Abstract
Inflammatory bowel disease is a chronic disorder characterized by inflammation of the gastrointestinal tract and an immune mediated attack against the commensal microbiota. Vitamin D is an essential vitamin which not only promotes calcium and phosphate absorption but regulates immune function. The active form of vitamin D (1,25(OH)2D) has been shown to suppress symptoms of inflammatory bowel disease by inhibiting T cell responses. Host protection from gastrointestinal infection depends on T cells. Paradoxically, vitamin D deficiency increases susceptibility to inflammatory bowel disease and gastrointestinal infection. Here we will review the roles of vitamin D in immune cells using a kinetic model of the vitamin D-mediated effects on infection to explain the sometimes, paradoxical effects of vitamin D on gastrointestinal immunity.
Keywords: vitamin D, infection, gastrointestinal tract, T cells
In the early 1980’s it was discovered that immune cells and other cells outside of those important in bone or mineral metabolism also expressed the vitamin D receptor (VDR, [1, 2]). Since this discovery, the role of vitamin D and the VDR in immune response regulation has been an active area of study. Early experiments added the active form of vitamin D (1,25(OH)2D, 1,25D) to human peripheral blood mononuclear cells in vitro and showed that 1,25D suppressed T cell proliferation, IL-2 and IFN-γ production [1, 2]. Later experiments in animal models of T cell mediated autoimmunity, demonstrated that 1,25D suppressed the development of experimental models of type-1 diabetes, multiple sclerosis and inflammatory bowel disease (IBD) [3–5]. The limitations for using 1,25D clinically to treat autoimmunity was hypercalcemia that developed at the therapeutic doses of 1,25D [6]. Interestingly limiting dietary calcium reduced the suppressive capacity of 1,25D on T cell function or autoimmunity, indicating that the effects of 1,25D on T cells are calcium dependent [6].
Immune cells express the VDR and produce 1,25D.
Vitamin D from the diet or following UV light exposure of skin is 25-hydroxylated by several enzymes found in the liver to produce 25(OH)D [7]. The kidney is the exclusive source of the 1alpha hydroxylase, Cyp27B1, for endocrine production of 1,25D [8]. Endocrine regulation of 1,25D production is through several P450 enzymes (Box 1). The VDR is a nuclear receptor, which is part of the steroid/thyroid super family of nuclear receptors that regulate transcription [9]. 1,25D is the high affinity VDR binding ligand [8].
Text Box 1.
Vitamin D regulation of 1,25D.
Endocrine regulation of 1,25D production is a critical regulator of calcium homeostasis. When dietary calcium is adequate, 1,25D increases calcium absorption from the small intestine. Prolonged hypocalcemia results in the production of 1,25D to signal the bones to release calcium [62]. Control of 1,25D production is regulated by a feedback loop that includes inhibition of Cyp27B1 that produces 1,25D from 25(OH)D and induction of Cyp24A1 that inactivates 25(OH)D and 1,25D [62, 63]. Other signals that induce Cyp27B1 production include the parathyroid hormone and hypocalcemia [62, 63]. The production of 1,25D suppresses Cyp27B1 and induces Cyp24A1 [63]. Mutations in Cyp27B1 result in secondary hyperparathyrodism and the development of rickets, and mutations in Cyp24A1 result in hypercalcemia [63]. The availability of 1,25D is carefully controlled by a feedback loop that includes regulation of Cyp27B1 and Cyp24A1 that maintain control of serum calcium.
In addition to the endocrine control of 1,25D, cells in the immune system also produce 1,25D and expresses the VDR. Local regulation of the production of 1,25D is controlled by expression of Cyp27B1 in extra-renal tissues including cells of the immune system [10, 11]. Induction of Cyp27B1 in immune cells requires activation via toll-like receptors or cytokines in macrophages and T cell receptor stimulation in T cells [10–13]. Macrophages, dendritic cells, B cells and T cells are all vitamin D targets since they express the VDR [14–16]. In addition to the systemic control of 1,25D, the immune system can both produce 1,25D and respond to 1,25D by expressing the VDR.
