Abstract
Purpose
Proton therapy is currently used in the management of pediatric tumors to decrease late toxicities. However, one of the criticisms of proton therapy is the limited data regarding efficacy on disease control. The purpose of this study was to examine local and distant control rates after proton therapy for neuroblastoma.
Methods and Materials
Eighteen patients with high-risk (n = 16) and locally recurrent neuroblastoma (n = 2) were treated with curative intent and received proton therapy to the primary site and up to three post-induction MIBG-avid metastatic sites. The primary sites (n = 18) were treated to 21 – 36 Gy(RBE) and the metastatic sites (n = 16) were treated to 21 – 24 Gy(RBE). Local control and survival rates were calculated using the Kaplan-Meier method.
Results
With a median follow-up of 60.2 months, 2- and 5-year local control (LC) rates at the irradiated primary site were 94% and 87% respectively. No failures at irradiated distant metastatic sites were observed. The 5-year progression-free survival (PFS) was 64% and the 5-year overall survival (OS) was 94%. Extent of surgical resection was not associated with LC, PFS, or OS. No radiation-related nephropathy or hepatopathy was reported.
Conclusions
Excellent local control was achieved using proton therapy to the primary and post-induction MIBG positive distant sites. The predominant site of failure is progression in post-induction non-MIBG avid distant sites. While proton therapy provides high rates of local control with acceptable toxicity for neuroblastoma, further advances in systemic therapy are needed for improved control of systemic disease.
Keywords: neuroblastoma, radiotherapy, proton therapy, local control
Introduction
High-risk neuroblastoma is currently treated with a multimodality approach including induction chemotherapy, surgical resection, high-dose chemotherapy with autologous stem cell rescue, radiotherapy (RT), cis-retinoic acid and immunotherapy. Locoregional control rates of 84 to 100% have been reported when RT is delivered to the primary and persistent MIBG-avid metastatic sites, and hence RT has been an essential part of therapy for this group of patients [1–4]. With the increasing number of proton centers, proton therapy has been used instead of photons to minimize the late effects of RT [5, 6]. Because of the lack of an exit dose compared to photons, proton therapy delivers less low-dose radiation to surrounding normal tissues beyond the target. In a recent study from the Pediatric Proton Consortium Registry, more than half of children treated with protons had brain tumors. For extracranial tumors, rhabdomyosarcoma was the most common tumor treated, and only 55 (3%) had a diagnosis of neuroblastoma [7]. Because of the small number of patients treated with protons, limited data exists on the effectiveness of proton therapy for local control in neuroblastoma at both the primary and treated metastatic sites. The purpose of this study is to review the local control outcomes of children with high-risk and locally recurrent neuroblastoma treated at our proton facility.
Methods
Eligibility criteria
Records of patients with high-risk neuroblastoma (age < 18 years) treated with proton therapy between 2007 to 2016 and entered in a prospective registry protocol at the University of Texas MD Anderson Cancer Center were analyzed. In order to be included in the current analysis, patients had to be treated with curative intent with a minimum 12-month follow-up after proton therapy. The minimum follow-up period was selected to allow for adequate duration to assess local control rates, the primary endpoint of interest in our study. Children with initial low or intermediate risk neuroblastoma were also eligible to be part of the study if they had local progression only, and the patient was treated at progression with surgery and systemic therapy with curative intent.
Pre-and Post-Radiotherapy Treatment
Prior to RT, high-risk patients had received induction chemotherapy and definitive resection followed by autologous stem cell transplant. Most patients were treated with induction chemotherapy per the Children’s Oncology Group protocol ANBL0532 (NCT00567567) (n = 9), while 5 received chemotherapy per an in-house institutional protocol (n = 5). As part of the institutional protocol, patients received 5 cycles of induction chemotherapy containing a total of 1,550 mg/m2 of etoposide, 200 mg/m2 of cisplatin, 1.5 mg/m2 of vincristine, 4,000 mg/m2 of cyclophosphamide, 60 mg/m2 of doxorubicin, 9,000 mg/m2 of ifosfamide, and 1,000 mg/m2 of carboplatin. For the 16 high-risk patients, 14 had a single stem cell transplant, 1 had a tandem stem cell transplant, and 1 patient did not undergo stem cell transplant due toxicity concerns related to chronic lung disease.
