Abstract
The collagenopathies are a diverse group of diseases caused primarily by mutations in collagen genes. The resulting disruptions in collagen biogenesis can impair development, cause cellular dysfunction, and severely impact connective tissues. Most existing treatment options only address patient symptoms. Yet, while the disease-causing genes and proteins themselves are difficult to target, increasing evidence suggests that resculpting the intracellular proteostasis network, meaning the machineries responsible for producing and ensuring the integrity of collagen, could provide substantial benefit. We present a proteostasis-focused perspective on the collagenopathies, emphasizing progress toward understanding how mechanisms of collagen proteostasis are disrupted in disease. In parallel, we highlight recent advances in small molecule approaches to tune endoplasmic reticulum proteostasis that may prove useful in these disorders.
1. Proteostasis and the Collagenopathies
1.1. Collagen Biogenesis
The twenty-eight types of collagen form the structural foundation of human tissues, ranging from skin and bone to cartilage and basement membranes. Beyond providing bulk material for extracellular matrices, collagens facilitate dynamic biological processes such as cell signaling, cell migration, and wound healing. Proper execution of the folding, modification, and quality control processes required for production of this complex protein is, therefore, critical for cell and organismal health.
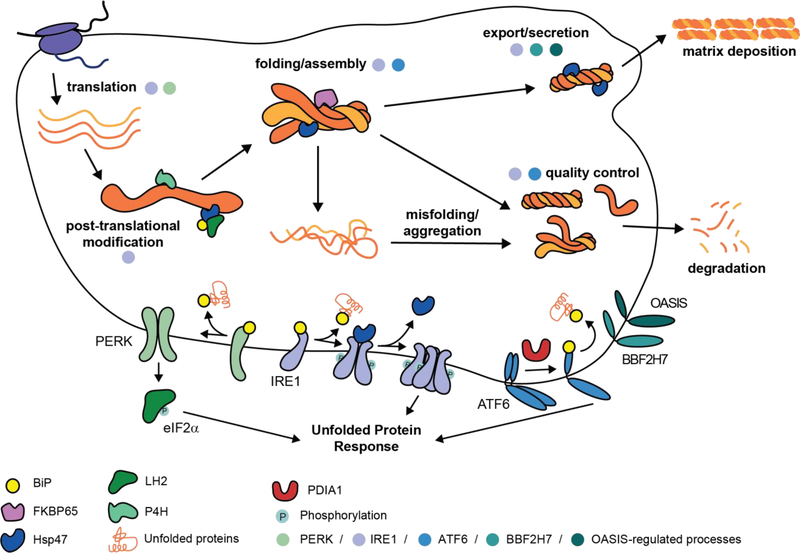
Collagen production, however, presents a unique problem to cells. Collagen is not only the most abundant protein produced by the secretory pathway, but also one of the most challenging to fold. As illustrated in Figure 1, collagen biogenesis encompasses all the issues of folding a large (typically >300 kDa), multi-domain, disulfide-containing protein combined with the added difficulties of correctly assembling three >1000 amino acid polypeptides, unusual rigidity owing to a lengthy triple-helical domain (up to ~1000 amino acids), slow folding due to high proline content, and a requirement for extensive post-translational modifications. This process is orchestrated by a large cohort of endoplasmic reticulum (ER) chaperones, quality control mechanisms, and collagen-modifying enzymes. Some of these proteostasis factors are specific to collagen, while others have broader roles in the folding of many different ER client proteins.
Figure 1 |. Collagen production.
Nascent procollagen polypeptides, comprised of N-propeptide (~15 kDa), triple-helical (up to ~100 kDa), and C-propeptide (~30 kDa) domains, are first co-translationally imported into the endoplasmic reticulum (ER). Within the ER, they undergo extensive co- and post-translational modifications prior to folding. These modifications include introduction of an N-glycan in the C-propeptide domain, extensive hydroxylation of proline residues that is required for thermal stability, and lysine hydroxylation that promotes extracellular crosslinking. Procollagen folding in the ER begins with the autonomous folding of the ~30 kDa C-propeptide domain on each monomeric strand, which can only begin after the entire protein is translated. The C-propeptide is a cysteine-rich domain whose folded state is stabilized by multiple intramolecular disulfide bonds. After the C-propeptide folds, individual C-propeptide domains recognize each other and assemble, a process that, at least for the fibrillar collagens, is mediated by Ca2+- and intermolecular disulfide bonds [62]. The resulting assembled C-propeptide trimer controls the triple helix register and initiates zipper-like folding of the proline- and glycine-rich triple-helical domain, a process that itself requires isomerization of hundreds of proline peptide bonds to the trans configuration. Triple-helix formation attenuates further procollagen hydroxylation, and sets the stage for secretion of the protein via a non-canonical pathway. For the fibrillar collagens, the mature protein is produced by cleavage of the propeptide domains, initiating extensive supramolecular assembly and the generation of hierarchical tissue architectures. This process is orchestrated by an extensive suite of ER chaperones and quality control mechanisms that are regulated by the three arms of the unfolded protein response (IRE1, ATF6, and PERK), as well as the related transcriptional responders OASIS and BBF2H7, which are highlighted in the lower portion of the figure.
