Abstract
Introduction
Reducing the level of nicotine in cigarettes is a regulatory strategy that has the potential to greatly improve public health. If nicotine levels are reduced in all commercially available cigarettes, current smokers might find it easier to quit and young people might be less likely to become dependent. However, it is not yet known whether age moderates subjective or behavioral responses to low-nicotine cigarettes.
Methods
Recently, a large, multisite randomized clinical trial was conducted to compare the effects of cigarettes differing in nicotine content (either usual-brand or research cigarettes containing 15.8, 5.2, 2.4, 1.3, or 0.4 mg nicotine/g tobacco) across 6 weeks of exposure. In this secondary analysis, we tested whether age moderated smokers’ subjective (measures of psychological reward, smoking satisfaction) and behavioral (cigarettes smoked per day, smoking topography, and nicotine exposure) responses to cigarettes varying in nicotine content after 2 and 6 weeks of use, while controlling for baseline dependence and demographic factors.
Results
Results indicated that younger adults (age 18–24) who smoked cigarettes with 2.4–0.4 mg/g nicotine reported significantly less smoking satisfaction and psychological reward, and smoked fewer cigarettes per day, than older adults (25+ years) after 2 weeks of use. No differences in topography were observed at either time point. After 6 weeks of use, differences had diminished on all measures.
Conclusions
The reduced positive effects of reduced-nicotine content cigarettes in young adults suggests that this regulatory policy may reduce smoking reinforcement in this vulnerable population.
Implications
As the FDA considers reducing the level of nicotine in cigarettes to make them less addictive, understanding the potential impact of this policy on young people is of crucial importance. We found that young adults had significantly lower positive subjective effects to very-low nicotine content (VLNC) cigarettes and smoked fewer VLNC cigarettes than older adults after 2 weeks of use, indicating that this policy may reduce smoking reinforcement more quickly in young adults. These data add to the growing body of evidence on the potential for this policy to positively affect public health.
Introduction
Despite recent reduction in smoking rates in the United States, the health effects of cigarette smoking continue to claim the lives of hundreds of thousands of Americans each year.1 The Family Smoking Prevention and Tobacco Control Act2 gave the FDA regulatory authority to dramatically reduce (though not eliminate) the amount of nicotine in cigarettes sold in the United States, with the goal of promoting public health. The FDA recently announced its intention to pursue a reduced-nicotine standard for cigarettes.3 By reducing the level of nicotine, the primary addictive agent in cigarettes, to a minimally addictive level, this policy could render cigarettes less reinforcing, facilitate quitting, and improve smokers’ responses to cessation treatments and other public health approaches to reducing smoking.4–6 Thus, this strategy has the potential to help current smokers quit and to prevent experimenting smokers from becoming addicted to combustible cigarettes.
Reinforcement from smoking is comprised of both primary reinforcement from nicotine and conditioned reinforcement from the sensorimotor aspects of smoking (eg, taste, smell, etc.) that have been associated with nicotine delivery.7 Thus, although verylow nicotine content (VLNC) cigarettes are rated as less satisfying than normal nicotine content cigarettes, they can partially alleviate craving and withdrawal symptoms and function as effective reinforcers for smokers in the absence of alternatives.8–11 These conditioned reinforcing effects likely undergo extinction with repeated use of VLNC cigarettes, resulting in fewer cigarettes smoked per day, decreases in dependence, and increases in cessation attempts.10,12,13
As regulatory authorities determine whether to move forward with a nicotine reduction policy, one important group that must be considered is young people. Young adults (18–24 years old) have the highest prevalence of smoking of any age group in the United States (26.7% in 201514). This developmental period is crucial in the lifetime trajectory of smoking, as many lighter smokers in this period of life quit (51%15), but those who persist through this period are likely to smoke well into adulthood. During this developmental stage smoking behavior may be particularly malleable, so such a policy may have its greatest impact among younger smokers.16,17 However, it is not yet known whether young adults may differ in their subjective and behavioral response to VLNC cigarettes relative to older adults.
Young adults (age 18–24) report smoking patterns that are quite different from older adults. Young adults smoke fewer cigarettes on average than older adults, and have shorter histories of smoking.16 A reduction in the nicotine content of cigarettes may differentially impact this group, as the strength of the conditioned reinforcing effects of smoking-related sensory stimuli may depend on length and intensity of smoking history.18 In support of this idea, smoking cue reactivity studies among young adults have shown greater cue-elicited craving among daily smokers relative to occasional smokers, and similar studies in adolescents have shown greater responses to smoking cues in adolescents with more smoking experience relative to their peers with less smoking experience.19,20 Because young adults have a shorter history of smoking than older adults, they may experience less conditioned reinforcement from the sensorimotor characteristics of smoking and find VLNC cigarettes less satisfying, which may hasten the transition away from smoking.
