Introduction
The regenerative capacity of damaged peripheral nerves enables the central nervous system (CNS) to regain neuromuscular connections and control over muscle contraction. Although necessary, muscle reinnervation alone is not sufficient to restore normal control of movement1-3. Limb movements during walking, for example, can exhibit persistent ataxia4-6 together with abnormal co-contraction of antagonist muscles, i.e. in-phase activation instead of the normal alternating or shifted-phase patterns of activation7-9. Unusual co-contraction would seem readily explained by the incompatible motor commands received by muscles that are non-selectively reinnervated by motoneurons from multiple, possibly antagonist motor pools1-3. The sufficiency of this explanation is challenged, however, on finding nearly continuous co-contraction of antagonist muscles in walking rats, even when severed nerves are surgically constrained to reinnervate corresponding flexor and extensor muscles10. The latter finding suggests that the CNS is complicit in antagonist discoordination, an inference supported by wide-ranging aberrations observed in CNS neurons and circuits following peripheral nerve injury and repair2. However, the circuit dysfunction responsible for abnormal antagonist coordination remains unknown.
Among the neural circuits distributed throughout the CNS that coordinate antagonist muscles11-14, reflex pathways in the spinal cord seem likely contributors to antagonist discoordination. Circuits in the spinal cord are the first to utilize mechanosensory information from primary afferents to regulate patterns of antagonist muscle activation. Acting on feedback from group Ia muscle spindle afferents, the disynaptic Ia inhibitory circuit mediates reciprocal inhibition that relaxes antagonists during contraction of agonists15-17. In normal animals, suppression of this pathway by central neural networks plays a permissive role in facilitating cocontraction of agonist and antagonist muscles18,19. Pathological reduction of reciprocal inhibition is associated with abnormal co-contraction of antagonists in human subjects with spasticity, spinal cord injury, stroke, and dystonia13,20-22. These observations led us to hypothesize a reduction in reflex inhibition between antagonist muscles after nerve injury and muscle reinnervation.
The present study was designed to test for abnormality in spinal reflex pathways that coordinate activation of muscles acting as mechanical antagonists at the ankle joint in adult rats. In a condition-test paradigm, stretch reflexes evoked in extensor muscles were conditioned by stretch activation of sensory receptors from flexor muscles reinnervated by the severed common fibular nerve. Acute decerebration in terminal experiments rendered rats reflexively responsive to mechanical activation of muscle sensory receptors and eliminated potential influences from several supraspinal regions. Results led us to reject our original hypothesis, because reflex suppression between antagonists was strengthened and not weakened as predicted. In addition, we found that reflex conditioning switched between suppression and facilitation from trial to trial with no predictable pattern, but with greater incidence and strength of facilitation than that also found, surprisingly, in control rats. Collectively, these findings suggest extensive dysregulation in spinal cord circuits that coordinate reflex activation of agonist and antagonist muscles. In discussion, we consider how dysregulation of spinal reflex circuits might yield abnormal expression of co-contraction among antagonist muscles following nerve injury and repair.
2. Methods
2.1. Animals
Data were obtained from 10, adult female Wistar rats aged 11-18 months (Charles Rivers Laboratories, Wilmington, MA), ranging in weight from 340-480 g. Rats were randomly assigned to one of two groups: an antagonist reinnervation group (5 rats) subjected to survival nerve surgery described below or a control group (5 rats), receiving no treatment prior to terminal experimentation. All rats were studied during single terminal experiments, after which they were euthanized by isoflurane overdose and exsanguination. All procedures for animal care and experimentation were approved by the Wright State University Institutional Animal Care and Use Committee.
2.2. Survival Surgery
Surgical nerve section and repair were performed during survival surgeries on 5 rats (ages 4.2-4.7 months) assigned to the antagonist reinnervation group. With rats deeply anesthetized by isoflurane delivered via nose cone (1.0-2.5% in 100% oxygen), the left common fibular peroneal nerve was surgically exposed by skin incision over the head of the fibula, cut through using microscissors, and immediately joined end-to-end through the epineurium with one to two sutures (10-0 ethilon). The skin incision was sutured closed, and a subcutaneous injection of buprenorphine (0.1 mg/kg) was given before anesthesia was discontinued. Animals were then returned to their cages and monitored throughout the recovery period. Additional buprenorphine was given prophylactically every 12 hours for the first 48 hours after surgery in order to alleviate the possibility of pain. Regularly scheduled veterinary observations and care revealed no signs of pain, distress, or infection of the treated rats throughout the 9.4-14.0 months following survival surgery.
2.3. Terminal Experiments: Preparation
Identical terminal experiments were performed on the reinnervation and control groups when the ages of member rats ranged, respectively from 13.4-18.6 months and 11.3-15.3 months. Experiments were similar to those described in earlier studies from this laboratory23. Briefly, rats were deeply anesthetized by isoflurane inhalation (1-3% in 100% oxygen) adjusted to maintain appropriate values for continuously-monitored vital signs, including heart rate, blood O2 saturation, expired CO2, temperature (37 ± 1° C), and respiratory rate. Surgical preparations under these conditions included freeing the TA and gastrocnemius (G) muscles (both lateral and medial gastrocnemii) and their nerves from surrounding tissues. After marking the resting lengths (Lr) of TA and G muscles with knee and ankle positioned at 90° flexion and plantar flexion, respectively, their tendons of insertion were severed. The rat was then fixed in a stereotaxic frame by clamps at the snout, lumbar spinous processes, and at the femur, just above the knee and ankle (both fixed at 90°). Next, the TA and G muscles were attached through their cut tendons directly to the lever arms of individual servo-motors which, in length servo mode, delivered computer driven muscle stretches while monitoring force and length (Fig. 1A). Bipolar wire electrodes were inserted into each muscle to record EMG. In final preparation for recording, the dorsal surface of the brain was surgically exposed for mid-collicular decerebration which rendered the rat insensate yet reflex responsive to muscle stretch when isoflurane was discontinued for the duration of data collection.
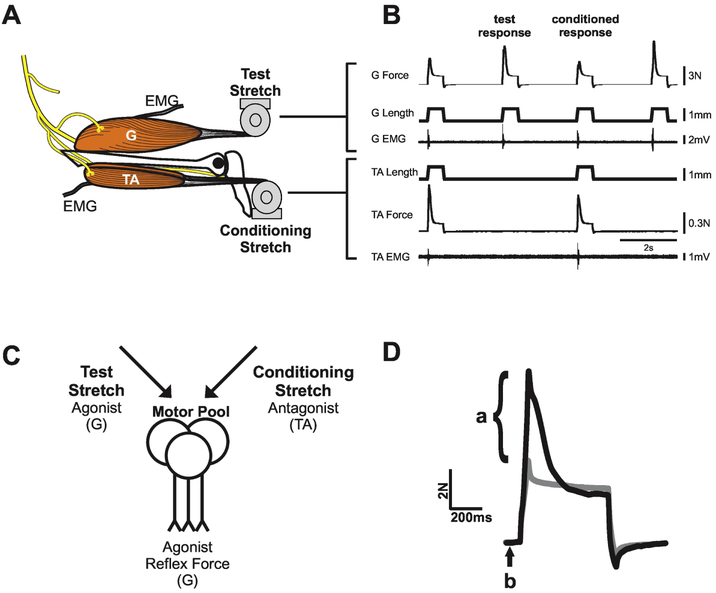
Figure 1.
