Abstract
Aims:
To examine the association between wine consumption and the prevalence of chronic kidney disease (CKD) and cardiovascular disease (CVD).
Data synthesis:
We performed a cross-sectional logistic regression analysis of National Health and Nutrition Examination Survey (NHANES) in participants 21 years of age or older from 2003-2006 in a large representative study of the U.S. population. Wine consumption was categorized as none (0 glass per day), light (< 1 glass per day), or moderate (≥ 1 glasses per day). Prevalent CKD was defined as a urine albumin/creatinine ratio (UACR) ≥30 mg/g or estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2. CVD was defined as history of CVD including angina, myocardial infarction, or stroke.
Only 27(0.5%) individuals reported moderate wine consumption, whereas 57.5% and 42% reported abstinence and light wine consumption, respectively. Light wine consumption was associated with a lower prevalence of CKD as opposed to abstinence in unadjusted analysis. After adjusting for demographics and CVD risk factors light wine consumption was associated with lower prevalence of CKD defined as UACR ≥30 mg/g but not with low eGFR. Furthermore, light wine consumption was associated with significantly lower rates of CVD in the general population and in subjects with CKD. The adjusted odd of CVD for those with light wine consumption was 0.72 (CI 0.52-0.99, p=0.046) for the subjects with CKD.
Conclusion:
These data suggest that light wine consumption (compared to abstinence) is associated with lower prevalence of CKD and a lower odd of CVD in those with CKD in the U.S. population.
Keywords: wine consumption, CKD, CVD
INTRODUCTION
The prevalence of chronic kidney disease (CKD) in the United States (U.S.) is high (estimated at approximately 11.5%).[1] While dialysis or transplantation may result from CKD, the majority of CKD patients will die prematurely from cardiovascular disease (CVD) prior to reaching this end-point.[2-4] Importantly, CKD is associated with increased cardiovascular mortality partially due to increased prevalence of traditional risk factors such as age, diabetes mellitus, hypertension, and hyperlipidemia in patients with CKD.[5, 6] However, CKD is also associated with an increased prevalence of nontraditional risk factors unique to this patient population such retention of uremic toxins, anemia, abnormalities in bone mineral metabolism, and increased inflammatory states which likely contribute to the higher risk of CVD in CKD.[7-10]
It has been speculated that variations in diet and lifestyle may contribute to an individual’s risk for CVD, and that light and moderate wine consumption may potentially alleviate such deleterious cardiovascular effects. Indeed, Renaud et al.’s seminal studies in the 1990s on the French paradox, found a relatively low incidence of cardiovascular disease in middle-aged French men, despite a relatively high dietary intake of saturated fats, potentially attributable to the consumption of red wine.[11] Subsequent observational studies, including in the U.S. and other populations, have demonstrated similar positive biological and clinical associations of light-to-moderate wine consumption on CVD and mortality.[12-14] Collectively, the available evidence suggest that light-to-moderate wine consumption may confer some degree of protection from CVD risk. Considering the strong link between CVD and CKD, we hypothesized that wine consumption would be associated with a lower odd of CKD and with a lower odd of CVD in patients with CKD. Therefore, the present study evaluated the prevalence of CKD according to wine consumption, and the odds of CVD in subjects with and without CKD using the National Health and Nutrition Examination Survey (NHANES), a large representative study of the U.S. population.