Data for the current study were drawn from a recent, large, multisite randomized controlled trial designed to evaluate the effects of reducing the nicotine content of cigarettes on smoking behavior.12 The study examined the effects of 6-week exposure to research cigarettes of varying levels of nicotine, as well as usual-brand cigarettes, across groups, and collected data on the behavioral and subjective effects of these cigarettes. Overall, relative to those in the control groups, smokers assigned to lower nicotine content cigarettes smoked fewer cigarettes per day and had lower levels of dependence at the end of 6 weeks. These results highlight the potential promise of this regulatory approach.
The aim of the current study was to determine the extent to which age would moderate relationships between nicotine content and smoking across six weeks of exposure in the Donny et al.12 study. We examined the extent to which age moderated the effects of nicotine content on subjective responses in the laboratory, which included measures of psychological reward, smoking satisfaction, and aversiveness of the cigarettes; as well as behavioral and biochemical responses to smoking the cigarettes ad lib in the natural environment, which included total cigarettes smoked per day (including both compliant use of study cigarettes and noncompliant use of nonstudy cigarettes), and total nicotine equivalents (TNEs), a measure of total exposure to nicotine. We were interested in determining whether subjective effects of cigarettes differing in nicotine content would be moderated by age, and whether any differences in subjective effects would be reflected in a difference in behavioral and/or biochemical response.
As a secondary aim, we also examined smoking topography in the laboratory, to determine whether age moderated any potential effects of nicotine group on total puff volume, a measure of compensatory smoking behaviors. Unlike ‘light’ cigarettes, the cigarettes used in this study contain less nicotine in the tobacco itself, and therefore complete ‘compensation’ for the lower amount of nicotine by altering puffing behavior is not achievable. The evidence to date indicates that very little compensatory smoking behavior occurs with these cigarettes.21 However, changes in nicotine content may lead to changes in cigarette subjective effects (eg, less “throat hit”) that could increase puffing behavior and therefore increase toxicant exposure. Given this important safety concern, we examined this outcome for evidence of a differential effect of age across nicotine groups.
Methods
The current study is a secondary analysis of data collected in a large, double-blind randomized controlled trial conducted at ten sites across the United States. Full methods and primary results are available in Donny et al.12
Participants
Participants in this study were 839 adults aged 18–65, recruited from the community and randomized to receive study cigarettes; 780 participants were retained to completion in the study. To be included in the study, participants had to report smoking a minimum of 5 cigarettes a day and have a confirmed expired breath carbon monoxide (CO) reading of 8 ppm or a urine cotinine level of at least 100 ng/mL. Exclusion criteria included serious, unstable medical and/or psychiatric conditions; pregnancy, breastfeeding or intention to become pregnant; evidence of current illicit drug use other than marijuana use on a urine toxicological screen; intention to quit smoking in the next 30 days; use of tobacco products other than cigarettes on more than 9 days in the past month and exclusive use of roll-your-own cigarettes. Of those screened and not excluded for alcohol or drug use, or for not being a smoker (ie, low CO value), 4.0% of young adults (18–24) and 4.3% of adults over age 24 were ineligible due to using other tobacco products on more than 9 of the last 30 days (p > .99).
Baseline Phase
Following an initial eligibility screening in the laboratory, participants underwent a week-long baseline period, during which they smoked as usual. A first baseline session (Baseline 1) was then conducted in which participants filled out questionnaires (detailed below). Following this session, participants continued to smoke their usual brand for another week, during which an interactive voice response (IVR) system (InterVision Media) called the participants daily and a recording asked participants to enter the number of cigarettes they had smoked the previous day. At the end of this week, a second baseline session (Baseline 2) was conducted, and during this session, participants filled out further questionnaires and reported on their craving, withdrawal and other symptoms. During this session, they also smoked a cigarette of their usual brand and reported on their subjective responses to their usual-brand cigarette.
Experimental Phase
At the end of the second baseline session, participants were randomized to one of seven groups for the duration of the study: a usual-brand condition or one of six research cigarette conditions. Each participant was asked to smoke only the cigarettes provided to them by the study for the next 6 weeks. In the current analysis, we focus on the data from the laboratory sessions that occurred after 2 and 6 weeks of research cigarette use, respectively. During these sessions, participants were asked to smoke one of their assigned study cigarettes, during which puff topography measures were collected, and to report on their subjective responses to the cigarette after smoking. Throughout the 6-week experimental phase, participants continued to receive daily calls from the IVR system, which asked them to report on their previous days’ use of study and nonstudy cigarettes. The IVR system was also used to collect self-reported withdrawal symptoms and craving during the first week after randomization.
Research Cigarettes
All cigarettes were Spectrum brand research cigarettes, produced by 22nd Century Group and provided free of cost by the National Institute of Drug Abuse (NOT-DA-14-004). Research cigarettes had the following nicotine contents expressed as milligrams of nicotine per gram of tobacco: 15.8, 5.2, 2.4, 1.3, and 0.4 (all tar yields were 9 ± 1.5 mg). An additional exploratory group received research cigarettes with 0.4 mg/g of nicotine and 13 ± 2 mg tar; however, this group was excluded from the current analyses as they differed from controls in both tar and nicotine yield. Participants were assigned to either menthol or nonmenthol cigarettes according to their usual-brand preference. Each week, participants were distributed a 14-day supply of cigarettes (average cigarettes per day reported at baseline times 14) to allow for detection of increases in consumption or in case visits occurred more than 7 days apart, and completed questionnaires and behavioral and cognitive testing in the laboratory.