Stretch reflex test-condition paradigm. A: Key features of in vivo recording: gastrocnemius (G) and tibialis anterior (TA) muscles attached through tendons to length servo motors controlling muscle stretch; electrodes in both muscles record electromyographic (EMG) signals. B: Continuous record segments of force, length, and EMG for G and TA muscles taken from control rat. Stimulus paradigm alternating test stretch of G muscle alone, with conditioning stretch to TA during G muscle stretch. C: Simplified illustration shows the convergence of test and conditioning stimuli onto G motor pools. Arrows indicate the sum of excitatory and inhibitory input from both test and conditioning stretches. D: Reflex force estimated at the peak of ramp stretch as the difference in force (a) measured with the muscle nerve intact (black line) vs cut (grey line). The force occurring immediately before stretch is referred to as the background force (b).
2.4. Terminal Experiments: Protocol
The function of antagonist reflex pathways was examined using a test-condition design (Fig. 1C), whereby the G muscle stretch reflex (test response) was conditioned in alternate trials by stretch applied to the ipsilateral (left) TA muscle (Figs 1A,B). Parameters of stretch were identical for each muscle: ramp-hold-release, with ramp and release each 50ms in duration at constant velocity (20mm/s), hold phase 500ms, amplitude 1mm, repeated at 2s intervals in order to minimize reflex history dependence and maximize data collection. Stretches were applied on a background of no muscle contraction preceding stretch, verified by the absence of EMG. Data were collected with the G muscles stretched out to different fixed lengths and corresponding levels of passive muscle tension (0.2 – 1.0N), in order to examine the effect of varying magnitude of the test stretch reflex. Several additional measures were taken at the end of each experiment. First, the nerves supplying the left G and TA muscles were cut and muscles were stretched at different fixed levels of background force and length just as they were with nerves intact, in order to estimate the passive force component of stretch responses24. Second, maximum force was recorded during tetanic electrical stimulation (0.04ms pulses at 50Hz) of the distal ends of the cut muscle nerves. Finally, muscle wet weights were obtained from TA and G muscles extracted bilaterally before rats were euthanized.
2.5. Measuring strength of test and conditioned reflexes
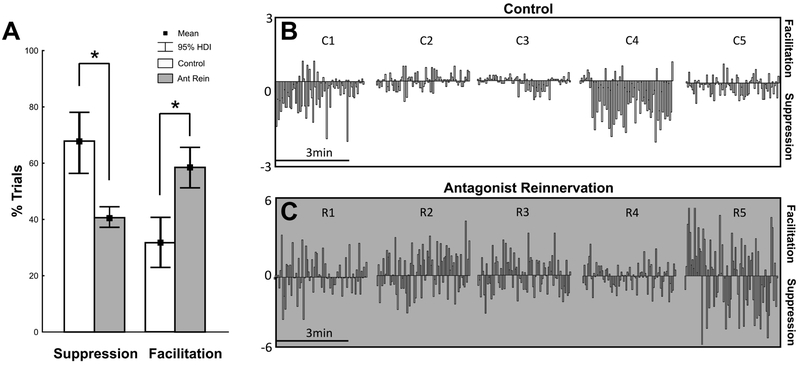
Muscle force, muscle length, and EMG obtained during the experiments were recorded, digitized (20 kHz) and stored on a computer for later analysis using CED Spike 2 software. The force muscle stretch attributable to reflex pathways was estimated by subtracting passive force measured when the nerve was cut (see above)25,26. Analysis was restricted to reflex force measured at the peak of ramp stretch (Fig. 1D). Reflex responses after ramp peak were not analyzed, because of high variability among individual rats in both control and antagonist reinnervation groups. These measures were taken from test and conditioned stretch trials. Each trial of G test stretch was compared with the succeeding TA conditioning trial. Conditioning that reduced test reflex force was labeled suppression, while increased test reflex force was labeled facilitation (Fig. 3).
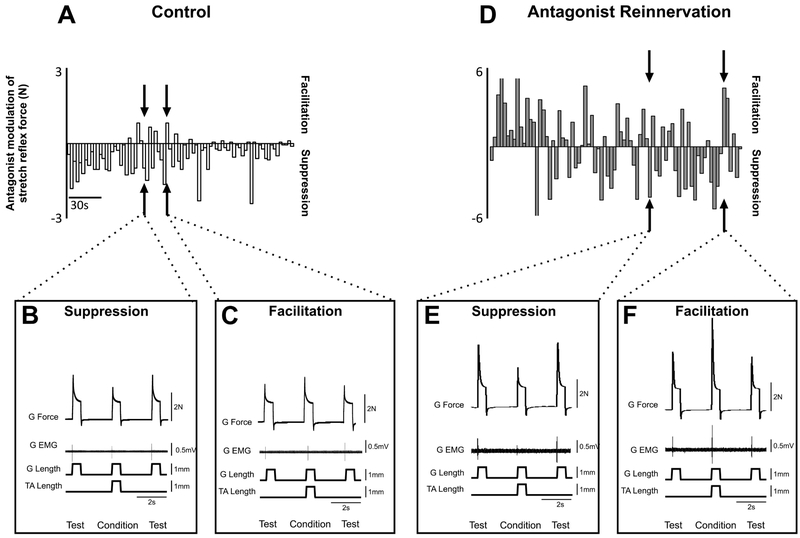
Figure 3.
Reflex Modulation by Antagonist Muscle Stretch. A: illustrates time-series (~4min) record from one control animal. Vertical bars represent the strength (amplitude) of TA conditioning of G. Negative values represent suppression while positive values represent facilitation. Individual examples of suppression (B) and facilitation (C). Isometric force, G EMG, and G length at matched background forces. Conditioned response flanked by test response. Note the occurrence of facilitation in some trials. D: illustrates time-series (~4min) record from one rat in antagonist reinnervation group. Note the stochastic pattern of facilitation and suppression occurring throughout the time-series. Individual examples of suppression (E) and facilitation (F). Isometric force, G EMG, and G length at matched background forces.
2.6. Bayesian Data Analysis
Analyses focused on the strength, proportion and force dependency of suppression and facilitation for both reinnervation and control groups. We employed Bayesian models to compute parameters in order to explicitly test our working hypothesis that nerve injury and repair reduces suppression in antagonist reflex circuits. We empirically derived the full joint posterior probability distributions of model parameters simultaneously (e.g. means, standard deviations, and effect sizes) and directly examined the posterior distributions. This enabled intuitive statistical judgments regarding the strength of the evidence in favor of either the alternative or null hypothesis27-29. Bayesian analytic techniques were chosen because they present noteworthy advantages over frequentist analysis such as null hypothesis statistical testing (NHST) e.g. t-test and ANOVA. Bayesian analytics do not require making assumptions that traditional ANOVA necessitates (e.g. normally distributed data, heteroscedasticity, multiple-test correction)28-30.
Bayesian inference was performed by inspecting the highest density interval (HDI), such that values inside the 95% of the HDI are more credible than outside values30. HDI was used to make unbiased decisions on parameter values. For example, when performing a mean comparison test (i.e. determining experimental group difference), an HDI of a credible difference distribution that does not span zero indicates that the model predictions for the two conditions of interest are different from each other. This reallocates evidential support in favor of the alternative hypothesis that the parameters for both populations are unequal. Alternatively, if an HDI of a credible difference distribution spans zero, that indicates the model predictions for the two conditions do not differ. By defining a region of practical equivalence (ROPE) range (values between −0.1 and 0.1), Bayesian analytic techniques afford the ability to accept the null hypothesis. In practice, when the 95% HDI falls completely within the ROPE region we declare the ROPE value accepted and determine that group differences are practically equivalent to zero.
All models were developed in fully Bayesian framework with the rstanarm package (2.18.1)31 in the R environment (3.5.0)32. Rstanarm implements regression models in stan33, which are fit using Hamiltonian Markov Chain Monte Carlo sampling to compute credible parameter values (θ) e.g. means, standard deviations, regression coefficients, effect sizes34,35. For intercepts and predictors we use Student’s t-distribution with mean zero and four degrees of freedom as the prior distribution. The scale of the prior distribution is 10 for the intercept and 2.5 for the predictors. Each model was run with four independent chains for 1,000 warm-up and 4,000 sampling steps. For all parameters, the number of effective samples was >500. Convergence was assessed and assumed to have reached the stationary distribution by ensuring that the Gelman–Rubin shrinkage statistic for all reported parameters was <1.0536. We report the expected mean parameter values alongside 95% credible intervals using (HDI). We estimate the percent of variance explained by regression models by computing the Bayesian equivalent to R2 37.
