Summary
Infections are still the main cause of mortality in burn patients. Multidrug resistant bacteria can cause outbreaks in critical care and burn units. We describe an outbreak of infection by extensively drug-resistant Pseudomonas aeruginosa in the Burn Unit of a University Hospital in Barcelona (Spain) between April and July 2016. A descriptive study of all cases, a bacterial colonization screening of all admitted patients and a microbiological environmental study were performed in order to detect a possible common focus. Contact isolation and cohortization of healthcare workers of all infected or colonized patients were applied. Environmental control measures were instituted for possible sources of infection. The outbreak was caused by a strain of P. aeruginosa only sensitive to colistin. Ten patients were infected or colonized and two of them died. The same strain was detected in several taps and drains in different rooms of the Unit. After applying control measures, changing faucets and drains, carrying out thermal disinfection of the hot water installation of the unit, disinfecting the rooms with ultraviolet radiation and placing antibacterial filtration devices in all the taps among other measures, an effective control of the outbreak was achieved.
Keywords: burns, Pseudomonas aeruginosa, outbreak, burn centre, extensively drug-resistant
Abstract
Les infections sont toujours une cause majeure de mortalité chez les brûlés. Des épidémies à bactéries multirésistantes (BMR) dans les CTB sont régulièrement rapportées. Nous décrivons une épidémie due à Pseudomonas æruginosa BMR, sensible uniquement à la colimycine, survenue dans le CTB d’un hôpital universitaire de Barcelone entre avril et juillet 2016. Elle a touché 10 patients dont 2 sont morts. Une étude de chaque cas, un dépistage chez tous les entrants et une étude environnementale ont été réalisées, afin de trouver d’éventuelles similitudes. Un isolement contact et un cohorting ont été mis en place. Des mesures de contrôle de l’environnement ont été implémentées. La souche incriminée a été retrouvée dans plusieurs robinets et siphons du service. Cette épidémie a été résolue après, outre les mesures précitées, changement des robinets et des siphons (avec mise en place d’ultrafiltres sur les robinets), choc thermique du réseau d’adduction d’eau, désinfection terminale UV des chambres.
Introduction
After the initial resuscitation of burned patients, up to 75% of patient deaths are related to an infectious process.1 Several factors contribute to the high frequency and severity of these infections and to their multiple possible locations: the destruction of the skin allows easy access of microorganisms, the presence of necrotic tissue provides an optimal growth medium for them, invasive monitoring facilitates other entry points and, finally, nonspecific alteration of immune function allows microbial proliferation.1
Pseudomonas aeruginosa is a gram-negative, aerobic and non-fermenting bacterium that is widely distributed in nature. Soil, plants or running water can act as a reservoir, with a predilection for humid, even nutrient-deficient environments.2,3 The ease with which P. aeruginosa grows both in nature and inside hospitals, and its capacity to acquire resistance mechanisms to antibiotics, make it one of the most significant causes of serious nosocomial infection, affecting mainly immunocompromised patients. 4,5 Infection between hospitalized patients occurs mainly through the hands of healthcare workers but also through contact with contaminated surfaces and objects.3
The number of published outbreaks caused by multidrug-resistant (MDR) bacteria in burn units over the last 30 years is relatively small. Of these, few come from developed countries, and diagnostic and screening techniques have changed in recent years.6 Early detection of the outbreak can facilitate its control and limit its spread. The objective of this work is to describe an outbreak caused by extensively drug-resistant P. aeruginosa (XDR-PA)7 in the Burn Unit of the Vall d’Hebron University Hospital in Barcelona (Spain) between April and July 2016, and the measures adopted to control it.
Materials and methods
The Vall d’Hebron University Hospital of Barcelona (Spain) is a health centre with more than 1100 beds. The Burn Unit is an integral unit that has six beds for critical patients, 16 beds for semi-critical or non-critical patients and four beds for pediatric patients. It also has its own operating room, a room for outpatient treatment, an emergency room, a rehabilitation area, a room for hydrotherapy and a games room, along with other rooms for storage and work areas for healthcare workers. It forms part of the Centres, Services and Reference Units (CSUR) of the Spanish National Health System for critically burned patients, and its direct area of influence includes Catalonia, the Balearic Islands and Andorra, covering a population of more than 8,000,000 people.