Patients were analyzed by the degree of surgical resection. Gross total resection (GTR) was defined as no residual tumor at the primary site by the surgeon’s assessment and on postoperative imaging (CT, MRI and MIBG) while subtotal resection (STR) was defined as residual tumor on postoperative imaging. Of the 18 patients, 12 had GTR and 6 had STR prior to proton therapy.
After completion of proton treatments, high-risk patients then received cis-retinoic acid with (n = 9) or without immunotherapy (n = 7). Acute and late toxicities were assessed using Common Terminology Criteria for Adverse Events (CTCAE) v4.0.
Radiation Therapy
Proton therapy was delivered to the primary site and up to three MIBG-avid metastatic sites after induction therapy and autologous stem cell transplant. CT simulation scans were obtained and were fused to the diagnostic CT or MRI prior to resection of the primary tumor. The clinical target volume (CTV) was defined as the tumor bed, determined on the pre-operative CT or MRI with an additional 1 to 1.5 cm. margin, respecting anatomical boundaries. Metastatic sites were also treated with a 0.5 to 1 cm. margin for the CTV. Because of the possibility of scoliosis or kyphosis with partial vertebral irradiation, the entire vertebra was treated to a dose of 15 to 18 Gy(RBE) in most cases, followed by a boost to the CTV. The majority of patients received 21 – 24 Gy(RBE) to the primary site, and in 2 cases, residual tumor with 1 cm margin received a boost up to a total dose of 36 Gy(RBE). Treatments were delivered using passively scattered protons (n = 17) or intensity-modulated proton therapy (n = 1). Mean and median doses to the heart, lungs, liver, and kidneys were recorded.
Statistics
Local control (LC), irradiated distant site control (IDSC), progression-free survival (PFS), and overall survival (OS) were calculated using the Kaplan-Meier method. Local recurrences were defined relative to the RT treatment fields as follows: in-field recurrence was defined as >80% of the recurrence volume within the 95% isodose line (IDL) of the initial treatment plan; marginal recurrence was defined as >20% and ≤80% of the recurrence volume within the 95% IDL of the initial plan; out-of-field recurrence was defined as ≤20% of the recurrence volume within the 95% IDL of the initial plan. LC, IDSC, PFS and OS were calculated from the time of initial diagnosis in high-risk patients or time of relapse in the locally recurrent cases to the event of interest. Statistical analysis was performed using GraphPad Prism (version 7.03).
Results
Patient and Tumor Characteristics
Eighteen patients (16 high-risk and 2 locally recurrent neuroblastoma) with median age at diagnosis of 3 years (range, 2 months – 7 years) were treated with proton therapy. There were 14 males and 4 females. International Neuroblastoma Staging System Stage was 3 in 6 (33%), Stage 4 in 9 (50%), Stage 4S in 1 (6%) and locally recurrent in 2 (11%). The two locally recurrent neuroblastoma patients were initially diagnosed with intermediate-risk disease and had received previous chemotherapy and resection prior to local recurrence. MYCN amplification was seen in 8 of 18 children (44%).
Primary tumor sites included the retroperitoneum/abdomen in 16 patients (89%) and thorax/mediastinum in 2 patients (11%). Overall, the 18 primary and 16 discrete metastatic sites were irradiated. The number of irradiated distant metastatic sites was 0 in 5 patients, 1 in 7 patients, 2 in 3 patients, and 3 in 1 patient. The treated metastatic sites included 11 in bone (69%), 4 in lymph nodes (25%), and 1 in soft tissue (6%). Table 1 shows the overall demographic, tumor and treatment characteristics for patients analyzed in this study.