Results
To test for reduced reflex suppression after nerve injury and repair, we measured the modulation of agonist muscle stretch reflexes by stretch applied to antagonist muscles in control and antagonist reinnervation groups 5 untreated and 5 treated reflex-reactive decerebrated rats. Focusing study on nerve injury and reinnervation restricted to pre-tibial flexors eliminated confounding influences introduced in previous studies of more extensive nerve injury10. This experimental design also gains clinical relevance, because damage to the common fibular nerve is among the most frequently occurring peripheral nerve injuries38.
3.1. Homonymous stretch reflexes of G and reinnervated TA muscles
G muscles when stretched alone consistently responded reflexively, as measured by force and EMG (e.g. test responses in Fig. 1B, D). Values for homonymous G stretch reflexes for control and antagonist reinnervation groups averaged, 0.448N and 0.761N, respectively. Posterior distributions of means of the test stretch reflex amplitudes were comparable between experimental groups at resting background force (0.2N). Test stretch reflex amplitudes from rats with reinnervated antagonist were on average 0.31N greater than control with a 95% probability of the effect residing between 0.261-0.363N.
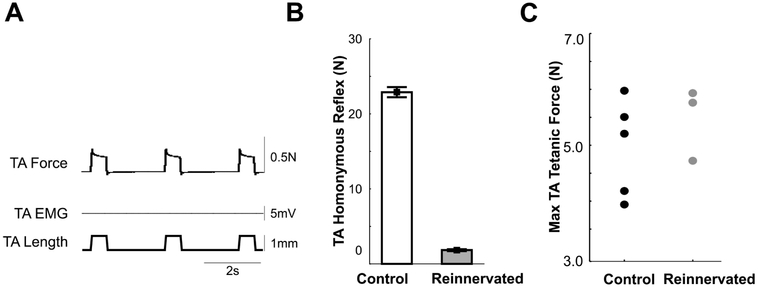
Consistent with earlier reports26,39-41, all rats with reinnervated TA muscles (5 out of 5) demonstrated the expected impotence of homonymous muscle stretch to evoke reflex contraction (Fig. 2A, B). Stretch areflexia occurred despite effective reinnervation evidenced by comparison with contralateral (normally innervated) TA muscles: wet weights were within 18% and tetanic contractions elicited by electrical stimulation were bilaterally comparable (Fig. 2C).
Figure 2.
Responses of reinnervated TA muscle. A: No detectable reflex response to homonymous stretch: absence of EMG and active TA contractile force (TA force identical before and after section of TA nerve (data not shown)). Data taken from one rat 12 months after common fibular nerve section and repair. B: Values for TA stretch reflex amplitude pooled from control (n=5 rats) and antagonist reinnervation (n=5 rats) groups (p<0.001, nested ANOVA with Tukey’s HSD). C: Maximum electrically-evoked tetanic force measured from left TA muscle in 5 rats in control and 3 rats in antagonist reinnervation groups (data not collected from 2 rats).
3.2. Antagonist Modulation of G Stretch Reflex
In control rats, conditioning stretches of TA muscles predominantly suppressed the G stretch reflex (Fig. 3A) during test-conditioned trials at given fixed levels of background G force (1N). Suppression was reflected in both force and EMG records (Fig. 3B). Note the unexpected occurrence of facilitation in some trials interspersed during the time series (Fig. 3A) as evidenced by a modest increase in both force and EMG responses (Fig. 3C).
Rats with reinnervated TA muscles exhibited conspicuous differences from controls. One was the emergence of strong G reflex facilitation produced by stretching the reinnervated antagonist (Fig. 3D, F). Another was an apparent increase in proportion of trials producing facilitation interspersed stochastically in the time series (Fig. 3D). Finally, the strengths of both facilitation and suppression were appreciably amplified (Fig. 3A vs 3D), and evident in comparison of force as well as EMG in paired single trials of test vs conditioned stretches (Fig. 3E, F).
3.3. Statistical analyses of group differences
Data were pooled from rats within groups and evaluated at a matched background force (1N) to investigate the population-level effects of reinnervation on stretch reflex modulation. Across all control rats and all trials, stretching TA predominantly suppressed the G stretch reflex, assign illustrated by the negative (left) shift in distribution (Fig. 4A). While suppression dominated control responses, a fraction of facilitatory responses were observed, as demonstrated by the control population to the right of red null line (Fig. 4A). By contrast, rats in the antagonist reinnervation group exhibited a substantial increase in facilitation over all trials. Figure 4B illustrates the relative positive (right) shift in population level response distribution (Fig. 4B), indicating greater facilitation.
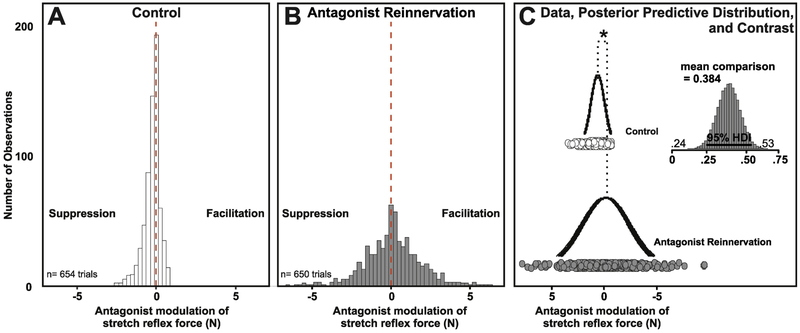
Figure 4.
Reflex Modulation Re-Distributed Following Nerve Regeneration. Histogram of pooled values of modulated reflex force for rats in control (A) and antagonist reinnervation (B) groups using 0.25N bins from all experiments over matched background ranges (1N). Results of Hierarchical One-way Bayesian ANOVA (C) reveal credible differences between empirically derived posterior distributions of group means were all greater than the null distribution 0+−0.1N and centered on .381N greater amplitude facilitation in antagonist reinnervation group. Asterisk denotes Bayesian Inference by Difference of Means Comparison where 95% highest density interval (HDI) does not cross zero.
For statistical inference, Bayesian parameter estimation was implemented to empirically derive the posterior distributions of means and standard deviations for both control and reinnervated antagonist groups (Fig. 4C). Inference by mean comparison testing gave evidence that reinnervation significantly disrupted antagonist conditioning of G stretch reflex. When compared to control, reinnervated TA conditioning of G resulted in a positive (0.381N) shift in conditioned response even when controlled for sample size (note number of observations). Detailed examination of the 95%HDI (0.234-0.529) revealed 0% of the posterior overlaps the null distribution indicating all credible parameter estimates support increased facilitation (Fig. 4C).
Next, to test for the reduced reflex suppression we hypothesized, we evaluated both the frequency of occurrence and strength of agonist reflex suppression from a reinnervated antagonist.