1.2. The Collagenopathies
Dysregulated collagen proteostasis occurs when cells fail to produce appropriate quantities of properly folded and functioning collagen and/or fail to minimize intra- and extra-cellular accumulation of defective collagens. The resulting diseases, often termed collagenopathies, are most commonly caused by autosomal dominant mutations in collagen genes themselves, although autosomal recessive mutations in specific collagen chaperones and modifying enzymes can also induce disease [1–3]. For example, hundreds of mutations in collagen type-I genes are associated with the archetypal collagenopathy, osteogenesis imperfecta (OI), which is also known as brittle bone disease [4]. Mutations in other collagen types are responsible for disorders as diverse as Ehlers-Danlos syndrome (type-IV collagen) and early onset osteoarthritis (type-II collagen).
The majority of current treatments for the collagenopathies address disease symptoms rather than underlying causes. In OI, these strategies include physical rehabilitation or pharmacological and biological approaches to increase bone mass [5] and minimize harmful signaling pathways [6]. Stem cell and gene therapies aimed at replacing or removing misfolded collagen offer long-term hope for substantial improvements to pathology [7,8]. The viability of these approaches remains unclear, however, in large part because questions of efficacy, donor availability, delivery, and potential toxicity are still unsolved. In summary, current therapies remain inadequate for alleviating pathologic manifestations of OI and the other collagenopathies, motivating an ongoing search for alternative treatment avenues [5,6].
1.3. A Proteostasis Perspective on the Collagenopathies
The traditional clinical view of OI and other collagenopathies focuses on addressing tissue dysfunction (e.g., increasing bone mass or treating inflammation) downstream of the intracellular processes related to collagen production. Mounting evidence, however, suggests that there could be substantial merit to intracellular, proteostasis-focused interventions. Indeed, the often observed breakdown of genotype–phenotype relationships (see, for instance, the OI-causing G352S mutation in Colα1(I) that can have moderate to lethal consequences [9,10]) suggests that the cellular environment in which collagen folds can be as important for disease outcomes as the specific mutation involved.
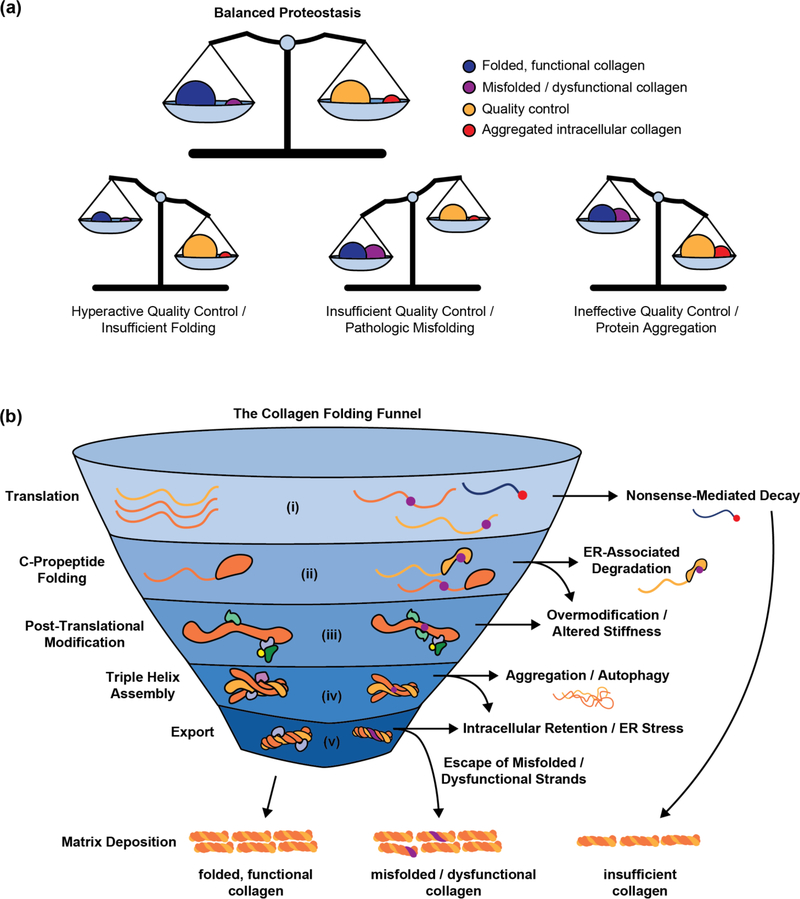
From the proteostasis perspective, disease-causing mutations can engender at least three defects that disrupt the collagen proteostasis balance (Figure 2a), all of which have been observed in OI: (1) Nonfunctional collagen may be allowed to escape the cell, disrupting matrix deposition, fibril organization, or interactions with other extracellular matrix components [11–13]. (2) Mutations might result in insufficient production of functional collagen-I, by directly lowering folding efficiency or by impacting the activity of key chaperones [14,15]. (3) Misfolding collagen could overwhelm the ER proteostasis network, resulting in intracellular collagen accumulation, chronic cell stress, and apoptotic signaling [11,16–18].
Figure 2 |. A proteostasis perspective on the collagenopathies.