The gastrointestinal immune system.
The immune system of the gastrointestinal tract serves as an important barrier between the host and the commensal microbes that colonize the gut. The intestine contains unique populations of immune cells with specialized functions that mount immune responses to pathogens but not to commensals. Epithelial cells form a single cell barrier between the microbiota and the lamina propria (LP) of the gut. The intraepithelial lymphocytes (IEL) are found interspersed between the epithelial cells. The IEL are mostly (80%) T cells, while the LP has T cells, B cells and myeloid cells (Box 2). A T and B cell response is critical for host protection from gastrointestinal infection [17]. Infection of mice with an enteropathogenic Escherichia coli like gastrointestinal infection (Citrobacter rodentium ) revealed that IL-22 and IL-17 are critical for survival as well as clearance of the infection [18, 19]. C. rodentium is a natural mouse pathogen, which models human gastrointestinal infections caused by the enteropathogenic E. coli [17]. Innate lymphoid type 3 (ILC3) cells are able to produce IL-22 and IL-17 immediately following injury or infection [20]. Later (7–10 days) the acquired immune system is engaged and Th17 cells also produce IL-22 and IL-17 [19]. Conversely, in IBD (Box 3) IL-17 is associated with disease and the IL-17 comes from Th17 cells that are responding to the dysbiotic commensal microbiota [21]. IL-22 is a cytokine that results in mucosal healing and is therefore thought to be an important therapeutic target for IBD [22]. The production of IL-22 is protective for IBD and GI infection, while Th17 cells are needed to clear infection but are pathogenic in IBD. Thus, constraining the IL-17 response following infection is necessary in order for the host to resolve inflammation and return to GI homeostasis.
Text Box 2.
The gastrointestinal immune system.
The unique features of the GI tract include a heterogenous mix of cells that are either absent or functionally different than immune cells in peripheral tissues.
Epithelial cells:
Provides a physical barrier between the host and the luminal contents [64].
Produces tight junction proteins and secretes mucins and antimicrobial peptides [64].
Expresses pattern recognition receptors (toll-like receptors) that identifies microbes [65].
Intraepithelial lymphocytes:
Most are T cells (80%) with very few B cells and myeloid cells [65, 66].
Most of the T cells express CD8αα which identifies the T cells as regulatory cells. The T cells that express CD8αα can also express CD4 or CD8αβ[67].
High frequency of γδ T cells and CD8+ T cells.
Lamina propria:
Dendritic cells and specialized CD103+ dendritic cells. The expression of CD103+ on dendritic cells reduces the ability of the dendritic cells to activate T cells [68].
Conventional CD4 cells: Th1, Th2, Th17 cells and conventional CD8 T cells.
Innate lymphoid cells (ILC) that produce IL-22 (ILC3) [20].
Regulatory (reg) T cells that recognize commensal microbes, FoxP3+/RORγt+ T reg [69].
Text Box 3.
Inflammatory bowel disease: Crohn’s disease or ulcerative colitis.
Crohn’s disease.
Affects the entire gastrointestinal tract from mouth to anus.
Dysbiosis of the microbiota, diarrhea and weight loss.
Vitamin D deficiency is associated with more severe Crohn’s disease and treatment escalation [70, 71].
Animal models of Crohn’s disease, Th1 and Th17 cell driven: IL-10 KO, T cell transfer to immunodeficient mice, acute dextran sodium induced colitis and trinitrobenzene sulfonic acid colitis [72].
Ulcerative colitis
Disease is limited to the colon
Dysbiosis of the microbiota, diarrhea and weight loss.
The role of vitamin D deficiency has not been well studied [70].
Animal models of ulcerative colitis, mixed Th1 and Th2: Chronic dextran sodium sulfate induced colitis and Oxazolone colitis [72].
Vitamin D regulation of immunity.