3.4. Suppression:Facilitation proportion decreased after nerve injury and repair
The percentage of trials in a time series at fixed G background force in which reflex suppression or facilitation occurred was calculated for control and antagonist reinnervation groups. Mean percentages were then aggregated across all background forces for initial comparison. Two-group comparison was used to derive the posterior distributions of all parameters for frequency effect comparisons. Examination of the control posterior distributions of mean, revealed that stretching TA suppressed the G stretch reflex in 72% of trials and facilitated the G stretch reflex in 28% of trials (Fig. 5A). Detailed examination of 95%HDIs reveals 0% overlap in probability distributions, indicating suppressive responses dominated TA conditioning of G. Examination of the reinnervation-group posterior distributions of mean frequency, revealed that stretching TA suppressed the G stretch reflex in 40% of trials and facilitated the G stretch reflex in 60% of trials. Detailed examination of 95%HDIs reveals 0% overlap indicating facilitation dominates responses. Extending inference to intergroup comparisons of means revealed substantial evidential support for a dramatic reduction in the frequency of suppression and increase in the frequency of facilitation, i.e. a reversal in proportion relative to control.
Figure 5.
Frequency of Facilitation Increased. A: The percentage of trials in which G experienced suppression or facilitation in response to TA conditioning was calculated for individual experiments over a matched background forces. One testconditioned test pair constitutes a single trial. The mean percentage was then calculated for both the control group (white bars, n=5) and the treated group (gray bars, n=5) and compared. Asterisk denotes empirically derived posterior distributions of means are credibly different from each other (Bayesian Inference by Difference of Means Comparison following Hierarchical Bayesian ANOVA). B & C illustrate raw time-series (~3min) records from all animals (N=5) in both control (B: individuals animals C1-5) and reinnervated antagonists groups (C: individuals animals R1-5). Vertical bars represent the modulated stretch reflex induced by antagonist (TA) stretch. Negative values represent suppression while positive values represent facilitation.
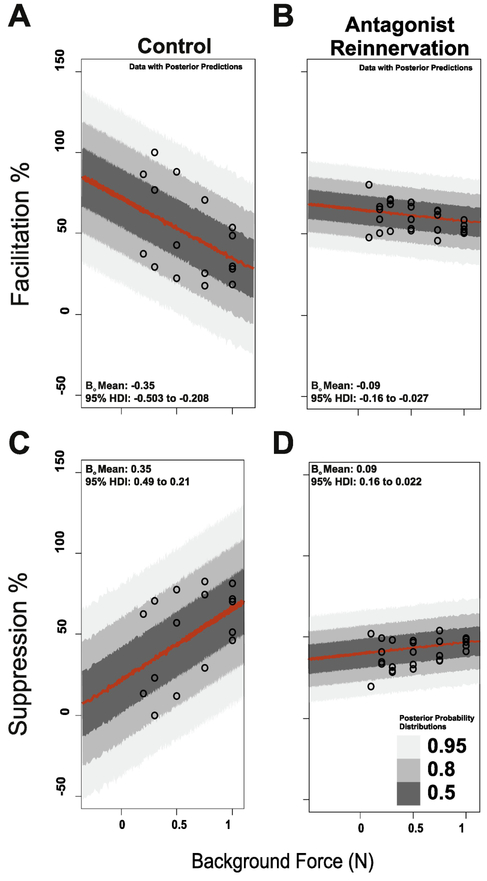
Data were examined carefully in an attempt to gain insight into inter-trial switching between suppression and facilitation (Fig. 5B, C), which, to our knowledge, has not been reported before. First, it was determined that switching was stochastic, i.e. that the sign of conditioning, facilitation positive and suppression negative, in successive trials was not predictable from earlier ones. Second, there was no evidence in force or EMG records that rats were in an oscillatory locomotor state. Third, whatever the mechanism of switching may be, its normal relationship with background force changes. Examination of force dependency in control rats, revealed force dependent increase in suppressive response and parallel decrease in facilitatory responses. All credible estimates of regression coefficients were substantially larger than the null distribution for suppression and less than the null for facilitation, indicating strong force dependent increase in the proportion of suppression, accompanied by a corresponding decrease in facilitation (Fig. 6). Rats with reinnervated antagonists demonstrated reduced force dependent sensitivity. Comparing 95% posterior probability distributions of mean regression coefficients between groups revealed no credible overlap, (Fig. 6) which provides strong support for a reduction, and possible loss of force dependent-sensitivity following reinnervation. This change is yet another expression of circuit dysregulation.
Figure 6.
Suppression and Facilitation Frequency Depend on Background G Force. (A) Expected force dependent decrease in facilitatory response (A) and parallel increase in suppressive responses in control group (C). Reduced dependence on force for facilitation (B) and suppression (D). Examining the posterior distributions of mean regression coefficients revealed no overlap in 95%HDI values between groups and indicates that force dependency sensitivity is blunted following reinnervation.
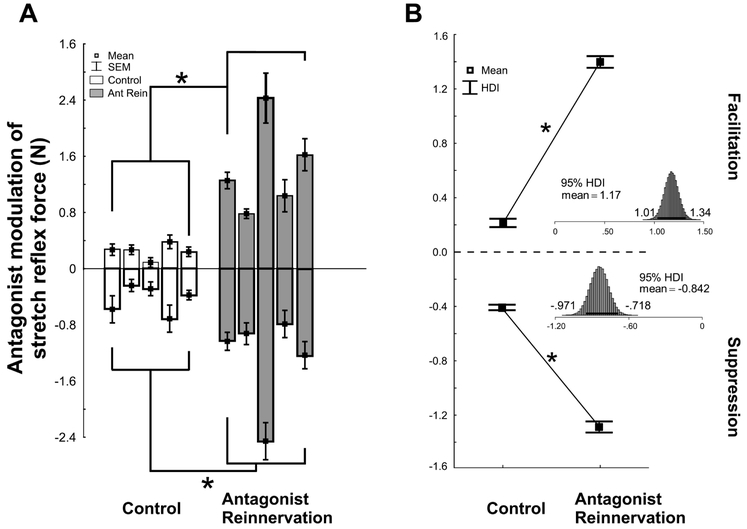
3.5. Strength of both Suppression and Facilitation increased after nerve injury and repair
Changes in strength of reflex modulation were evaluated using Bayesian parameter estimation to empirically derive the posterior distributions for both experimental groups across all rats at a matched background force (1N). Figure 7A illustrates suppressive and facilitatory responses for individual rats in both groups and documents that the strength of both facilitation and suppression are substantially amplified following reinnervation. Group means for facilitation and suppression are documented in Figure 7B. Inference by mean comparison reveals a credibly non-zero positive amplification in the strength of facilitation by 1.17N following reinnervation (Fig. 7B). Detailed examination reveals strong evidential support for this effect residing between a 1.04-1.31N increase (Fig. 7B top inset).
Figure 7.
Strength of both suppression and facilitation increased in antagonist reinnervation group. A: Means and SEs of modulated G stretch reflex force due to TA conditioning for individual experiments over a matched range of background forces (1N) in both control (white bars, n=5) and antagonist reinnervation (gray bars, n=5) groups. Negative values represent suppression while positive values represent facilitation. B: Pooled means for control and antagonist reinnervation groups for the data set in A for both facilitation (top) and suppression (bottom). Connecting lines are for visual aid only. Results of Hierarchical One-way Bayesian ANOVA (B) reveals empirically derived posterior distributions of means are credibly different from each other. Asterisk denotes Bayesian Inference by Difference of Means Comparison where 95% highest density interval (HDI) does not cross zero.
Inference by mean comparison reveals a credibly non-zero negative amplification in the strength of suppression by 0.839N following reinnervation (Fig. 7B). Detailed examination reveals strong evidential support (95% probability) for this effect residing between a 0.692-0.982N amplification (Fig. 7B bottom inset). Data presented in Figure 7 provide strong evidential support to explicitly reject our hypothesis, by documenting substantially stronger reflex suppression evoked by reinnervated as compared with normally innervated muscles. Coincident with strengthened suppression, was the unexpected emergence of strong facilitation following reinnervation.