(a) Maintaining the balance between production of folded, functional collagen-I and quality control/clearance of misfolded, dysfunctional collagen-I is essential for health. In OI and other collagenopathies, insufficient quality control, hyperactive quality control and failed folding, or failed clearance leading to intracellular collagen aggregation can all cause a loss of collagen proteostasis balance. Modulating proteostasis network activities holds potential to resolve such defects. (b) The process that takes a collagen chain from unfolded polypeptide to mature triple helix requires multiple steps, each of which may present a target for therapeutic intervention. In optimal conditions (left), collagen molecules proceed smoothly towards their final folded and functional form. By contrast (right), mutations in enzymes that facilitate these steps or in collagen genes themselves cause nascent proteins to fail folding or quality control and exit the funnel. From top to bottom: (i) Premature stop codons (red circle) can lead to mRNA subjected to nonsense-mediated decay. (ii) Other mutations (purple circles) are translated, but interfere with folding and later stages in collagen biosynthesis. (iii) Mutant procollagen chains that are not cleared by ER-associated degradation are often slow to fold and therefore over-modified, leading to altered matrix stiffness. (iv) Meanwhile, procollagen chains that fail to fold may be cleared by autophagy, or persist as intracellular aggregates. (v) Defects in collagenous tissues can result from either insufficient collagen or escape of misfolded collagen molecules that then disrupt matrix integrity.
Because proper folding and function of collagen depends on numerous ER proteostasis pathways that together tailor collagen output to physiological demand, there are many potential opportunities to intervene (Figure 2b). For example, it may be possible to identify and manage dysfunctional or misfolded collagen-I by enhancing quality control mechanisms, to restore collagen-I folding efficiency by increasing chaperone availability, and/or to improve misfolded collagen clearance to avoid chronic cellular dysfunction. Such proteostasis-focused interventions have shown substantial promise in other misfolding-related genetic diseases [19–21]. Moreover, the heterogeneous nature of OI, which impacts not only bone structure, but also skin, hearing, teeth, heart tissue, and lung function [4], suggests that improving cellular capacity to handle misbehaving collagen strands may prove more effective than tissue- or patient-specific approaches.
2. Targeting Collagen Proteostasis in Disease
2.1. Identifying Potential Proteostasis Network Targets
Decades of work on both the genetics of the collagenopathies and the biochemistry of ER proteostasis have provided substantial insight into how ER chaperones and enzymes promote collagen folding. The peptidyl-prolyl isomerases [22], proline and lysine hydroxylases, and collagen-specific chaperones such as Hsp47 [23] are all well-established players. In addition, both wild-type and mutant procollagens are known to engage ATP-dependent ER chaperones such as BiP and Grp94 [24,25], although no clear function for this interaction has been demonstrated.
Until recently, systematic studies to define the collagen proteostasis network had not been performed, resulting in an incomplete picture of how the ER orchestrates collagen folding and quality control. However, two groups have now employed mass spectrometry-based proteomics to more comprehensively map the ER proteostasis network components that engage wild-type collagen-I [24] and collagen-VII [26], leading to the identification of >40 putative new collagen interactors. Among other findings, DiChiara et al showed that the protein Erp29 recognizes and retains immature procollagen in the ER [24]. They also discovered a previously unknown collagen-I post-translational modification, aspartyl hydroxylation, which has been linked to extracellular matrix disease [27]. Kuttner et al reported a possible role for the multi-functional protein TGM2 in collagen type-VII maturation, as well as potentially disrupted autophagy, both of which may be involved in recessive dystrophic epidermolysis bullosa [26].
These studies highlight the power of interactome studies to provide new and unexpected insights into collagen proteostasis. They also set the stage for systematic efforts to define how ER proteostasis networks differentially engage wild-type versus mutant, disease-causing collagens [28]. Related -omics approaches focused on transcript and miRNA profiling have also helped to identify candidates whose tissue- or disease-specific expression suggests their involvement in chondrocyte development or pathology, respectively [29,30]. As these methods mature and mechanistic follow-up studies emerge, we anticipate many exciting discoveries in the field of collagen proteostasis, as well as the identification of potential therapeutic targets.
Another valuable tool that has recently been applied to the collagen proteostasis problem is cell-based high-throughput screening. Wong et al described the use of a Gaussia luciferase fusion to assay how a compound library impacted collagen-I secretion from Saos-2 cells [31]. Follow-up studies with one screening hit, the broad-spectrum Hsp90 inhibitor 17-allylamino geldanamycin, identified an unexpected role for cytosolic isoforms of Hsp90 in collagen-I secretion [31]. Meanwhile, Omachi et al used a sophisticated strategy based on fusions of collagen type-IV strands to split-NanoLuc to specifically assess assembly of Alport Syndrome-causing variants of collagen-IV [32]. With these new assays now available, the application of Cas9-based tools in genome-wide screens provides unique opportunities to discover, in an unbiased fashion, new factors involved in collagen proteostasis – especially in the folding and quality control of mutant, disease-causing collagens.
Fully assessing the therapeutic potential of pathways involved in collagen proteostasis will require not just discovery of collagen interactors, but also understanding how regulatory partners may contribute to disease phenotypes. In tandem with biochemical studies, the development of improved compounds to target collagen chaperones, either alone or in the context of relevant complexes, is another key step for translating interactome and screening-based findings into treatment options. There now are selective inhibitors for prolyl-4-hydroxylase [33], Hsp47 [23], and protein disulfide isomerase A1 [34], all of which are involved in collagen biogenesis. Application of these tools to disease models, and the development of selective activating compounds for these and other chaperones, will help determine if modulating these proteostasis factors can favorably influence collagen folding and/or quality control outcomes.
