Abstract
Recent large-scale genetic studies have allowed for the first glimpse of the effects of common genetic variability in dementia with Lewy bodies (DLB), identifying risk variants with appreciable effect sizes. However, it is currently well established that a substantial portion of the genetic heritable component of complex traits is not captured by genome-wide significant SNPs. To overcome this issue, we have estimated the proportion of phenotypic variance explained by genetic variability (SNP heritability) in DLB using a method that is unbiased by allele frequency or linkage disequilibrium properties of the underlying variants. This shows that the heritability of DLB is nearly twice as high as previous estimates based on common variants only (31% vs 59.9%). We also determine the amount of phenotypic variance in DLB that can be explained by recent polygenic risk scores from either Parkinson’s disease (PD) or Alzheimer’s disease (AD), and show that, despite being highly significant, they explain a low amount of variance. Additionally, to identify pleiotropic events that might improve our understanding of the disease, we performed genetic correlation analyses of DLB with over 200 diseases and biomedically relevant traits. Our data shows that DLB has a positive correlation with education phenotypes, which is opposite to what occurs in AD. Overall, our data suggests that novel genetic risk factors for DLB should be identified by larger GWAS and these are likely to be independent from known AD and PD risk variants.
Introduction
Recent studies have highlighted the role of genetics in the common, but often underappreciated, form of dementia that is dementia with Lewy bodies (DLB). Associations with GBA, APOE and SNCA have all been reproducibly reported by independent groups (Bras et al., 2014; Hardy et al., 1994; Nalls et al., 2013), and a recent genome-wide association study (GWAS) identified several risk and candidate variants associated with the disease (Guerreiro et al., 2018). However, GWAS significant single nucleotide polymorphisms (SNPs) often explain only a small proportion of the total heritability estimated (usually from family-based studies) for a given trait, which results in the ‘missing heritability’ issue (Manolio et al., 2009). One of the possible explanations for this issue is that, for complex diseases, SNPs with small effect sizes and well below genome-wide statistical significance account for most of the heritability of those traits (Boyle et al., 2017; Lee et al., 2011; Yang et al., 2013). However, given that each individual associated marker explains only a small proportion of the genetic variation with little predictive power, methods have been developed to test disorder prediction by summarizing variation across many loci (regardless of association p-values) into quantitative scores. One such approach is the generation of polygenic risk scores (PRSs). PRSs have been successfully applied to Parkinson’s (PD) (Escott-Price et al., 2015a) and Alzheimer’s diseases (AD) (Escott-Price et al., 2015b) and their usefulness will continue to increase as discovery datasets are augmented.
A separate, but related, concept is that of genetic correlation of traits. Here, what is estimated is the genetic covariance between traits that is tagged by common genome-wide SNPs (Lee et al., 2012). This allows us to identify pleiotropic effects between traits that might be unrelated by any other measurement. We have performed a preliminary study of genetic correlation between DLB and both PD and AD (Guerreiro et al., 2016), however performing similar analyses with other (even apparently unrelated) traits might provide novel insights for the underlying pathobiology of disease and perhaps for treatments across diseases.
The phenotypic variance of most complex human traits combines the genetic with the environmental variance (Mackay, 2001). While the effects of the environment are difficult to ascertain given their complexity and lack of adequate measurements, we are able to determine the genetic variance more accurately. Classically, genetic variance has been partitioned into sources of variation due to additive, dominance and epistatic effects. Additive genetic variance (h2SNP) relates to an allele’s independent effect on a phenotype; dominance variance (δ2SNP) refers to the effect on a phenotype caused by interactions between alternative alleles at a specific locus; epistatic variance refers to the interaction between different alleles in different loci. Most available cohorts for studies of human biology and disease are still underpowered to identify epistatic events, however, additive and dominance variance can be estimated from standard genome-wide genotyping data (Zhu et al., 2015).
Here, using data from the first GWAS in DLB that included haplotype reference consortium (HRC)-imputed genotypes (McCarthy et al., 2016), we have estimated the total heritability of this disease. We used a method (GCTA-LDMS) that is unbiased regardless of the minor allele frequency (MAF) and linkage disequilibrium (LD) properties of variants and thus greatly improves on previous estimates (Yang et al., 2015). Since it has been suggested that heritability estimates may be inflated by non-additive variation (Eichler et al., 2010), we have also estimated the dominance genetic variation in DLB. Additionally, to measure the proportion of variance explained by PRSs from PD and AD in a large DLB cohort, we measured the ability of PRS to discriminate case from control subjects. Lastly, to attempt to derive novel biological insights from unrelated traits, we have performed pairwise genetic correlation analysis of DLB with 235 phenotypes, including cognitive, anthropometric and education traits.
Materials and Methods
Sample description
The DLB dataset was previously published (Guerreiro et al., 2018) and is comprised of 1,216 cases and 3,791 controls matched for ancestry, imputed with HRC v1.1 and includes variants with minor allele frequency >= 0.001 and R2>=0.3, for a total number of 18.4 million variants (median R2=0.92). We used AD summary statistics from the International Genomics of Alzheimer’s Project (IGAP) (Lambert et al., 2013), which is a large two-stage study based upon genome-wide association studies (GWAS) on individuals of European ancestry. In stage 1, IGAP used genotyped and imputed data on 7,055,881 single nucleotide polymorphisms (SNPs) to meta-analyse four previously-published GWAS datasets consisting of 17,008 Alzheimer’s disease cases and 37,154 controls (the European Alzheimer’s disease Initiative – EADI the Alzheimer Disease Genetics Consortium – ADGC, the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium – CHARGE, the Genetic and Environmental Risk in AD consortium – GERAD). PD summary statistics were derived from the International Parkinson’s Disease Genomics Consortium (IPDGC) previously published data and included 13,708 cases and 95,282 controls (Nalls et al., 2014).
DLB heritability estimates
We used the GCTA-LDMS method to estimate heritability based on imputed data (Yang et al., 2015, 2011) using an imputation quality above 0.3 and a disease prevalence of 0.1%. This method considers the LD-bias that occurs in the SNP-based estimates and is unbiased regardless of the properties of the underlying variants. We calculated segment-based LD scores using a segment length of 200kb (with 100kb overlap between two adjacent segments), which were used to stratify the SNPs into quartiles. We then estimated the genetic relationship matrix (GRM) for each sample using the SNPs in each quartile separately and further stratified by minor allele frequency bins (0.001–0.01, 0.01–0.1, 0.1–0.2, 0.2–0.3, 0.3–0.4, 0.4–0.5). Lastly, we performed restricted maximum likelihood (REML) analysis using the multiple GRMs.
DLB dominance variance estimates
To estimate the dominance GRM between pairs of individuals, we used genome-wide imputed SNPs as implemented in GCTA-GREMLd (Zhu et al., 2015). This method calculates the additive and dominance GRMs and fits both GRMs in a mixed linear model to estimate additive and dominance variance simultaneously.
PRS analyses
Determining the polygenic risk of a given phenotype and applying it to another trait is an approach that allows to determine shared genetic aetiology between traits. We calculated PRSs on the base phenotypes (PD and AD), using GWAS summary statistics, and used these as predictors of the target phenotype (DLB) in a regression test. To construct and apply the PRSs we used PRSice v2.1 (Euesden et al., 2015). We performed clumping on the target data by retaining the SNP with the smallest p-value from each LD block (excluding SNPs with r2 > 0.1 in 250kb windows). Each allele was weighted by its effect-size as estimated in the respective study (for PD and AD). Association of PRSs with case-control status was performed with logistic regression, and Nagelkerke’s pseudo-R2 was calculated to measure the proportion of variance explained.
Genetic correlation analysis
To estimate the genetic correlation between DLB and other complex traits and diseases, we used a method based on LD score regression and implemented in the online web utility LDHub v1.9.0 (Bulik-Sullivan et al., 2015; Zheng et al., 2017). The LD score regression method uses summary statistics from the DLB GWAS and the other available traits, calculates the cross-product of test statistics at each SNP, and then regresses the cross-product on the LD score. After identifying the most significant correlations for DLB (p<0.01), we estimated the correlation of those traits with PD and AD.