Vitamin D controls GI homeostasis by regulating epithelial cells, innate immune cells and acquired immune cells. Vitamin D status and expression of the VDR are important regulators of the epithelial barrier in the intestine [23]. VDR knockout mice have increased permeability of the intestine and expression of the VDR only in the GI epithelial cells rescues epithelial integrity and protects the mice from experimental colitis [23–25]. The innate immune cells (macrophages, dendritic cells and ILCs) are also important vitamin D targets. In dendritic cells and macrophages, 1,25D inhibits IL-12, toll-like receptor expression and the ability of dendritic cells to activate T cells [26–28]. Conversely, 1,25D induces the production of IL-10 in dendritic cells and cathelicidin in macrophages [11, 29,30]. Vitamin D deficient mice have fewer ILC3 cells and produced significantly less IL-22 than vitamin D sufficient mice [14]. In the acquired immune system and following activation, 1,25D inhibits B cells and T cells from proliferating [1, 31]. In addition, 1,25D inhibits IL-2, interferon (IFN)-γ, IL-17 and TNF-α production from T cells [31, 32]. Conversely, 1,25D induces regulatory T cells that produce IL-10 and Th2 cells that produce IL-4 [31–33]. It was because of the inhibitory properties of 1,25D on T cells that the early research focused on the effects of vitamin D in diseases where T cells caused pathology [34, 35]. In the gastrointestinal tract there is clear evidence of the immunosuppressive effects of 1,25D on experimentally-induced IBD [36]. However, T cells and the production of IL-17 are also critical for an anti-infectious response to C. rodentium [37]. Paradoxically however, vitamin D deficient mice are more susceptible to experimental IBD and a GI infection with C. rodentium [14, 36]. The recent data demonstrates that the very mechanisms whereby vitamin D inhibits IBD are also critical for the effects of vitamin D to control a GI infection and these ideas will be the focus of this review.
Vitamin D and 1,25D suppress T-cell mediated disease.
The inhibitory effects of 1,25D on T cells suggested that immune-mediated diseases such as multiple sclerosis and IBD could be affected by vitamin D status. 1,25D treatments suppress the development of experimental models of multiple sclerosis (experimental autoimmune encephalomyelitis, EAE) and IBD [3–5, 38]. In addition, vitamin D deficient and VDR knockout (KO) mice develop severe experimental IBD [39–41]. In fact, the transfer of VDR KO T cells into immuno-deficient mice resulted in more severe IBD than the transfer of WT T cells [39]. T cell-specific VDR KO exacerbates EAE and eliminates the capacity of 1,25D to suppress EAE [42]. Furthermore, 1,25D did not suppress EAE in mice without invariant natural killer T (iNKT) cells, suggesting that vitamin D can also directly regulate iNKT cells [43]. T cells are also indirectly regulated by 1,25D. Macrophages and dendritic cells have reduced abilities to stimulate T cells in the presence of 1,25D [27]. Furthermore, treating dendritic cells with 1,25D prior to transfer induced T regs that suppressed allograft rejection in mice [27]. Vitamin D deficient or VDR KO mice have fewer CD8αα expressing regulatory T cells in the gastrointestinal mucosa, suggesting that vitamin D is important in the development or function of CD8αα T cells [44]. The mechanisms by which vitamin D regulates IBD include both the direct inhibition of T cells that produce IL-17 and IFN-γ, and induction of regulatory cells (T regs, CD8αα and Tand iNKT cells) that produce IL-10 [36].
Vitamin D and infection.