TABLE 1.
Host, tumor and treatment characteristics in patients treated with proton therapy for neuroblastoma
| Characteristic | No. of Patients (%) |
|---|---|
| Sex | |
| Female | 4 (22) |
| Male | 14 (78) |
| Age | |
| Median in years (range) | 3 (2 months – 7 years) |
| Primary Tumor Site | |
| Retroperitoneum/Abdomen | 16 (89) |
| Thorax/Mediastinum | 2 (11) |
| Stage (INSS) | |
| 3 | 6 (33) |
| 4 | 9 (50) |
| 4-S | 1 (6) |
| Locally recurrent | 2 (11) |
| MYCN Amplification | |
| Yes | 8 (44) |
| No | 10 (56) |
| Chemotherapy Regimen (High Risk Patients) | |
| COG ANBL0532 | 9 (56) |
| THIN | 5 (31) |
| COG ANBL12P1 | 1 (6) |
| SIOPEN | 1 (6) |
| Degree of Resection | |
| GTR | 12 (67) |
| STR | 6 (33) |
| Post-Induction MIBG Curie Score (High Risk Patients) | |
| 0 | 5 (31) |
| 1 | 3 (19) |
| 2 | 7 (44) |
| 3 | 1 (6) |
| Stem Cell Transplant (High Risk Patients) | |
| Single | 14 (88) |
| Tandem | 1 (6) |
| None | 1 (6) |
| Irradiated Metastatic Sites | |
| 0 | 5 (31) |
| 1 | 7 (44) |
| 2 | 3 (19) |
| 3 | 1 (6) |
| Treated Metastatic Sites | |
| Bone | 11 (69) |
| Lymph Node | 4 (25) |
| Soft Tissue | 1 (6) |
| Proton Therapy Modality | |
| PSPT | 17 (94) |
| IMPT | 1 (6) |
| Immunotherapy (High-Risk Patients) | |
| Yes | 9 (56) |
| No | 7 (44) |
COG = Children’s Oncology Group; THIN = Texas Children’s Hospital In-House Institutional Protocol; SIOPEN = High-Risk Neuroblastoma Study 1.8 of International Society of Pediatric Oncology-Europe; GTR = gross total resection; STR = subtotal resection; PSPT = passive scatter proton therapy; IMPT = intensity modulated proton therapy; MIBG = metaiodobenzylguanidine
Radiotherapy Treatment
The median radiation doses to the primary and metastatic sites were 21.6 Gy(RBE) (range, 21 – 36 Gy(RBE)) and 21.6 Gy(RBE) (range, 21 – 24 Gy(RBE)), respectively. The median number of fractions to the primary site and metastatic sites was 12 (range, 12 – 22) and 12 (range, 12 – 14), respectively. Seventeen patients (94%) were treated with passively scattered protons and 1 patient (6%) received intensity-modulated proton therapy. The specific radiation characteristics for each high-risk patient is included in Table 2. To consider potential acute and late effects on normal tissues, doses to the heart, lungs, liver, and kidneys were evaluated. The mean dose to the heart was 1.1 Gy(RBE) (range, 0.001 – 5.6 Gy(RBE)), lungs was 2.3 Gy(RBE) (range, 0.001 – 12.6 Gy(RBE)), liver was 2.8 Gy(RBE) (range, 0.001 – 10.9 Gy(RBE)), and total kidneys was 10.6 Gy(RBE) (range, 2.7 – 15.7 Gy(RBE)).
TABLE 2.