Results
Quantifying the genetic heritability of DLB
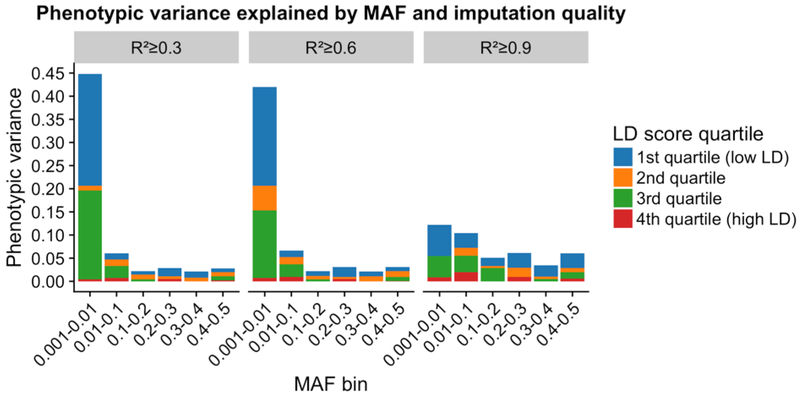
We applied the GREML-LDMS approach to estimate the proportion of phenotypic variance explained by the HRC-imputed variants for DLB. Results from this approach showed that imputed variants with R2 greater than or equal to 0.3 and frequency above 0.1% explained 59.9% (s.e.= 2.1%; p=6.8×10−6) of phenotypic variance for DLB. Lower frequency variants explained a large proportion of the phenotypic variance in DLB. This pattern was maintained for the higher quality imputed variants as well (Figure 1, Supplementary Table 1).
Figure 1:
Estimate of the DLB variance explained by HRC-imputed variants by MAF and LD. Segmental LD score increases from the 1st to 4th quartiles. Negative scores are not shown for simplicity but are present in Supplementary Table 1. The estimates of variance explained are from the GREML-LDMS analyses of fitting all the 24 genetic components simultaneously.
To determine if non-additive variance in DLB would explain a subset of the total disease heritability, we calculated the disease dominance variance as implemented in the tool GCTA-GREMLd. This method uses genome-wide data to estimate the additive and dominance genetic relationship matrices (GRMs) and fits both GRMs in a mixed linear model to estimate h2SNP and δ2SNP simultaneously. Our results suggest that DLB does not show significant dominance variance with an overall estimate δ2SNP=−0.05 (s.e. = 0.02).
Polygenic Prediction of Case-Control Status
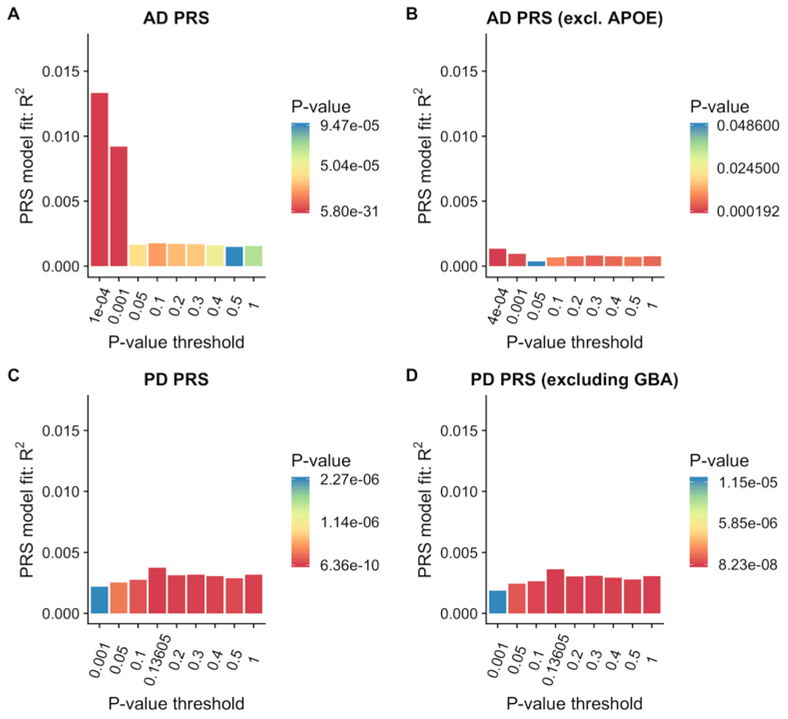
We applied the PRSs derived from AD and PD data to determine if these would discriminate between DLB and controls. The AD score explained 1.33% of the variance (Nagelkerke’s pseudo-R2) and was highly significant (p = 5.8×10−31). Performing the same analysis while excluding the APOE locus brought the estimate down to 0.14%, while reaching only nominal significance. Using the PD polygenic risk score, we obtained an estimate of 0.37% of the variance in DLB being explained by that score, a result that was also significant (p=6.4×10−10). Interestingly, removing the GBA locus resulted in only a small reduction in the variance explained by the PD PRS (0.36%; p=1.23×10−9) at the best p-value threshold.
The bar plots of DLB variance explained by the AD and PD polygenic risk scores are presented in Figure 2.
Figure 2:
Proportion of variance of DLB case-control status explained by PRSs from AD (A), AD excluding the APOE locus (B), PD (C) and PD excluding the GBA locus (D). The bars represent PRSs calculated for 9 subsets of markers at different p-value thresholds in the original GWAS publications. Best scores for each PRS are presented in (D). R2: Nagelkerke’s pseudo-R2; Threshold: P-value threshold in original GWAS.
Unbiased genetic correlation
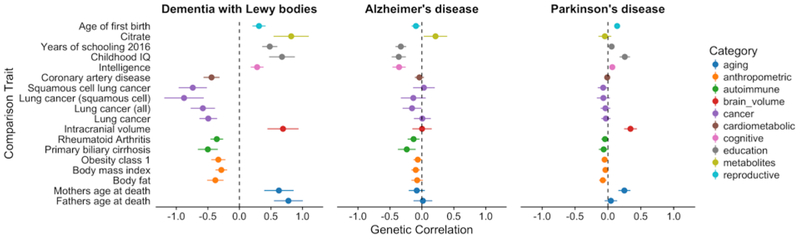
To test whether DLB has a shared genetic etiology with any of 235 other diseases or biomedical relevant traits, we used LD score regression as implemented in LDHub (http://ldsc.broadinstitute.org/ldhub/). This method estimates the degree to which genetic risk factors are shared between pairs of diseases or traits, although it should be noted that it does not inform regarding how this shared genetic etiology arises. We selected the correlations with a p-value <0.01 in DLB and tested these in AD and PD (Figure 4).
The most significant correlation identified, and the only surpassing Bonferroni correction, between DLB and each of the 235 tested traits was with “Years of schooling” (Okbay et al., 2016) reaching a p-value of 6.32×10−5 (Bonferroni corrected p-value=0.015) and a correlation estimate (rg) of 0.48 (s.e. = 0.12) (Table 2). Interestingly, these scores were found to be in the opposite direction in AD, but in the same direction in PD (AD: rg=−0.33, p-value=8.87×10−5; PD: rg=0.05, p-value=0.07) (Figure 3). A positive correlation was also obtained for “Childhood IQ” (Benyamin et al., 2014) in DLB and PD, whereas a negative correlation was identified in AD (DLB: 0.68, p-value=0.0009; AD: rg=−0.36, p-value=0.0011; PD: rg=0.25, p-value=0.0013). Similarly, “Intracranial volume” (Hibar et al., 2015) presented a positive correlation with both DLB and PD, but no discernible correlation with AD (DLB: 0.69, p-value=0.0052; AD: rg=−0.003, p-value=0.96; PD: rg=0.34, p-value=0.0005). Conversely, “Citrate” (Kettunen et al., 2016) was positively correlated with both DLB and AD, but had no correlation with PD (DLB: 0.82, p-value=0.0033; AD: rg=−0.21, p-value=0.25; PD: rg=−0.05, p-value=0.63).
Figure 3:
Correlation scores with p-value <0.01 in DLB. Shown are also the scores for those same traits in PD and AD.