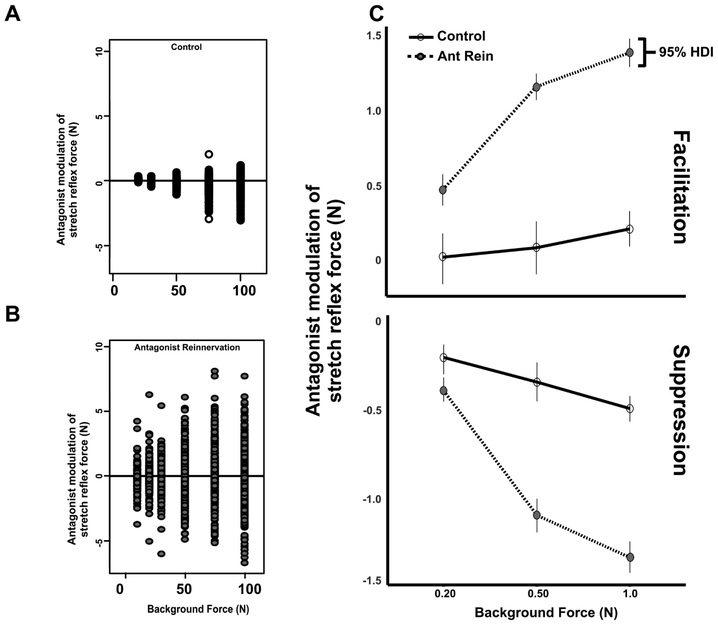
By extending analysis to multiple background forces, we tested whether the amplification of suppression42 and facilitation described above depends on G background force. All data from conditioned trials were pooled per experimental group and plotted vs. background force in Fig. 8. Two-factor, hierarchical Bayesian ANOVA was implemented to derive the independent and combinatorial effects of background force and treatment on the strength of suppression and facilitation. Interaction effects are illustrated by dramatic amplification of the strength of facilitation and suppression as background force increases between control and antagonist reinnervation groups (Fig. 8). Note the highly non-linear interaction of reinnervation as background force increases, highlighting further evidence of circuit dysregulation.
Figure 8.
Strength of Suppression and Facilitation Depend on Background G Force in Antagonist Reinnervation Group. All data from conditioned trials were pooled per control (A) and antagonist reinnervation (B) groups and plotted vs. background force in Fig 8. C: Effects of background G force on the strength of facilitation (top) and suppression (bottom). Lines represent change from observed pooled means of control to reinnervated antagonist groups for three background forces. Results of Hierarchical Factorial Bayesian ANOVA reveals empirically derived posterior distributions of means are only credibly different between 0.2 to 1N background G forces for control as compared to all contrasts in antagonist reinnervation groups.
3.6. Partial dependence of conditioning effects on test amplitude
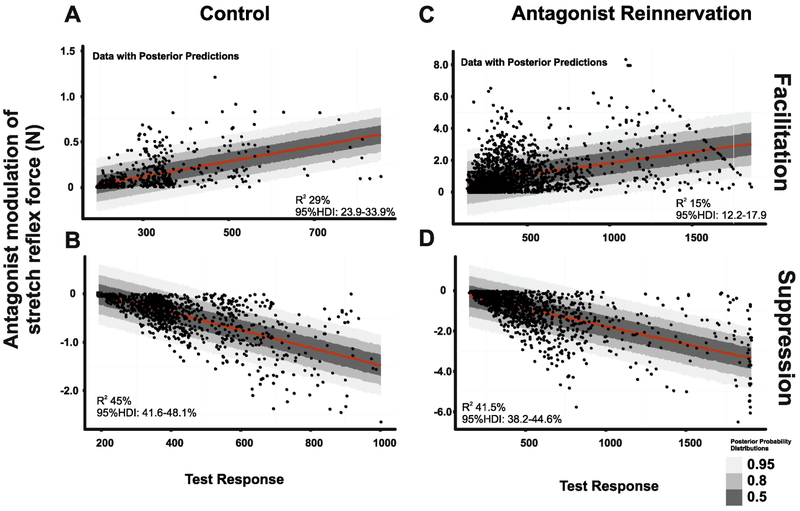
The magnitude of reflex suppression or facilitation is expected to depend on G motor pool excitability43,44, which we estimated from amplitudes of the unconditioned (test) stretch reflex. The Robust Bayesian linear regression model was employed to calculate percent of variance (Bayesian equivalent to R2 37of strength of suppression and facilitation explained by unconditioned (test) stretch reflex amplitudes. In all instances, test stretch reflex amplitudes (excitability of G motor pools) predicted only a minority of the strength of conditioned stretch reflex. Predictions for control and antagonist reinnervation groups respectively were: (a) for facilitation 29% (95% HDI: 23.9-33.9%) (Fig. 9A) and 15% (95% HDI:12.2-17.9%) (Fig. 9B) and (b) for suppression, 45% (95% HDI:41.6-48.1%) (Fig. 9C) and 41.5% (95% HDI:38.2-44.6%) (Fig. 9D).
Figure 9.
Partial Dependence of Conditioning on Test Response Amplitude. All data from conditioned trials were pooled for facilitation and suppression strength in control (A,B) and treated rats (C,D) and plotted vs. test reflex amplitude. Robust Bayesian linear regression model was employed to calculate percent of variance (Bayesian equivalent to R2) of strength of suppression and facilitation explained by test response amplitude. In all instances, test response amplitudes predicted <50% of the strength of conditioned stretch reflex. The test reflex amplitude contributions were as follows: (a) for the strength of facilitation, 29% (95% HDI: 23.9-33.9%) of control (A) and 15% (95% HDI:12.2-17.9%) of treated rats (B) and for the strength of suppression, 45% (95% HDI:41.6-48.1%) of control (C) and 41.5% (95%HDI:38.2-44.6%) of treated rats (D).
Discussion
Findings presented here demonstrated severe dysregulation in mechanosensory reflex coordination of agonist and antagonist muscles following peripheral nerve injury and muscle reinnervation in rats. Stretch applied to reinnervated antagonists produced much greater than normal levels in both suppression and facilitation, of agonist muscle activity, together with a reversal in the normal dominance in occurrence of suppression over facilitation. These findings led us to reject our hypothesized reduction in spinal reflex inhibition between agonist and antagonist motor pools, and to suggest candidate origins of dysregulation in spinal reflex pathways that govern antagonist motor pool activation.
Dysregulation of Reflex Conditioning
Discussion of possible mechanisms underlying dysregulated reflex conditioning builds on Figure 10. The figure highlights a simplified version of spinal reflex pathways relevant to test and conditioned stretch reflexes. We confine inferences to ones supported by findings presented here, while recognizing the potential importance of various other possible contributors, e.g. presynaptic inhibition and interneuronal excitability not examined in this study.
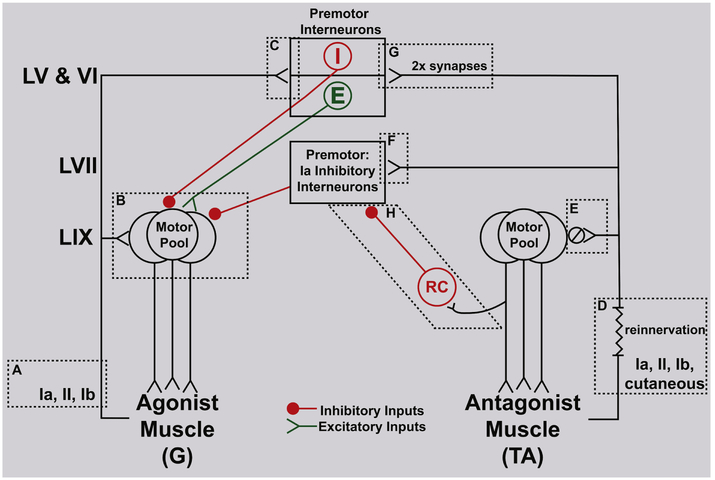
Figure10.