Epidemiological investigation
A case was defined as any patient admitted to the Burn Unit who, as of April 2016, presented infection or colonization by P. aeruginosa with “in vitro” resistance to all the antibiotics evaluated except colistin. Weekly screening by rectal smear was performed on all patients admitted to the Unit to detect possible colonization, in addition to the cultures that were performed in case of suspected infection. This screening was extended to patients who went to the outpatient and rehabilitation treatment rooms and who had been admitted as of April 2016.
Microbiological study
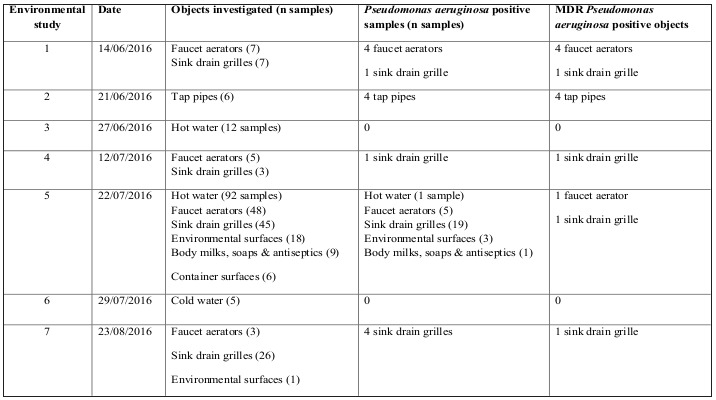
In order to detect possible reservoirs and environmental sources from which the pathogen could have been transmitted, seven rounds of environmental sampling were performed in which samples were taken with a swab, and on three occasions, water samples were taken. A total of 290 samples were collected, which included hot and cold water, moisturizers, soaps and antiseptic solutions, the aerators from all taps, the internal surfaces of the tap pipes and all of the drain grates, the surfaces of containers and other environmental surfaces (Table I). The surfaces were sampled using wet sponges with neutralizing buffer (3MTM Sponge-Stick, USA). Likewise, samples of hot and cold water were obtained from all of the taps in the Unit. The samples of hot water were obtained following the recommendations published in “Health Technical Memorandum 04-01: Safe water in healthcare premises: Part C - Pseudomonas aeruginosa - advice for augmented care units”. In the hot water taps, a 2-L water sample was obtained after 2 hours of not using the tap, and another 2-L sample was obtained after 2 min of letting the water circulate.
Table I. Summary of environmental studies, and objects from which the outbreak strain was isolated.
The sponges were incubated overnight at 37ºC and were then planted in selective and differential media to investigate the presence of P. aeruginosa and multidrug-resistant gram-negative bacilli. The water samples were filtered through a 0.45-μm Millipore filter and cultured in the appropriate selective media.
The clinical samples were seeded in selective and differential culture media, some of which specific for the search for resistant microorganisms. In the case of urine and respiratory samples, quantitative cultures were inoculated. All media were incubated at 35ºC in suitable atmospheres, with reading and interpretation after at least 24 hours had elapsed.
As regards monitoring samples (stool samples taken by rectal swab) for colonization, active searches for multidrug-resistant gram-negative bacilli were made with specific selective media.