Patient, tumor and radiotherapy characteristics in high-risk neuroblastoma patients
| Patient | Age (years) | Gender | Primary site | Metastatic site (N) | Dose to primary (Gy(RBE)) | Dose to metastases (Gy(RBE)) | Proton modality | Local recurrence |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | M | Thorax | - | 36.0 | - | PSPT | None |
| 2 | 2 | M | Abdomen | Bone (1) | 23.4 | 23.4 | PSPT | None |
| 3 | 3 | F | Abdomen | Bone (1) | 21.6 | 21.6 | PSPT | None |
| 4 | 3 | M | Abdomen | - | 21.6 | - | IMPT | None |
| 5 | 7 | M | Abdomen | Bone (2) | 21.6 | 21.6 | PSPT | None |
| 6 | 3 | M | Abdomen | Bone (1) | 21.6 | 21.6 | PSPT | Primary |
| 7 | 2 | M | Abdomen | - | 21.6 | - | PSPT | Primary |
| 8 | 3 | M | Abdomen | LN (2) | 21.6 | 21.6 | PSPT | None |
| 9 | 4 | F | Abdomen | LN (1) | 21.0 | 21.0 | PSPT | None |
| 10 | 3 | M | Abdomen | ST (1) | 24.0 | 24.0 | PSPT | None |
| 11 | 1 | M | Abdomen | LN (1) | 21.6 | 21.6 | PSPT | None |
| 12 | 0 (2 months) | M | Abdomen | - | 21.6 | - | PSPT | None |
| 13 | 3 | F | Abdomen | Bone (3) | 21.6 | 21.6 | PSPT | None |
| 14 | 2 | M | Abdomen | Bone (2) | 21.6 | 21.6 | PSPT | None |
| 15 | 1 | M | Abdomen | - | 36.0 | - | PSPT | None |
| 16 | 4 | F | Abdomen | Bone (1) | 21.6 | 21.6 | PSPT | None |
M = male; F = female; LN = lymph node; ST = soft tissue; N = number of sites; RBE = relative biological effectiveness; PSPT = passive scatter proton therapy; IMPT = intensity modulated proton therapy
Follow-up, Local Control and Survival Outcomes
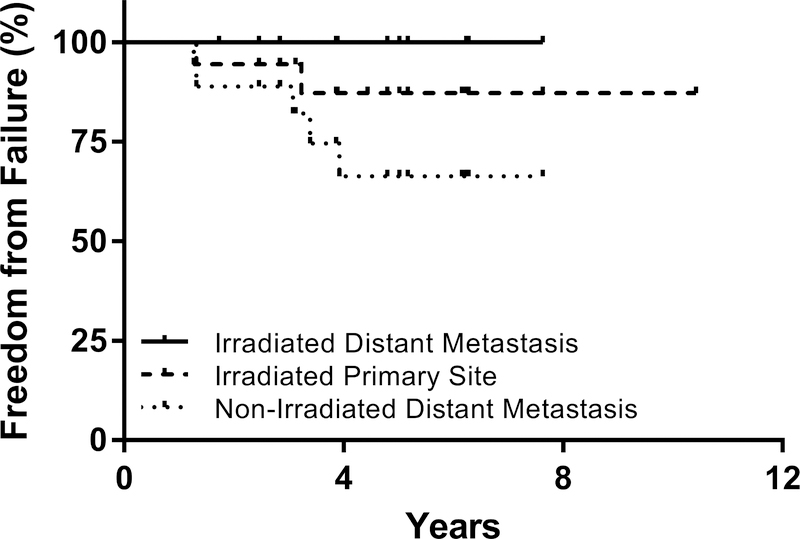
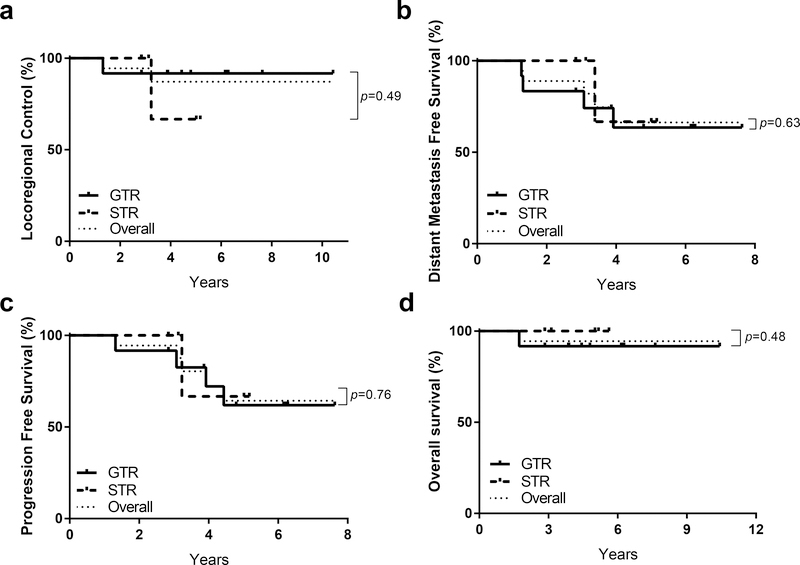
The median follow-up was 60.2 months (range, 20.8 to 125.1 months). Two patients had local recurrences occurring at 7 and 25 months after completing radiation therapy. Both of the patients with local recurrence had primary disease in the abdomen and received 21.6 Gy(RBE) to the primary site; one patient underwent gross total resection and one underwent subtotal resection. One patient who developed local recurrence did