Dysregulation in mechanosensory spinal circuits coordinating agonist-antagonist motor pools following muscle reinnervation. Simplified diagram identifies pathways producing (a) homonymous test stretch reflex in agonist (gastrocnemii, G) and (b) conditioning by stretch of reinnervated antagonist (tibialis anterior, TA). Diagram depicts (from top to bottom) pre-motor interneurons in LV,VI and VII, motor pools in LIX, and muscles supplied; lines represent established synaptic interconnections among afferents, neurons and muscles. Elements of test reflex (G stretch reflex): (A) afferents activated by passive muscle stretch; (B) monosynaptic input to G motor pool from G group Ia and II afferents; (C) monosynaptic input to LV,VI inhibitory (red I) and excitatory (green E) premotor interneurons from G group Ia, II, Ib afferents. Conditioning reflex (stretch reinnervated TA): (D) regenerated afferents activated by TA stretch; (E) monosynaptic input from TA stretch-activated afferents, Ia synapses reduced by 80% ( ); (F) monosynaptic input to LVII Ia inhibitory interneurons from TA stretch-activated afferents; (G) monosynaptic input (synapses 2x normal) to LV,LVI I and E pre-motor interneurons; LV,VI, and VII pre-motor interneurons synapse with motoneurons in G motor pool; (H) stretch of reinnervated TA muscle fails to activate TA motor pool and Renshaw cells (RC) in turn.
Test Reflex:
Figure 10A identifies afferent contributors to the test stretch reflex in uninjured agonists, i.e. gastrocnemii muscles. All three types of large-diameter muscle proprioceptors, groups Ia and II muscle spindle afferents and group Ib tendon organ afferents had potential to contribute to the net strength of the test reflex. Each afferent type exhibits robust firing when the ramp-hold-release stretch parameters used in the present study are applied to passive, i.e. non-contracting gastrocnemius muscles in rats23. During the eccentric muscle contraction evoked by the stretch reflex, evidence shows that firing continues both for muscle spindle afferents25 and for tendon organ afferents, which are most responsive to active contraction45. Muscle spindle afferents, primarily group Ia, contribute prominently to stretch reflex force through their monosynaptic connections with the homonymous motor pool in LIX (Fig. 10B). All three afferent types had the potential to influence stretch reflex force through oligosynaptic pathways converging inhibition and excitation to the G motor pool (Fig. 10B) via premotor interneurons in LV,VI (Fig. 10C)23. Transmission through segmental oligosynaptic pathways to motor pools introduces central synaptic delays of no more than 5ms46,47, making it possible for them to influence reflex force within the 50ms duration of the ramp portion of stretch eliciting the test reflex. The test stretch reflex potentially reflected, therefore, the sum of reflex force initiated or modified by combinations of three afferent types and transmitted to the agonist motor pool via both mono- and oligosynaptic pathways.
Test reflex amplitudes were larger in the antagonist reinnervation group compared to control. The reason for increased responsiveness of the uninjured G muscles to homonymous stretch in animals with reinnervated antagonists is thought provoking, but its meaning requires speculation outside the scope of the present study. Important to interpretation presented below is the result that test reflex amplitude was partially predictive of the magnitude of reflex conditioning: relatively enlarged test reflexes in the antagonist reinnervation group corresponded with amplified suppression and facilitation (see Results, sect. 3.1; Fig 9). This dependency of conditioning on test reflex magnitude has been observed and attributed to a variety of factors e.g. variation in excitability within a motor pool43,44. Unlike many, but not all48 previous studies in human and cat43, however, we found that test reflex amplitude explained less than half of the increase in strength of facilitation and suppression (see Results, sect 3.6), and we assign the major portion of amplification to changes in pre-motor interneuronal circuits discussed below.
Conditioned Reflex:
The conditioning effects of antagonist muscle stretch on the G stretch reflex were qualitatively similar in both control and antagonist reinnervation groups. If not by novel circuits, then amplified suppression and facilitation would result from adjustments to normal spinal pathways. Referring to Fig. 10D, we begin by considering the afferents activated by stretch of the reinnervated TA muscles. Recovery of sensory signaling was verified by the strong reflex conditioning effects, both suppression and facilitation evoked by stretch of the reinnervated TA muscle (e.g. Fig. 7). Although the modality and tissue origin of the regenerated afferents were not determined here, ample evidence from earlier studies supports the following suppositions. Partial recovery of sensory signaling from groups Ia, II and Ib was likely49-52, but, owing to non-selective reinnervation3 by the common fibular nerve, the TA muscle undoubtedly acquired a mix of sensory neurons, both original and foreign, i.e. from other muscles and from skin52. Importantly, axons conveyed through the common fibular nerve normally innervate proprioceptors in muscles, which mostly share actions at the ankle that are mechanically antagonistic to the gastrocnemius muscles10,53. Cutaneous afferents are also shown to reinnervate muscle and respond to stretch52, and ones conveyed by the common fibular nerve would commonly transmit inhibition to gastrocnemius muscles54.
In addition to peripheral alterations, regenerated afferents also undergo substantial central changes2. Figures 10 E,F,G illustrate changes in the synaptic terminations of regenerated primary afferents, specifically Ia afferents55 and possibly others56,57. Following section and regeneration of their major limb nerve trunk, group Ia afferents lose ca. 80% of their synaptic connections with homonymous motoneurons and retract their collateral axons from LIX (Fig. 10D)58. These central synaptic loses of Ia afferents in LIX, together with peripheral deficits described above, rendered stretch of the reinnervated TA motor pool insufficient to activate the homonymous motor pool, as evidenced by a complete absence of stretch-evoked force or EMG (see Fig. 2). Significant to interpretation of the conditioning effects discussed below, the failure of stretch to activate the TA motor pool would also release Ia inhibitory interneurons from Renshaw cell (RC) inhibition of Ia inhibitory interneurons (Figure 10H)59. Other segmental targets of Ia afferents are retained. In LVII, which contains Ia inhibitory interneurons, synapses of regenerated Ia afferents are conserved, and in LV,VI, where inhibitory and excitatory premotor interneurons are located, the number of Ia synapses doubles compared to control58. Adding these changes in afferent input to a simplified model of known spinal circuits in Fig. 10 supports provisional explanations presented below for amplified suppression and facilitation.
To explain the three-fold increase in reflex suppression conveyed to an agonist from its reinnervated antagonist muscle, we forward three possible mechanisms acting individually or in concert. One potential mechanism is an increase in reciprocal inhibition, which would be disinhibited by the failure of reinnervated muscle stretch to activate RC’s (Fig. 10H, see above). Another possible source of enhanced suppression arises from erroneous reinnervation of the TA muscle by common-fibular cutaneous afferents. Through their anomalous activation by muscle stretch (see above), these cutaneous afferents might initiate the suppression of ankle extensor motor pools found in normal rats54, routed through pre-motor interneurons in dorsal horn, including those in LV,VI. One further pre-motor pathway for inhibition includes inhibitory interneurons in LV,LVI60,61, and this known source of reflex suppression16,62 might be amplified by the doubling of synaptic input it receives from regenerated primary afferents (Fig. 10H)58. All of these mechanisms would be expected to oppose co-contraction of agonists and antagonists, which must result, therefore, from additional mechanisms.