Antimicrobial susceptibility to beta-lactam antibiotics with antipseudomonal activity (piperacillintazobactam, aztreonam, ceftazidime, cefepime, imipenem and meropenem), aminoglycosides (gentamicin, tobramicin and amikacin), quinolones (ciprofloxacin), fosfomycin and colistin was determined by disk diffusion in Mueller-Hinton agar and antibiotic gradient strip test, following the EUCAST guidelines (www.eucast.org). The presence of metallobeta-lactamase production (MBL) was screened by the modified Hodges test and double-disk synergy test using imipenem and imipenem+EDTA and confirmed by Polymerase Chain Reaction (PCR). In this case specific primers for the detection of the MBL-encoding genes blaVIM, blaIMP and blaNDM were used.8
Clonal relatedness
Pulsed-field gel electrophoresis (PFGE) of the restriction fragments of total bacterial DNA, obtained by means of the restriction enzyme Spel (Roche, USA) was performed to confirm the clonal relationships of the P. aeruginosa isolates. All of the isolates from the culture of the clinical samples, along with those from the previously mentioned colonization and environmental samples, were studied using this technique. DNA separation was performed in the CHEF-DRII pulsed field electrophoresis system (Bio-Rad, USA) according to the conditions described previously.9 The gel images were captured using the Gel DocTM XR system (Bio-Rad, USA), and the analysis of the macro-restriction profiles was performed with the GelCompar II program (Applied Maths, Belgium) using the Dice similarity index, which is based on UPGMA (unweighted pair group method using arithmetic averages). For the generation of the dendrograms, a position tolerance value of 1.0% was accepted. The clonal relationship was determined by comparisons of the macrorestriction profiles of each of the isolates studied. Thus, two isolates belonging to the same clone were defined based on a similarity higher than 85%.10
Control measures taken
All infected or colonized patients were subjected to contact precautions. Isolation precautions were adopted by the healthcare workers (personnel dedicated exclusively to infected or non-infected patients).
Compliance with basic infection prevention measures (hand hygiene, aseptic measures, cleaning and disinfection of surfaces and materials) was reinforced through training sessions with the professionals of the unit.
A review of the antimicrobial treatment protocols and antibiotic policy of the unit was conducted, with the aim of reducing antibiotic pressure and limiting the development of multidrug-resistance. We do not perform empirical systemic antibiotic treatment in a generalized way on admission. In cases where infection is suspected in the first 72 hours of admission, in patients who have had no previous relationship with the health system and no colonization by Pseudomonas aeruginosa is suspected, amoxycillin/clavulanic acid is preferentially administered, after obtaining samples for microbiological culture. In contrast, in patients with criteria of possible colonization or infection by Pseudomonas aeruginosa or with more than 72 hours of admission, broad-spectrum antibiotic therapy is initiated, preferably piperacillin-tazobactam. The use of carbapenems, aminoglycosides, glycopeptides or other antimicrobials is reserved for patients with septic shock or prolonged admis sion, starting from the week of admission. Subsequently, therapeutic de-escalation is carried out depending on the antibiogram of the isolated germ, using directed treatment of the minimum possible spectrum according to the infection focus.
Thermal disinfection of the facilities supply- ing hot water to the Burn Unit was per- formed (circulating the water at a temperature exceeding 60°C for a minimum time of 5 min at the taps), along with a daily purge of all of the taps in the Unit. All of the taps were replaced, the drainpipes were replaced, and H2Otap3000 and H2Ower3500 (Biogen Technologies, Spain) antibacterial filters were installed in all of the rooms, the nurses' station, the surgical area, the rehabil- itation area, the outpatient treatment rooms, the emergency room and the hydrotherapy room..
The rooms were disinfected with ultraviolet radiation (Xenex® Germ-Zapping RobotsTM system, USA), and the critical care hospital beds were disinfected according to the man- ufacturer's instructions..
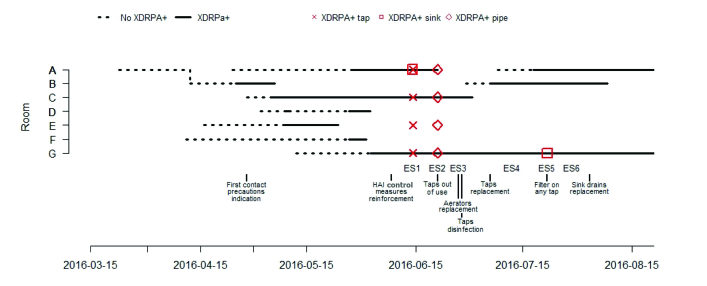
This intervention ended in September 2016 when the outbreak was controlled (Fig. 1).