Discussion
With this study we provide more accurate estimates of genetic heritability for DLB, quantify the variance explained by AD and PD polygenic risk and estimate pleiotropy between DLB and over 200 diseases and biomedical relevant traits.
Previous heritability estimates for DLB were calculated based on a smaller cohort genotyped at a relatively smaller number of sites and using GCTA’s GREML-SC (based on a single genetic relationship matrix). These earlier studies provided an estimate of 31% heritability for this disease (Guerreiro et al., 2016). It is now recognised that GREML-SC may, under certain circumstances (such as causal variants being enriched in regions with higher or lower LD than average or if the causal variants had a different MAF spectrum than the variants sampled), be biased (Yang et al., 2015). Because of this, we used a recently developed approach that corrects for the LD bias in the estimated SNP-based heritability and that is unbiased regardless of the properties (e.g. MAF and LD) of the underlying causal variants (GCTA GREML-LDMS) (Yang et al., 2015). We applied this tool to a larger cohort, that was imputed with the most recent imputation panel, providing more detailed genetic information. Using this approach we estimated that all HRC-imputed variants with MAF >0.001 explained 59.9% (s.e= 2.1%) of phenotypic variance for DLB, which is nearly double the previous estimate (Guerreiro et al., 2016). Our results also show that a large proportion of the variance is explained by variants with lower frequency (MAFs from 0.001 to 0.01). Given that the current version of HRC allows for imputation of variants with frequencies as low as 0.0005 and aggregate R2 above 0.5 (McCarthy et al., 2016), this indicates that performing GWAS in DLB with increased sample sizes will allow us to identify novel loci involved in conferring risk for disease without the need for large-scale whole-genome sequencing.
One of the explanations for the common issue of “missing heritability” is that non-additive heritability (such as dominance variance or epistatic variance) represents a substantial component of a trait’s total heritable genetic component. Our results suggest that dominance variance has a negligible effect on the genetic heritability of DLB, in line with findings from 79 unrelated traits (Zhu et al., 2015). However, we cannot exclude that epistatic variance plays a role in DLB, given that our cohort is underpowered to detect epistatic events.
Recently, there has been growing interest in the use of PRSs as a way to perform risk prediction in various diseases and these have successfully been applied to AD (Escott-Price et al., 2015b) and PD (Escott-Price et al., 2015a). To determine how much of the phenotypic variance in our DLB cohort can be caused by AD and PD known genetic risk factors, we used PRSs from recent GWAS from each of these diseases. In both cases scores explained only relatively small proportions of variance (0.37–1.33%). In AD, excluding the APOE locus greatly reduced the amount of variance explained in DLB (0.14%), which is in accordance with the strong effect that locus has in the risk of both diseases (Guerreiro et al., 2018; Lambert et al., 2013). Conversely, excluding the GBA locus in PD had only a modest effect, which likely results from the lower frequency in the general population of the variants that comprised this signal compared to APOE. Since the amount of variance explained by each of the PRS is relatively small, this adds to the growing body of evidence that suggests that, genetically, DLB is a unique condition and not simply a mix of PD and AD risk factors. These data also confirm the polygenic nature of DLB as well as quantify the amount of variance that polygenic risk from each of those diseases accounts for in DLB.
Given the large number of pleiotropic events that are being identified for a variety of diseases and traits (Guerreiro et al., 2014; Visscher et al., 2017), finding correlated conditions opens the door to a better understanding of disease pathobiology and perhaps may even suggest novel therapeutic targets. Assessing the genetic correlation of DLB with over 200 diseases and traits showed correlations that were in the same direction of those seen in PD while others were in the same direction as in AD. Due to the relatively small sample size in our cohort, the only correlation surpassing Bonferroni correction was for “Years of schooling”. It is interesting to note that these scores were positively correlated with DLB, while they have a well-established negative correlation with AD (Barnes and Yaffe, 2011; Norton et al., 2014). Similar positive correlations have been identified for bipolar disorder and autism spectrum disorders (Bulik-Sullivan et al., 2015), as well as for PD in the present data. Also in PD, there is evidence for the presence of increased intracranial volumes when compared to controls (Krabbe et al., 2005). Here, supporting those findings, we identify a positive genetic correlation between both PD and DLB (although not statistically significant) with intracranial volume, whereas in AD no evidence for genetic correlation was identified. Interestingly, the anthropometric characteristics obesity, body mass index (BMI) and body fat were negatively correlated with all 3 diseases. For BMI and PD, recent Mendelian randomization results have shown a negative effect (Noyce et al., 2017) which our results replicate and suggest they extend to both AD and DLB. A similar finding was obtained for cancer traits, where lung cancer showed a general negative correlation with the three traits. This agrees with transcriptomic studies that showed that the cancer gene expression profile is almost an opposite mirror image to that of neurodegenerative disease (Aramillo Irizar et al., 2018). A positive correlation between both DLB and AD with citrate (Kettunen et al., 2016) was identified, although this was not the case for PD, where no evidence of correlation was found. Increased plasma levels of citrate have been shown to be associated with increased levels of oxidative stress (Convertini et al., 2016), making it tempting to speculate that in AD and DLB oxidative stress may be involved in the neurodegenerative processes, while in PD it may be more akin to a consequence.
We note several limitations in our study. First, the DLB dataset, despite being the largest to date, is relatively small when compared to other recently published GWAS in PD or AD. This is reflected in a lower statistical power to identify novel associations; while the recent PD and AD GWAS were sufficiently powered to detect variants with effects as low as 1.4 and frequencies of only 1%, at this frequency, the DLB dataset is only sufficiently powered to detect variants with large effects of 3 and above. The lower statistical power is also reflected in the standard errors of the analyses performed in this work. We are underpowered to detect rare variants and certainly rare variants with small effect sizes. Second, we are unable to provide definitive biological mechanisms underlying the genetic correlations identified. This means that it is possible that for some of the correlations observed, what we are seeing are proxy effects and not direct correlations. Lastly, this study focused on individuals of European/North American descent. It is likely that studies of populations of different ancestries will reveal not only novel loci, but perhaps also novel pleiotropic effects, which could improve our understanding of the pathobiology of DLB.
Conclusion
In summary, we provide updated estimates of the genetic heritability of DLB and show that dominance variance is not a substantial part of the heritability of this disease. We quantify the amount of phenotypic variance in DLB that can be attributed to PD and AD polygenic risk scores and show that this is relatively small. Lastly, we estimate genetic correlations between DLB and over 200 diseases and medically relevant traits, shedding light into the complex relationship between DLB and both PD and AD.
Supplementary Material
Table 1.
Significant genetic correlations for DLB, PD and AD.