METHODS
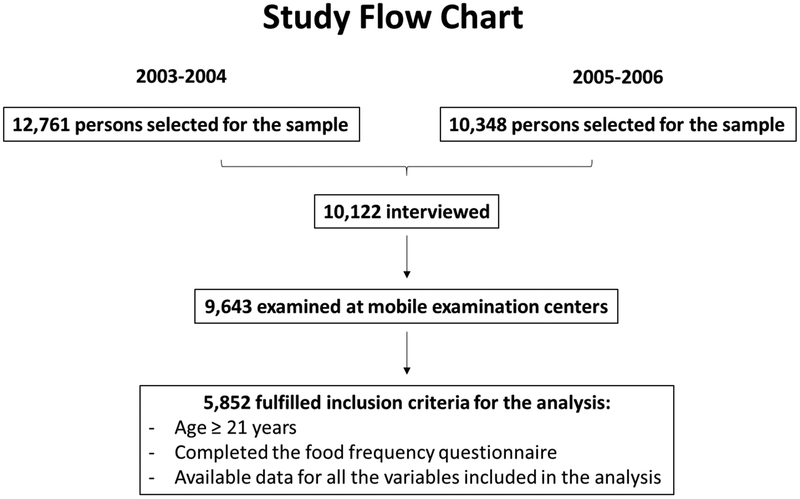
We performed a cross-sectional analysis of adult NHANES participants between the years 2003 to 2006 with the primary aim of evaluating the association between wine consumption and the prevalence of CKD. A secondary goal of the analysis was to evaluate the association between wine intake and cardiovascular disease in subjects with and without CKD. As shown in the flow chart in Figure 1, NHANES identified 12,761 persons for the sample in 2003-2004 and 10,248 for the sample in 2005-2006. Of those, 9,643 persons had completed the interview and physical examination and were eligible for inclusion in the analysis. [15, 16] In order to meet inclusion criteria for the analysis, subjects were required: 1) to be 21 years of age or older, 2) to have responded to the food frequency questionnaire, and 3) to have data available to be labeled for sex, race, age, education, household income, waist circumference, hypertension, HDL cholesterol, triglycerides, and kidney disease. Of the original sample, 5,852 subjects fulfilled these inclusion criteria. Diabetes mellitus was defined as diagnosed by a physician or the use of diabetic medications. CVD was defined as a positive response to the questions regarding history of CVD including angina, myocardial infarction, and stroke. Prevalent CKD was defined as urinary albumin/creatinine ratio (UACR) of ≥30 mg/g or eGFR of <60 mL/min/1.73m2 calculated using the Modification of Diet in Renal Disease study formula. [1] Hypertension was defined as taking blood pressure medications or as an average systolic blood pressure of >140 mmHg or average diastolic blood pressure of >90 mmHg. Wine intake was extracted from the NHANES food frequency questionnaire data and included wine and wine coolers. For purposes of this investigation, wine consumption was categorized as none (0 glass/day), light (< 1 glass per day), or moderate (≥ 1 glasses per day). [17]
Figure 1: Study flow chart.
Of the 9,643 persons eligible for the inclusion in the analysis, 5,852 met inclusion criteria, which included an age >21 years, completion of the food frequency questionnaire and data on all anthropometric and socioeconomic characteristics
To account for the complex sample strategy of NHANES, appropriate 4 year weights and strata were applied. Statistical analysis software 9.3 PROC SURVEYREG and SURVEYFREQ was used to obtain descriptive statistics for the population. All statistical tests were two-tailed (α = 0.05). Characteristics of the population were compared among wine consumption categories using the Rao-Scott Chi Square for survey-adjusted categorical variables and ANOVA for continuous variables. Parametric analysis was utilized as the data is normally distributed. We performed logistical regression analysis to determine if light wine consumption was associated with prevalent CKD and CVD. Due to the small number of subjects with moderate wine consumption (n=27), this group was not included in the regression analysis. If the overall test was significant for variables with more than 2 levels, p-values for comparisons between groups are presented as odds ratios with 95% confidence intervals.
RESULTS
General characteristics according to wine consumption:
A total of 5,852 participants met inclusion criteria for the study. The majority (n=3,370; 57.5%) reported no wine intake, whereas 2,455 (42%) participants reported light wine intake. Compared to individuals who did not drink wine, light wine drinkers were significantly younger, had lower prevalence of diabetes, hypertension, and CVD, had a higher mean waist circumference, and lower HDL-cholesterol (Table 1).
Table 1.