We consider three possible explanations for the 7-fold increase in reflex facilitation mediated through spinal pathways linking antagonists. First, we rule out the pathway known as reciprocal facilitation63. In normal animals, controlled reduction of Ia inhibition has been a focus of attention for its potential to promote co-activation of antagonists59,60,64,65. In the present experiments, however, this route of facilitation was lost when Ia inhibitory interneurons were released from RC inhibition (see above and Figure 10E). Second, facilitation observed in test-conditioning protocols might have been amplified, hypothetically, as a result from stimuli interacting non-linearly within the G motoneurons (Figure 10A and see Figure 1C)44. However, TA afferents are not monosynaptically coupled to G motoneurons, and TA muscle stretch as the sole stimulus does not activate the G motoneurons43,44. These observations rule out the G motor pool as the site of interaction and shift attention to interaction at pre-motoneuronal levels, which becomes a third candidate locus for amplified facilitation43,44. In this case, excitatory interneurons gain favor, possibly those in LV,VI60,61, that respond to mechanosensory primary afferents, group Ib most acknowledged for conveying reflex excitation from agonists to antagonists (see Figure 10I).16,62,66 With this as the only established pathway capable of explaining the reflex facilitation from antagonists that we observed here, we turn to the doubling of synaptic connections with LV,VI excitatory interneurons as a possible basis for amplification of facilitation (Figure 10G).
Inter-trial Fluctuation between Facilitation and Suppression
The stochastic switching found here between suppression and facilitation in sequential test-condition trials of muscle stretch was unexpected and, to our knowledge, not previously reported. Switching might have originated from any portion of the neuraxis, supraspinal or spinal, that remained in continuity after acute decerebration. We observed no temporal structure in fluctuating reflex sign, and no correlation with other measured parameters, e.g. test or conditioned reflex amplitude, that might provide mechanistic clues. It is possible that reflex fluctuation originated from rhythmic modulation of spinal circuits operating in a background spinal locomotor state17,67. While we found no evidence of locomotor activity in our records of muscle force and EMG, we cannot rule out possible influences of subthreshold locomotor activity.
Notwithstanding its unidentified mechanism, fluctuation in reflex sign and its modification after muscle reinnervation should significantly affect muscle coordination. Suppression that dominated in the control group (72% of trials) reversed to more facilitation but greater variability (60% of trials). With its doubling incidence in combination with the 7-fold amplification in strength, reflex facilitation emerges from nerve injury and repair as the predominant functional coupling of antagonist muscles evoked by mechanosensory stimulation. The contrast with constrained expression of facilitation in normal animals reflects severe dysregulation of the coordination after nerve injury and repair.
Functional Implications
The dysregulation described here for segmental spinal circuits linking agonist and antagonist motor pools has the potential to profoundly change the expression of limb movement and its neural control. Coordination among muscles acting as mechanical antagonists normally plays a major role in controlling the timing, speed, accuracy and precision of movement, as well as stiffness and impedance of limbs68-73. The normal reliance of coordination upon mechanosensory feedback from muscle proprioceptors would be seriously challenged by the emergence of antagonist reflex facilitation that outweighs suppression in strength and occurrence. On average, co-contraction would result from the overbalance of facilitation, instead of by weakening suppression in spinal circuits held responsible for co-contraction in dystonia and Parkinson’s disease, and after trauma in spinal cord injury and stroke13,20-22,74,75. For individual movements, the unpredictable switching between facilitation and suppression that we found should significantly interfere with CNS control of movement. This unpredictability might even be the driver for co-contraction employed as an intentional strategy by behaving animals to regain control of joint stiffness and stability. Present findings indicate that any of these or other possibilities assign a decisive role of spinal reflex circuits in determining the movement disabilities that persist following nerve injury and repair.
Highlights.
Dysregulation of spinal circuits regulating inhibition between antagonist motor pools
Increase strength and frequency of reflex facilitation
Concurrent amplification of reflex suppression
Aberrant dependence of reflex suppression and facilitation on agonist muscle stretch
Spinal circuits exposed as likely contributors to abnormal co-contraction of antagonist muscles
Acknowledgments
Funding
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant P01NS057228.
Abbreviations
- CNS
central nervous system
- TA
tibialis anterior
- G
gastrocnemius
- EMG
electromyography
- SR
stretch reflex
- HDI
highest density interval
- ROPE
region of practical equivalence
- N
newton unit of measure for force
- L
laminae
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosure statement
The authors have no competing financial interests.
References
- 1.Sunderland S. Nerves and nerve in juries. Edinburgh, E& S Livingstone Ltd; 1968. [Google Scholar]
- 2.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Progress in neurobiology. 2007;82(4):163–201. [DOI] [PubMed] [Google Scholar]
- 3.Brushart TM. Nerve repair. Oxford University Press; 2011. [Google Scholar]
- 4.Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84(5):2709–2714. [DOI] [PubMed] [Google Scholar]
- 5.Bauman JM, Chang Y-H. Rules to limp by: joint compensation conserves limb function after peripheral nerve injury. Biology letters. 2013;9(5):20130484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y-H, Housley SN, Hart KS, et al. Progressive adaptation of whole-limb kinematics after peripheral nerve injury. Biology open. 2018;7(8):bio028852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasserschaff M Coordination of reinnervated muscle and reorganization of spinal cord motoneurons after nerve transection in mice. Brain research. 1990;515(1-2):241–246. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. Journal of neuroscience methods. 2000;96(2):89–96. [DOI] [PubMed] [Google Scholar]
- 9.Boeltz T, Ireland M, Mathis K, et al. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol. 2013;109(11):2645–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabatier MJ, To BN, Nicolini J, English AW. Effect of axon misdirection on recovery of electromyographic activity and kinematics after peripheral nerve injury. Cells Tissues Organs. 2011;193(5):298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetts R, Kalaska JF, Smith AM. Cerebellar nuclear cell activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. Journal of Neurophysiology. 1985;54(2):231–244. [DOI] [PubMed] [Google Scholar]
- 12.Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Current biology. 2001;11(23):R986–R996. [DOI] [PubMed] [Google Scholar]
- 13.Haruno M, Ganesh G, Burdet E, Kawato M. Differential neural correlates of reciprocal activation and cocontraction control in dorsal and ventral premotor cortices. Journal of neurophysiology. 2011;107(1):126–133. [DOI] [PubMed] [Google Scholar]
- 14.Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. Journal of Neurophysiology. 1990;64(3):782–795. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg A Multisensory control of spinal reflex pathways In: Progress in brain research. Vol 50 Elsevier; 1979:11–28. [DOI] [PubMed] [Google Scholar]
- 16.Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. Handbook of Physiology The Nervous System Motor Control. 1981:509–595. [Google Scholar]
- 17.Geertsen SS, Stecina K, Meehan CF, Nielsen JB, Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. The Journal of physiology. 2011;589(1):119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen J, Kagamihara Y. The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. The Journal of physiology. 1992;456(1):373–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia R, Rymer W. Reflex reciprocal facilitation of antagonist muscles in spinal cord injury. Spinal Cord. 2005;43(1):14. [DOI] [PubMed] [Google Scholar]
- 20.Mirbagheri M, Barbeau H, Ladouceur M, Kearney R. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Experimental brain research. 2001;141(4):446–459. [DOI] [PubMed] [Google Scholar]
- 21.Crone C, Johnsen L, Biering-Sørensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126(2):495–507. [DOI] [PubMed] [Google Scholar]
