Abstract
Background and Aims:
Older individuals are susceptible to the loss of muscle and accumulation of fat. To address this problem, we have compared protein kinetics following consumption of an essential amino acid (EAA)-enriched meal replacement (EMR) to consumption of a high-protein meal replacement beverage (Bariatric Advantage, BA) using stable isotope methodology.
Methods:
Eight older (67±2), obese (35±2 kg/m2) female and male participants completed two studies using a randomized, crossover design in which they ingested each meal replacement. The isotopic tracers L-[2H5]phenylalanine & L-[2H2]tyrosine were delivered via primed, continuous intravenous infusion throughout a basal period and following consumption of EMR or BA. We determined changes in whole body protein synthesis (PS), protein breakdown (PB), and net protein balance (NB) from fasted states via analysis of plasma samples by LC-ESI-MS.
Results:
PS was higher (P=0.03) and PB was less (P=0.005) with EMR in comparison to BA. As a result, NB was much greater (P=0.00003) following the ingestion of EMR as compared to BA.
Conclusions:
In comparison with BA, which has a higher amount of intact protein that any other meal replacement, EMR promoted a greater increment in NB. These data support the potential efficacy of EMR as a meal replacement for the preservation of lean tissue mass during weight loss in older, overweight individuals.
Keywords: Essential Amino Acids, Protein Kinetics, Skeletal Muscle, Stable isotope tracers, liver, muscle, insulin resistance, protein intake
INTRODUCTION
Sarcopenic obesity is characterized by a gradual loss of lean tissue and progressive increase in fat mass [1]. About ten years ago, 33% of the older obese population would have been classified as sarcopenic [2], and the rate of obesity in the older age group has now increased by at least 48% [3]. This detrimental alteration in body composition has been linked to impairments in functional capacity and the ability to perform the activities of daily living in the elderly [4].
Weight loss in obese elderly would be beneficial not only in terms of health outcomes [5], but also because the amount of work that must be done to perform simple activities would be reduced [6]. Unfortunately, current methods employing caloric restriction-induced weight loss (CRWL) have often been considered counterproductive when measured against the acceleration of muscle atrophy that occurs with weight loss in older individuals [7]. While the feasibility of exercise-induced weight loss has been demonstrated in previous studies in middle-aged individuals [8], the practicality of this approach is limited in obese individuals who usually have very low capacity for exercise [9]. It is also understood that supervised, well-controlled combinations of exercise training (resistance and aerobic) in combination with caloric restriction may preserve lean tissue during weight loss [8], but the cost-effectiveness of these interventions may limit adequate implementation in the elderly [10]. Nonetheless, an increase in physical activity should follow any weight loss paradigm to maintain metabolic health, preserve muscle and optimize functional independence.
In order to address the conundrum presented by the need to maintain muscle mass during weight loss in the elderly, we developed a meal replacement, including a precise blend of essential amino acids (EMR) that was three times more effective than whey protein-based meal replacement in stimulating muscle protein synthesis in older individuals [11]. Utilizing a previous meal replacement version (TMR) based on minimal protein requirements (0.8 g protein/kg/day), we demonstrated a greater reduction in adipose tissue compared to a competitive meal replacement, but participants still lost some lean tissue mass [11]. The loss of muscle mass using the EAA-based beverage despite the increased stimulation of muscle protein synthesis was likely due to the fact that the amount of protein intake necessary to maintain lean tissue mass in older individuals is significantly greater than 0.8 g/kg/day and the hypocaloric condition of CRWL increased the protein intake necessary to maintain lean tissue mass to an even greater extent [12]. Given the physiological consideration of negative caloric balance and the existence of “anabolic resistance” in older individuals [13–15], we have increased the absolute amount of EAAs in our meal replacement. We now hypothesize that our revised formulation (i.e., EMR) will promote higher whole-body net protein balance (NB) above fasted states as determined by stable isotope methodology than a meal replacement with highest amount of intact protein on the market (Bariatric Advantage).
METHODS
Subjects.