Characteristics of participants according to wine consumption
| None (n = 3,370) |
Light (n = 2,455) |
Moderate (n = 27) |
p-value* | |
|---|---|---|---|---|
| Age, mean (years) | 49 ± 0.5 | 48 ± 0.5 | 48 ± 2.7 | 0.02 |
| Male gender (%) | 50 ± 1.0 | 44 ± 0.6 | 50 ± 11.7 | 0.0009 |
| Non-Hispanic white (%) | 73 ± 2.6 | 79 ± 1.8 | 91 ± 4.7 | <0.0001 |
| Diabetes (%) | 11 ± 0.7 | 6 ± 0.6 | 3 ± 3.0 | <0.0001 |
| Hypertension (%) | 43 ± 1.7 | 35 ± 1.2 | 40 ± 11.3 | <0.0001 |
| Waist circumference (cm) | 52 ± 0.4 | 57 ± 0.4 | 65 ± 5.4 | <0.0001 |
| HDL (mg/dL) | 100 ± 0.4 | 96 ± 0.5 | 95 ± 2.2 | <0.0001 |
HLD = high-density lipoprotein, CVD = cardiovascular disease
Categorical variables are reported as survey weighted %. Continuous variables are reported as mean ± SD. None, defined as 0 glass/day; light wine consumption, defined as <1 glass/day; moderate wine consumption, defined as ≥1 glass/day
p-values presented test for overall differences across wine-consumption categories
The prevalence of CKD based on wine consumption categories:
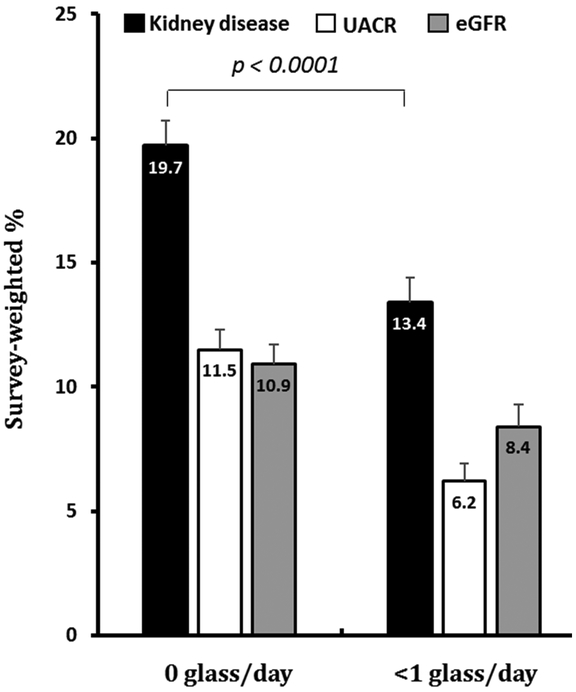
1,031 (18%) participants had CKD. The prevalence of CKD was significantly lower in subjects who reported light wine consumption compared to non-drinkers (13% vs 20%, p < 0.0001). Light wine drinkers in CKD were also less likely to have UACR ≥30 mg/g or eGFR <60 mL/min/1.73m2 (Figure 2). In the unadjusted analysis, the odds ratio (OR) of having CKD was 0.63 lower for those with light wine consumption compared to those who did not drink any wine (CI 0.54-0.74, p < 0.0001). This association remained significant after adjusting for demographics (OR 0.67, CI 0.55-0.81, p = 0.0001) and CV risk factors (OR 0.75, 0.62-0.91, p = 0.02). Other variables that were associated with CKD in the multivariate model are shown in Supplemental Table 1 and include: sex (females), age, diabetes, and hypertension. As shown in Table 2, when analyzing the components used to diagnose CKD separately, participants who reported wine consumption were significantly less likely to have UACR <30 mg/g independently of demographics and other CV risk factors (OR 0.62, CI 0.44-0.87, p = 0.006). Although light wine consumption was associated with lower odds of eGFR < 60 mL/min/1.73m2 in unadjusted analysis, this association was attenuated and no longer significant after adjusting for demographics or CV risk factors.
Figure 2: Light wine intake is associated with a lower prevalence of CKD relative to non-wine drinkers.
CKD = chronic kidney disease, UACR = urine albumin/creatinine ratio (≤30 mg/g), eGFR = estimated glomerular filtration rate (<60 mL/min/1.73m2). Variables are shown as a survey weighted %. None is defined as 0 glass/day, light wine consumption is defined as <1 glass/day and moderate wine consumption is defined as ≥1 glass/day. Overall-effect p value < 0.0001. Values are mean ± SD.
Table 2.