Measures
Cigarette Evaluation Scale (CES)
The CES22 is a 12-item questionnaire measuring aspects of cigarette subjective effects. Each question is measured on a Likert scale from 1 to 7 (not at all to extremely). The CES is comprised of five subscales: Smoking Satisfaction (was smoking satisfying, did smoking taste good, did you enjoy smoking); Psychological Reward (did smoking calm you down, did smoking make you feel more awake, feel less irritable, help you concentrate, reduce your hunger); Aversion (did smoking make you dizzy or nauseous), as well as the single-item assessments of Enjoyment of Respiratory Tract Sensations (did you enjoy the sensations in your throat and chest); and Craving Reduction (did smoking immediately reduce your craving for smoking). The CES was administered at Baseline 2 after participants smoked a single cigarette of their usual brand, and participants were instructed to respond about that cigarette. At Week 2 and Week 6, the CES was administered after participants smoked one of their assigned study cigarettes, and they were instructed to answer the questions about their study cigarette.
Cigarettes per Day
The number of study and total (study plus nonstudy) cigarettes was calculated from the IVR telephone reports.
Demographics
At baseline, participants were asked questions about their race, gender, and age.
Fagerström Test for Nicotine Dependence (FTND)
At baseline, participants were administered the seven-item FTND.23
Total Nicotine Equivalents (TNEs)
At Weeks 2 and 6, participants provided first void urine samples which were later analyzed for total nicotine equivalents (nicotine exposure).24
Total Puff Volume
During the Baseline 2, Week 2 and Week 6 visits, participants were asked to smoke a cigarette through a handheld smoking topography capturing device (CReSS, Borgwaldt KC, Richmond VA). The primary measure was total puff volume, which is the sum of the volume of all puffs taken while smoking the cigarette. During Baseline 2, the cigarette smoked was their usual brand; during Week 2 and 6, the cigarette smoked was their assigned study cigarette. Thus, at Week 2, when topography measures from the study cigarette were initially collected, participants had prior experience with the device.
Statistical Analysis
There were 716 subjects included in this analysis, which excludes the participants enrolled in the low-nicotine/high-tar group. Of these, 664 participants completed all 6 weeks of the study. To maximize statistical power to detect interactions between age and nicotine content, data from the three very-low nicotine content conditions (2.4–0.4 mg/g nicotine), which had similar effects in Donny et al.,12 were combined (VLNC condition) and compared to the 5.2 mg/g, 15.8 mg/g (normal nicotine content; NNC), and usual-brand (UB) conditions. The NNC and UB control conditions were both included because the NNC condition helps control for the effects of brand switching generally—that is, participants in the VLNC cigarette conditions were not only exposed to cigarettes with lower levels of nicotine, but also to cigarettes with different carton and pack labels, flavor, sensory characteristics, etc. than their usual brand. Demographic characteristics (age, FTND) and other variables (CES subscales, CPD and TNEs) were summarized by standard descriptive statistical methods and compared across age group category using t-tests.
Linear regression was used to analyze the interaction between age (dichotomized as 18–24 years old vs. 25+ years old) and cigarette condition for each outcome variable. The outcome variables were comprised the following subscales: Smoking Satisfaction, Psychological Reward, Enjoyment of Respiratory Tract Sensations, Aversion, and Craving Reduction; as well as total (study plus nonstudy) CPD, total puff volume, and total nicotine equivalents (TNEs). All outcomes were analyzed at both Week 2 and Week 6. TNE values were analyzed on the natural log scale due to their highly skewed distribution; no other outcome variables were transformed.24 The linear regression model for each outcome variable at each time point included nicotine group, FTND score at baseline, race, gender, nicotine metabolite ratio (NMR) at baseline, the corresponding score for each outcome at baseline in order to increase precision, and interaction between age and nicotine group. FTND at baseline was included as a covariate because of significant differences between younger and older adults along this dimension, and because we planned to examine differences between age groups beyond the effect of differences in dependence. NMR was included as a covariate as it is associated with smoking topography, as well as with VLNC response in young adults.25,26 As a complementary approach, all models were also analyzed with age entered as a continuous variable, as opposed to dichotomizing between younger and older adults; as the pattern of results were similar these analyses are not reported. Finally, given that compliance with the study cigarettes may be differentially affected by age, data were re-analyzed using only the subset of participants who self-reported compliance with the study cigarettes across all groups.
Moderation by age was indicated by a significant overall age by nicotine group interaction. The effect of age was summarized in tables and figures by the difference in least squares means between age groups within nicotine groups. All statistical analyses were implemented using Statistical Analysis System software version 9.3 (SAS Institute Inc., Cary, NC). The cut-off significance level for all p values was α = 0.05.