Eight older, overweight female and male subjects between the ages 60 and 80, BMI of 30–40 kg/m2 were recruited from the Fairbanks, AK area using local newspaper advertisements and flyers posted around the University of Alaska Fairbanks (UAF) campus (Table 1). They were required to have access to transportation to the site clinic for performance of the study tests. All materials given to volunteers who were properly consented and procedures related to the study were reviewed and approved by the UAF Institutional Review Board (IRB). The confidentiality of all participants was ensured and protected. Subjects visited the clinic located in the Clinical Research and Imaging Facility at UAF on three separate occasions: a) consenting and determination of eligibility status, b) metabolic study #1, and c) metabolic study #2. The metabolic studies were performed in a randomized, double blind fashion to evaluate the response to the ingestion of isocaloric amounts of either BA or EMR. The ingredients of EMR and BA have been listed in Table 2 and the list of essential amino acids have been provided in descending order of concentration (Supplemental File 1).
Table 1.
Clinical characteristics
| Sex | 4F/4M |
| Age (years) | 67±7 |
| Body Mass (kg) | 102±17 |
| Height (m) | 1.7±0.1 |
| BMI (kg/m2) | 35.2±4.3 |
| Lean Body Mass (kg) | 54.9±10.2 |
| Fat Mass (kg) | 42.7±8.9 |
| BMC (kg) | 3.1±0.8 |
| RSMI (kg/m2) | 8.8±1.3 |
| % Fat | 42±6 |
Table 2.
Composition of Meal Replacement
| Component | BA | EMR |
|---|---|---|
| Whey Protein Isolate | 27 | 6 |
| Carbohydrate | 7 | 11 |
| Fat | 2 | 2 |
| Free Essential Amino Acids | 0 | 17 |
| Total weight | 36 | 36 |
During the initial consent and screening visit, participants completed several assessments to determine eligibility. The exclusion criteria included creatinine >1.4, and a serum glutamate pyruvate transaminase >2 times normal. All volunteers with a resting blood pressure above 160/90 mmHg were excluded. We excluded any participant with previously diagnosed diabetes (fasting blood glucose ≥ 126 mg/dl), a history of kidney or liver disease, heart disease as indicated by interventional procedures, a recent history of alcoholism, and active cancer. We excluded volunteers with a chronic inflammatory condition or any patient taking corticosteroids. Finally, any participant who had a medical condition or was taking a medication that, in the opinion of the study physician represented an unacceptable risk was excluded from the study.
Eligible participants performed a dual-energy X-ray absorptiometry (GE iDXA) scan for determination of body composition prior to metabolic studies (Table 1).
Experimental protocol.
After determination of eligibility and body composition assessment, participants completed two metabolic studies in a randomized double-blind fashion that included the isocaloric ingestion of EMR and BA in conjunction with isotope methodology designed to determine whole body protein synthesis (PS), protein breakdown (PB) and net protein balance (NB).
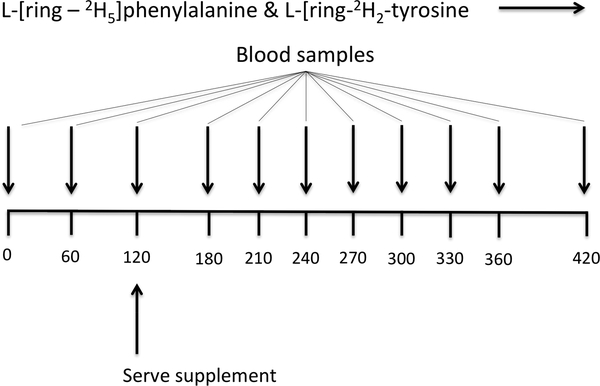
Participants reported for the isotope infusion/feeding studies after an overnight (after 2200 hr) fast on two occasions that were at least three days apart for the metabolic studies. Two polyethylene catheters were placed into each lower arm; one for the infusion of stable isotope tracers and the other for “arterialized” blood sampling via a heated hand technique [21]. Prior to the initiation of the tracer infusion, a baseline blood sample was collected to determine background isotopic enrichments. Primed continuous infusions of L-[2H5]phenylalanine (prime, 5.04 μmol/kg; rate, 5.04 μmol·kg−1·h−1) and L-[2H2]tyrosine (prime, 2.16 μmol/kg; rate, 1.995 μmol·kg−1·h−1) were performed in order to determine rates of PS, PB, and NB at the whole body level. To appropriately reach isotopic equilibrium of L-[2H4]tyrosine enrichment derived from L-[2H5]phenylalanine tracer infused, a priming dose of L-[2H4]tyrosine was also injected (prime: 5.04 μmol/kg) (Figure 1). All isotope tracers were purchased from Cambridge Isotope Laboratories (Andover, MA). Blood samples were taken at 0, 60, 120 min (fasted blood samples), at t=180, 210, 240, 270, 300, 330, 360, and 420 min following randomized ingestion of BA and EMR at 120 min to measure tracer enrichment and plasma responses of AAs. Blood samples were taken at t=120, 210, and 270 min for insulin and growth hormone analysis. A total of 12 blood samples were taken during the study.