Association between light wine consumption and CKD
| UACR ≥30 | eGFR <60 | CKD* | ||||
|---|---|---|---|---|---|---|
| Odds ratio (CI) |
p-value | Odds ratio (CI) |
p-value | Odds ratio (CI) |
p-value | |
| Unadjusted | 0.51 (0.38-0.68) |
<0.0001 | 0.75 (0.61-0.92) |
0.006 | 0.63 (0.54-0.74) |
<0.0001 |
| Adjusted for demographics** |
0.56 (0.40-0.77) |
0.0004 | 0.83 (0.65-1.07) |
0.35† | 0.67 (0.55-0.81) |
<0.0001 |
| Adjusted for demographics and CV risk factors‡ |
0.62 (0.44-0.87) |
0.006 | 0.94 (0.72-1.22) |
0.87† |
0.75 (0.62-0.91) |
0.004 |
Light wine consumption defined as <1 glass per day; reference defined as no wine consumption
UACR = urine albumin/creatinine ratio, eGFR = estimated glomerular filtration rate
CKD, defined as either UACR ≥30 or eGFR <60
Demographics: age, gender, race
CV risk factors: waist size, HDL, triglycerides, diabetes, hypertension
Odds of CVD with wine consumption:
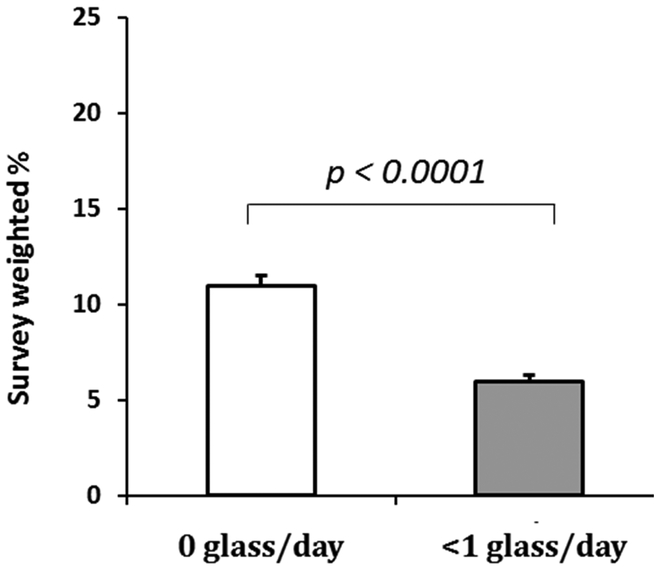
Of those with CKD, 666 (11%) participants reported a history of CVD. As shown in Figure 3, the prevalence of CVD was lower in those individuals who drank light wine compared to non-drinkers (5.8% vs 10.7%, p < 0.0001). In the unadjusted analysis, those who reported light wine consumption had approximately half the odds of CVD compared to those who did not drink wine (CI 0.42-0.64, p < 0.0001) as shown in Table 3. This association remained significant after adjusting for demographics and CV risk factors as shown in Table 3. In the final multivariate model (data not shown), male gender, older age, diabetes, and hypertension were significantly associated with self-reported CVD.
Figure 3: Light wine intake is associated with a lower prevalence of CVD in CKD relative to non-wine drinkers.
Variables are shown as a survey weighted %. None is defined as 0 glass/day, light wine consumption is defined as <1 glass/day and moderate wine consumption is defined as ≥1 glass/day. Overall-effect p value < 0.0001. Values are mean ± SD.
Table 3.
Association between light wine consumption and CVD
| All participants OR (CI, p value) |
CKD absent OR (CI, p value) |
CKD present OR (CI, p value) |
|
|---|---|---|---|
| Unadjusted | 0.51 (0.42-0.64, p <0.0001) |
0.50 (0.37-0.6, p <0.0001) |
0.72 (0.52-0.99, p = 0.046) |
| Adjusted for demographics** |
0.55 (0.44-0.69, p <0.0001) |
0.52 (0.39-0.71, p <0.0001) |
0.65 (0.48-0.89, p = 0.008) |
| Adjusted for demographics and CV risk factors† |
0.63 (0.61-0.79, p <0.0001) |
0.60 (0.45-0.81, p = 0.001) |
0.71 (0.53-0.95, p = 0.02) |
Light wine consumption defined as <1 glass per day; reference defined as no wine consumption
CKD, defined as either UACR ≥30 or eGFR <60
Demographics: age, gender, race
CV risk factors: waist size, HDL, triglycerides, diabetes, hypertension
Odds of CVD with wine consumption based on CKD status:
Results after stratifying subjects by CKD status are presented in Table 3. Findings for those without CKD were similar to the overall group where light wine consumption was associated with lower odds of CVD compared to reported abstinence even after adjusting for demographics and CV risk factors (OR 0.60, CI 0.45-0.81, p = 0.001). For subjects with CKD, light wine consumption was similarly associated with lower odds of CVD after adjusting for demographics and CV risk factors. Other variables that were associated with increased odds of self-reported CVD in the multivariate model included: age, diabetes, and hypertension. Female sex was associated with significantly lower odds of CVD in those with CKD in the multivariate model. These data are shown in Supplemental Table 2. Of note, in subjects with CKD, the odds of CVD with light wine consumption remained significantly lower than in those who did not drink any wine after adjusting for level of education and household income (OR 0.78, p = 0.04).