- 22.Mirbagheri MM, Duffell LD, Kotsapouikis D, Rogers LM. Reciprocal inhibition becomes facilitation after spinal cord injury: Clinical application of a system identification approach. Paper presented at: Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE2014. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JA, Gabriel HM, Deardorff AS, et al. Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. Journal of neurophysiology. 2017;118(5):2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols T, Houk J. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. journal of Neurophysiology. 1976;39(1):119–142. [DOI] [PubMed] [Google Scholar]
- 25.Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. Journal of Neurophysiology. 1999;81(2):467–478. [DOI] [PubMed] [Google Scholar]
- 26.Haftel VK, Bichler EK, Wang Q-B, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive afferents. Journal of Neuroscience. 2005;25(19):4733–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagenmakers E-J. A practical solution to the pervasive problems ofp values. Psychonomic bulletin & review. 2007;14(5):779–804. [DOI] [PubMed] [Google Scholar]
- 28.Kruschke J Doing Bayesian data analysis: A tutorial with R, JAGS, and Stan. Academic Press; 2014. [Google Scholar]
- 29.Kruschke JK. Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General. 2013;142(2):573. [DOI] [PubMed] [Google Scholar]
- 30.Kruschke JK. What to believe: Bayesian methods for data analysis. Trends in cognitive sciences. 2010;14(7):293–300. [DOI] [PubMed] [Google Scholar]
- 31.Gabry J, Goodrich B. rstanarm: Bayesian applied regression modeling via Stan. R package version. 2018;2.18.1. [Google Scholar]
- 32.Team RC. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Austria, 2015. In: ISBN 3-900051-07-0: URL http://www.R-project.org; 2018. [Google Scholar]
- 33.Carpenter B, Gelman A, Hoffman MD, et al. Stan: A probabilistic programming language. Journal of statistical software. 2017;76(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks S, Gelman A, Jones G, Meng X-L. Handbook of markov chain monte carlo. CRC press; 2011. [Google Scholar]
- 35.Hoffman MD, Gelman A. The No-U-turn sampler: adaptively setting path lengths in Hamiltonian Monte Carlo. Journal of Machine Learning Research. 2014;15(1):1593–1623. [Google Scholar]
- 36.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical science. 1992;7(4):457–472. [Google Scholar]
- 37.Gelman A, Goodrich B, Gabry J, Vehtari A. R-squared for Bayesian regression models. The American Statistician. 2018(just-accepted):1–6. [Google Scholar]
- 38.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. American journal of physical medicine & rehabilitation. 2008;87(5):381–385. [DOI] [PubMed] [Google Scholar]
- 39.Cope TC, Bonasera S, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. Journal of neurophysiology. 1994;71(2):817–820. [DOI] [PubMed] [Google Scholar]
- 40.Huyghues-Despointes CM, Cope TC, Nichols TR. Intrinsic properties and reflex compensation in reinnervated triceps surae muscles of the cat: effect of activation level. Journal of neurophysiology. 2003;90(3):1537–1546. [DOI] [PubMed] [Google Scholar]
- 41.Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res. 2007;181(2):377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichols TR, Koffler-Smulevitz D. Mechanical analysis of heterogenic inhibition between soleus muscle and the pretibial flexors in the cat. Journal of Neurophysiology. 1991;66(4):1139–1155. [DOI] [PubMed] [Google Scholar]
- 43.Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Experimental brain research. 1990;81(1):35–45. [DOI] [PubMed] [Google Scholar]
- 44.Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiologie Clinique/Clinical Neurophysiology. 2000;30(2):67–80. [DOI] [PubMed] [Google Scholar]
- 45.Jami L Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiological reviews. 1992;72(3):623–666. [DOI] [PubMed] [Google Scholar]
- 46.Stauffer EK, Watt D, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. Journal of Neurophysiology. 1976;39(6):1393–1402. [DOI] [PubMed] [Google Scholar]
- 47.Watt D, Stauffer EK, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. Journal of Neurophysiology. 1976;39(6):1375–1392. [DOI] [PubMed] [Google Scholar]
- 48.Lavoie BA, Devanne H, Capaday C. Differential control of reciprocal inhibition during walking versus postural and voluntary motor tasks in humans. Journal of Neurophysiology. 1997;78(1):429–438. [DOI] [PubMed] [Google Scholar]
- 49.Banks R, Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. The Journal of physiology. 1989;408(1):345–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown M, Butler R. Regeneration of afferent and efferent fibres to muscle spindles after nerve injury in adults cats. The Journal of physiology. 1976;260(2):253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregory J, Luff A, Proske U. Muscle receptors in the cross-reinnervated soleus muscle of the cat. The Journal of physiology. 1982;331(1):367–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewin GR, McMahon SB. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skin in the adult rat. Journal of neurophysiology. 1991;66(4):1205–1217. [DOI] [PubMed] [Google Scholar]
- 53.Nichols TR. Distributed force feedback in the spinal cord and the regulation of limb mechanics. Journal of neurophysiology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagbarth K-E. Excitatory and inhibitory skin areas for flexor andextensor motoneurons. Acta Physiol Scand. 1952;26(94):1–58. [PubMed] [Google Scholar]
- 55.Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. Journal of Neuroscience. 2014;34(10):3475–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koerber H, Mirnics K, Brown P, Mendell L. Central sprouting and functional plasticity of regenerated primary afferents. Journal of Neuroscience. 1994;14(6):3655–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koerber H, Mirnics K, Mendell L. Properties of regenerated primary afferents and their functional connections. Journal of neurophysiology. 1995;73(2):693–702. [DOI] [PubMed] [Google Scholar]
- 58.Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. Journal of neurophysiology. 2011;106(5):2450–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hultborn H, Lindström S, Wigström H. On the function of recurrent inhibition in the spinal cord. Experimental Brain Research. 1979;37(2):399–403. [DOI] [PubMed] [Google Scholar]
- 60.Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. European Journal of Neuroscience. 2010;32(6):881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levine AJ, Hinckley CA, Hilde KL, et al. Identification of a cellular node for motor control pathways. Nature neuroscience. 2014;17(4):586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison P interneuronal system contributing to the coordination of the cat hind limb. Paper presented at: Seminar series-Society for Experimental Biology1985. [Google Scholar]
- 63.Hultborn H Spinal reflexes, mechanisms and concepts: from Eccles to Lundberg and beyond. Progress in neurobiology. 2006;78(3-5):215–232. [DOI] [PubMed] [Google Scholar]
- 64.Knikou M The H-reflex as a probe: pathways and pitfalls. Journal of neuroscience methods. 2008;171(1):1–12. [DOI] [PubMed] [Google Scholar]
- 65.Hultborn H, Jankowska E, Lindstrom S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. The Journal of physiology. 1971;215(3):591–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pratt CA. Evidence of positive force feedback among hindlimb extensors in the intact standing cat. Journal of Neurophysiology. 1995;73(6):2578–2583. [DOI] [PubMed] [Google Scholar]
- 67.McCrea D, Shefchyk S, Stephens M, Pearson K. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. The Journal of Physiology. 1995;487(2):527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghez C, Martin J. The control of rapid limb movement in the cat. Experimental Brain Research. 1982;45(1-2):115–125. [DOI] [PubMed] [Google Scholar]
- 69.Rasmussen S, Chan A, Goslow G Jr. The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. Journal of Morphology. 1978;155(3):253–269. [DOI] [PubMed] [Google Scholar]
- 70.Marsden C, Obeso J, Rothwell J. The function of the antagonist muscle during fast limb movements in man. The journal of physiology. 1983;335(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gottlieb GL, Latash ML, Corcos DM, Liubinskas TJ, Agarwal GC. Organizing principles for single joint movements: V. Agonist-antagonist interactions. Journal of Neurophysiology. 1992;67(6):1417–1427. [DOI] [PubMed] [Google Scholar]
- 72.Bonasera SJ, Nichols TR. Mechanical actions of heterogenic reflexes among ankle stabilizers and their interactions with plantarflexors of the cat hindlimb. Journal of neurophysiology. 1996;75(5):2050–2070. [DOI] [PubMed] [Google Scholar]
- 73.Bernstein N The co-ordination and regulation of movements. The co-ordination and regulation of movements. 1966. [Google Scholar]
- 74.Boorman G, Lee R, Becker W, Windhorst U. Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control. 1996;101(2):84–92. [DOI] [PubMed] [Google Scholar]
- 75.Meunier S, Pol S, Houeto J-L, Vidailhet M. Abnormal reciprocal inhibition between antagonist muscles in Parkinson's disease. Brain. 2000;123(5):1017–1026. [DOI] [PubMed] [Google Scholar]