Results
From April 25 to July 18, 2016, 10 patients with positive culture for P. Aeruginosa carrying a VTM-type MBL were detected among the patients admitted to the Burn Unit of the Vall d'Hebron University Hospital. The monthly XDR-PA infection rate cal- culated per stay during the outbreak period ranged between 0.23 and 1.70 per 100 days of stay (0.23 in April, 1.70 in May, 0.22 in June and 0.55 in July).
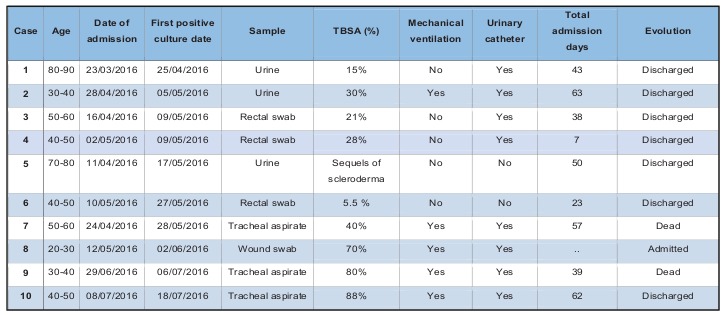
The index case was a woman older than 80 years, with no pathological history of interest, admitted for second-degree burns on 15% of her total body sur- face area. Secondary cases are described in Table II: six in May, one in June and two in July. Nine pa- tients were directly admitted to our Unit from the accident location and one patient came from the emergency department of another intermediate hos- pital where he stayed less than 6 hours. As seen in Table II and Fig. 1, in the last two cases detected in July, the interval between entry into the Unit and detection of P. aeruginosa was shorter than in the previous cases; these two patients were admitted to rooms that had previously been occupied by other colonized or infected patients. Of the 10 patients, seven had infection (three respiratory, three urinary and one soft tissue), and three had colonization by XDR-PA. Two patients died due to septic shock that progressed to multi-organ failure and death; in one of them, the septic shock was secondary to a respiratory infection by XDR-PA, and in the other, it was secondary to an infection of the burns by XDR-PA. The results of the environmental study are shown in Table I. The strain of XDR-PA that caused the outbreak was detected in the tap aerator and the tap plumbing of four rooms occupied with patients colonized or infected by said bacteria. This strain was also detected in the grates of the basin drains of two rooms occupied by infected patients (in one of these rooms, two consecutive patients acquired XDR-PA; in the drain of the basin in the other room, XDR-PA was detected only in the second sampling), in the aerator of the tap of the hydrotherapy room and in the grate of the basin drain of the nurses’ station (Fig. 2).
Fig. 1. Timeline of hospital stay of patients colonized or infected with the Pseudomonas aeruginosa outbreak strain, results from environmental samples, and outbreak control measures. The dashed lines represent patients over time and located in the different key rooms when the results of the cultures were negative for extensively drug-resistant P. aeruginosa (XDRPA). The continuous lines show when these results were positive. The red figures represent positive environmental samples (ES).
Fig. 2. Schematic diagram of the burn unit. The rooms with positive environmental samples to XDR-PA are indicated by a circle and the patients infected or colonized by XDR-PA are represented with a star.