Results
An overall description of the study sample is available in Donny et al.12 Demographic characteristics and descriptive summaries of each covariate and outcome variable by age category at baseline are shown in Table 1. Overall, as is commonly reported, younger adults in this sample smoked significantly fewer cigarettes relative to older adults and had correspondingly lower TNEs at baseline, as well as lower FTND scores. Younger adults also differed from older adults on some CES variables at baseline, with younger adults reporting greater psychological reward, enjoyment of respiratory tract sensations, and craving reduction from their usual-brand cigarettes than older adults.
Table 1.
Baseline Characteristics by Age Group Category
| Age Group | |||
|---|---|---|---|
| Characteristic | 18–24 | ≥25 | p value |
| N | 93 | 595 | |
| Gender (Number, % male) | 54 (58%) | 339 (57%) | 0.84 |
| Race—(Number, %) | <0.01 | ||
| White | 65 (70%) | 278 (47%) | |
| Black | 13 (14%) | 260 (44%) | |
| Other | 15 (16%) | 57 (10%) | |
| Pack years (Mean (SD)) | 2.9 (2.1) | 22.0 (16.6) | <0.01 |
| Cigarettes/day—no. (Study) | 12.2 (5.6) | 16.1 (7.8) | <0.01 |
| FTND (Mean (SD)) | 4.1 (2.0) | 5.3 (2.2) | <0.01 |
| Total puff volume at baseline (Mean (SD)) | 672 (268) | 748 (314) | 0.04 |
| Psychological Reward at baseline (Mean (SD)) | 3.0 (1.3) | 2.2 (1.5) | <0.01 |
| Smoking Satisfaction at baseline (Mean (SD)) | 4.0 (1.3) | 4.0 (1.3) | 0.97 |
| Enjoyment of Respiratory Tract Sensations at baseline (Mean (SD)) | 3.3 (1.8) | 2.7 (1.8) | 0.01 |
| Craving Reduction at baseline (Mean (SD)) | 4.1 (1.4) | 3.7 (1.9) | 0.04 |
| Aversion (Mean (SD)) | 0.5 (0.8) | 0.4 (0.7) | 0.12 |
| TNEs at baseline—geometric mean (range) | 27.1 (0.6–812.4) | 36.6 (0.1–992.3) | <0.01 |
| Nicotine Metabolite Ratio at Baseline (Mean (SD)) | 0.2 (0.1) | 0.3 (0.2) | 0.06 |
P values represent t-tests of differences across group. Subjective response scores were in reference to participants’ usual-brand cigarettes at baseline. Bold text indicates significant p value. FTND = Fagerström Test for Nicotine Dependence.
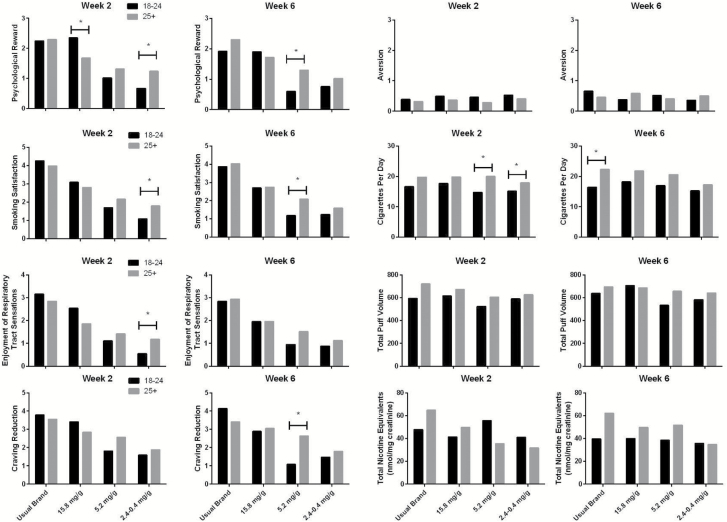
Week 2 Results
Results for outcomes collected at Week 2 are summarized in Table 2 and presented in Figure 1. The results showed significant interactions between age and nicotine condition on the CES Smoking Satisfaction, Psychological Rewards, and Enjoyment of Respiratory Sensations subscales, but no significant interactions on the Aversion or Craving Reduction subscales were found. Post hoc tests revealed that the younger and older adults differed in their responses to the 2.4–0.4 mg/g nicotine (VLNC) cigarettes on the Smoking Satisfaction, Psychological Reward and Enjoyment of Respiratory Tract Sensations subscales, such that younger adults had significantly lower scores on these scales than older adults in the 2.4–0.4 mg/g nicotine (VLNC) condition, whereas there was no difference or younger adults scored higher in the 15.8 mg/g (NNC) condition. Younger and older adults also differed in their responses to the NNC cigarettes on the Psychological Reward subscale, with younger adults having higher scores on this measure than older adults. In addition, post hoc tests revealed that the younger and older adults differed in their behavioral responses to the 2.4–0.4 mg/g nicotine (VLNC) cigarettes and 5.2 mg/g nicotine cigarettes on total cigarettes per day (study plus nonstudy), with the younger adults smoking fewer CPD, although the interaction between nicotine group and age on CPD was not significant (Table 2). No moderation of nicotine group effect by age was evident for total nicotine equivalents or total puff volume.