The ability of vitamin D and 1,25D to inhibit Th1/Th17 cells and induce T regs should compromise the hosts’ ability to fight infection. This hypothesis was tested in mice following a systemic fungal or viral infection [45]. Mice were treated with 1,25D or the T cell suppressor cyclosporin A, and then infected with either a herpes virus or Candida albicans [45]. The cyclosporin A treated mice had reduced survival, while mice treated with 1,25D had no reduction in survival [45]. Vitamin D deficiency had no effect on Mycobacterium tuberculosis, Streptococcus pneumoniae or Pseudomonas aeruginosa infections in mice [46, 47]. However, Listeria monocytogenes and Salmonella infections were cleared more slowly in mice that could not respond to vitamin D (VDR KO) [48, 49]. Some infections like L. monocytogenes and M. tuberculosis replicate inside of macrophages, and other infections Salmonella, S. pneumoniae and P. aeruginosa are extracellular infections. The other possible difference between the effects of vitamin D on infection is the tissues being infected, with L. monocytogenes and Salmonella infecting the GI tract, and M. tuberculosis infecting the lung. The final issue that might be affecting the result of vitamin D on infection is the suitability of the models and how well the model mirrors the human effects of 1,25D. The 1,25D induction of cathelicidin, that is important for inhibiting M. tuberculosis growth in human cultured macrophages, is not regulated by 1,25D in the mouse [50]. Thus the effect of vitamin D on infection may be tissue, pathogen or model specific.
Immune cells express the VDR and produce 1,25D.
Expression of the VDR depends on the tissue and cell type. In the kidney of vitamin D sufficient hosts the VDR is expressed constitutively [51]. Calcium and 1,25D are in vivo positive regulators of the VDR in the kidney but not the intestine [51]. Tissue specific regulation of the VDR by 1,25D has also been demonstrated by Chip-Seq analysis of VDR expression in the kidney, intestine and bone [52]. All cells of the immune system that have been evaluated express the VDR. The signals that regulate VDR expression in immune cells are different than those in kidney, intestine and bone. Cytokines, toll-like receptor ligands, T cell receptor stimulation, and non-specific activation using PMA and ionomycin all increase the VDR expression in immune cells [12, 15, 16]. Unlike the kidney that constitutively expresses high amounts of the VDR, the immune system maximally expresses the VDR 2–3 days after activation. T cell activation for 48–72h upregulates the VDR in mouse and human CD8+ and CD4+ T cells [12, 15, 16]. Other immune cells including macrophages also require activation for maximal expression of the VDR [16]. VDR is constitutively expressed in the kidney and inducible in immune cells.
Like the VDR, the level of 1,25D is tissue specific. In the kidney the expression of the 1-alpha hydroxylase (Cyp27B1) is induced by hypocalcemia, and the parathyroid hormone [13, 53]. In a feedback loop 1,25D inhibits Cyp27B1 expression in the kidney and induces Cyp24A1 to eliminate excess 1,25D (Box 1). The immune system can also be a source for locally produced 1,25D; however, the amount of Cyp27B1 made by immune cells is extremely low compared to the amount produced in the kidney [11, 13]. In addition, the signals that regulate the Cyp27B1 in immune cells are not the same as those that regulate renal Cyp27B1 [11, 13]. Lipopolysacharide (LPS) activation of human macrophages is required for induction of Cyp27B1 [11, 54]. Conversely, LPS has no effect on renal production of Cyp27B1[13]. In vitro, human macrophages produce 1,25D when activated with toll-like receptors (LPS) and cytokines [11, 54]. Activated T cells are also a source of Cyp27B1 [13]. Immune cells produce 1,25D locally following 2–3 days of activation.
When the immune system is in homeostasis, the VDR and Cyp27B1 are expressed at low levels (i.e. absence of infection). In the first several days following activation, the immune system does not utilize or respond to vitamin D [31]. Early post-infection the macrophages and innate cells that are activated immediately following infection and are the first to produce 1,25D. Later the acquired T cells are activated 5–7 days post-infection and then 2–3 days after activation the VDR and Cyp27B1 gene are expressed in T cells. Therefore, it is not until 7–10 days post-infection that T cells express the VDR and are targets for 1,25D mediated inhibition of proliferation, IL-17 and IFN-γ [36]. Within a few weeks of infection the T and B cells clear the infection and antigen is eliminated. If the host is vitamin D deficient they would still be able to eliminate the infection. In fact, vitamin D deficient mice cleared C. rodentium infection but with delayed kinetics compared to vitamin D sufficient mice [14]. Conversely, in IBD the antigen cannot be eliminated and the T cells become chronically activated. In activated T cells the availability of 1,25D would be essential to inhibit IL-17, IFN-γ and T cell proliferation. In addition, 1,25D induces regulatory T cells that produce IL-10 [33, 36]. These functions of 1,25D constrain the chronically activated Th1 and Th17 responses and therefore suppress IBD symptoms [55]. If the host is vitamin D deficient the Th1 and Th17 cells remain chronically activated [36]. 1,25D is therefore critical for the induction of regulatory T cells that together with the direct effects of 1,25D on Th1/Th17 cells restrains the IFN-γ and IL-17 response and resolves inflammation.