| DLB | AD | PD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TRAIT | Category | rg | s.e. | p-value | rg | s.e. | p-value | rg | s.e. | p-value |
| FATHERS AGE AT DEATH; PMID:27015805 | aging | 0.777 | 0.230 | 0.001 | ||||||
| MOTHERS AGE AT DEATH; PMID:27015805 | aging | 0.626 | 0.234 | 0.007 | 0.250 | 0.092 | 0.007 | |||
| BODY FAT; PMID:26833246 | anthropometric | −0.382 | 0.130 | 0.003 | ||||||
| BODY MASS INDEX; PMID:20935630 | anthropometric | −0.287 | 0.093 | 0.002 | ||||||
| HEIGHT; FEMALES AT AGE 10 AND MALES AT AGE 12; PMID:23449627 | anthropometric | −0.282 | 0.107 | 0.008 | ||||||
| OBESITY CLASS 1; PMID:23563607 | anthropometric | −0.334 | 0.113 | 0.003 | ||||||
| PRIMARY BILIARY CIRRHOSIS; PMID:26394269 | autoimmune | −0.501 | 0.159 | 0.002 | ||||||
| RHEUMATOID ARTHRITIS; PMID:24390342 | autoimmune | −0.358 | 0.101 | 0.000 | ||||||
| ICV; PMID:25607358 | brain_volume | 0.691 | 0.247 | 0.005 | 0.343 | 0.098 | 0.001 | |||
| MEAN ACCUMBENS; PMID:25607358 | brain_volume | 0.402 | 0.143 | 0.005 | ||||||
| MEAN CAUDATE; PMID:25607358 | brain_volume | 0.266 | 0.075 | 0.000 | ||||||
| MEAN PUTAMEN; PMID:25607358 | brain_volume | 0.251 | 0.082 | 0.002 | ||||||
| LUNG CANCER; PMID:27488534 | cancer | −0.493 | 0.141 | 0.001 | ||||||
| LUNG CANCER (ALL); PMID:24880342 | cancer | −0.579 | 0.193 | 0.003 | ||||||
| LUNG CANCER (SQUAMOUS CELL); PMID:24880342 | cancer | −0.878 | 0.313 | 0.005 | ||||||
| SQUAMOUS CELL LUNG CANCER; PMID:27488534 | cancer | −0.739 | 0.224 | 0.001 | ||||||
| CORONARY ARTERY DISEASE; PMID:26343387 | cardiometabolic | −0.442 | 0.128 | 0.001 | ||||||
| INTELLIGENCE; PMID:28530673 | cognitive | 0.281 | 0.102 | 0.006 | −0.357 | 0.104 | 0.001 | |||
| CHILDHOOD IQ; PMID:23358156 | education | 0.675 | 0.204 | 0.001 | −0.362 | 0.111 | 0.001 | 0.256 | 0.080 | 0.001 |
| COLLEGE COMPLETION; PMID:23722424 | education | −0.364 | 0.100 | 0.000 | ||||||
| YEARS OF SCHOOLING (PROXY COGNITIVE PERFORMANCE); PMID:25201988 | education | −0.300 | 0.081 | 0.000 | 0.138 | 0.048 | 0.004 | |||
| YEARS OF SCHOOLING 2013; PMID:23722424 | education | −0.290 | 0.092 | 0.002 | 0.137 | 0.048 | 0.005 | |||
| YEARS OF SCHOOLING 2016; PMID:27225129 | education | 0.481 | 0.120 | 0.000 | −0.330 | 0.084 | 0.000 | |||
| CITRATE; PMID:27005778 | metabolites | 0.821 | 0.280 | 0.003 | ||||||
| ISOLEUCINE; PMID:27005778 | metabolites | −0.547 | 0.208 | 0.009 | ||||||
| AGE OF FIRST BIRTH; PMID:27798627 | reproductive | 0.311 | 0.105 | 0.003 | 0.140 | 0.042 | 0.001 | |||
Genetic heritability of DLB is nearly 60%, double of previous estimates.
Polygenic risk scores from PD and AD explain a low amount of variance in DLB.
DLB has a positive correlation with education phenotypes, which is contrary to AD.
Acknowledgements
Jose Bras and Rita Guerreiro’s work is funded by research fellowships from the Alzheimer’s Society. Tatiana Orme is supported by a scholarship from the Lewy Body Society. This work was supported in part by the National Institutes of Neurological Disease and Stroke. For the neuropathologically confirmed samples from Australia, tissues were received from the Sydney Brain Bank, which is supported by Neuroscience Research Australia and the University of New South Wales, and Dr Halliday is funded by an NHMRC senior principal research fellowship. We would like to thank the South West Dementia Brain Bank (SWDBB) for providing brain tissue for this study. The SWDBB is supported by BRACE (Bristol Research into Alzheimer’s and Care of the Elderly), Brains for Dementia Research and the Medical Research Council. We acknowledge the Oxford Brain Bank, supported by the Medical Research Council (MRC), Brains for Dementia Research (BDR) (Alzheimer Society and Alzheimer Research UK), Autistica UK and the NIHR Oxford Biomedical Research Centre. The brain samples and/or bio samples were obtained from The Netherlands Brain Bank, Netherlands Institute for Neuroscience, Amsterdam (open access: www.brainbank.nl). All Material has been collected from donors for or from whom a written informed consent for a brain autopsy and the use of the material and clinical information for research purposes had been obtained by the NBB. This study was also partially funded by the Wellcome Trust, Medical Research Council (Dr. St. George-Hyslop) and the Canadian Consortium on Neurodegeneration in Aging (E. Rogaeva). Work from Dr. Compta was supported by the CERCA Programme / Generalitat de Catalunya, Barcelona, Catalonia, Spain. The Nottingham Genetics Group is supported by ARUK and The Big Lottery Fund. The effort from Columbia University was supported by the Taub Institute, the Panasci Fund, the Parkinson’s Disease Foundation, and NIH grants NS060113 (Dr Clark), P50AG008702 (P.I. Scott Small), P50NS038370 (P.I.R. Burke), and UL1TR000040 (P.I.H. Ginsberg). Dr Ross is supported by the Michael J. Fox Foundation for Parkinson’s Research, NINDS R01# NS078086. The Mayo Clinic Jacksonville is a Morris K. Udall Parkinson’s Disease Research Center of Excellence (NINDS P50 #NS072187) and is supported by The Little Family Foundation and by the Mangurian Foundation Program for Lewy Body Dementia research and the Alzheimer Disease Research Center (P50 AG016547). The work from the Mayo Clinic Rochester is supported by the National Institute on Aging (P50 AG016574 and U01 AG006786). This work has received support from The Queen Square Brain Bank at the UCL Institute of Neurology; where Dr Lashley is funded by an ARUK senior fellowship. Some of the tissue samples studied were provided by the MRC London Neurodegenerative Diseases Brain Bank and the Brains for Dementia Research project (funded by Alzheimer’s Society and ARUK). This research was supported in part by both the NIHR UCLH Biomedical Research Centre and the Queen Square Dementia Biomedical Research Unit. This work was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project AG000951–12. The University of Pennsylvania case collection is funded by the Penn Alzheimer’s Disease Core Center (AG10124) and the Penn Morris K. Udall Parkinson’s Disease Research Center (NS053488). Tissue samples from UCSD are supported by NIH grant AG05131. The authors thank the brain bank GIE NeuroCEB, the French program “Investissements d’avenir” (ANR-10-IAIHU-06). Dr Tienari and Dr Myllykangas are supported by the Helsinki University Central Hospital, the Folkhälsan Research Foundation and the Finnish Academy. This work was in part supported by the Canadian Consortium on Neurodegeneration in Aging (ER). The authors acknowledge the contribution of data from Genetic Architecture of Smoking and Smoking Cessation accessed through dbGAP. Funding support for genotyping, which was performed at the Center for Inherited Disease Research (CIDR), was provided by 1 X01 HG005274–01. CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. Assistance with genotype cleaning, as well as with general study coordination, was provided by the Gene Environment Association Studies (GENEVA) Coordinating Center (U01 HG004446). Funding support for collection of datasets and samples was provided by the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392) and the University of Wisconsin Transdisciplinary Tobacco Use Research Center (P50 DA019706, P50 CA084724). The data used for the analyses described in this paper were obtained from the database of Genotypes and Phenotypes (dbGaP), at http://www.ncbi.nlm.nih.gov/gap. Genotype and phenotype data for the Genetic Analysis of Psoriasis and Psoriatic Arthritis study were provided by Dr. James T. Elder, University of Michigan, with collaborators Dr. Dafna Gladman, University of Toronto and Dr. Proton Rahman, Memorial University of Newfoundland, providing samples. This work was supported in part by the Intramural Research Program of the National Institutes of Health (National Institute of Neurological Disorders and Stroke; project ZIA NS003154). Tissue samples for genotyping were provided by the Johns Hopkins Morris K. Udall Center of Excellence for Parkinson’s Disease Research (NIH P50 NS38377) and the Johns Hopkins Alzheimer Disease Research Center (NIH P50 AG05146). This study was supported by grants from the National Institutes of Health, the Canadian Institute for Health Research, and the Krembil Foundation. Additional support was provided by the Babcock Memorial Trust and by the Barbara and Neal Henschel Charitable Foundation. JTE is supported by the Ann Arbor Veterans Affairs Hospital.