Table 2.
Mean and Standard Deviations for Differences at Week 2 Between Age Groups (Age 18–24 and Age ≥25) as a Function of Treatment Group
| Outcome | Interaction test p value |
Usual brand | 15.8 mg/g | 5.2 mg/g | 2.4–0.4 mg/g | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference | p value | Mean difference | p value | Mean difference | p value | Mean difference | p value | ||
| CES PR a | <0.01 | −0.05 (0.31) | 0.87 | 0.67 (0.29) | 0.02 | −0.29 (0.32) | 0.36 | −0.57 (0.17) | <0.01 |
| CES SS a | 0.03 | 0.29 (0.39) | 0.46 | 0.29 (0.35) | 0.42 | −0.47 (0.40) | 0.24 | −0.70 (0.21) | <0.01 |
| CES ERTS a | 0.01 | 0.31 (0.40) | 0.44 | 0.69 (0.36) | 0.06 | −0.31 (0.41) | 0.45 | −0.64 (0.22) | <0.01 |
| CES CR a | 0.25 | 0.24 (0.54) | 0.44 | 0.57 (0.49) | 0.25 | −0.76 (0.56) | 0.18 | −0.28 (0.30) | 0.34 |
| CES AV a | 0.99 | 0.06 (0.21) | 0.76 | 0.12 (0.19) | 0.52 | 0.18 (0.21) | 0.41 | 0.12 (0.11) | 0.30 |
| Total CPD a | 0.62 | −3.11 (1.89) | 0.10 | −2.12 (1.69) | 0.21 | −5.22 (1.87) | 0.01 | −2.75 (1.01) | 0.01 |
| Total puff volume a | 0.71 | −128 (70) | 0.07 | −57 (64) | 0.74 | −81 (91) | 0.37 | −37 (39) | 0.34 |
| Log TNEs a | 0.27 | 0.01 (0.30) | 0.95 | −0.32 (0.26) | 0.22 | 0.01 (0.32) | 0.97 | 0.27 (0.16) | 0.09 |
Interaction Test p values represent the outcome of tests for overall significant interactions between age category and nicotine content and mean differences between age groups at Visit 2. Contrast p values represent the outcome of post hoc contrast test probing the interaction for significant differences by age group. Positive mean difference values indicate higher values in the younger adults compared to older adults.
aRegression model adjusted for age group (18–24, ≥25), nicotine content group, value of the given outcome at baseline, FTND at baseline, race (White, AA, other), NMR at baseline and gender.
Bold text indicates significant p value.
Figure 1.
The figure presents the mean predicted values data from all outcome variables listed in Table 1. Asterisks represent a significant mean difference. Note that TNEs are presented as geometric means in their raw units, though these were log transformed prior to analysis.
Week 6 Results
The results for outcomes collected at Week 6 are summarized in Table 3. The linear regression outcomes showed a significant interaction between age and nicotine condition on the Craving Reduction subscale, with post-hoc tests indicating that in the 5.2 mg/g nicotine condition, younger adults had lower craving reduction scores than the older adults. There were no significant differences between age groups on responses to the 2.4–0.4 mg/g nicotine (VLNC) cigarettes in terms of Psychological Reward, Smoking Satisfaction (Figure 1, top and middle right panels), Enjoyment of Respiratory Sensations or Aversion subscales. In the Usual Brand condition, there was a significant effect of age on total cigarettes per day in post-hoc tests, such that younger people smoked significantly fewer cigarettes than older adults. There was no indication that age moderated the effect of nicotine content on total puff volume or total nicotine equivalents.
Table 3.