Vitamin D and host-resistance to gastrointestinal infection.
Vitamin D has been shown to promote gastrointestinal homeostasis. Infections are often linked to autoimmunity and infections in the gastrointestinal tract contribute to the pathogenesis of IBD. At the peak of infection with C. rodentium a strong Th17 response is observed, which is also characteristic of colitis in IBD [17]. 1,25D treatment of mice already infected with C. rodentium suppressed the IL-17 response in the colon which increased the number of C. rodentium shed in the feces [56]. Paradoxically, vitamin D deficient and VDR KO mice also developed more severe infections with C. rodentium [14, 57]. Thus, it is unclear how vitamin D deficiency and 1,25D treatment both led to more severe infection with C. rodentium.
To date, we know that the innate immune system is required for early protection from C. rodentium and mice without T and B cells use the innate immune system to control infection for two weeks but then develop a systemic infection that is fatal [17]. IL-22 KO mice develop an early and lethal infection within several days of C. rodentium infection and the IL-22 is produced by innate lymphoid cells (ILC3) [42]. Both T cells and B cells are important for protection from C. rodentium since mice without T cells (CD4 KO) or B cells (IgμM KO) also develop systemic infection [41]. IL-17 production by Th17 cells is required for clearance of C. rodentium [19]. ILC3 cells that produce IL-22, Th17 cells and B cells are all critical for host defense against C. rodentium.
The effects of vitamin D status on the mucosal immune response to C. rodentium were recently examined. Vitamin D deficient mice develop a severe infection with C. rodentium that resulted in the rapid mortality of the vitamin D deficient mice [14]. IL-22 production was lower in vitamin D deficient than vitamin D sufficient mice [14]. Treating vitamin D deficient mice with IL-22 completely protected them from infection, replacing the need for vitamin D early post-C. rodentium infection [14]. IL-17 mRNA expression was higher in the gastrointestinal tract of C. rodentium infected vitamin D deficient mice and 1,25D suppressed IL-17 at the peak of C. rodentium infection [14, 56]. However, vitamin D deficient mice failed to expand the numbers of Th17 cells following C. rodentium infection [14]. Tissue specific KO of the VDR in T cells (T-VDR KO) or in B cells (B-VDR KO) had no effect on the kinetics of C. rodentium clearance, which is consistent with a model where vitamin D in T and B cells is not required for the ability to eliminate an acute infection [14]. Vitamin D is required for early IL-22 production and the expansion of infection induced Th17 cells that protect the gastrointestinal tract from C. rodentium.
Downregulating immunity to increase resistance to infection.