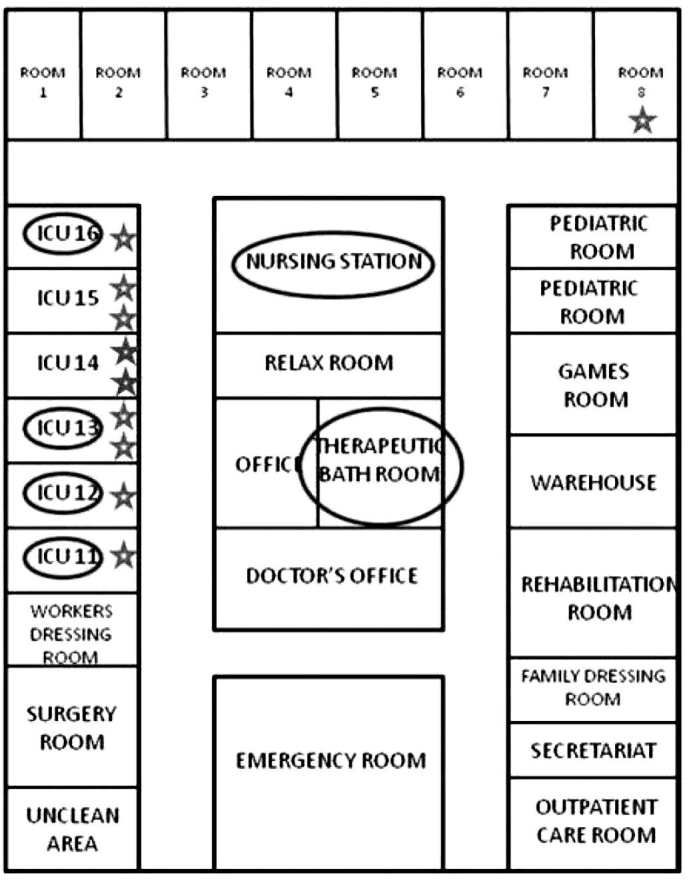
Table II. Characteristics of the cases of infection or colonization by extremely resistant Pseudomonas aeruginosa that occurred in the outbreak of the Burn Unit (TBSA: total body surface area).
The molecular epidemiology study using the PFGE technique showed that all the isolates of XDR-PA obtained from clinical and environmental samples were identical.
With the progressive application of the control measures mentioned above, no more cases were detected, and all of the environmental samples were negative for XDR-PA (Fig. 1); therefore, the outbreak was considered controlled in September 2016. Patient no. 8 remained hospitalized and colonized by XDR-PA until October 2016. For the early detection of a possible recontamination, a protocol was established that includes the monthly sampling of the Unit’s water and the taps and plumbing. On 1st November 2018, no new episodes of XDR-PA infection had been detected, nor were positive environmental samples detected.
Discussion
P. aeruginosa is an opportunistic bacterium with a large environmental reservoir that can cause serious systemic infections of any type (respiratory, urinary, cutaneous or soft-tissue infections, or bacteremia, among others) in patients with severe comorbidities or some type of immunosuppression, such as occurs with extensive burns. Its multiple structural (capsular exopolysaccharides, adhesins, pili, diffusible pigments, endotoxins), toxigenic (exotoxins A, S and T) and enzymatic (elastase, alkaline protease, rhamnolipid, phospholipase C) virulence factors largely explain why it can cause a wide variety of infections.2 The described outbreak affected 10 patients, three with respiratory infection, three with urinary tract infection, one with soft tissue infection and three colonized in the gastrointestinal tract.
The high degree of resistance of this pathogen is due to the coexistence of multiple mechanisms.2 Although the presence of carbapenemase-producing strains of P. aeruginosa in our hospital is less than 2%, in this case the production of a VIM-type MBL was detected from the beginning and was a good marker for the phenotypic detection of new cases or reservoirs. The XDR-PA that originated the outbreak in our Unit was only susceptible to colistin. Intravenous treatment with colistin ceased to be used in the 1980s due to its nephrotoxic and neurotoxic potential. Recently, this treatment has presented favorable clinical results in 72% of cases, with confirmed eradication of MDR-PA in 35%.11 According to data from this study, there were no cases of neurotoxicity, and nephrotoxicity was present in 8.3% (related to the history of chronic renal failure, diabetes mellitus and the use of aminoglycosides); therefore, colistin could be a safe option for the treatment of MDR-PA infections.