Mean and Standard Deviations for Differences at Week 6 Between Age Groups (Age 18–24 and Age ≥25) as a Function of Treatment Group
| Outcome | Interaction test p value |
Usual brand | 15.8 mg/g | 5.2 mg/g | 2.4–0.4 mg/g | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference | p value | Mean difference | p value | Mean difference | p value | Mean difference | p value | ||
| CES PR a | 0.32 | −0.38 (0.36) | 0.28 | 0.18 (0.32) | 0.57 | −0.70 (0.36) | 0.05 | −0.27 (0.19) | 0.17 |
| CES SS a | 0.50 | −0.15 (0.43) | 0.72 | −0.05 (0.39) | 0.89 | −0.89 (0.44) | 0.04 | −0.36 (0.23) | 0.13 |
| CES ERTS a | 0.81 | −0.09 (0.45) | 0.85 | 0.01 (0.40) | 0.99 | −0.56 (0.46) | 0.22 | −0.25 (0.24) | 0.31 |
| CES CR a | 0.03 | 0.73 (0.55) | 0.19 | −0.15 (0.50) | 0.76 | −1.55 (0.56) | 0.01 | −0.31 (0.30) | 0.30 |
| CES AV a | 0.61 | 0.19 (0.28) | 0.49 | −0.21 (0.26) | 0.41 | 0.11 (0.29) | 0.71 | −0.15 (0.15) | 0.34 |
| Total CPD a | 0.54 | −5.94 (2.50) | 0.02 | −3.62 (2.52) | 0.09 | −3.65 (2.47) | 0.14 | −2.01 (1.31) | 0.13 |
| Total puff volume a | 0.64 | −57 (74) | 0.44 | 20.79 (68) | 0.76 | −123 (99) | 0.22 | −59 (44) | 0.17 |
| Log TNEs a | 0.53 | 0.31 (0.32) | 0.33 | −0.16 (0.29) | 0.58 | 0.10 (0.34) | 0.77 | 0.31 (0.17) | 0.08 |
Interaction test p values represent the outcome of tests for overall significant interactions between age category and nicotine content and mean differences between age groups at Visit 6. Contrast p values represent the outcome of post-hoc contrast test probing the interaction for significant differences by nicotine group. Positive mean differences values indicate higher values in the younger adults compared to older adults.
aRegression model adjusted for age group (18–24, ≥25), nicotine content group, value of the given outcome at baseline, FTND at baseline, race (White, AA, other), NMR at baseline, and gender.
Bold text indicates significant p value.
Effects of Compliance With Study Cigarettes on Outcomes
As younger age has been found to be associated with lower rates of biologically verified compliance in the VLNC group in this sample,27 we conducted further analyses based on self-reported rates of compliance. Though not significantly different across group, young adults in the VLNC group were less likely to self-report compliance with study cigarettes compared with older adults at Week 2 (29% of young adults versus 44% of older adults in the VLNC group); this difference was much smaller at Week 6 (51% of young adults in the VLNC group vs. 60% of older adults self-reporting compliance), which is consistent with the biological data and thus further justified this analysis. Among participants who self-reported compliance with the study cigarettes (N = 42 young adults, N = 326 older adults at Week 2, N = 55 young adults, N = 394 older adults at Week 6), the pattern of results remained generally the same: at Week 2, young adults showed significantly less psychological reward in the VLNC group relative to older adults, and showed decreased study cigarettes per day at Week 2; and smoking satisfaction trended in the same direction though it was only marginally significant. These differences were not evident at Week 6, though there was a significant difference in aversion at Week 6, with young adults in the VLNC groups reporting more aversion than older adults. Thus, it appears that the overall effect of decreased reward for VLNC cigarettes in young adults relative to older adults remains even when only investigating those individuals who self-reported compliance.
Discussion
The aim of the current study was to investigate whether age moderates the effects of nicotine dose on subjective and behavioral responses to cigarettes. We found that age moderated subjective responses to nicotine content of cigarettes, such that younger smokers had lower levels of smoking satisfaction, psychological reward, and enjoyment of respiratory sensations after two weeks of use in the VLNC group; whereas younger smokers had higher scores or there was no effect in the other conditions. Age did not moderate response to the aversive effects of these cigarettes at either time point; endorsement of aversive effects were low overall. However, many of these interactive effects had diminished by Week 6, with younger participants reporting similar levels of smoking satisfaction and psychological reward from cigarettes in the very-low nicotine groups as older participants. This effect was largely due to older participants’ decrease in satisfaction and reward levels, indicating perhaps that the conditioned reinforcing effects of these VLNC cigarettes may have begun to extinguish in older adults by this time point. However, younger adults in the moderate (5.2 mg/g) nicotine group continued to report lower psychological reward, craving reduction and satisfaction at week 6 relative to older adults.
In addition to subjective responses to these cigarettes, we also examined age effects on total cigarettes per day. Unlike the pattern of results for the Psychological Reward and Smoking Satisfaction, in which younger adults showed greater sensitivity to the effect of nicotine reduction on these measures than older adults, a slightly different pattern held for total CPD. Younger participants smoked fewer cigarettes per day than their older counterparts in all nicotine groups; however, this difference was relatively greater in the VLNC group relative to the control group at Week 2. This difference between age groups diminished somewhat by Week 6 due to a decrease in cigarettes per day among the older participants.
We also investigated a biochemical measure of total nicotine exposure in order to evaluate whether age affected the actual amount of nicotine participants were exposed to. In this measure there was no evidence that age moderated the effect of nicotine condition at Week 6; TNEs were decreased across all participants in the VLNC group relative to control, and though younger adults had higher average TNEs than older adults this was not statistically significant. This comports with findings from the same parent study reported by Nardone et al.27 that age was associated with TNE levels in the context of an analysis designed to better understand compliance rates with study cigarettes. The Nardone et al. study examined only the lowest nicotine groups, and looked at age as a predictor of TNE values that would suggest noncompliance; in contrast, the current study examined the effect of nicotine group on TNEs, and whether age moderated this effect across all nicotine groups. We also found that younger adults in the VLNC condition had higher TNEs than older adults (Table 3, positive mean difference); however, the moderation term was not significant, which indicates that this effect was not different across groups by age. Finally, there was no indication that age moderated the potential effect of cigarette group on total puff volume, our measure of compensatory smoking, at either time point. In short, VLNC cigarettes did not have a greater negative impact on compensatory smoking or total nicotine exposure in younger participants, and both younger and older participants experienced an overall decrease in cigarettes per day in the VLNC groups, with younger participants smoking fewer cigarettes overall at Week 6.