2.2. Systemic Approaches to Resolve Collagen Proteostasis Defects
‘Chemical chaperones’ such as 4-phenylbutyric acid (4-PBA) have shown promise in OI and in Alport Syndrome, apparently by improving the assembly and secretion of disease-causing variants while simultaneously decreasing intracellular accumulation and cell stress [35,36]. However, such chemical chaperones typically require treatment with very high concentrations, display pleiotropic effects, and operate by unknown mechanisms, highlighting a need for more focused strategies to adapt ER proteostasis.
One promising alternative is small molecule-mediated transcriptional remodeling of the ER proteostasis network via the unfolded protein response (UPR). The UPR (see lower portion of Figure 1) is generally responsible for maintaining ER proteostasis (reviewed in [37]). Upon detection of protein misfolding stress, the transmembrane signaling proteins IRE1 and ATF6 generate the downstream transcription factors XBP1s and ATF6(f), respectively. These transcription factors remodel the ER proteostasis network by increasing levels of ER chaperones, quality control factors, and secretion machineries. A third stress sensor, PERK, activates signaling pathways that reduce non-essential protein translation. Further highlighting the potential merit of UPR-focused strategies in the collagenopathies, in addition to the established benefits of arm-specific UPR activation for other misfolding proteins [19,38], many collagen chaperones are also UPR target genes [24,38]. Moreover, results of a recent study suggest that the collagen chaperone Hsp47 can directly regulate activation of the IRE1α isoform [39]. Finally, mutations that disrupt the DNA binding site or prevent processing of the ATF6 family members OASIS and BBF2H7 can cause OI and chondrocyte-related defects, apparently by reducing collagen secretion [40–42].
Several chemical tools are now available to induce the IRE1 and ATF6 branches of the UPR in a selective, stress-independent manner. Although the compounds likely require further optimization, various strategies have been employed to confer small molecule activation of IRE1 [43–45]. Recent screening campaigns have also identified small molecules capable of selectively activating endogenous ATF6 [46,47]. Finally, disease phenotypes may benefit from chemical inhibition of PERK, which was found to alleviate chondrodysplasia in a mouse model system [48]. The ability of the small molecule ISRIB to productively modify PERK/ATF4/CHOP signaling in that study, in contrast to genetic deletion of CHOP, underscores the advantages that chemical strategies can offer in terms of dosability, delivery, and specificity. Tuning translation via the PERK-eIF2α signaling pathway may also be useful, once the specificity and mechanism of current compounds is established [49–51].
Testing the potential of UPR pathway activation (as well as OASIS and BBF2H7 activation) for ameliorating collagenopathies using these and other emergent chemical methods should be a priority for the field. Robust evaluation of these small molecule UPR modulators may benefit from the increasing availability of animal models of the collagenopathies [52], although whether the differences between such models and human tissues limits their relevance to disease remains to be seen.
2.3. Targeting Collagen Quality Control
While UPR-based remodeling of the ER proteostasis network could provide a general strategy to improve collagen proteostasis, another more focused possibility is to specifically adapt collagen quality control. Depending on the genotype, the objective could be to (1) reduce intracellular accumulation of misfolded procollagen, (2) limit disruption of the extracellular matrix by misfolded or dysfunctional collagen molecules, or (3) promote the folding and secretion of collagen by inhibiting premature procollagen degradation.
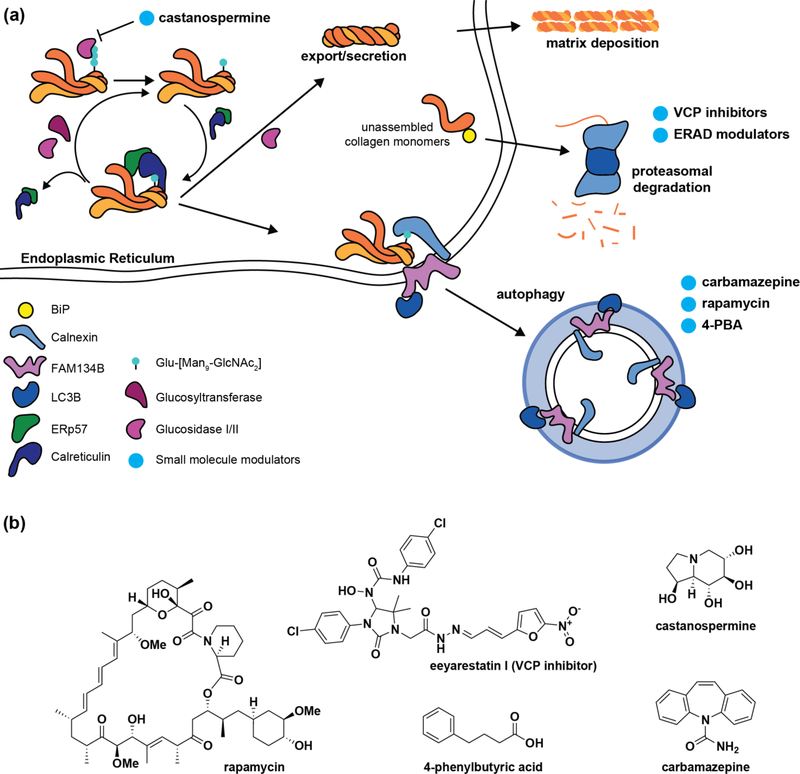
Monomeric procollagen polypeptides can be subjected to proteasome-mediated clearance via ER-associated degradation (ERAD) (Figure 3) [25,53]. How the misfolded monomers are recognized for proteasomal targeting remains unclear, although Erp29 may be involved [24]. Interactome studies of relevant misfolding collagen variants should provide additional targets for tuning ERAD. Alternatively, treatment with extant ERAD inhibitors could potentially have merit.