The correct implementation of isolation and control measures against the transmission of multidrugresistant bacteria is of vital importance in the face of an outbreak with multidrug-resistant bacteria. The horizontal transmission of strains has been demonstrated to be one of the most frequent causes of acquiring P. aeruginosa in Intensive Care Units (ICU) since only between 20% and 33% of patients are colonized at admission.3 In addition, cross-transmission between patients has been identified in between 8% and 50% of infections or colonizations acquired during admission.3 In these cases, healthcare workers would be the most likely vector. Reinforcing hand hygiene of healthcare workers is one of the most essential measures to prevent cross-transmission of infections. An educational program to promote hand hygiene among healthcare workers is well established in our centre following the World Health Organization (WHO) multimodal hand hygiene strategy12 and annual monitoring of hand hygiene compliance is performed by direct observational method. Alcohol-based handrub is the most used method for routine hand antisepsis in our setting, using soap and water when hands are visibly dirty or soiled with blood or body fluids. In 2016, the observed adherence of hand hygiene in the burn unit of our hospital was about 70.4%.
However, for 30% to 60% of the remaining cases, there is plausible evidence from previous studies that strains of P. aeruginosa found in washbasins, hydrotherapy tanks, taps and water from these taps can be a source for colonization and infection of patients, especially in ICUs.3,13-15 The persistence of P. aeruginosa in adverse media is due to its capacity to form biofilms.5 Transmission might be caused by splashing of water droplets from contaminated sinks to healthcare personnel’s hands, which may become transiently colonized;16then these hands might come into contact with the patient or with invasive devices such as an orotracheal tube, a vascular catheter or a urinary catheter. Although in outbreaks related to water environment the routes of transmission have been difficult to characterize, in most of them the reinforcement of hand hygiene and contact precautions has been associated with a significant reduction in transmission.17 In the present outbreak, XDR-PA was found in the tap aerators of four rooms and in the grates of the basin drains of two additional rooms where patients colonized or infected by XDR-PA were admitted. To relate the possible sources with the cases, an epidemiological study is required, and currently only genotypic methods are considered adequate to relate the isolates,3 such as PFGE, as used in the present outbreak.
The transmission of strains from patients to drains has also been described in the literature.13 This observation emphasizes the importance of minimizing room changes of patients infected or colonized by XDR-PA as much as possible to avoid the spread of these microorganisms. For all of these reasons, the disinfection of the taps, basins and drains of the ICUs and Burn Units is of vital importance. However, Garvey et al.15 demonstrated that following the national guidelines of the British Department of Health on the use and maintenance of the water supply system was not sufficient since transmission persisted. This assessment has led to the description of alternative methods for cleaning and disinfecting the surfaces of the basins, grates and drains.18,19 Finally, it has been described that the use of point-of-use tap filters is associated with a decrease in number of colonizations and infections,3,20 with a lower cost than a strategy involving the use of sterile bottled water.20
However, in our outbreak, after having identified the facility that supplies sanitary hot water as a potential source and having adopted corrective measures (replacement of taps and aerators, disinfection), two more cases of XDR-PA infection were detected. In addition, the times between admission and acquisition of XDR-PA were shorter in these two cases compared with the others. These two cases corresponded to patients who were admitted to rooms in which other infected patients had been admitted. In a previous prospective observational study,21 it was found that admission to an ICU room previously occupied by a patient with MDR-PA increases the risk of acquisition of this bacterium in subsequent patients, and the time to acquire the bacterium was also shorter. This has been related to the fact that disinfection and cleaning of these rooms does not completely eliminate these pathogens22 and suggests that the contamination of surfaces of the rooms, and of the basins and drains, can play a role in the transmission of MDR-PA.21 Interestingly, in the present study, XDR-PA was detected in the drain grate of a basin from one of the two rooms where two patients were positive for XDR-PA. Another recent study23 associated a more intense terminal disinfection of the rooms with a decrease in the risk of infection of the organisms studied (methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and multidrug-resistant Clostridium difficile and Acinetobacter spp.); however, this work did not include MDR-PA as an object of study. This observation suggests that objects and surfaces may play a role in the spread of certain microorganisms and, therefore, that the use of the ultraviolet radiation disinfection system used in the present outbreak may have played an important role in their control.