We further investigated the role of compliance with study cigarettes across age in the current sample by conducting a subgroup analysis including only those individuals who self-reported compliance, as the pattern of results were generally the same. We acknowledge that self-reported rates of noncompliance are problematic, as other reports from this data set have indicated much higher rates of biologically verified noncompliance in the VLNC group than was self-reported;27 however, biological verification is only possible in the lowest nicotine groups, so in order to analyze all groups we relied on self-reported compliance. Overall, our data are consistent with that paper in that young adults are more likely to self-report noncompliance than adults; and the idea that young adults were less likely to be compliant is consistent with the conclusion that they found these cigarettes less reinforcing and therefore were more likely to seek out alternative sources of nicotine.
There are several possible reasons why younger people were more sensitive to the effects of nicotine reduction on reductions in cigarette satisfaction and reward. Due to their shorter smoking histories, the conditioned reinforcing effects of sensorimotor smoking stimuli may be less salient for younger adults, and thus less likely to sustain reinforcement when nicotine is reduced. Other interpretations are possible. The developmental period of adolescence, extending into young adulthood, is characterized by profound changes in brain function as neural pathways develop and are strengthened.28 Nicotine in particular is known to have a differential effect on adolescents relative to adults in terms of brain function29; and therefore, differences in the effects of nicotine on the brain across neurodevelopment may be driving observed differences between younger and older adults.30 Overall, more research remains to be done to determine the mechanisms underlying differences between young adults and older adults in terms of their response to nicotine, and how these differences may impact tobacco regulatory policy. For example, studies that investigate how smoking cue reactivity changes over time in people randomized to cigarettes varying in nicotine content, and how this relationship is affected by smoking history, would help to clarify whether the lower levels of smoking satisfaction with VLNC cigarettes in younger adults observed in this study are due to nicotine vs. nonnicotine factors.
The strengths of this study lie in the large sample size and 6-week exposure period; however, limitations exist. Young adults were not specifically recruited for this study, so there were relatively few adults in the 18–24 age range (N = 98 overall). These young adults were also subject to the same inclusion criteria as older adults: they had to self-report smoking at least five cigarettes daily. However, many young adults are nondaily or social smokers, and often report fewer than five cigarettes smoked per day.31 Thus, these young adults are not representative of all young adult smokers, but rather of young adult heavy smokers. Furthermore, recent data have suggested that compliance with VLNC cigarettes is often much lower than what is self-reported32 and continuing to smoke usual-brand cigarettes in addition to their study cigarettes would delay the extinction of the pairing between nicotine and smoking stimuli during the study. Indeed, as noted above, using data from the same parent study and examining only the VLNC group Nardone et al.27 found that younger participants were more likely to continue to smoke their usual-brand cigarettes while in the study. Thus, there exists a partial confound between age and noncompliance that remains to be explored when determining how age affects response to nicotine dose in cigarettes.
Overall, these data suggest that reducing the nicotine content of cigarettes to a minimally addictive level may have beneficial effects for both younger and older adults, as both younger and older participants in the VLNC groups experienced an overall decrease in positive subjective response, total CPD and TNEs relative to the NNC group. Furthermore, younger participants did not show a greater tendency to engage in compensatory smoking. This study provides further evidence that less-positive subjective response to lower levels of nicotine in cigarettes emerged more quickly among younger adults. Lower positive reaction can signal less potential for abuse, so these data are encouraging in that respect.33 Reduced positive effects of VLNC cigarettes in the context of a nationwide regulatory policy could also increase demand for alternative sources of nicotine, either from alternative sources such as e-cigarettes and snus or, potentially, from black market sources of cigarettes.34,35 As smoking behavior during this time period may be more malleable, public health interventions such as a reduction in nicotine levels in cigarettes have the potential to greatly impact the future health of these smokers. Furthermore, while this study is restricted to those over 18, adolescent smokers will also be impacted by such a policy. As earlier age of initiation of smoking is highly correlated with greater dependence and heavier smoking later in life, it is crucial to understand whether a reduced-nicotine policy will help arrest the transition from experimental smoking to stable, daily smoking in young people. As VLNC cigarettes will likely be less reinforcing for young people, such policy may aid in that goal; though more data specifically on adolescent populations are urgently needed. Continuing research on the potential effects of such a policy on vulnerable populations will help the FDA make decisions about the viability and safety of a nationwide policy on reducing nicotine in cigarettes.