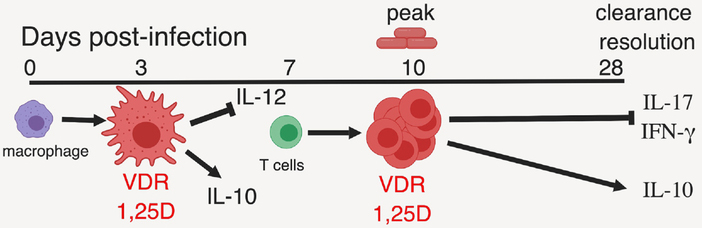
The immune system is designed to eliminate invading pathogens without causing collateral damage to healthy tissue. Therefore, dampening immune responses post-infection is equally important as initiating the immune response to infection. The benefits of vitamin D in experimental models of Streptococcus pneumoniae, Pseudomonas aeruginosa, M. tubercolosis and malaria were shown to be via the reduction of the immunopathology from the infection and not more rapid elimination of the pathogen [46, 58, 59]. Vitamin D interventions enhanced resolution of inflammation in patients infected with M. tuberculosis and 1,25D treatment of peripheral blood mononuclear cells from patients with pulmonary tuberculosis downregulated IFN-γ, and cytotoxic cell mediators (perforin, granzyme-B and granulysin) [60, 61]. Based on the available data we have developed a model to describe the effects of vitamin D in host resistance to gastrointestinal infection (Fig. 1). The host mounts a robust innate immune response to infection (Fig. 1). The macrophages and other innate immune cells express the VDR and produce 1,25D at 3 days post-infection (Fig. 1). The 1,25D regulates the innate cells that present antigen to the T cells that expand following infection (Fig. 1). At the peak of the infection the T cells now express the VDR and can produce 1,25D (Fig. 1). The ability of the immune system to both produce 1,25D and express the VDR requires activation and time (Fig. 1). Since the innate immune system is activated prior to the T cells, macrophages and other innate cells are early targets of vitamin D following infection, while Th1 and Th17 cells are later targets of vitamin D following infection (Fig. 1). In the absence of vitamin D the damage from poorly controlled immune activation causes immune pathology, while infections are cleared. The same mechanisms whereby vitamin D and 1,25D regulate Th1/Th17 cell in IBD are used to control the resolution of immunity following infection and to protect from immunopathology.
Figure 1. A model for the kinetics of the immune mediated effects of 1,25D following infection.
The innate immune system responds first to an infection. Macrophages and other innate immune cells become activated by sensing the presence of the pathogen. The VDR and 1,25D is produced within 3 days of infection in macrophages and innate immune cells. At day 3 post-infection, the 1,25D regulates the activated macrophages to modify the ability to present antigen to T cells and to reduce IL-12 and induce IL-10. At day 7 post-infection T cells begin to be activated and Th1 and Th17 cells proliferate rapidly. After 10 days of infection the activated T cells will begin to clear the infection and will express the VDR and produce 1,25D. As the infection is cleared the 1,25D will decrease IL-17 and IFN-γ and induce regulatory cells to produce IL-10. The 1,25D mediated inhibition of IL-17 and IFN-γ and induction of IL-10 helps to resolve the inflammation and prevents further damage.
Concluding Remarks and Future Perspectives.
Vitamin D is an important regulator of immunity, and in particular critical for the down-regulation of the immune system after infection. The immune response to infection requires rapid recognition of the infectious threat, elimination of the pathogen and resolution of the immune response. There is strong evidence demonstrating that vitamin D and 1,25D inhibit Th1/Th17 mediated immunity directly, and indirectly by the induction of IL-10 producing regulatory T cells which contribute to the resolution of an immune response. Vitamin D does not regulate innate immune responses before day 3 of infection and T cells before day 10 of infection, since immune cells do not express the VDR or produce 1,25D until activated. An important role of vitamin D in the anti-infectious immune response is to limit the inflammation and tissue injury. The same vitamin D regulated mechanisms that are effective in the protection from IBD are in play and provide protection from gastrointestinal infection.