Figure 1.
Meal ingestion and tracer infusion protocol
Calculations.
Whole-body protein kinetics were calculated based on the determinations of the rate of appearance (Ra) into the plasma of phenylalanine and tyrosine and the fractional Ra of endogenous tyrosine derived from the metabolism of phenylalanine [17]. The area under the curve (AUC) of plasma enrichments of phenylalanine and tyrosine tracers was calculated using Graphpad Prism 5 for Mac (Graphpad Software, La Jolla, CA) to account for variations in postprandial tracer kinetics [17,18]. Whole body protein kinetics were calculated by dividing kinetic values of phenylalanine by its fractional contribution to protein (4%) and were expressed as changes from the fasted to fed states [19]. For the calculations for whole body protein breakdown rate, contribution from exogenous meal and tracers infused were subtracted from total Ra. The following equations were used for the calculations of whole-body protein kinetics:
Enrichment (E) was expressed as the tracer-to-tracee ratio (TTR) or mole percent excess (MPE), calculated at TTR/(TTR + 1). TTR was used for calculations of NB whereas MPE was used for calculations of PS. E was used as the enrichment of respective tracers. F is the tracer infusion rate into an intravenous infusion site: FPhe for phenylalanine tracer. ETyr M+4 and EPhe M+5 were the plasma enrichments of tyrosine tracers at M+4 and M+5 relative to M+0 where M represents mass, respectively. The correction of 25 was utilized for the conversion value for phenylalanine to protein based on the assumption that the contribution of phenylalanine to protein was 4% (100/4 = 25) [18]. PRO is the amount of phenylalanine and tyrosine appearing in the circulation due to digestion of exogenous protein [20]. Phe hydroxylation rate was the rate of appearance of tyrosine derived from phenylalanine through the process of hydroxylation. Based on 4 mol ATP utilized per mole of amino acids incorporated into protein [21], and 1 mol of ATP turnover = 20 kcal energy expenditure (including the energy cost of resynthesis of ATP) [22], we calculated the theoretical difference in the energy cost between the post-absorptive to post-prandial stimulation of PS between EMR and BA.
Analytical methods.
Plasma samples were deproteinized using dry sulfosalicylic acid, and frozen at −80°C until analysis. Enrichment analysis was performed by Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC-ESI-MS) (QTrap 5500 MS; AB Sciex) with ExpressHT Ultra LC (Eksigent Div.; AB Sciex) after derivatization with 9-fluoren-9-ylmethoxycarbonyl (Fmoc) [23]. Ions of mass to charge ratios of 234, 235, and 239 for phenylalanine and 466, 467, 468, and 470 were utilized. Plasma insulin concentrations were measured by using commercially available human insulin ELISA kit (Alpco Diagnostics, Salem, MA). Plasma human growth hormone concentrations were measured using a commercially available ELISA kit (Invitrogen; Thermo Fisher Scientific, Carlsbad, CA). Plasma amino acid concentrations were measured using liquid chromatography-mass spectrometry (QTrap 5500 MS; AB Sciex) using the internal standard method [24].
Statistical Methods.
Two-tailed independent t-tests were used to compare differences in PS, PB, and NB between in EMR and BA. This method of statistical analysis was also used to determine differences in plasma insulin and growth hormone. A two-way analysis of variance with repeated measures was utilized to determine differences in the plasma amino acids between EMR and BA. All data were analyzed using the Graphpad Prism 6 for Mac (Graphpad Software, Inc. La Jolla CA) and presented as mean ± SEM.
