. 2007;85(3):816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 22.El-Ghazely MH, Mahmoud WH, Atia MA, Eldip EM. Effect of probiotic administration in the therapy of pediatric thermal burn. Ann Burns Fire Disasters. 2016;29(4):268–272. [PMC free article] [PubMed] [Google Scholar]
- 23.Sanaie S, Ebrahimi-Mameghani M, Hamishehkar H, Mojtahedzadeh M. Effect of a multispecies probiotic on inflammatory markers in critically ill patients: a randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(9):827–833. [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh FL, Lin WL, Shen HD, Fang RH. Changes in circulating levels of interleukin 6 in burned patients. Burns. 1999;25:131–136. doi: 10.1016/s0305-4179(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 25.Babu RJ, Karthikeyan G, Ponnambalam N. Could serum cytokines serve as predictors in outcome of thermal burn injuries. Indian J Burns. 2017;25:18–22. [Google Scholar]
- 26.Nitsten MW, Hack CE, Helle M, ten Duis HJ. Interleukin 6 and its relation to the humoral immune response and clinical parameters in burned patients. Surgery. 1991;109(6):761–767. [PubMed] [Google Scholar]
- 27.McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 28.Mayes T, Gottshlich MM, James LE, Allgeler C. Clinical safety and efficacy of probiotic administration following burn injury. J Burn Care Res. 2015;36(1):92–99. doi: 10.1097/BCR.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 29.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A. Benefits of a synbiotic formula in critically ill trauma patients: early results of a randomised controlled trial. World J Surg. 2006;30:1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]