not receive stem cell transplant due to toxicity concerns related to chronic lung disease. None had a marginal recurrence. The 2- and 5-year LC rates were 94% and 87%, respectively. The 2- and 5-year PFS rates were 94% and 64%, while 2- and 5-year OS rates were both 94%. Patients treated with proton therapy to metastatic sites experienced excellent LC at the irradiated distant sites, with no failures observed among the 16 total metastatic sites treated with proton therapy (Figure 1). The predominant mode of failure in these patients was progression at non-irradiated distant metastases which were initially MIBG positive at diagnosis and MIBG negative at the end of induction, with 2- and 5-year distant metastasis-free survival rates of 89% and 66%, respectively (Figure 1). The majority of treatment-related acute toxicities were Grade 1, and minimal late toxicities were observed using available follow-up data from the study population. No radiation-related hepatopathy or nephropathy was reported (Table 3). In this population of high-risk and locally recurrent neuroblastoma patients, no significant differences were observed between patients who underwent GTR or STR with respect to local control (p=0.49), distant metastasis-free survival (p=0.63), progression-free survival (p=0.76), or overall survival (p=0.48) (Figure 2a–d).
Figure 1.
Local Control at Irradiated Primary and Distant and Non-Irradiated Distant Sites
TABLE 3.
Acute toxicities for high-risk neuroblastoma patients treated with proton therapy
| Acute Toxicity | Grade | Number of patients (%) |
|---|---|---|
| Fatigue | 1 | 3 (17%) |
| 2 | 0 (0%) | |
| 3 | 0 (0%) | |
| Dermatitis | 1 | 7 (39%) |
| 2 | 0 (0%) | |
| 3 | 0 (0%) | |
| Nausea | 1 | 1 (6%) |
| 2 | 0 (0%) | |
| 3 | 0 (0%) | |
| Diarrhea | 1 | 1 (6%) |
| 2 | 1 (6%) | |
| 3 | 0 (0%) | |
| Pain | 1 | 1 (6%) |
| 2 | 0 (0%) | |
| 3 | 0 (0%) | |
| Mucositis | 1 | 2 (11%) |
| 2 | 0 (0%) | |
| 3 | 0 (0%) | |
| Anorexia | 1 | 3 (17%) |
| 2 | 0 (0%) | |
| 3 | 0 (0%) | |
Figure 2.