Figure 3 |. Procollagen quality control.
(a) While folding-competent procollagen molecules exit folding and modification cycles for secretion, slow-folding or aggregated procollagen is recognized by calnexin and targeted to autophagy, at least in part by the ER-phagy receptor FAM134B. Meanwhile, unassembled and/or misfolding procollagen monomers can be targeted to the proteasome via ER-associated degradation (ERAD), a process mediated by the transitional endoplasmic reticulum ATPase VCP. Reducing degradation of procollagen chains by using VCP inhibitors to prevent ERAD targeting or treatment with the calnexin cycle entry inhibitor castanospermine could prove beneficial for collagenopathies stemming from hyperactive quality control. In other cases, the persistence of misfolded collagen aggregates can overwhelm cellular clearance pathways, leading to escape of misfolded collagen molecules or stress-induced cellular dysfunction. Treatment with the autophagy activators carbamazepine, rapamycin, and 4-PBA may hold promise in reducing the disease burden of specific collagen variants that accumulate inside cells. (b) Structures of small molecule modulators highlighted in (a).
It seems likely, however, that the more important pathway for procollagen quality control is autophagy (Figure 3). Autophagy has been reported to clear assembled procollagens from the ER [54] and to play critical roles in collagen production [55]. Forrester et al recently reported the discovery of a pathway by which ER-localized, assembled procollagen is targeted to autophagy [56]. In particular, they found that calnexin specifically engages misfolded procollagens, which are then targeted to autophagy by the ER-phagy receptor FAM134B. This autophagic targeting can be prevented by disrupting any aspect of the calnexin cycle, as well as by Bafilomycin A1 treatment. Another recent study also confirmed autophagic targeting of aggregating procollagen molecules [57], although the degradation in that case was Bafilomycin A1-insensitive. While this discrepancy remains to be resolved, these two studies motivate efforts to target autophagy for addressing the collagenopathies, especially as impaired autophagy has been linked to higher disease severity in both osteoblasts and articular chondrocytes [16,58].
Although 4-PBA is primarily known as a chemical chaperone, it has also been proposed to regulate autophagy via inhibition of histone deacetylases. Thus, the beneficial effects of extended 4-PBA treatment in OI and Alport Syndrome models could be related to improved collagen clearance via autophagy [36,59]. Rapamycin, another autophagy activator, was able not only to promote clearance of misfolded collagen aggregates and restore ER morphology, but also to rescue collagen secretion and deposition in osteoblasts derived from an OI mouse model, although growth suppression of OI bones has also been observed in vivo [16,60]. Meanwhile, the autophagy activator carbamazepine alleviated several features of disease in a metaphyseal chondrodysplasia type Schmid (MCDS) mouse model, as indicated by reduced protein levels of UPR markers, reduced collagen type-X retention, and restored chondrocyte organization in hypertrophic zones [61]. As the molecules currently used to activate autophagy have potentially deleterious pleiotropic effects, improved small molecule-based autophagy activators with greater specificity and potency are required to more fully understand the potential of autophagy activators in the collagenopathies. Further development of such small molecules should be catalyzed by these observations.
3. Concluding Remarks
We conclude that a proteostasis-focused approach to the collagenopathies may hold substantial merit. The proteostasis lens provides a unifying perspective from which to view these diseases, despite their origins in dozens of distinctive genes and symptoms impacting a broad range of tissues. Critically, approaches targeting proteostasis network mechanisms to rebalance collagen proteostasis are likely to prove broadly applicable across the collagenopathies. As our understanding of the mechanisms of collagen proteostasis emerges in tandem with advances in the chemical targeting of ER proteostasis mechanisms, we believe this area holds great promise for effective treatment of the collagenopathies.
4. Acknowledgements
This work was supported by the National Institutes of Health (Grant 5R01AR071443) and a Research Grant from the G. Harold and Leila Y. Mathers Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jobling R, D’Souza R, Baker N, Lara-Corrales I, Mendoza-Londono R, Dupuis L, Savarirayan R, Ala-Kokko L, Kannu P: The collagenopathies: Review of clinical phenotypes and molecular correlations. Curr Rheum Rep (2014) 16:394. [DOI] [PubMed] [Google Scholar]
- 2.Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bachinger HP et al. : CRTAP is required for prolyl 3-hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell (2006) 127:291–304. [DOI] [PubMed] [Google Scholar]
- 3.Barnes AM, Cabral WA, Weis M, Makareeva E, Mertz EL, Leikin S, Eyre D, Trujillo C, Marini JC: Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum Mut (2012) 33:1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forlino A, Marini JC: Osteogenesis imperfecta. Lancet (2016) 387:1657–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen CM, Schwartz MA, Roberts HJ, Lim K-E, Spevak L, Boskey AL, Zurakowski D, Robling AG, Warman ML: Enhanced Wnt signaling improves bone mass and strength, but not brittleness, in the Col1a1+/Mov13 mouse model of type I Osteogenesis Imperfecta. Bone (2016) 90:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, Ishikawa Y et al. : Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med (2014) 20:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rousseau J, Gioia R, Layrolle P, Lieubeau B, Heymann D, Rossi A, Marini JC, Trichet V, Forlino A: Allele-specific Col1a1 silencing reduces mutant collagen in fibroblasts from Brtl mouse, a model for classical osteogenesis imperfecta. Eur J Hum Genet (2014) 22:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T: Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA (2002) 99:8932–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateman JF, Moeller I, Hannagan M, Chan D, Cole WG: Characterization of three osteogenesis imperfecta collagen α1(I) glycine to serine mutations demonstrating a position-dependent gradient of phenotypic severity. Biochem J (1992) 288:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay K, Byers PH, Dalgleish R: An RT-PCR-SSCP screening strategy for detection of mutations in the gene encoding the alpha 1 chain of type I collagen: Application to four patients with osteogenesis imperfecta. Hum Mol Genet (1993) 2:1155–1160. [DOI] [PubMed] [Google Scholar]
- 11.Makareeva E, Sun G, Mirigian LS, Mertz EL, Vera JC, Espinoza NA, Yang K, Chen D, Klein TE, Byers PH, Leikin S: Substitutions for arginine at position 780 in triple helical domain of the α1(I) chain alter folding of the type I procollagen molecule and cause osteogenesis imperfecta. PLoS One (2018) 13:e0200264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. •.Qiu Y, Mekkat A, Yu H, Yigit S, Hamaia S, Farndale RW, Kaplan DL, Lin Y-S, Brodsky B: Collagen Gly missense mutations: Effect of residue identity on collagen structure and integrin binding. J Struct Biol (2018) 203:255–262.Recent report from an important line of work examining how non-synonymous mutations influence collagen’s interactions with other extracellular matrix components.