IGAP: We thank the International Genomics of Alzheimer’s Project (IGAP) for providing summary results data for these analyses. The investigators within IGAP contributed to the design and implementation of IGAP and/or provided data but did not participate in analysis or writing of this report. IGAP was made possible by the generous participation of the control subjects, the patients, and their families. The i–Select chips was funded by the French National Foundation on Alzheimer’s disease and related disorders. EADI was supported by the LABEX (laboratory of excellence program investment for the future) DISTALZ grant, Inserm, Institut Pasteur de Lille, Université de Lille 2 and the Lille University Hospital. GERAD was supported by the Medical Research Council (Grant n° 503480), Alzheimer’s Research UK (Grant n° 503176), the Wellcome Trust (Grant n° 082604/2/07/Z) and German Federal Ministry of Education and Research (BMBF): Competence Network Dementia (CND) grant n° 01GI0102, 01GI0711, 01GI0420. CHARGE was partly supported by the NIH/NIA grant R01 AG033193 and the NIA AG081220 and AGES contract N01–AG–12100, the NHLBI grant R01 HL105756, the Icelandic Heart Association, and the Erasmus Medical Center and Erasmus University. ADGC was supported by the NIH/NIA grants: U01 AG032984, U24 AG021886, U01 AG016976, and the Alzheimer’s Association grant ADGC–10–196728.
IPDGC: We would like to thank all of the subjects who donated their time and biological samples to be a part of this study. This work was supported in part by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Aging (NIA), and the National Institute of Environmental Health Sciences both part of the National Institutes of Health, Department of Health and Human Services; project numbers 1ZIANS003154, Z01-AG000949–02 and Z01-ES101986. In addition this work was supported by the Department of Defense (award W81XWH-09-2-0128), and The Michael J Fox Foundation for Parkinson’s Research. This work was supported by National Institutes of Health grants R01NS037167, R01CA141668, P50NS071674, American Parkinson Disease Association (APDA); Barnes Jewish Hospital Foundation; Greater St Louis Chapter of the APDA; Hersenstichting Nederland; the Prinses Beatrix Fonds. The KORA (Cooperative Research in the Region of Augsburg) research platform was started and financed by the Forschungszentrum für Umwelt und Gesundheit, which is funded by the German Federal Ministry of Education, Science, Research, and Technology and by the State of Bavaria. This study was also funded by the National Genome Research Network (NGFNplus number 01GS08134, German Federal Ministry for Education and Research), the German Federal Ministry of Education and Research (NGFN 01GR0468, PopGen) and 01EW0908 in the frame of ERA-NET NEURON. In addition, this work was supported by the EU Joint Programme - Neurodegenerative Diseases Research (JPND) project under the aegis of JPND (www.jpnd.eu) through Germany, BMBF, funding code 01ED1406 and iMed - the Helmholtz Initiative on Personalized Medicine. The French GWAS work was supported by the French National Agency of Research (ANR-08-MNP-012). This study was also funded by France-Parkinson Association, the French program “Investissements d’avenir” funding (ANR-10-IAIHU-06) and a grant from Assistance Publique-Hôpitaux de Paris (PHRC, AOR-08010) for the French clinical data. This study was also sponsored by the Landspitali University Hospital Research Fund (grant to SSv); Icelandic Research Council (grant to SSv); and European Community Framework Programme 7, People Programme, and IAPP on novel genetic and phenotypic markers of Parkinson’s disease and Essential Tremor (MarkMD), contract number PIAP-GA-2008–230596 MarkMD (to HP and JHu). The McGill study was funded by the Michael J. Fox Foundation and the Canadian Consortium on Neurodegeneration in Aging (CCNA). This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, Md. (http://biowulf.nih.gov), and DNA panels, samples, and clinical data from the National Institute of Neurological Disorders and Stroke Human Genetics Resource Center DNA and Cell Line Repository. People who contributed samples are acknowledged in descriptions of every panel on the repository website. We thank the French Parkinson’s Disease Genetics Study Group and the Drug Interaction with genes (DIGPD) study group: Y Agid, M Anheim, A-M Bonnet, M Borg, A Brice, E Broussolle, JC Corvol, P Damier, A Destée, A Dürr, F Durif, A Elbaz, D Grabil, S Klebe, P. Krack, E Lohmann, L. Lacomblez, M Martinez, V Mesnage, P Pollak, O Rascol, F Tison, C Tranchant, M Vérin, F Viallet, and M Vidailhet. We also thank the members of the French 3C Consortium: A Alpérovitch, C Berr, C Tzourio, and P Amouyel for allowing us to use part of the 3C cohort, and D Zelenika for support in generating the genome-wide molecular data. We thank P Tienari (Molecular Neurology Programme, Biomedicum, University of Helsinki), T Peuralinna (Department of Neurology, Helsinki University Central Hospital), L Myllykangas (Folkhalsan Institute of Genetics and Department of Pathology, University of Helsinki), and R Sulkava (Department of Public Health and General Practice Division of Geriatrics, University of Eastern Finland) for the Finnish controls (Vantaa85+ GWAS data). We used genome-wide association data generated by the Wellcome Trust Case-Control Consortium 2 (WTCCC2) from UK patients with Parkinson’s disease and UK control individuals from the 1958 Birth Cohort and National Blood Service. Genotyping of UK replication cases on ImmunoChip was part of the WTCCC2 project, which was funded by the Wellcome Trust (083948/Z/07/Z). UK population control data was made available through WTCCC1. This study was supported by the Medical Research Council and Wellcome Trust disease centre (grant WT089698/Z/09/Z to NW, JHa, and ASc). As with previous IPDGC efforts, this study makes use of data generated by the Wellcome Trust Case-Control Consortium. A full list of the investigators who contributed to the generation of the data is available from www.wtccc.org.uk. Funding for the project was provided by the Wellcome Trust under award 076113, 085475 and 090355. This study was also supported by Parkinson’s UK (grants 8047 and J-0804) and the Medical Research Council (G0700943 and G1100643). Sequencing and genotyping done in McGill University was supported by grants from the Michael J Fox Foundation and the Canadian Consortium on Neurodegeneration in Aging (CCNA). We thank Jeffrey Barrett and Jason Downing for assistance with the design of the ImmunoChip and NeuroX arrays. DNA extraction work that was done in the UK was undertaken at University College London Hospitals, University College London, who received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding. This study was supported in part by the Wellcome Trust/Medical Research Council Joint Call in Neurodegeneration award (WT089698) to the Parkinson’s Disease Consortium (UKPDC), whose members are from the UCL Institute of Neurology, University of Sheffield, and the Medical Research Council Protein Phosphorylation Unit at the University of Dundee. We thank the Quebec Parkinson’s Network (http://rpq-qpn.org) and its members. Mike A. Nalls’ participation is supported by a consulting contract between Data Tecnica International and the National Institute on Aging, NIH, Bethesda, MD, USA, as a possible conflict of interest Dr. Nalls also consults for Illumina Inc, the Michael J. Fox Foundation, University of California Healthcare and Genoom Health among others. This work was supported by the Medical Research Council grant MR/N026004/1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr Shepherd and Halliday from Australia report no conflict of interests for this work.