The investigation of the survival mechanisms of inanimate environments and the dispersion mechanisms of these microorganisms is fundamental to understanding and stopping these outbreaks. For example, it would be useful to investigate the designs of taps and drains to reduce their contamination and to avoid spreading, since one of the postulated forms of transmission is related to splashing from the drains, possibly due to basins designed in such a way that the stream of water directly hits the drain.19
Several studies have been published on the impact that multidrug-resistant P. aeruginosa has on the increase in mortality rates, the hospitalization period and, to a lesser extent, the economics. In general, it is estimated that infections caused by multidrug-resistant P. aeruginosa are associated with a higher mortality rate, a prolonged period of hospitalization and increased costs compared with infections caused by antibiotic-susceptible bacteria, especially in terms of increased pharmaceutical costs.24-27 The secondary expense of the implementation of the different control measures (replacement of contaminated taps and drains, bacterial filtration systems in taps, ultraviolet irradiation, among other measures) could be justified if the eradication of the outbreak and a decrease in the number of baseline cases with multidrug-resistant microorganisms are achieved.
On February 27, 2017, WHO published a list of the 12 bacteria that require priority research for the development of new antibiotics.28 P. aeruginosa resistant to carbapenems is one of the critical priority group. These multidrug-resistant bacteria constitute a serious public health problem.
Conclusion
In April 2016, there was an outbreak of infection by extensively drug-resistant P. aeruginosa in the Burn Unit of a university hospital in Barcelona (Spain), with 10 patients affected. The epidemiological investigation revealed the existence of environmental reservoirs in the taps and drains of the Unit. The application of strict control measures against transmission, the strict adherence by healthcare workers to infection control policy, the use of an antibiotic protocol, along with the replacement of taps and drains and the installation of bacterial filters were associated with an effective control of the outbreak.
P. aeruginosa infections continue to be a major concern among burn patients because they drastically worsen the prognosis of these patients, and outbreaks are not uncommon in burn units. We believe that research should be promoted in this field, as should the exchange of experiences to control one of the greatest threats to modern medicine: microorganisms resistant to multiple antibiotics.
References
- 1.Arsemino M, Hemsley C. ABC of burns: Intensive care management and control of infection. BMJ Br Med J. 2004;239(7459):220–223. doi: 10.1136/bmj.329.7459.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montero MM. Pseudomonas aeruginosa multiresistente: aspectos epidemiológicos, clínicos y terapêuticos [dissertation] Bellaterra, Universitat Autònoma de Barcelona. 2013 Available at: http//hdl.handle.net/10803/107902 . [Google Scholar]
- 3.Trautmann M, Lepper PM, Haller M. Ecology of Pseudomonas aeruginosa in the intensive care unit and the evolving role of water outlets as a reservoir of the organism. Am J Infect Control. 2005;33(5 SUPPL. 1):41–49. doi: 10.1016/j.ajic.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Kasper DL, Braunwald E, Fauci AS, Hauser SL. 17. New York: 2008. Harrison’s Principles of Internal Medicine; pp. 202–208. [Google Scholar]
- 5.Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Cell Frontal Infect Microbiol. 2017;7:39. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girerd-Genessay I, Benet T, Vanhems P. Multidrug-resistant bacterial outbreaks in Burn Units: a synthesis of the literature according to the ORION statement. J Burn Care Res. 2016;37(3):172–180. doi: 10.1097/BCR.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 7.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y. Multidrugresistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance: international standard definitions for acquired resistance. Clin Microbiol Infecti. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Tortola MT, Lavilla S, Miró E, González JJ. First detection of a carbapenem-hydrolyzing metalloenzyme in two Enterobacteriaceae isolates in Spain. Antimicrob Agents Chemother. 2005;49:3492–3494. doi: 10.1128/AAC.49.8.3492-3494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann ME. Pulsed-field gel electrophoresis. Methods Mol Med. 1998;15:33–50. doi: 10.1385/0-89603-498-4:33. [DOI] [PubMed] [Google Scholar]