Funding
This work was supported by the National Institutes of Health (R01AT005378), United State Department of Agriculture (2914-38420-21822) and United States Department of Agriculture National Institute of Food and Agriculture/Hatch Appropriations (under Project: #PEN04605, Accession #1018545).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tsoukas CD et al. (1984) 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science 224 (4656), 1438–40. [DOI] [PubMed] [Google Scholar]
- 2.Rigby WF et al. (1984) Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest 74 (4), 1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemire JM and Archer DC (1991) 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 87 (3), 1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantorna MT et al. (2000) 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130 (11), 2648–52. [DOI] [PubMed] [Google Scholar]
- 5.Cantorna MT et al. (1996) 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A 93 (15), 7861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantorna MT et al. (1999) Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr 129 (11), 1966–71. [DOI] [PubMed] [Google Scholar]
- 7.Hoel DG et al. (2016) The risks and benefits of sun exposure 2016. Dermatoendocrinol 8 (1), e1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prosser DE and Jones G (2004) Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29 (12), 664–73. [DOI] [PubMed] [Google Scholar]
- 9.Pike JW (2011) Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Mol Cell Endocrinol 347 (1–2), 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewison M et al. (2007) Extra-renal 25-hydroxyvitamin D3–1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103 (3–5), 316–21. [DOI] [PubMed] [Google Scholar]
- 11.Liu PT et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311 (5768), 1770–3. [DOI] [PubMed] [Google Scholar]
- 12.Kongsbak M et al. (2014) Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS One 9 (5), e96695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ooi JH et al. (2014) Murine CD8+ T cells but not macrophages express the vitamin D 1alpha-hydroxylase. J Nutr Biochem 25 (1), 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YD et al. (2019) Vitamin D Is Required for ILC3 Derived IL-22 and Protection From Citrobacter rodentium Infection. Front Immunol 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahon BD et al. (2003) The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 89 (5), 922–32. [DOI] [PubMed] [Google Scholar]
- 16.Veldman CM et al. (2000) Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374 (2), 334–8. [DOI] [PubMed] [Google Scholar]
- 17.Mundy R et al. (2005) Citrobacter rodentium of mice and man. Cell Microbiol 7 (12), 1697–706. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y et al. (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14 (3), 282–9. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov II et al. (2009) Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139 (3), 485–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonnenberg GF and Artis D (2012) Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity 37 (4), 601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moschen AR et al. (2019) IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 16 (3), 185–196. [DOI] [PubMed] [Google Scholar]
- 22.Mizoguchi A et al. (2018) Clinical importance of IL-22 cascade in IBD. J Gastroenterol 53(4), 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He L et al. (2018) Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 159(2), 967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ooi JH et al. (2013) Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate-Induced Colitis. J Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W et al. (2013) Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 123 (9), 3983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadeghi K et al. (2006) Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 36 (2), 361–70. [DOI] [PubMed] [Google Scholar]
- 27.Griffin MD et al. (2001) Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A 98 (12), 6800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Ambrosio D et al. (1998) Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest 101 (1), 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma R and Kim JY (2016) 1,25-Dihydroxyvitamin D3 Facilitates M2 Polarization and Upregulates TLR10 Expression on Human Microglial Cells. Neuroimmunomodulation 23 (2), 75–80. [DOI] [PubMed] [Google Scholar]
- 30.Korf H et al. (2012) 1,25-Dihydroxyvitamin D(3) curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. [DOI] [PubMed] [Google Scholar]
- 31.Cantorna MT et al. (2015) Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 7(4), 3011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baeke F et al. (2010) Vitamin D: modulator of the immune system. Curr Opin Pharmacol 10 (4), 482–96. [DOI] [PubMed] [Google Scholar]
- 33.Mathieu C and Adorini L (2002) The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 8 (4), 174–9. [DOI] [PubMed] [Google Scholar]
- 34.Cantorna MT (2000) Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med 223 (3), 230–3. [DOI] [PubMed] [Google Scholar]
- 35.Cantorna MT (2012) Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys 523 (1), 103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantorna MT et al. (2014) Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 239 (11), 1524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valeri M and Raffatellu M (2016) Cytokines IL-17 and IL-22 in the host response to infection. Pathog Dis 74 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zella JB et al. (2003) Oral administration of 1,25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys 417 (1), 77–80. [DOI] [PubMed] [Google Scholar]