References
- Aramillo Irizar P, Schäuble S, Esser D, Groth M, Frahm C, Priebe S, Baumgart M, Hartmann N, Marthandan S, Menzel U, Müller J, Schmidt S, Ast V, Caliebe A, König R, Krawczak M, Ristow M, Schuster S, Cellerino A, Diekmann S, Englert C, Hemmerich P, Sühnel J, Guthke R, Witte OW, Platzer M, Ruppin E, Kaleta C, 2018. Transcriptomic alterations during ageing reflect the shift from cancer to degenerative diseases in the elderly. Nat. Commun 9, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, 2011. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamin B, Pourcain B, Davis OS, Davies G, Hansell NK, Brion M-JA, Kirkpatrick RM, Cents RAM, Franic S, Miller MB, Haworth CMA, Meaburn E, Price TS, Evans DM, Timpson N, Kemp J, Ring S, McArdle W, Medland SE, Yang J, Harris SE, Liewald DC, Scheet P, Xiao X, Hudziak JJ, de Geus EJC, Wellcome Trust Case Control Consortium 2 (WTCCC2), Jaddoe VWV, Starr JM, Verhulst FC, Pennell C, Tiemeier H, Iacono WG, Palmer LJ, Montgomery GW, Martin NG, Boomsma DI, Posthuma D, McGue M, Wright MJ, Davey Smith G, Deary IJ, Plomin R, Visscher PM, 2014. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol. Psychiatry 19, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EA, Li YI, Pritchard JK, 2017. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell 169, 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, Hernandez DG, Nalls MA, Clark LN, Honig LS, Marder K, Van Der Flier WM, Lemstra A, Scheltens P, Rogaeva E, St George-Hyslop P, Londos E, Zetterberg H, Ortega Cubero S, Pastor P, Ferman TJ, Graff-Radford NR, Ross OA, Barber I, Braae A, Brown K, Morgan K, Maetzler W, Berg D, Troakes C, Al-Sarraj S, Lashley T, Compta Y, Revesz T, Lees A, Cairns N, Halliday GM, Mann D, Pickering-Brown S, Dickson DW, Singleton A, Hardy J, 2014. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum. Mol. Genet 23, 6139–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, ReproGen Consortium, Psychiatric Genomics Consortium, Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3, Duncan L, Perry JRB, Patterson N, Robinson EB, Daly MJ, Price AL, Neale BM, 2015. An atlas of genetic correlations across human diseases and traits. Nat. Genet 47, 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertini P, Menga A, Andria G, Scala I, Santarsiero A, Castiglione Morelli MA, Iacobazzi V, Infantino V, 2016. The contribution of the citrate pathway to oxidative stress in Down syndrome. Immunology 149, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH, 2010. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet 11, 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escott-Price V, International Parkinson’s Disease Genomics Consortium, Nalls MA, Morris HR, Lubbe S, Brice A, Gasser T, Heutink P, Wood NW, Hardy J, Singleton AB, Williams NM, IPDGC consortium members, 2015a. Polygenic risk of Parkinson disease is correlated with disease age at onset. Ann. Neurol 77, 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, Badarinarayan N, GERAD/PERADES, IGAP consortia, Morgan K, Passmore P, Holmes C, Powell J, Brayne C, Gill M, Mead S, Goate A, Cruchaga C, Lambert J-C, van Duijn C, Maier W, Ramirez A, Holmans P, Jones L, Hardy J, Seshadri S, Schellenberg GD, Amouyel P, Williams J, 2015b. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain 138, 3673–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euesden J, Lewis CM, O’Reilly PF, 2015. PRSice: Polygenic Risk Score software. Bioinformatics 31, 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Brás J, Hardy J, Singleton A, 2014. Next generation sequencing techniques in neurological diseases: redefining clinical and molecular associations. Hum. Mol. Genet 23, R47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Escott-Price V, Darwent L, Parkkinen L, Ansorge O, Hernandez DG, Nalls MA, Clark L, Honig L, Marder K, van der Flier W, Holstege H, Louwersheimer E, Lemstra A, Scheltens P, Rogaeva E, St George-Hyslop P, Londos E, Zetterberg H, Ortega-Cubero S, Pastor P, Ferman TJ, Graff-Radford NR, Ross OA, Barber I, Braae A, Brown K, Morgan K, Maetzler W, Berg D, Troakes C, Al-Sarraj S, Lashley T, Compta Y, Revesz T, Lees A, Cairns NJ, Halliday GM, Mann D, Pickering-Brown S, Powell J, Lunnon K, Lupton MK, International Parkinson’s Disease Genomics Consortium, Dickson D, Hardy J, Singleton A, Bras J, 2016. Genome-wide analysis of genetic correlation in dementia with Lewy bodies, Parkinson’s and Alzheimer’s diseases. Neurobiol. Aging 38, 214.e7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, Shepherd CE, Parkkinen L, Darwent L, Heckman MG, Scholz SW, Troncoso JC, Pletnikova O, Ansorge O, Clarimon J, Lleo A, Morenas-Rodriguez E, Clark L, Honig LS, Marder K, Lemstra A, Rogaeva E, St George-Hyslop P, Londos E, Zetterberg H, Barber I, Braae A, Brown K, Morgan K, Troakes C, Al-Sarraj S, Lashley T, Holton J, Compta Y, Van Deerlin V, Serrano GE, Beach TG, Lesage S, Galasko D, Masliah E, Santana I, Pastor P, Diez-Fairen M, Aguilar M, Tienari PJ, Myllykangas L, Oinas M, Revesz T, Lees A, Boeve BF, Petersen RC, Ferman TJ, Escott-Price V, Graff-Radford N, Cairns NJ, Morris JC, Pickering-Brown S, Mann D, Halliday GM, Hardy J, Trojanowski JQ, Dickson DW, Singleton A, Stone DJ, Bras J, 2018. Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol. 17, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Crook R, Prihar G, Roberts G, Raghavan R, Perry R, 1994. Senile dementia of the Lewy body type has an apolipoprotein E epsilon 4 allele frequency intermediate between controls and Alzheimer’s disease. Neurosci. Lett 182, 1–2. [DOI] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, Toro R, Wittfeld K, Abramovic L, Andersson M, Aribisala BS, Armstrong NJ, Bernard M, Bohlken MM, Boks MP, Bralten J, Brown AA, Chakravarty MM, Chen Q, Ching CRK, Cuellar-Partida G, den Braber A, Giddaluru S, Goldman AL, Grimm O, Guadalupe T, Hass J, Woldehawariat G, Holmes AJ, Hoogman M, Janowitz D, Jia T, Kim S, Klein M, Kraemer B, Lee PH, Olde Loohuis LM, Luciano M, Macare C, Mather KA, Mattheisen M, Milaneschi Y, Nho K, Papmeyer M, Ramasamy A, Risacher SL, Roiz-Santiañez R, Rose EJ, Salami A, Sämann PG, Schmaal L, Schork AJ, Shin J, Strike LT, Teumer A, van Donkelaar MMJ, van Eijk KR, Walters RK, Westlye LT, Whelan CD, Winkler AM, Zwiers MP, Alhusaini S, Athanasiu L, Ehrlich S, Hakobjan MMH, Hartberg CB, Haukvik UK, Heister AJGAM, Hoehn D, Kasperaviciute D, Liewald DCM, Lopez LM, Makkinje RRR, Matarin M, Naber MAM, McKay DR, Needham M, Nugent AC, Pütz B, Royle NA, Shen L, Sprooten E, Trabzuni D, van der Marel SSL, van Hulzen KJE, Walton E, Wolf C, Almasy L, Ames D, Arepalli S, Assareh AA, Bastin ME, Brodaty H, Bulayeva KB, Carless MA, Cichon S, Corvin A, Curran JE, Czisch M, de Zubicaray GI, Dillman A, Duggirala R, Dyer TD, Erk S, Fedko IO, Ferrucci L, Foroud TM, Fox PT, Fukunaga M, Gibbs JR, Göring