- 13.Bishop N: Bone material properties in osteogenesis imperfecta. J Bone Miner Res (2016) 31:699–708. [DOI] [PubMed] [Google Scholar]
- 14. •.Cabral WA, Ishikawa M, Garten M, Makareeva EN, Sargent BM, Weis M, Barnes AM, Webb EA, Shaw NJ, Ala-Kokko L, Lacbawan FL et al. : Absence of the ER cation channel TMEM38B/TRIC-B disrupts intracellular calcium homeostasis and dysregulates collagen synthesis in recessive osteogenesis imperfecta. PLoS Genet (2016) 12:e1006156.Evidence that general proteostasis defects such as impaired intracellular Ca2+ homeostasis can manifest as collagen-misfolding diseases.
- 15.Ito S, Nagata K: Mutants of collagen-specific molecular chaperone Hsp47 causing osteogenesis imperfecta are structurally unstable with weak binding affinity to collagen. Biochem Biophys Res Comm (2016) 469:437–442. [DOI] [PubMed] [Google Scholar]
- 16.Mirigian LS, Makareeva E, Mertz EL, Omari S, Roberts-Pilgrim AM, Oestreich AK, Phillips CL, Leikin S: Osteoblast malfunction caused by cell stress response to procollagen misfolding in α2(I)-G610C mouse model of osteogenesis imperfecta. J Bone Miner Res (2016) 31:1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheiber AL, Guess AJ, Kaito T, Abzug JM, Enomoto-Iwamoto M, Leikin S, Iwamoto M, Otsuru S: Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem Biophys Res Comm (2019) 509:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron TL, Bell KM, Gresshoff IL, Sampurno L, Mullan L, Ermann J, Glimcher LH, Boot-Handford RP, Bateman JF: Xbp1-independent upr pathways suppress C/EBP-β mediated chondrocyte differentiation in ER-stress related skeletal disease. PLoS Genet (2015) 11:e1005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooley CB, Ryno LM, Plate L, Morgan GJ, Hulleman JD, Kelly JW, Wiseman RL: Unfolded protein response activation reduces secretion and extracellular aggregation of amyloidogenic immunoglobulin light chain. Proc Natl Acad Sci USA (2014) 111:13046–13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankow S, Bamberger C, Calzolari D, Martinez-Bartolome S, Lavallee-Adam M, Balch WE, Yates JR: ΔF508 CFTR interactome remodelling promotes rescue of cystic fibrosis. Nature (2015) 528:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu YL, Han DY, Wang YJ, Di XJ, Yu HB, Mu TW: Remodeling the endoplasmic reticulum proteostasis network restores proteostasis of pathogenic GabaA receptors. PLoS One (2018) 13:e0207948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa T, Kashima M, Nagano AJ, Ishikawa-Fujiwara T, Kamei Y, Todo T, Mori K: Unfolded protein response transducer IRE1-mediated signaling independent of XBP1 mRNA splicing is not required for growth and development of medaka fish. eLife (2017) 6:e26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito S, Ogawa K, Takeuchi K, Takagi M, Yoshida M, Hirokawa T, Hirayama S, Shin-ya K, Shimada I, Doi T, Goshima N et al. : A small-molecule compound inhibits a collagen-specific molecular chaperone and could represent a potential remedy for fibrosis. J Biol Chem (2017) 292:20076–20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. •.DiChiara AS, Taylor RJ, Wong MY, Doan ND, Rosario AM, Shoulders MD: Mapping and exploring the collagen-I proteostasis network. ACS Chem Biol (2016) 11:1408–1421.A strategy for comparative collagen-I interactomics as well as the resulting identification of novel collagen-I interactors and post-translational modifications.