Degree of Resection and Impact on (a) Local Control, (b) Distant Metastasis Free Survival, (c) Progression Free Survival and (d) Overall Survival
Discussion
There is limited data reporting long-term disease control with proton therapy in high-risk neuroblastoma (Table 4). At Massachusetts General Hospital, 9 children with high-risk neuroblastoma treated with proton therapy were locally controlled at the primary site at a median follow-up of 38 months [8]. At the University of Pennsylvania, all 11 children treated with proton therapy to the primary site were locally controlled at a median follow-up of 16 months [9]. In another study from Japan, 14 patients (8 high-risk and 6 recurrent neuroblastoma) received proton therapy with a 3-year locoregional control rate of 82% [10]. Compared to the above, our study has a longer median follow-up of 60.2 months, with larger number of patients and looked at local control at both primary and irradiated metastatic sites. Of 34 sites treated (18 primary, 16 distant), local recurrence occurred only in 2 sites, both at the primary site. We used a minimum follow-up of 12 months in this study to provide an adequate duration to assess local control rates. We did not exclude any patients with local failures occurring within 12 months. Our study adds to the scant proton literature and is consistent with the existing proton and photon literature with longer follow-up [1–4, 8, 10]. The predominant site of failure is progression in post-induction non-MIBG avid distant sites, similar to previously published reports [3, 11, 12]. While proton therapy provides high rates of local control with acceptable toxicity for neuroblastoma, our results suggest that advances in systemic therapy are needed for improved control of systemic disease.
TABLE 4.
Long-term disease control with proton therapy in high-risk neuroblastoma patients
| First Author Institution | Reference | Treatment Period | Number of Patients | Median Follow-up (months) | Local Control - Primary Site (%) | Local Control – Irradiated Distant Metastases (%) |
|---|---|---|---|---|---|---|
| Hattangadi Massachusetts General Hospital | [8] | 2005–2010 | 9 | 38 | 100 | - |
| Hill-Kayser University of Pennsylvania | [9] | 2011–2012 | 11 | 16 | 100 | - |
| Oshiro University of Tsukuba | [10] | 1984–2010 | 14 | 36 | 82 | - |
| Bagley MD Anderson | Current study | 2007–2016 | 18 | 60.2 | 87 | 100 |
Achieving gross total resection has been reported to improve outcomes in some studies of high-risk neuroblastoma and is influenced by several factors including the site and extent of primary disease, patient comorbidities, and surgical expertise [13, 14]. In the NB97 trial, gross total resection did not improve overall survival or local control; however in the same study some patients with STR received 36 Gy RT to the tumor bed while none of the GTR patients received RT. There has been speculation whether the presence of gross disease after surgery can be controlled by a higher dose of radiation (36 Gy) as employed in the NB97 trial [13]. A recent publication reviewed the published locoregional control rates after standard dose radiation (21 to 24 Gy) versus higher dose radiation (30 to 36 Gy); in children with STR, local failure was found in 17 to 43% of patients receiving standard and 0% in those receiving higher dose RT [15]. We did not find a difference in locoregional control, progression-free or overall survival according to the degree of resection.
Because high-risk neuroblastoma has a propensity to metastasize, it is important to understand the role of proton radiation in managing distant metastases. Patients in our study had up to three MIBG avid distant metastatic sites treated with proton radiation. Importantly, with this oligometastatic treatment approach, we did not observe any local failures at irradiated distant metastatic sites. Others have reported less favorable outcomes after radiotherapy for metastatic disease, with 23 to 26% failure rates in the irradiated sites [3, 16].