HHH, Green RC, Guelfi S, Hansell NK, Hartman CA, Hegenscheid K, Heinz A, Hernandez DG, Heslenfeld DJ, Hoekstra PJ, Holsboer F, Homuth G, Hottenga J-J, Ikeda M, Jack CR Jr, Jenkinson M, Johnson R, Kanai R, Keil M, Kent JW Jr, Kochunov P, Kwok JB, Lawrie SM, Liu X, Longo DL, McMahon KL, Meisenzahl E, Melle I, Mohnke S, Montgomery GW, Mostert JC, Mühleisen TW, Nalls MA, Nichols TE, Nilsson LG, Nöthen MM, Ohi K, Olvera RL, Perez-Iglesias R, Pike GB, Potkin SG, Reinvang I, Reppermund S, Rietschel M, Romanczuk-Seiferth N, Rosen GD, Rujescu D, Schnell K, Schofield PR, Smith C, Steen VM, Sussmann JE, Thalamuthu A, Toga AW, Traynor BJ, Troncoso J, Turner JA, Valdés Hernández MC, van ’t Ent D, van der Brug M, van der Wee NJA, van Tol M-J, Veltman DJ, Wassink TH, Westman E, Zielke RH, Zonderman AB, Ashbrook DG, Hager R, Lu L, McMahon FJ, Morris DW, Williams RW, Brunner HG, Buckner RL, Buitelaar JK, Cahn W, Calhoun VD, Cavalleri GL, Crespo-Facorro B, Dale AM, Davies GE, Delanty N, Depondt C, Djurovic S, Drevets WC, Espeseth T, Gollub RL, Ho B-C, Hoffmann W, Hosten N, Kahn RS, Le Hellard S, Meyer-Lindenberg A, Müller-Myhsok B, Nauck M, Nyberg L, Pandolfo M, Penninx BWJH, Roffman JL, Sisodiya SM, Smoller JW, van Bokhoven H, van Haren NEM, Völzke H, Walter H, Weiner MW, Wen W, White T, Agartz I, Andreassen OA, Blangero J, Boomsma DI, Brouwer RM, Cannon DM, Cookson MR, de Geus EJC, Deary IJ, Donohoe G, Fernández G, Fisher SE, Francks C, Glahn DC, Grabe HJ, Gruber O, Hardy J, Hashimoto R, Hulshoff Pol HE, Jönsson EG, Kloszewska I, Lovestone S, Mattay VS, Mecocci P, McDonald C, McIntosh AM, Ophoff RA, Paus T, Pausova Z, Ryten M, Sachdev PS, Saykin AJ, Simmons A, Singleton A, Soininen H, Wardlaw JM, Weale ME, Weinberger DR, Adams HHH, Launer LJ, Seiler S, Schmidt R, Chauhan G, Satizabal CL, Becker JT, Yanek L, van der Lee SJ, Ebling M, Fischl B, Longstreth WT Jr, Greve D, Schmidt H, Nyquist P, Vinke LN, van Duijn CM, Xue L, Mazoyer B, Bis JC, Gudnason V, Seshadri S, Ikram MA , Alzheimer’s Disease Neuroimaging Initiative, CHARGE Consortium, EPIGEN, IMAGEN, SYS, Martin NG, Wright MJ, Schumann G, Franke B, Thompson PM, Medland SE, 2015. Common genetic variants influence human subcortical brain structures. Nature 520, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen J, Demirkan A, Würtz P, Draisma HHM, Haller T, Rawal R, Vaarhorst A, Kangas AJ, Lyytikäinen L-P, Pirinen M, Pool R, Sarin A-P, Soininen P, Tukiainen T, Wang Q, Tiainen M, Tynkkynen T, Amin N, Zeller T, Beekman M, Deelen J, van Dijk KW, Esko T, Hottenga J-J, van Leeuwen EM, Lehtimäki T, Mihailov E, Rose RJ, de Craen AJM, Gieger C, Kähönen M, Perola M, Blankenberg S, Savolainen MJ, Verhoeven A, Viikari J, Willemsen G, Boomsma DI, van Duijn CM, Eriksson J, Jula A, Järvelin M-R, Kaprio J, Metspalu A, Raitakari O, Salomaa V, Slagboom PE, Waldenberger M, Ripatti S, Ala-Korpela M, 2016. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun 7, 11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe K, Karlsborg M, Hansen A, Werdelin L, Mehlsen J, Larsson HBW, Paulson OB, 2005. Increased intracranial volume in Parkinson’s disease. J. Neurol. Sci 239, 45–52. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, Lin CF, Gerrish A, Schmidt H, Kunkle B, Dunstan ML, Ruiz A, Bihoreau MT, Choi SH, Reitz C, Pasquier F, Cruchaga C, Craig D, Amin N, Berr C, Lopez OL, De Jager PL, Deramecourt V, Johnston JA, Evans D, Lovestone S, Letenneur L, Morón FJ, Rubinsztein DC, Eiriksdottir G, Sleegers K, Goate AM, Fiévet N, Huentelman MW, Gill M, Brown K, Kamboh MI, Keller L, Barberger-Gateau P, McGuiness B, Larson EB, Green R, Myers AJ, Dufouil C, Todd S, Wallon D, Love S, Rogaeva E, Gallacher J, St George-Hyslop P, Clarimon J, Lleo A, Bayer A, Tsuang DW, Yu L, Tsolaki M, Bossù P, Spalletta G, Proitsi P, Collinge J, Sorbi S, Sanchez-Garcia F, Fox NC, Hardy J, Deniz Naranjo MC, Bosco P, Clarke R, Brayne C, Galimberti D, Mancuso M, Matthews F, European Alzheimer’s Disease Initiative (EADI), Genetic and Environmental Risk in Alzheimer’s Disease, Alzheimer’s Disease Genetic Consortium, Cohorts for Heart and Aging Research in Genomic Epidemiology, Moebus S, Mecocci P, Del Zompo M, Maier W, Hampel H, Pilotto A, Bullido M, Panza F, Caffarra P, Nacmias B, Gilbert JR, Mayhaus M, Lannefelt L, Hakonarson H, Pichler S, Carrasquillo MM, Ingelsson M, Beekly D, Alvarez V, Zou F, Valladares O, Younkin SG, Coto E, Hamilton-Nelson KL, Gu W, Razquin C, Pastor P, Mateo I, Owen MJ, Faber KM, Jonsson PV, Combarros O, O’Donovan MC, Cantwell LB, Soininen H, Blacker D, Mead S, Mosley TH Jr, Bennett DA, Harris TB, Fratiglioni L, Holmes C, de Bruijn RF, Passmore P, Montine TJ, Bettens K, Rotter JI, Brice A, Morgan K, Foroud TM, Kukull WA, Hannequin D, Powell JF, Nalls MA, Ritchie K, Lunetta KL, Kauwe JS, Boerwinkle E, Riemenschneider M, Boada M, Hiltuenen M, Martin ER, Schmidt R, Rujescu D, Wang LS, Dartigues JF, Mayeux R, Tzourio C, Hofman A, Nöthen MM, Graff C, Psaty BM, Jones L, Haines JL, Holmans PA, Lathrop M, Pericak-Vance MA, Launer LJ, Farrer LA, van Duijn CM, Van Broeckhoven C, Moskvina V, Seshadri S, Williams J, Schellenberg GD, Amouyel P, 2013. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet 45, 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Wray NR, Goddard ME, Visscher PM, 2011. Estimating missing heritability for disease from genome-wide association studies. Am. J. Hum. Genet 88, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yang J, Goddard ME, Visscher PM, Wray NR, 2012. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics 28, 2540–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TF, 2001. The genetic architecture of quantitative traits. Annu. Rev. Genet 35, 303–339. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TFC, McCarroll SA, Visscher PM, 2009. Finding the missing heritability of complex diseases. Nature 461, 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, Luo Y, Sidore C, Kwong A, Timpson N, Koskinen S, Vrieze S, Scott LJ, Zhang H, Mahajan A, Veldink J, Peters U, Pato C, van Duijn CM, Gillies CE, Gandin I, Mezzavilla M, Gilly A, Cocca M, Traglia M, Angius A, Barrett JC, Boomsma D, Branham K, Breen G, Brummett CM, Busonero F, Campbell H, Chan A, Chen S, Chew E, Collins FS, Corbin LJ, Smith GD, Dedoussis G, Dorr M, Farmaki A-E, Ferrucci L, Forer L, Fraser RM, Gabriel S, Levy S, Groop L, Harrison T, Hattersley A, Holmen OL, Hveem K, Kretzler M, Lee JC, McGue M, Meitinger T, Melzer D, Min JL, Mohlke KL, Vincent JB, Nauck M, Nickerson D, Palotie A, Pato M, Pirastu N, McInnis M, Richards JB, Sala C, Salomaa V, Schlessinger D, Schoenherr S, Slagboom PE, Small K, Spector T, Stambolian D, Tuke M, Tuomilehto J, Van den Berg LH, Van Rheenen W, Volker U, Wijmenga C, Toniolo D, Zeggini E, Gasparini P, Sampson MG, Wilson JF, Frayling T, de Bakker PIW, Swertz MA, McCarroll S, Kooperberg C, Dekker A, Altshuler D, Willer C, Iacono W, Ripatti S, Soranzo N, Walter K, Swaroop A, Cucca F, Anderson CA, Myers RM, Boehnke M, McCarthy MI, Durbin R, Haplotype Reference Consortium, 2016. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet 48, 1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, Morris CM, Theuns J, Crosiers D, Cras P, Engelborghs S, De Deyn PP, Van Broeckhoven C, Mann DMA, Snowden J, Pickering-Brown S, Halliwell N, Davidson Y, Gibbons L, Harris J, Sheerin U-M, Bras J, Hardy J, Clark L, Marder K, Honig LS, Berg D, Maetzler W, Brockmann K, Gasser T, Novellino F, Quattrone A, Annesi G, De Marco EV, Rogaeva E, Masellis M, Black SE, Bilbao JM, Foroud T, Ghetti B, Nichols WC, Pankratz N, Halliday G, Lesage S, Klebe S, Durr A, Duyckaerts C, Brice A, Giasson BI, Trojanowski JQ, Hurtig HI, Tayebi N, Landazabal C, Knight MA, Keller M, Singleton AB, Wolfsberg TG, Sidransky E, 2013. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 70, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson’s Disease Genomics Consortium (IPDGC), Parkinson’s Study Group (PSG) Parkinson’s Research: The Organized GENetics Initiative (PROGENI), 23andMe, GenePD, NeuroGenetics Research Consortium (NGRC), Hussman Institute of Human Genomics (HIHG), Ashkenazi Jewish Dataset Investigator, Cohorts for Health and Aging Research in Genetic Epidemiology (CHARGE), North American Brain Expression Consortium (NABEC), United Kingdom Brain Expression Consortium (UKBEC), Greek Parkinson’s Disease Consortium, Alzheimer Genetic Analysis Group, Ikram MA, Ioannidis JPA, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB, 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C, 2014. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 13, 788–794. [DOI] [PubMed] [Google Scholar]
- Noyce AJ, Kia DA, Hemani G, Nicolas A, Price TR, De Pablo-Fernandez E, Haycock PC, Lewis PA, Foltynie T, Davey Smith G, International Parkinson Disease Genomics Consortium, Schrag A, Lees AJ, Hardy J, Singleton A, Nalls MA, Pearce N, Lawlor DA, Wood NW, 2017. Estimating the causal influence of body mass index on risk of Parkinson disease: A Mendelian randomisation study. PLoS Med. 14, e1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, Turley P, Chen G-B, Emilsson V, Meddens SFW, Oskarsson S, Pickrell JK, Thom K, Timshel P, de Vlaming R, Abdellaoui A, Ahluwalia TS, Bacelis J, Baumbach C, Bjornsdottir G, Brandsma JH, Pina Concas M, Derringer J, Furlotte NA, Galesloot TE, Girotto G, Gupta R, Hall LM, Harris SE, Hofer E, Horikoshi M, Huffman JE, Kaasik K, Kalafati IP, Karlsson R, Kong A, Lahti J, van der Lee SJ, deLeeuw C, Lind PA, Lindgren K-O, Liu T, Mangino M, Marten J, Mihailov E, Miller MB, van der Most PJ, Oldmeadow C, Payton A, Pervjakova N, Peyrot WJ, Qian Y, Raitakari O, Rueedi R, Salvi E, Schmidt B, Schraut KE, Shi J, Smith AV, Poot RA, St Pourcain B, Teumer A, Thorleifsson G, Verweij N, Vuckovic D, Wellmann J, Westra H-J, Yang J, Zhao W, Zhu Z, Alizadeh BZ, Amin N, Bakshi A, Baumeister SE, Biino G, Bønnelykke K, Boyle PA, Campbell H, Cappuccio FP, Davies G, De Neve J-E, Deloukas P, Demuth I, Ding J, Eibich P, Eisele L, Eklund N, Evans DM, Faul JD, Feitosa MF, Forstner AJ, Gandin I, Gunnarsson B, Halldórsson BV, Harris TB, Heath AC, Hocking LJ, Holliday EG, Homuth G, Horan MA, Hottenga J-J, de Jager PL, Joshi PK, Jugessur A, Kaakinen MA, Kähönen M, Kanoni S, Keltigangas-Järvinen L, Kiemeney LALM, Kolcic I, Koskinen S, Kraja AT, Kroh M, Kutalik Z, Latvala A, Launer LJ, Lebreton MP, Levinson DF, Lichtenstein P, Lichtner P, Liewald DCM, LifeLines Cohort Study, Loukola A, Madden PA, Mägi R, Mäki-Opas T, Marioni RE, Marques-Vidal P, Meddens GA, McMahon G, Meisinger C, Meitinger T, Milaneschi Y, Milani L, Montgomery GW, Myhre R, Nelson CP, Nyholt DR, Ollier WER, Palotie A, Paternoster L, Pedersen NL, Petrovic KE, Porteous DJ, Räikkönen K, Ring SM, Robino A, Rostapshova O, Rudan I, Rustichini A, Salomaa V, Sanders AR, Sarin A-P, Schmidt H, Scott RJ, Smith BH, Smith JA, Staessen JA, Steinhagen-Thiessen E, Strauch K, Terracciano A, Tobin MD, Ulivi S, Vaccargiu S, Quaye L, van Rooij FJA, Venturini C, Vinkhuyzen AAE, Völker U, Völzke H, Vonk JM, Vozzi D, Waage J, Ware EB, Willemsen G, Attia JR, Bennett DA, Berger K, Bertram L, Bisgaard H, Boomsma DI, Borecki IB, Bültmann U, Chabris CF, Cucca F, Cusi D, Deary IJ, Dedoussis GV, van Duijn CM, Eriksson JG, Franke B, Franke L, Gasparini P, Gejman PV, Gieger C, Grabe H-J, Gratten J, Groenen PJF, Gudnason V, van der Harst P, Hayward C, Hinds DA, Hoffmann W, Hyppönen E, Iacono WG, Jacobsson B, Järvelin M-R, Jöckel K-H, Kaprio J, Kardia SLR, Lehtimäki T, Lehrer SF, Magnusson PKE, Martin NG, McGue M, Metspalu A, Pendleton N, Penninx BWJH, Perola M, Pirastu N, Pirastu M, Polasek O, Posthuma D, Power C, Province MA, Samani NJ, Schlessinger D, Schmidt R, Sørensen TIA, Spector TD, Stefansson K, Thorsteinsdottir U, Thurik AR, Timpson NJ, Tiemeier H, Tung JY, Uitterlinden AG, Vitart V, Vollenweider P, Weir DR, Wilson JF, Wright AF, Conley DC, Krueger RF, Davey Smith G, Hofman A, Laibson DI, Medland SE, Meyer MN, Yang J, Johannesson M, Visscher PM, Esko T, Koellinger PD, Cesarini D, Benjamin DJ, 2016. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533, 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J, 2017. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet 101, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AAE, Lee SH, Robinson MR, Perry JRB, Nolte IM, van Vliet-Ostaptchouk JV, Snieder H, LifeLines Cohort Study, Esko T, Milani L, Mägi R, Metspalu A, Hamsten A, Magnusson PKE, Pedersen NL, Ingelsson E, Soranzo N, Keller MC, Wray NR, Goddard ME, Visscher PM, 2015. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat. Genet 47, 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM, 2011. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet 88, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee T, Kim J, Cho M-C, Han B-G, Lee J-Y, Lee H-J, Cho S, Kim H, 2013. Ubiquitous polygenicity of human complex traits: genome-wide analysis of 49 traits in Koreans. PLoS Genet. 9, e1003355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Erzurumluoglu AM, Elsworth BL, Kemp JP, Howe L, Haycock PC, Hemani G, Tansey K, Laurin C, Early Genetics and Lifecourse Epidemiology (EAGLE) Eczema Consortium, Pourcain BS, Warrington NM, Finucane HK, Price AL, Bulik-Sullivan BK, Anttila V, Paternoster L, Gaunt TR, Evans DM, Neale BM, 2017. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bakshi A, Vinkhuyzen AAE, Hemani G, Lee SH, Nolte IM, van VlietOstaptchouk JV, Snieder H, LifeLines Cohort Study, Esko T, Milani L, Mägi R, Metspalu A, Hill WG, Weir BS, Goddard ME, Visscher PM, Yang J, 2015. Dominance genetic variation contributes little to the missing heritability for human complex traits. Am. J. Hum. Genet 96, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.