- 25.Lamandé SR, Chessler SD, Golub SB, Byers PH, Chan C, Cole WG, Sillence DO, Bateman JF: Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the proα1(I) chain carboxyl-terminal propeptide which impair subunit assembly. J Biol Chem (1995) 270:8642–8649. [DOI] [PubMed] [Google Scholar]
- 26.Kuttner V, Mack C, Gretzmeier C, Bruckner-Tuderman L, Dengjel J: Loss of collagen VII is associated with reduced transglutaminase 2 abundance and activity. J Invest Dermatol (2014) 134:2381–2389. [DOI] [PubMed] [Google Scholar]
- 27.Patel N, Khan AO, Mansour A, Mohamed JY, Al-Assiri A, Haddad R, Jia X, Xiong Y, Megarbane A, Traboulsi EI, Alkuraya FS: Mutations in ASPH cause facial dysmorphism, lens dislocation, anterior-segment abnormalities, and spontaneous filtering blebs, or Traboulsi syndrome. Am J Hum Genet (2014) 94:755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doan N-D, DiChiara AS, Del Rosario AM, Schiavoni RP, Shoulders MD: Mass spectrometry-based proteomics to define intracellular collagen interactomes. Methods Mol Biol (2019) 1944: in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Balasubramanian K, Krakow D, Cohn DH: Genes uniquely expressed in human growth plate chondrocytes uncover a distinct regulatory network. BMC Genomics (2017) 18:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kung LHW, Ravi V, Rowley L, Angelucci C, Fosang AJ, Bell KM, Little CB, Bateman JF: Cartilage microRNA dysregulation during the onset and progression of mouse osteoarthritis is independent of aggrecanolysis and overlaps with candidates from end-stage human disease. Arth Rheum (2018) 70:383–395. [DOI] [PubMed] [Google Scholar]
- 31.Wong MY, Doan N-D, DiChiara AS, Papa LJ, Cheah JH, Soule CK, Watson N, Hulleman JD, Shoulders MD: A high-throughput assay for collagen secretion suggests an unanticipated role for Hsp90 in collagen production. Biochemistry (2018) 57:2814–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ••.Omachi K, Kamura M, Teramoto K, Kojima H, Yokota T, Kaseda S, Kuwazuru J, Fukuda R, Koyama K, Matsuyama S, Motomura K et al. : A split-luciferase-based trimer formation assay as a high-throughput screening platform for therapeutics in Alport syndrome. Cell Chem Biol (2018) 25:634–643.An elegant luminescence-based screen for assaying trimer formation of collagen-IV chains that should also prove applicable to other collagen types and screening platforms.
- 33.Vasta JD, Andersen KA, Deck KM, Nizzi CP, Eisenstein RS, Raines RT: Selective inhibition of collagen prolyl 4-hydroxylase in human cells. ACS Chem Biol (2016) 11:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole KS, Grandjean JMD, Chen K, Witt CH, O’Day J, Shoulders MD, Wiseman RL, Weerapana E: Characterization of an A-site selective protein disulfide isomerase A1 inhibitor. Biochemistry (2018) 57:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray LS, Lu Y, Taggart A, Van Regemorter N, Vilain C, Abramowicz M, Kadler KE, Van Agtmael T: Chemical chaperone treatment reduces intracellular accumulation of mutant collagen IV and ameliorates the cellular phenotype of a Col4A2 mutation that causes haemorrhagic stroke. Hum Mol Genet (2014) 23:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Besio R, Iula G, Garibaldi N, Cipolla L, Sabbioneda S, Biggiogera M, Marini JC, Rossi A, Forlino A: 4-PBA ameliorates cellular homeostasis in fibroblasts from osteogenesis imperfecta patients by enhancing autophagy and stimulating protein secretion. Biochim Biophys Acta (2018) 1864:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong MY, DiChiara AS, Suen PH, Chen K, Doan N-D, Shoulders MD: Adapting secretory proteostasis and function through the unfolded protein response. Curr Top Microbiol Immunol (2018) 414:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, Yates JR 3rd, Su AI, Kelly JW, Wiseman RL: Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep (2013) 3:1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepulveda D, Rojas-Rivera D, Rodriguez DA, Groenendyk J, Kohler A, Lebeaupin C, Ito S, Urra H, Carreras-Sureda A, Hazari Y, Vasseur-Cognet M et al. : Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1α. Mol Cell (2018) 69:238–252. [DOI] [PubMed] [Google Scholar]
- 40.Keller RB, Tran TT, Pyott SM, Pepin MG, Savarirayan R, McGillivray G, Nickerson DA, Bamshad MJ, Byers PH: Monoallelic and biallelic CREB3L1 variant causes mild and severe osteogenesis imperfecta, respectively. Genet Med (2018) 20:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindert U, Cabral WA, Ausavarat S, Tongkobpetch S, Ludin K, Barnes AM, Yeetong P, Weis M, Krabichler B, Srichomthong C, Makareeva EN et al. : MBTPS2 mutations cause defective regulated intramembrane proteolysis in X-linked osteogenesis imperfecta. Nat Comm (2016) 7:e11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito A, Hino S, Murakami T, Kanemoto S, Kondo S, Saitoh M, Nishimura R, Yoneda T, Furuichi T, Ikegawa S, Ikawa M et al. : Regulation of endoplasmic reticulum stress response by a BBF2H7-mediated SEC23A pathway is essential for chondrogenesis. Nat Cell Biol (2009) 11:1197–1204. [DOI] [PubMed] [Google Scholar]