In the current study, doses to the liver, heart and lungs were low with the use of 3-D proton therapy. The location of the CTV may be the most important determinant of whether proton therapy will have an advantage over photon therapy with regard to the kidney. In some cases, intensity modulated radiation therapy (IMRT) might be better compared to 3-D proton therapy in reducing mean kidney dose [9]. The mean dose to the kidney in the current cohort is 10.6 Gy(RBE). Our patients were treated primarily with passively scattered protons, and thus it is possible that the doses to the normal surrounding organs can further be reduced by intensity modulated proton techniques. A recent study of 49 children with neuroblastoma treated using IMRT delivered mean doses of 10.7 and 14.8 Gy to the bilateral kidneys with no chronic renal insufficiency [17]. Thus, further reduction in mean kidney doses with more sophisticated proton techniques might not be clinically relevant. In the modern era with intensity modulated proton therapy, however, improved dosimetry to other organs with effective local control at the primary and metastatic sites suggests that proton therapy will play an increasing role in the future for patients with high-risk neuroblastoma. As the survival of high-risk neuroblastoma continues to improve with advances in multimodality therapy, minimizing treatment-related late toxicities including nephropathy, hepatopathy, cardiac toxicity, and secondary malignancies is of paramount importance in this population. Proton therapy, by reducing dose to surrounding tissues without compromising local disease control as this study and others demonstrate, is therefore a promising approach to reduce late toxicities.
Patients in this study were referred to the Proton Therapy Center at the UT MD Anderson Cancer Center, and all patients were dispositioned to receive proton therapy. We identified three high-risk neuroblastoma patients during the study period treated with IMRT who were not included in the current study; we therefore estimate that this study describes the majority (86%) of patients treated at our center. The decision regarding the use of proton therapy versus conventional radiation for high-risk neuroblastoma has been discussed previously [9]. For selected patients with lateralized abdominal disease, conventional radiation techniques such as IMRT may reduce ipsilateral kidney dose relative to passively scattered proton therapy. While the above authors note that most patients had improved sparing of the contralateral kidney, liver, and bowel with proton therapy, certain patients may not have sparing of contralateral kidney dose with proton therapy due patient anatomy and the location of the tumor bed [9]. In these patients, treatment with conventional radiation therapy may therefore be advantageous versus passively scattered proton therapy. Because all patients received proton therapy we cannot directly compare dosimetry between proton therapy and conventional radiation therapy in the current study. Given the variability in primary tumor sites, patient anatomy, and other clinical factors, however, all treatment decisions regarding radiation modality and dose should ultimately be individualized for each patient.
There are limitations to the current study. Estimates of progression-free and overall survival may be biased in this retrospective study because only patients treated for definitive intent with at least twelve months of available follow-up were included in this study. Patients failing to respond to induction therapy would be expected to have less favorable outcomes than those included in our analysis, contributing to the high overall survival observed in this study. In addition, the treatment for high-risk neuroblastoma is multimodal involving chemotherapy, surgery, stem cell transplantation, radiation, and immunotherapy. Variations in the non-radiation components of therapy, such as degree of resection and use of immunotherapy may contribute to survival outcomes. Notably, three distinct chemotherapy regimens were used in this population, seven patients did not receive immunotherapy, and only one patient received tandem stem cell transplant; we attribute this primarily to the study’s time span, as experimental chemotherapy protocols were not yet developed and immunotherapy and tandem stem cell transplant were not standard of care at the time when some patients received treatment. This study includes a limited number of patients with variable clinical and treatment parameters accrued over a nearly 10-year period at a single center, which may limit the generalizability of the findings to patients treated at other centers. Lastly, continued follow-up will be necessary to more definitively assess late toxicities following proton therapy. Nevertheless, our study of high-risk and locally recurrent neuroblastoma patients treated with proton therapy for curative intent shows that proton therapy does not compromise locoregional outcomes when compared to other series using photon treatments.
Our study demonstrates that for high-risk neuroblastoma patients, proton therapy provides excellent local and locoregional disease control both at the primary tumor site and irradiated distant metastatic sites with acceptable rates of toxicity. However, the majority of patients develop distant failures at non-irradiated sites, suggesting that further advances in systemic therapy are needed in parallel to further improve the disease control and survival for patients with high-risk neuroblastoma.
Acknowledgments
Source of Financial Support / Funding Statement
This work is supported by the NIH/NCI under award number P30CA016672.