- 10.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montero M, Horcajada JP, Sorlí L, Alvarez-Lezma F. Effectiveness and safety of colistin for the treatment of multidrug-resistant Pseudomonas aeruginosa infections. Infection. 2009;37(5):461–465. doi: 10.1007/s15010-009-8342-x. [DOI] [PubMed] [Google Scholar]
- 12.WHO. Geneva: 2009. World Health Organization: Patient safety a world alliance for safer health care. WHO guidelines on hand hygiene in health care. First global patient safety challenge clean care is safer care. [PubMed] [Google Scholar]
- 13.Tredget EE, Shankowsky A, Joffe AM, Inkson TI. Epidemiology of infections with Pseudomonas aeruginosa in burn patients: the role of hydrotherapy. Clin Infect Dis. 1992;15:941–949. doi: 10.1093/clind/15.6.941. [DOI] [PubMed] [Google Scholar]
- 14.Reuter S, Sigge A, Wiedeck H, Trautmann M. Analysis of transmission pathways of Pseudomonas aeruginosa between patients and tap water outlets. Crit Care Med. 2002;30:2222–2228. doi: 10.1097/00003246-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Garvey MI, Bradley GW, Tracey J, Oppenheim B. Continued transmission of Pseudomonas aeruginosa from a wash hand basin tap in a critical care unit. J Hosp Infect. 2016;94(1):8–102. doi: 10.1016/j.jhin.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis. 2016;62(11):1423–1435. doi: 10.1093/cid/ciw122. [DOI] [PubMed] [Google Scholar]
- 17.Kizny Gordon AE, Mathers AJ, Cheong EYL,, Gottlieb T. The hospital water environment as a reservoir for Carbapenem-resistant organisms causing hospital-acquired infections - a systematic review of the literature. Clin Infect Dis. 2017;64(10):1435–1444. doi: 10.1093/cid/cix132. [DOI] [PubMed] [Google Scholar]
- 18.Garvey MI, Bradley CR, Bradley CW. Evaluating the risks of wash hand basin tap disinfection. J Hosp Infect. 2016;94(1):21–22. doi: 10.1016/j.jhin.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Stjärne Aspelund A, Sjöström K, Olsson Liljequist B, Mörgelin M. Acetic acid as a decontamination method for sink drains in a nosocomial outbreak of metallo-β-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect. 2016;94(1):13–20. doi: 10.1016/j.jhin.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Hall J, Hodgson G, Kerr KG. Provision of safe potable water for immune- compromised patients in hospital. J Hosp Infect. 2004;58(2):155–158. doi: 10.1016/j.jhin.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Nseir S, Blazejewski C, Lubret R, Wallet F. Risk of acquiring multidrug-resistant gram-negative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect. 2011;17:1201–1208. doi: 10.1111/j.1469-0691.2010.03420.x. [DOI] [PubMed] [Google Scholar]
- 22.Otter JA. Interpreting the Joint Working Party Guidelines for cleaning up after carbapenemase-producing organisms: the devil’s in the dilution. J Hosp Infect. 2016;94:1058–109. doi: 10.1016/j.jhin.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DJ, Chen LF, Weber DJ, Moehring RW. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile: a clusterrandomized, multicentre, crossover study. Lancet. 2017;389:805–814. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bou R, Lorente L, Aguilar A, Perpiñán J. Hospital economic impact of an outbreak of Pseudomonas aeruginosa infections. J Hosp Infect. 2009;71:138–142. doi: 10.1016/j.jhin.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42:S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 26.Morales E, Cots F, Sala M, Comas M. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Services Research. 2012;12:122. doi: 10.1186/1472-6963-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmeli Y, Troillet N, Karchmer AW, Samore MH. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med. 1999;159:1127–1132. doi: 10.1001/archinte.159.10.1127. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: WHO Press. 2017:1–7. [Google Scholar]