RESULTS
Clinical Characteristics.
Data related to body composition for all participants are listed in Table 1.
Amino Acids.
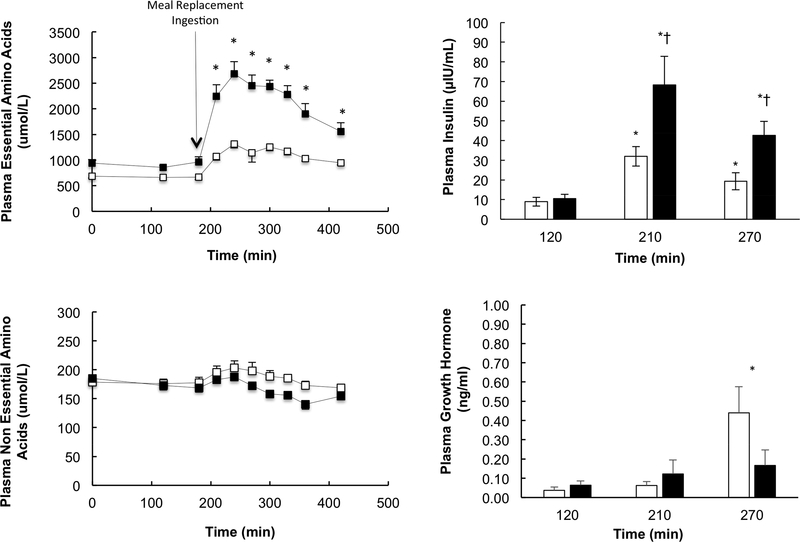
Plasma essential amino acids were significantly higher at all post-prandial time points in EMR compared to BA (Figures 2). On the other hand, plasma non-essential amino acids were significantly higher during the post-prandial period (i.e., t=270, 300, 330 and 360 min) in BA compared to EMR (Figure 2).
Figure 2.
Plasma essential and non-essential amino acid, and plasma insulin and growth hormone concentrations in response to BA and EMR. *Represents a significant difference between EMR and BA and EX (P<0.05). † = BA and EMR, (p<0.02).
Plasma insulin was similar in BA and EMR during the post-absorptive state, and rose significantly in both EMR and BA with the ingestion of the meal replacements (Figure 2). The increase in plasma insulin was significantly higher with the ingestion of EMR compared to BA. Plasma growth hormone was similar in both groups except for a higher level at t=270 min in BA compared to EMR (Figure 2). Growth hormone concentration did not rise above the basal level in EMR.
Protein Kinetics.
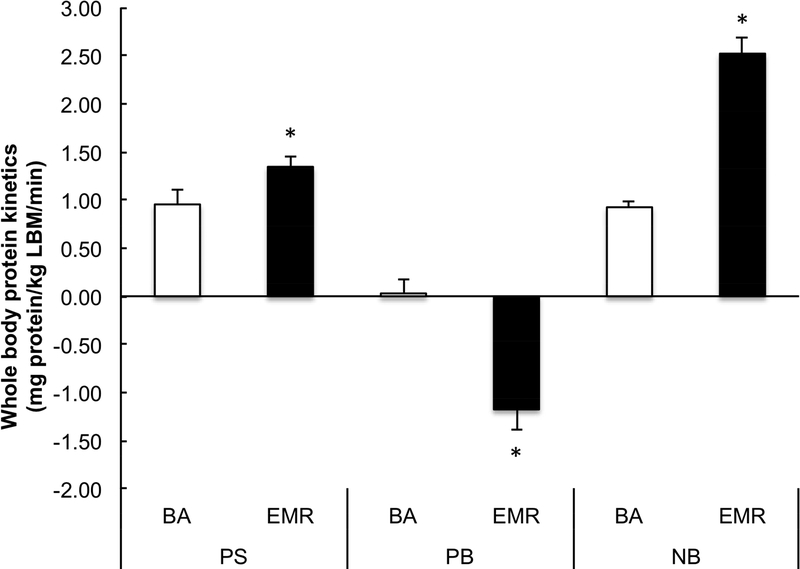
There were no significant differences in post-absorptive values for PS, PB or NB or whole-body phenylalanine oxidation rates (Supplemental File 2) between EMR and BA. The changes in PS (1.35±0.11 and 0.95±0.15 mg protein•kg LBM−1• min−1), PB (−1.18±0.21 0.03±0.14 mg protein•kg LBM−1• min−1) and NB (2.52±0.17 and 0.92±0.05 mg protein•kg LBM−1 • min−1) from the post-absorptive to the post-prandial state was greater in EMR compared to BA, respectively (P<0.01) (Figure 3).