DISCUSSION
In this analysis, we evaluated self-reported intake of wine in NHANES, a large representative sample of the U.S. population. We found that the majority of the U.S. population reported either no or light intake of wine (defined as < 1 glass per day). Our analysis, furthermore, indicated that light wine consumption is associated with lower odds of CKD. Lastly, and most notably, in subjects with CKD, light wine consumption was associated with significantly lower odds of CVD independent of demographics, CVD risk factors and socio-economic factors. Collectively, these findings are important considering the high risk of CVD in patients with CKD, as they highlight that light wine consumption (i.e. < 1 glass per day) may reduce CVD in CKD. [2-4]
Previous data have indicated moderate wine consumption associates a lower risk of CVD.[11-14, 18] These epidemiological observations have often been attributed to the cardiovascular benefit of red wine; specifically the polyphenol content of red wine.[19-21] Polyphenol compounds may decrease oxidative stress, enhance cholesterol efflux from vessel walls (mainly by increasing levels of HDL), and inhibit lipoproteins oxidation, macrophage cholesterol accumulation, and foam-cell formation.[22] They may also increase nitric oxide bioavailability, thereby antagonizing the development of endothelial dysfunction. [23] However, it is important to note that most of the published epidemiological studies did not distinguish between red and white wine intake.[11, 13, 18] In addition, studies that did examine red and white wine intake reported similar risk reduction for white and red wines, suggesting that other factors than red wine components may be at play.[14, 24] Furthermore, although phenolic compounds exist in white wine in smaller amounts, there is data to suggest that the quantity/volume of ingested polyphenols may be less important than the biological activity.[25, 26] Our results appear to raise an intriguing question; that is, does light (<1 glass/day) wine consumption elicit a greater cardiovascular benefit and reduced all-cause mortality compared to the widely accepted, and highly investigated, moderate (≥1 glass/day) wine consumption? Future studies will be needed to fully elucidate this running hypothesis.
With regards to the association between light wine intake and CKD, we are unaware of other studies that have explored this potential association. Earlier analyses from NHANES (1976-1980) reported no association between alcohol consumption and CKD.[27] However, the earlier study did not evaluate wine consumption specifically, as we have done, which may explain our discrepant findings. Much like the link between wine intake and CVD, some data suggest that the polyphenol content of the wine may modify the disease process in the kidney itself. Resveratrol, for example, has been shown to reduce fibrosis in animal models of CKD.[28-30] Additionally, in animal models of diabetic kidney disease, resveratrol has been shown to reduce hyperglycemia-mediated oxidative stress and inflammation leading to slower kidney disease progression in these models.[31-34] Of interest, in human subjects with stage 3 and 4 CKD, there is data to suggest white wine compounds may reduce markers of inflammation including C-reactive protein and inlerleukin-6. [35] Unfortunately, we were unable to evaluate the potential association between wine consumption and markers of inflammation in this analysis. In addition, due to the cross sectional nature of the study, we are unable to evaluate CKD progression. Rather, we found an independent association between wine intake and albuminuria. Considering that albuminuria is a marker of endothelial dysfunction, it is likely that our findings reflect the potential benefit of wine intake on the vasculature rather than on the kidney. Consistent with that, the observed lower odds of CVD with light wine consumption was observed in both groups with and without CKD, suggesting that this effect may be independent of CKD. Alternatively, wine compounds have been shown to inhibit common mediators of both CVD and CKD such as platelet activating factor [36, 37]. As such, it is possible that the effects of wine compounds include both CVD and CKD by targeting common pathways between both conditions.