Funding
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. Research supported by a grant from the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products (U54DA031659). Manuscript preparation supported by NCI K01CA189300 (PI Cassidy) and NIDA P50DA036114.
Declaration of Interests
None declared.
References
- 1. Jha P, Ramasundarahettige C, Landsman V, et al. . 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. [DOI] [PubMed] [Google Scholar]
- 2. Congress. Family Smoking Prevention and Tobacco Control Act (H.R. 1256). Washington: U.S.G.P.O; 2009. [Google Scholar]
- 3. Gottlieb S, Zeller M. A nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- 4. Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction -- the implications for tobacco regulation. N Engl J Med. 1994;331(2):123–125. [DOI] [PubMed] [Google Scholar]
- 5. Benowitz NL, Henningfield JE. Reducing the nicotine content to make cigarettes less addictive. Tob Control. 2013;22(suppl 1):i14–i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M. Nicotine reduction: Strategic research plan. Nicotine Tob Res. 2013;15(6):1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl). 2006;184(3-4):274–285. [DOI] [PubMed] [Google Scholar]
- 8. Dallery J, Houtsmuller EJ, Pickworth WB, Stitzer ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology (Berl). 2003;165(2):172–180. [DOI] [PubMed] [Google Scholar]
- 9. Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: The role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100(4):550–559. [DOI] [PubMed] [Google Scholar]
- 10. Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. [DOI] [PubMed] [Google Scholar]
- 11. Shahan TA, Bickel WK, Badger GJ, Giordano LA. Sensitivity of nicotine-containing and de-nicotinized cigarette consumption to alternative non-drug reinforcement: A behavioral economic analysis. Behav Pharmacol. 2001;12(4):277–284. http://www.ncbi.nlm.nih.gov/pubmed/11548113. Accessed December 1, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Donny EC, Denlinger RL, Tidey JW, et al. . Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatsukami DK, Kotlyar M, Hertsgaard LA, et al. . Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction. 2010;105(2):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Vol HHS Public. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 15. Wetter DW, Kenford SL, Welsch SK, et al. . Prevalence and predictors of transitions in smoking behavior among college students. Health Psychol. 2004;23(2):168–177. [DOI] [PubMed] [Google Scholar]
- 16. Biener L, Albers AB. Young adults: Vulnerable new targets of tobacco marketing. Am J Public Health. 2004;94(2):326–330. http://www.ncbi.nlm.nih.gov/pubmed/14759950. Accessed June 24, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. USDHHS. Preventing Tobacco Use Among Youth and Young Adults 2016. https://www.surgeongeneral.gov/library/reports/preventing-youth-tobacco-use/factsheet.html. Accessed August 29, 2017.
- 18. Kelleher RT, Gollub LR. A review of positive conditioned reinforcement. J Exp Anal Behav. 1962;5(4):543–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carpenter MJ, Saladin ME, Larowe SD, et al. . Craving, cue reactivity, and stimulus control among early-stage young smokers: Effects of smoking intensity and gender. Nicotine Tob Res. 2014;16(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM. Cue reactivity in adolescents: Measurement of separate approach and avoidance reactions. J Stud Alcohol. 2005;66(3):332–343. http://www.ncbi.nlm.nih.gov/pubmed/16047522. Accessed September 27, 2016. [DOI] [PubMed] [Google Scholar]
- 21. Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24(2):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 23. Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3–4):235–241. http://www.ncbi.nlm.nih.gov/pubmed/735910. Accessed December 1, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47(2):171–183. [DOI] [PubMed] [Google Scholar]
- 25. Strasser AA, Benowitz NL, Pinto AG, et al. . Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20(2):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faulkner P, Ghahremani DG, Tyndale RF, et al. . Reduced-nicotine cigarettes in young smokers: Impact of nicotine metabolism on nicotine dose effects. Neuropsychopharmacology. 2017;42(8):1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nardone N, Donny EC, Hatsukami DK, et al. . Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016;111(12):2208–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. http://www.ncbi.nlm.nih.gov/pubmed/10817843. Accessed July 19, 2017. [DOI] [PubMed] [Google Scholar]
- 29. Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. 2015;593(16):3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lydon DM, Wilson SJ, Child A, Geier CF. Adolescent brain maturation and smoking: What we know and where we’re headed. Neurosci Biobehav Rev. 2014;45:323–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran S, Wechsler H, Rigotti NA. Social smoking among US college students. Pediatrics. 2004;114(4):1028–1034. [DOI] [PubMed] [Google Scholar]
- 32. Benowitz NL, Nardone N, Hatsukami DK, Donny EC. Biochemical estimation of noncompliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2015;24(2):331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoedel KA, Sellers EM. Assessing abuse liability during drug development: Changing standards and expectations. Clin Pharmacol Ther. 2008;83(4):622–626. [DOI] [PubMed] [Google Scholar]
- 34. Benowitz NL, Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game. Addiction. 2017;112(1): 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kozlowski LT. Cigarette prohibition and the need for more prior testing of the WHO TobReg’s global nicotine-reduction strategy. Tob Control. 2017;26(e1):e31–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]