Figure 3.
Whole body protein synthesis, protein breakdown, and net balance in response to BA and EMR. *Represents a significant difference between BA and EMR (P<0.05).
DISCUSSION
To provide a “proof of concept” for future weight loss intervention studies, we hypothesized that EMR would promote a more positive feeding-induced increase in NB when compared to BA. BA was chosen for the comparison since it contains the highest amount of whey protein of any other meal replacement currently on the market. Notably, whey protein has been touted as more effective than casein or soy in maintaining skeletal muscle [25]. We found that consumption of EMR resulted in a superior insulinotropic response (P=0.02), a greater increase in PS (P=0.03), and reduction in PB (P=0.005) that contributed to a 2.5-fold higher increase (P=0.00003) in NB compared to BA. These results support the hypothesis that EMR stimulates the greater anabolic response when compared to a meal replacement with the highest amount of high-quality protein. From this result, we can speculate that use of EMR in a CRWL program over a prolonged period of weight reduction would maintain lean tissue mass while eliciting a reduction in fat mass.
The insulinotropic effect of amino acids was initially described ~50 years ago [26]. The results of the present study clearly demonstrate higher feeding-induced plasma insulin concentrations with EMR. Leucine comprises ~32% of the overall EAAs in EMR, and previous studies have specifically demonstrated that leucine promotes glutamate dehydrogenase activity. This enzyme ultimately augments oxygen consumption in pancreatic ß-cells and increases insulin secretion [27]. The amount of carbohydrate was also slightly higher in BA and could have influenced the insulin response as well [27].
The superior insulintropic effect of EMR is relevant to the response of protein kinetics because insulin activates the mammalian target of rapamycin (mTOR) [28,29]. This factor plays a role in the molecular events necessary for the initiation of protein synthesis [30,31]. In addition, an increased availability of EAAs in plasma, particularly leucine, can activate mTOR [32]. Consequently, it seems unlikely that the greater insulin response following EMR is primarily responsible for the higher rate of synthesis with EMR consumption. It is more likely that both beverages activated the initiation process, and that the greater availability of EAA precursors resulted in a higher rate of PS following EMR. It is plausible that insulin played a role in suppressing PB in the EMR study. The suppressive effect of insulin on breakdown of splanchnic and muscle protein has been established [33]. It is also possible that the greater amounts of EAAs in EMR played a role in the suppression of PB. We recently showed that PB was reduced to a greater extent in response to a mixed meal containing a greater amount of protein than in an isocaloric meal, despite similar insulin responses to the two meals [34]. Based on the results of studies that combined the measurement of muscle FSR and whole body protein synthesis, similar alterations in NB likely occurred across tissues other than muscle including those in the splanchnic region [35]. The magnitude of reduction in PB primarily explained the greater increase in NB following EMR. This has been the case in our previous studies of the responses of PS and PB at the whole-body level following consumption of mixed meals as well [17].
The most important endpoint of this study was NB, as that is the kinetic parameter that corresponds to increases or decreases in protein mass [35]. Therefore, in theory it should make no difference how a positive NB is achieved. An anabolic response (i.e., increase in NB) can be attained by a stimulation of PS, a suppression of PB, or a combination of the two [17]. From an energy balance standpoint, it would be advantageous to achieve an increase in NB by a stimulation of protein synthesis because there is an energy cost associated with protein synthesis [36]. In contrast, the decrease in PB following EMR, while benefitting the NB response, did not contribute to an increase in energy expenditure. On the other hand, EMR also stimulated PS to a greater extent than BA. For every 1 mol of amino acids incorporated into the synthesis of tissue protein, 4 mol of ATP are utilized [21]. This comes at a caloric cost of 20 kcal for every 1 mol of ATP turnover [22]. Therefore, the energy cost of the relevant to the acute ingestion of EMR and BA can be calculated, not only for a single event, but that value can be extrapolated to estimate anticipated differences in the rate of weight loss over a prolonged period of weeks. In our study, this extrapolation yielded a theoretical calculation of 21,707±2337 kcal for BA and 31,931±2856 kcal for EMR directly linked to feeding induced alterations in PS over a 12-week period. If this estimation were accurate, the calculation corresponds to 1.3 kg of additional weight loss with EMR that would be most likely linked to the reduction of adipose tissue [11].