Our study has some limitations, including its cross-sectional design from which we cannot derive causation. In addition, because we relied on participants’ reports, recall and reporting bias have the potential to introduce spurious associations.[27] Moreover, we used three categories of wine consumption, but we could not account for the exact amount of wine consumption due to the limitations of the questionnaire nor were we able to compare light with moderate wine consumption due to the small number of subjects in the latter group. Finally, we did not account for other forms of alcohol intake, as some studies have suggested alcohol intake in general (not just wine) may associate with cardiovascular benefits. [38] However, recent data indicate no net benefit from alcohol intake itself, rather such data are likely attributable to inappropriate selection of “abstainers” who had a higher burden of disease in general.[38, 39] Notwithstanding these limitations, strengths of the present study include a large population consisting of light wine drinkers, generalizability to the overall population, and the ability to define CKD and adjust for socioeconomic factors. Lastly, we should acknowledge that, while we found light drinkers to have lower odds of CKD and CVD than non-drinkers, the results should be interpreted with caution, as it may be unwise for non-drinkers to take up drinking wine as a means of lowering their risk. Data on the renal effects of light to moderate alcohol consumption in general are scarce, however, heavier alcohol use is associated with negative outcomes including hypertension and end stage renal disease.[40, 41]
In conclusion, our data demonstrate that light wine consumption (compared to abstinence) is associated with lower prevalence of CKD and a lower odd of CVD in those with CKD within the U.S. population. Additionally, we have found that light wine consumption associates with lower odds of albuminuria. Collectively, these findings suggest that the potential cardiovascular benefits of light wine consumption may extend to those with CKD. Future prospective cohort studies will be needed to better understand the potential mechanisms that may underlie these findings.
Supplementary Material
Highlights.
Analysis of the National Health and Nutrition Examination Survey, a large representative sample of the U.S. population
Evaluation of the association between light wine consumption and CVD and CKD
Light wine consumption is associated with a lower prevalence of CKD
Light wine consumption is associated with a lower odd of CVD in those with CKD
Acknowledgments
We would like to thank Dr. Kim McFann and Dr. Pamela Mettler for conducting the statistical analysis for this manuscript.
Funding
This work was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare that there are no conflicts of interest
References
- [1].Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659–63. [DOI] [PubMed] [Google Scholar]
- [3].Gargiulo R, Suhail F, Lerma EV. Cardiovascular disease and chronic kidney disease. Disease-a-month : DM. 2015;61:403–13. [DOI] [PubMed] [Google Scholar]
- [4].Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- [5].Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol 2005;16:529–38. [DOI] [PubMed] [Google Scholar]
- [6].Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clinic proceedings. 2005;80:1270–7. [DOI] [PubMed] [Google Scholar]
- [7].Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney international. 2002;62:1524–38. [DOI] [PubMed] [Google Scholar]
- [8].Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999;34:125–34. [DOI] [PubMed] [Google Scholar]
- [9].Tomiyama C, Higa A, Dalboni MA, Cendoroglo M, Draibe SA, Cuppari L, et al. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:2464–71. [DOI] [PubMed] [Google Scholar]
- [10].Arici M, Walls J. End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney international. 2001;59:407–14. [DOI] [PubMed] [Google Scholar]
- [11].Renaud SC, Gueguen R, Siest G, Salamon R. Wine, beer, and mortality in middle-aged men from eastern France. Arch Intern Med 1999;159:1865–70. [DOI] [PubMed] [Google Scholar]
- [12].Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, De Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation. 2002;105:2836–44. [DOI] [PubMed] [Google Scholar]
- [13].Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, et al. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med 2000;133:411–9. [DOI] [PubMed] [Google Scholar]
- [14].Klatsky AL, Friedman GD, Armstrong MA, Kipp H. Wine, liquor, beer, and mortality. Am J Epidemiol 2003;158:585–95. [DOI] [PubMed] [Google Scholar]
- [15].Health USDo, Control HSCfD, Statistics PNCfH. National Health and Nutrition Examination Survey (NHANES), 2005-2006 Inter-university Consortium for Political and Social Research [distributor]; 2012. [Google Scholar]