Abbreviations Key
- LC
Local Control
- IDSC
Irradiated Distant Site Control
- PFS
Progression free survival
- OS
Overall Survival
- RT
Radiotherapy
- GTR
Gross total resection
- STR
Subtotal resection
- CTV
Clinical target volume
- IDL
Isodose line
Footnotes
Conflict of Interest Disclosure
The authors report the following disclosures: A.C.P. receives book royalties from Elsevier, Inc.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Ferris MJ, et al. , Favorable Local Control From Consolidative Radiation Therapy in High-Risk Neuroblastoma Despite Gross Residual Disease, Positive Margins, or Nodal Involvement. Int J Radiat Oncol Biol Phys, 2017. 97(4): p. 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey DL, et al. , Local Control With 21-Gy Radiation Therapy for High-Risk Neuroblastoma. Int J Radiat Oncol Biol Phys, 2016. 96(2): p. 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazloom A, et al. , Radiation therapy to the primary and postinduction chemotherapy MIBG-avid sites in high-risk neuroblastoma. Int J Radiat Oncol Biol Phys, 2014. 90(4): p. 858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pai Panandiker AS, et al. , Intensity modulated radiation therapy provides excellent local control in high-risk abdominal neuroblastoma. Pediatr Blood Cancer, 2013. 60(5): p. 761–5. [DOI] [PubMed] [Google Scholar]
- 5.Paulino AC and Fowler BZ, Risk factors for scoliosis in children with neuroblastoma. Int J Radiat Oncol Biol Phys, 2005. 61(3): p. 865–9. [DOI] [PubMed] [Google Scholar]
- 6.Paulino AC, et al. , Locoregional control in infants with neuroblastoma: role of radiation therapy and late toxicity. Int J Radiat Oncol Biol Phys, 2002. 52(4): p. 1025–31. [DOI] [PubMed] [Google Scholar]
- 7.Hess CB, et al. , An Update From the Pediatric Proton Consortium Registry. Front Oncol, 2018. 8: p. 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattangadi JA, et al. , Proton radiotherapy for high-risk pediatric neuroblastoma: early outcomes and dose comparison. Int J Radiat Oncol Biol Phys, 2012. 83(3): p. 1015–22. [DOI] [PubMed] [Google Scholar]
- 9.Hill-Kayser C, et al. , Proton versus photon radiation therapy for patients with high-risk neuroblastoma: the need for a customized approach. Pediatr Blood Cancer, 2013. 60(10): p. 1606–11. [DOI] [PubMed] [Google Scholar]
- 10.Oshiro Y, et al. , Clinical results of proton beam therapy for advanced neuroblastoma. Radiat Oncol, 2013. 8: p. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, et al. , Patterns of Relapse in High-Risk Neuroblastoma Patients Treated With and Without Total Body Irradiation. Int J Radiat Oncol Biol Phys, 2017. 97(2): p. 270–277. [DOI] [PubMed] [Google Scholar]
- 12.Polishchuk AL, et al. , Likelihood of bone recurrence in prior sites of metastasis in patients with high-risk neuroblastoma. Int J Radiat Oncol Biol Phys, 2014. 89(4): p. 839–45. [DOI] [PubMed] [Google Scholar]
- 13.von Allmen D, et al. , Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma. J Clin Oncol, 2017. 35(2): p. 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer J, et al. , Complete surgical resection improves outcome in INRG high-risk patients with localized neuroblastoma older than 18 months. BMC Cancer, 2017. 17(1): p. 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey DL, et al. , Dose-escalation is needed for gross disease in high-risk neuroblastoma. 2018. 65(7): p. e27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandula S, et al. , Outcomes After Radiation Therapy to Metastatic Sites in Patients With Stage 4 Neuroblastoma. J Pediatr Hematol Oncol, 2015. 37(3): p. 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckham TH, et al. , Renal Function Outcomes of High-risk Neuroblastoma Patients Undergoing Radiation Therapy. Int J Radiat Oncol Biol Phys, 2017. 99(2): p. 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]