The beneficial responses in protein metabolism with EMR occurred despite a greater plasma growth hormone response in BA. This calls into question the relevance of growth hormone with regard to feeding induced changes in whole body protein kinetics. While initial studies in the elderly posited beneficial improvements in lean tissue mass, bone mineral density and reductions in adipose tissue from biosynthetic growth hormone administration [37], replication of these studies has proven difficult [38]. Perhaps more importantly, no study in middle aged to elderly individuals have linked the administration of growth hormone to improvements in strength unless the individuals were also performing some type of resistance training [39]. In addition to the questionable anabolic effect of growth hormone in elderly, there is evidence that an increase in growth hormone can induce insulin resistance [40]. Moreover, the exclusion of arginine from EMR seemed to reduce the feeding-induced increment in growth hormone but had no detrimental influence on NB.
Conclusion
Using stable isotope methodology to determine whole body protein kinetics in older, overweight adults, we have demonstrated that EMR surpassed BA with regard to the promotion of NB. While the delivery of leucine most likely triggered the molecular alterations necessary to promote PS in both EMR and BA, the unique proportion and enhanced availability of EAAs provided by EMR were likely responsible for the significant advantage in PS compared to BA. The superior insulin response elicited by EMR likely prompted the greater suppression of PB; combined with a superior stimulation PS that resulted in an overall advantage in NB when compared to BA. Adequate consumption of protein to offset the loss of lean tissue mass during CRWL presents a conundrum in older adults due to concomitant ingestion of excess nonprotein calories required to overcome anabolic resistance (10). Therefore, the utilization of EMR as part of a lifestyle intervention designed to promote weight loss would have a distinct advantage with regard to the caloric cost of protein turnover and the preservation of whole-body lean tissue mass, especially in older individuals.
Supplementary Material
What is already known about this subject?
Most overweight individuals utilize caloric restriction for weight loss
Caloric restriction usually results in muscle atrophy.
Older individuals are already at high risk for sarcopenia.
What does this study add?
Caloric restriction may be the most effective strategy for weight loss in older individuals.
Unique profiles of essential amino acids may improve anabolism/kcal ingested.
Improvements in net protein balance may allow preservation of lean tissue mass during weight loss.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institutes of Health Older American Independence Center Grant PG30-AG-028718, and by the National Institute of Diabetes and Digestive and Kidney Diseases Small Business Innovations in Research (R43 AG051298-01), the National Institute of General Medical Sciences (5 U54 GM104944), and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
Footnotes
Disclosure: Drs. Coker and Wolfe are Managing Partners and Co-Owners of Essential Blends, LLC that has received funding through the Small Business Innovations in Research from the National Institutes of Health to develop clinical nutrition products. We declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Evans W What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50: 5–8. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner R Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. [DOI] [PubMed] [Google Scholar]
- 3.Ogden C, Yanovski S, Carroll M, Flegal K. The epidemiology of obesity. Gastroenterology 2007;13:2087–2102. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard D, Dionne I, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the nutrition as a determinant of successful aging (NuAge)-the Quebec Longitudinal Study. Obesity 2009;17:2082–2088. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi S, Suga H, Sasaki S. Diet with a combination of high protein and high total antioxidant capacity is strongly associated with low prevalence of frailty among old Japanese women: a multicenter cross-sectional study. Nutr J. 2017;12;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frimel T, Sinacore D, Villareal D. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc 2008;40:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller S, Wolfe R. The danger of weight loss in the elderly. J Nutr Health Aging 2008; 12:487–491. [DOI] [PubMed] [Google Scholar]
- 8.Coker RH, Williams RH, Yeo SE, Kortebein PM, Bodenner DL, Kern PA, Evans WJ. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab. 2009;94:4258–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeCaria J, Sharp C, Petrella R. Scoping review report: obesity in older adults. Int J Obes (Lond). 2012;36:1141–1150. [DOI] [PubMed] [Google Scholar]