- [16].Health USDo, Control HSCfD, Statistics PNCfH. National Health and Nutrition Examination Survey (NHANES), 2003-2004 Inter-university Consortium for Political and Social Research [distributor]; 2016. [Google Scholar]
- [17].Agriculture UDoHaHSaUDo. 2015-2020 Dietary Guidelines for Americans Appendix 9. Alcohol In: Agriculture UDoHaHSaUDo, editor. 8th ed. Washington, D.C.2015. [Google Scholar]
- [18].St Leger AS, Cochrane AL, Moore F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet. 1979;1:1017–20. [DOI] [PubMed] [Google Scholar]
- [19].Cruz MN, Luksha L, Logman H, Poston L, Agewall S, Kublickiene K. Acute responses to phytoestrogens in small arteries from men with coronary heart disease. Am J Physiol Heart Circ Physiol 2006;290:H1969–75. [DOI] [PubMed] [Google Scholar]
- [20].Leikert JF, Rathel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation. 2002;106:1614–7. [DOI] [PubMed] [Google Scholar]
- [21].Opie LH, Lecour S. The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J 2007;28:1683–93. [DOI] [PubMed] [Google Scholar]
- [22].Kerry NL, Abbey M. Red wine and fractionated phenolic compounds prepared from red wine inhibit low density lipoprotein oxidation in vitro. Atherosclerosis. 1997;135:93–102. [DOI] [PubMed] [Google Scholar]
- [23].Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–8. [DOI] [PubMed] [Google Scholar]
- [24].Fragopoulou E, Choleva M, Antonopoulou S, Demopoulos CA. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism. 2018;83:102–19. [DOI] [PubMed] [Google Scholar]
- [25].Fragopoulou E, Antonopoulou S, Demopoulos CA. Biologically active lipids with antiatherogenic properties from white wine and must. J Agric Food Chem 2002;50:2684–94. [DOI] [PubMed] [Google Scholar]
- [26].Migliori M, Cantaluppi V, Mannari C, Bertelli AA, Medica D, Quercia AD, et al. Caffeic acid, a phenol found in white wine, modulates endothelial nitric oxide production and protects from oxidative stress-associated endothelial cell injury. PLoS One. 2015;10:e0117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology (Cambridge, Mass). 2003;14:479–87. [DOI] [PubMed] [Google Scholar]
- [28].Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 2010;177:1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang XZ, Wen D, Zhang M, Xie Q, Ma L, Guan Y, et al. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-beta/Smad3 pathway. Journal of cellular biochemistry. 2014;115:996–1005. [DOI] [PubMed] [Google Scholar]
- [30].Chen KH, Hung CC, Hsu HH, Jing YH, Yang CW, Chen JK. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-beta/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem Biol Interact 2011;190:45–53. [DOI] [PubMed] [Google Scholar]
- [31].Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact 2010;186:200–10. [DOI] [PubMed] [Google Scholar]
- [32].Palsamy P, Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Chem Biol Interact 2009;179:356–62. [DOI] [PubMed] [Google Scholar]
- [33].Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol 2010;224:423–32. [DOI] [PubMed] [Google Scholar]
- [34].Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta 2011;1812:719–31. [DOI] [PubMed] [Google Scholar]
- [35].Migliori M, Panichi V, de la Torre R, Fito M, Covas M, Bertelli A, et al. Anti-inflammatory effect of white wine in CKD patients and healthy volunteers. Blood Purif. 2015;39:218–23. [DOI] [PubMed] [Google Scholar]
- [36].Argyrou C, Vlachogianni I, Stamatakis G, Demopoulos CA, Antonopoulou S, Fragopoulou E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins Other Lipid Mediat 2017;130:23–9. [DOI] [PubMed] [Google Scholar]
- [37].Wang Y, Li SS, Na SP, Yu CY, Ji Y, Zhao SL, et al. Characterization of Lipoprotein-associated Phospholipase A2 in Serum in Patients With Stage 3-5 Chronic Kidney Disease. Am J Med Sci 2016;352:348–53. [DOI] [PubMed] [Google Scholar]
- [38].Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ (Clinical research ed). 2015;350:h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stockwell T, Zhao J, Panwar S, Roemer A, Naimi T, Chikritzhs T. Do "Moderate" Drinkers Have Reduced Mortality Risk? A Systematic Review and Meta-Analysis of Alcohol Consumption and All-Cause Mortality. J Stud Alcohol Drugs. 2016;77:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Klatsky AL, Friedman GD, Siegelaub AB, Gerard MJ. Alcohol consumption and blood pressure. Kaiser-Permanente Multiphasic Health Examination data. N Engl J Med 1977;296:1194–200. [DOI] [PubMed] [Google Scholar]
- [41].Perneger TV, Whelton PK, Puddey IB, Klag MJ. Risk of end-stage renal disease associated with alcohol consumption. American journal of epidemiology. 1999;150:1275–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.