- 43.Wiseman RL, Zhang Y, Lee KP, Harding HP, Haynes CM, Price J, Sicheri F, Ron D: Flavonol activation defines an unanticipated ligand-binding site in the kinase-rnase domain of IRE1. Mol Cell (2010) 38:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR: IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell (2009) 138:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendez AS, Alfaro J, Morales-Soto MA, Dar AC, McCullagh E, Gotthardt K, Li H, Acosta-Alvear D, Sidrauski C, Korennykh AV, Bernales S et al. : Endoplasmic reticulum stress-independent activation of unfolded protein response kinases by a small molecule ATP-mimic. eLife (2015) 4:e05434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallagher CM, Garri C, Cain EL, Ang KK-H, Wilson CG, Chen S, Hearn BR, Jaishankar P, Aranda-Diaz A, Arkin MR, Renslo AR et al. : Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. eLife (2016) 5:e11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plate L, Cooley CB, Chen JJ, Paxman RJ, Gallagher CM, Madoux F, Genereux JC, Dobbs W, Garza D, Spicer TP, Scampavia L et al. : Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. eLife (2016) 5:e15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Tan Z, Niu B, Tsang KY, Tai A, Chan WCW, Lo RLK, Leung KKH, Dung NWF, Itoh N, Zhang MQ et al. : Inhibiting the integrated stress response pathway prevents aberrant chondrocyte differentiation thereby alleviating chondrodysplasia. eLife (2018) 7:e37673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, Sigurdardottir A, Bertolotti A: Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science (2015) 348:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carrara M, Sigurdardottir A, Bertolotti A: Decoding the selectivity of eIF2α holophosphatases and PPP1R15A inhibitors. Nat Struct Mol Biol (2017) 24:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crespillo-Casado A, Chambers JE, Fischer PM, Marciniak SJ, Ron D: PPP1R15A-mediated dephosphorylation of EIF2α is unaffected by sephin1 or guanabenz. eLife (2017) 6:e26109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gistelinck C, Kwon RY, Malfait F, Symoens S, Harris MP, Henke K, Hawkins MB, Fisher S, Sips P, Guillemyn B, Bek JW et al. : Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies. Proc Natl Acad Sci USA (2018) 115:E8037–E8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fitzgerald J, Lamandé SR, Bateman JF: Proteasomal degradation of unassembled mutant type I collagen pro-α 1(I) chains. J Biol Chem (1999) 274:27392–27398. [DOI] [PubMed] [Google Scholar]
- 54.Ishida Y, Yamamoto A, Kitamura A, Lamandé SR, Yoshimori T, Bateman JF, Kubota H, Nagata K: Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell (2009) 20:2744–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cinque L, Forrester A, Bartolomeo R, Svelto M, Venditti R, Montefusco S, Polishchuk E, Nusco E, Rossi A, Medina DL, Polishchuk R et al. : FGF signalling regulates bone growth through autophagy. Nature (2015) 528:272–275. [DOI] [PubMed] [Google Scholar]
- 56. ••.Forrester A, De Leonibus C, Grumati P, Fasana E, Piemontese M, Staiano L, Fregno I, Raimondi A, Marazza A, Bruno G, Iavazzo M et al. : A selective ER-phagy exerts procollagen quality control via a calnexin-FAM134B complex. EMBO J (2019) 38:e99847.Mechanistic elucidation of an ER-phagy pathway for procollagen turnover, providing the first clear insight into how misassembled procollagen is cleared from the ER.
- 57. •.Omari S, Makareeva E, Roberts-Pilgrim A, Mirigian L, Jarnik M, Ott C, Lippincott-Schwartz J, Leikin S: Noncanonical autophagy at ER exit sites regulates procollagen turnover. Proc Natl Acad Sci USA (2018) 115:E10099–E10108.Discovery of a novel, bafilomycin-insensitive autophagic pathway capable of clearing mutant procollagen aggregates from the ER.
- 58.Alvarez-Garcia O, Matsuzaki T, Olmer M, Plate L, Kelly JW, Lotz MK: Regulated in development and DNA damage response 1 deficiency impairs autophagy and mitochondrial biogenesis in articular cartilage and increases the severity of experimental osteoarthritis. Arth Rheum (2017) 69:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gioia R, Tonelli F, Ceppi I, Biggiogera M, Leikin S, Fisher S, Tenedini E, Yorgan TA, Schinke T, Tian K, Schwartz JM et al. : The chaperone activity of 4PBA ameliorates the skeletal phenotype of Chihuahua, a zebrafish model for dominant osteogenesis imperfecta. Hum Mol Genet (2017) 26:2897–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bateman JF, Sampurno L, Maurizi A, Lamandé SR, Sims NA, Cheng TL, Schindeler A, Little DG: Effect of rapamycin on bone mass and strength in the α2(I)-G610C mouse model of osteogenesis imperfecta. J Cell Mol Med (2019) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forouhan M, Sonntag S, Boot-Handford RP: Carbamazepine reduces disease severity in a mouse model of metaphyseal chondrodysplasia type schmid caused by a premature stop codon (Y632X) in the Col10a1 gene. Hum Mol Genet (2018) 27:3840–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DiChiara AS, Li RC, Suen PH, Hosseini AS, Taylor RJ, Weickhardt AF, Malhotra D, McCaslin DR, Shoulders MD: A cysteine-based molecular code informs collagen C-propeptide assembly. Nat Comm (2018) 9:e4206. [DOI] [PMC free article] [PubMed] [Google Scholar]