- 10..Coker RH, Wolfe RR, Weight loss strategies in the elderly: a clinical conundrum. Obesity (Silver Spring) 2018. 26(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coker R, Miller S, Schutzler S, Deutz NE, Wolfe RR. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J. 2012; 11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe RR, Miller SL. Miller K. Optimal protein intake in the elderly. Clin Nutr. 2008; 27:675–684. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Selby A, Rankin D. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009; 587:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P. et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. [DOI] [PubMed] [Google Scholar]
- 15.Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B, et al. Blunting of adaptive responses to resistance exercise training in women over 75 years. Exp Gerontol. 2011; 46:884–890. [DOI] [PubMed] [Google Scholar]
- 16.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metab Clin Exp. 1981;30: 936–940. [DOI] [PubMed] [Google Scholar]
- 17.Kim I-Y Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015;308:E21–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe RR, Chinkes D. Isotope tracers in metabolic research. 2nd edn, (John Wiley and Sons, 2005). [Google Scholar]
- 19.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab. 1995; 268:E75–E84. [DOI] [PubMed] [Google Scholar]
- 20.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101(9):2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterlow J, Garlick P, Millward D. Protein turnover in mammalian tissues and in the whole body. (North Holland Publishing Co., 1978). [Google Scholar]
- 22.Newsholme E Substrate cycles: their metabolic, energetic and thermic consequences in man. Biochem Soc Symp 1978;43:183–205. [PubMed] [Google Scholar]
- 23.van Eijk MH, Suylen DPL, Dejong CHC, Luiking YC, Deutz NE. Measurement of amino acid isotope enrichment by liquid chromatography mass spectroscopy after derivatization with 9-fluorenylmethylchloroformate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:48–56. [DOI] [PubMed] [Google Scholar]
- 24.de Betue CT, Joosten KF, Deutz NE, Vreugdenhil AC, van Waardenburg DA. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am J Clin Nutr. 2013;98:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devries MC, Philips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015. 80 Suppl 1:A8–A15. [DOI] [PubMed] [Google Scholar]
- 26.Floyd J Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sener A, Malaisse WJ, L-Leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature 1980;187–189. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–2143. [DOI] [PubMed] [Google Scholar]
- 29.Proud CG. mTOR-mediated regulation of translation factors by amino acids. BIochem Biophys Res Commun. 2004;313:429–436. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. [DOI] [PubMed] [Google Scholar]
- 31.Drummond MJ, Rasmussen BB. Leucine-enriched nutrients and the regulation of mTOR signalling and human muscle protein synthesis. Curr Opin Clin Nutr Metab Care 2008; 11(3):222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graber TG, Borack MS, Reidy PT, Volpi E, Rasmussen BB. Essential amino acid ingestion alters expression of genes associated with amino acid sensing, transport, and mTORC1 regulation in human skeletal muscle. Nutr Metab (Lond). 2017;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meek SE, Persson M, Ford GC, Nair KS. Differential regulation of amino acid exchange and protein dynamics across splanchnic and skeletal muscle beds by insulin in healthy human subjects. Diabetes 1998;47:1824–1835. [DOI] [PubMed] [Google Scholar]
- 34.Kim I-Y, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, Wolfe RR. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab. 2016;310:E73–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr. 2013;32:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfe R The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. [DOI] [PubMed] [Google Scholar]
- 37.Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;5:1–6. [DOI] [PubMed] [Google Scholar]
- 38.Rennie MJ. Claims for the anabolic effects of growth hormone: a case for the Emperor’s new clothes? B J Sports Med. 2003;37:100–105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarasheski KE, Zachwieja JJ. Growth hormone therapy for the elderly: the fountain of youth proves toxic. JAMA 1993;270:1694. [PubMed] [Google Scholar]
- 40.Brammert M, Segerlantz M, Laurila E, Daugaard JR, Manhem P, Groop L. Growth hormone replacement therapy induces insulin resistance by activating the glucose-fatty acid cycle. J Clin Endocrinol Metab. 2003;88:1455–1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.