- 39.Froicu M et al. (2003) A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17 (12), 2386–92. [DOI] [PubMed] [Google Scholar]
- 40.Froicu M et al. (2006) Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology 117 (3), 310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Froicu M and Cantorna MT (2007) Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayne CG et al. (2011) 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur J Immunol 41(3), 822–32. [DOI] [PubMed] [Google Scholar]
- 43.Waddell A et al. (2015) NKT cells can help mediate the protective effects of 1,25-dihydroxyvitamin D3 in experimental autoimmune encephalomyelitis in mice. Int Immunol 27(5), 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruce D and Cantorna MT (2011) Intrinsic requirement for the vitamin D receptor in the development of CD8alphaalpha-expressing T cells. J Immunol 186 (5), 2819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantorna MT et al. (1998) 1,25-Dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation 66 (7), 828–31. [DOI] [PubMed] [Google Scholar]
- 46.Reeme AE and Robinson RT (2016) Dietary Vitamin D3 Suppresses Pulmonary Immunopathology Associated with Late-Stage Tuberculosis in C3HeB/FeJ Mice. J Immunol 196 (3), 1293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niederstrasser J et al. (2016) Vitamin D Deficiency Does Not Result in a Breach of Host Defense in Murine Models of Pneumonia. Infect Immun 84 (11), 3097–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruce D et al. (2009) Elevated non-specific immunity and normal Listeria clearance in young and old vitamin D receptor knockout mice. Int Immunol 21 (2), 113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu S et al. (2010) Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol 177 (2), 686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu PT and Modlin RL (2008) Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol 20 (4), 371–6. [DOI] [PubMed] [Google Scholar]
- 51.Healy KD et al. (2003) Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D3 and calcium. Proc Natl Acad Sci U S A 100 (17), 9733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SM et al. (2018) The impact of VDR expression and regulation in vivo. J Steroid Biochem Mol Biol 177, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenza HL and DeLuca HF (2000) Regulation of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys 381 (1), 143–52. [DOI] [PubMed] [Google Scholar]
- 54.Adams JS et al. (2014) Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol 144 Pt A, 22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantorna MT and Waddell A (2014) The vitamin D receptor turns off chronically activated T cells. Ann N Y Acad Sci. [DOI] [PubMed] [Google Scholar]
- 56.Ryz NR et al. (2012) Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses. Am J Physiol Gastrointest Liver Physiol 303 (12), G1299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J et al. (2015) Dysbiosis caused by vitamin D receptor deficiency confers colonization resistance to Citrobacter rodentium through modulation of innate lymphoid cells. Mucosal Immunol 8 (3), 618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He X et al. (2014) Vitamin D inhibits the occurrence of experimental cerebral malaria in mice by suppressing the host inflammatory response. J Immunol 193 (3), 1314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niederstrasser J et al. (2016) Vitamin D deficiency does not result in a breach of host defense in murine models of pneumonia. Infect Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coussens AK et al. (2012) Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A 109 (38), 15449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Afsal K et al. (2018) 1, 25-dihydroxyvitamin D3 downregulates cytotoxic effector response in pulmonary tuberculosis. Int Immunopharmacol 62, 251–260. [DOI] [PubMed] [Google Scholar]
- 62.Goltzman D et al. (2018) Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front Horm Res 50, 1–13. [DOI] [PubMed] [Google Scholar]
- 63.Jones G et al. (2017) Genetic Diseases of Vitamin D Metabolizing Enzymes. Endocrinol Metab Clin North Am 46 (4), 1095–1117. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad R et al. (2017) Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol 10 (2), 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eberl G (2005) Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol 5 (5), 413–20. [DOI] [PubMed] [Google Scholar]
- 66.Mowat AM (2003) Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 3 (4), 331–41. [DOI] [PubMed] [Google Scholar]
- 67.Gangadharan D et al. (2006) Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity 25 (4), 631–41. [DOI] [PubMed] [Google Scholar]
- 68.Johansson-Lindbom B and Agace WW (2007) Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev 215, 226–42. [DOI] [PubMed] [Google Scholar]
- 69.Sefik E et al. (2015) Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 349 (6251), 993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cantorna MT (2016) IBD: Vitamin D and IBD: moving towards clinical trials. Nat Rev Gastroenterol Hepatol 13 (6), 322–3. [DOI] [PubMed] [Google Scholar]
- 71.Kabbani TA et al. (2016) Association of Vitamin D Level With Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am J Gastroenterol. [DOI] [PubMed] [Google Scholar]
- 72.Kiesler P et al. (2015) Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol 1 (2), 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]