Abstract
In the course of investigating how common clinical treatments and adaptive technologies affect recovery after spinal cord injury (SCI), we discovered that a clinically-modeled hindlimb stretching protocol dramatically, but transiently, reduces locomotor function. Nociceptive sensory input is capable of altering motor output at the spinal level, and nociceptive neurons are sensitized after SCI. Here we tested the hypotheses that stretch-induced locomotor deficits are dependent on nociceptive afferents by depleting TRPV1+ sensory afferents using capsaicin injections in neonatal rats. Following maturation, animals received 25g-cm contusive SCI at T10. After plateau of locomotor recovery at 6 weeks, daily stretching was performed for 3 weeks, followed by 2 weeks without stretch, and again for two additional weeks. Animals were sacrificed 2h after the last stretching session for histological assessments. Consistent with previous findings, stretch-induced drops in locomotor function were observed in nociceptor-intact animals but were nearly absent in nociceptor-depleted animals. These functional changes were accompanied by corresponding increases in the number of c-Fos+ nuclei throughout the lumbar enlargement. As expected, nociceptor-depleted animals had very little CGRP+ axonal innervation of the dorsal horn. Nociceptor-intact stretched animals had significantly higher levels of CGRP+ as compared to non-stretched SCI rats, suggesting that stretching promoted intraspinal CGRP+ sprouting. These results indicate that stretch-induced locomotor dysfunction in animals with incomplete SCI involves C-fibers, adding a negative post-SCI role to their adaptive roles (e.g., bladder control), and suggesting that the clinical use of muscle stretching to combat contractures and spasticity may be unintentionally detrimental to locomotor function.
Keywords: spinal cord injury, physical therapy, nociceptors, locomotor function, muscle stretch
Introduction.
The most obvious manifestation of a severe spinal cord injury (SCI) is paralysis of the musculature below the lesion level. Patients with severe SCIs become dependent on wheelchairs for their mobility. Lack of active loading and/or weight bearing in the limbs results in muscle atrophy and pathological changes in joints and their supporting structures that, with time, can result in contractures, characterized by dramatically decreased range of motion (Strommen, 2013) and increased stiffness (Dalyan et al., 1998; Moriyama et al., 2006). In addition, neurobiological changes within the spinal cord below the level of injury leads to the development of spasticity (Roy and Edgerton, 2012), characterized by velocity-dependent increases in resistance to muscle stretch and hyperreflexia (Katz and Rymer, 1989; Nielsen et al., 1995; Morita et al., 2001). Stretching remains one of the leading therapies intended to prevent and treat muscle contractures and spasticity (Harvey et al., 2011; Strommen, 2013). The rationale for therapeutic stretching comes largely from studies of neurologically intact animals with joint contractures that develop as a result of cast immobilization. In these studies, stretching was found to be effective at preventing range of motion (ROM) decreases when applied intermittently during cast-immobilization (Williams, 1988; Williams et al., 1988; Williams, 1990). In addition, stretching is the cornerstone of any flexibility training program for healthy intact human subjects and is effective at increasing ROM acutely after a single 30–90 second stretch (Magnusson et al., 1998; Nishikawa et al., 2015) and chronically, after multiple weeks of flexibility training utilizing various frequencies and durations of stretch (Bandy and Irion, 1994; Bandy et al., 1997, 1998).
In an effort to treat muscle contractures in wheelchair-immobilized rats with mild SCIs we collaborated with trained and licensed physical therapist (PTs) to develop a hindlimb stretching protocol (Caudle et al., 2011). The protocol consists of two, one-minute (static) stretch and hold maneuvers applied to each of the six major hindlimb muscle groups and is modeled after clinical therapeutic stretching. Stretching did not reduce the prevalence of contractures or the profile of recovery/dysfunction in wheelchair-immobilized rats. However, control rats with mild SCIs that received stretching had significantly reduced locomotor recovery as compared to unstretched controls. This dramatic effect of stretching on locomotor function is true also for rats with more severe injuries at either acute or chronic time points (Keller et al., 2017b).
Currently, the physiological mechanisms underlying this function-reducing phenomenon are unknown. We have found no signs of overt muscle damage (Keller et al., 2017b) that could account for the disruption in locomotor function and animals show a substantial recovery over 1–2 weeks when daily stretching ceases.
In the absence of muscle damage it is logical to think that the stretch-induced locomotor deficits have a neurologic basis via the sensory-system. Skeletal muscle is heavily innervated by group IV sensory afferents (unmyelinated nociceptors) which comprise about 66% of the total sensory innervation (Stacey, 1969). Group III and IV afferents are known to mediate decreased motor output during fatiguing exercise in humans (Amann et al., 2013), and activation of stretch- sensitive free nerve endings produces a rapid inhibition of motor output (Cleland et al., 1990). It is also known that C-fiber strength electrical stimulation, delivered randomly to the hindpaw, disrupts instrumental learning in rats with complete transections (Joynes et al., 2003) and locomotor recovery in rats with incomplete contusions (Grau et al., 2004). Therefore, we hypothesized that the negative effects of stretching are mediated by nociceptive afferents. We addressed this hypothesis by examining the locomotor function and response to our daily hindlimb muscle stretching protocol in rats depleted of TRPV1+ unmyelinated afferents via neonatal capsaicin treatment.
Material and Methods.
Ethical statement concerning animal research.
All experimental procedures involving animals were approved by the University of Louisville Institutional Animal Care and Use Committee.
Neonatal capsaicin injections.
In order to test our hypothesis we used neonatal capsaicin administration (D’Amour, 1941), the well-established method for depleting nociceptive afferents expressing TRPV1, the ion channel activated by capsaicin. Six pregnant Sprague-Dawley rats of known gestational age were checked several times a day to ensure an accurate record of birth time. Every pup in two of the litters received capsaicin injections, pups in two other litters received vehicle injections and the remaining two litters received no injections. Injections were done at 2 days of age. The rat pups were taken out of their cages (half of the litter at a time, along with some bedding) and received intraperitoneal injections of either capsaicin (50 mg/kg dissolved in 10% Tween 80 and 10% ethanol (v/v) in 0.9% saline) or vehicle injections (same solution without capsaicin). Anesthesia was achieved by wrapping pups in gauze and placing them on ice for 4–5 minutes. The animals were monitored continuously and the injections were done when all movements ceased and animals were not responsive to touch or pinch. After the injection, the animals were placed on the removed bedding until they warmed up and regained movement. The animals were returned to their mothers for the next 4 weeks after which they were weaned. The weaned animals were sexed so that males and female could be housed separately. Only female rats were used in the current study. The animals were allocated to 3 groups: capsaicin-treated (CAP, n=8), vehicle- treated (VEH, n=8) and control animals that received no injections (CON, n=8). All three groups received SCIs; CAP and VEH rats received stretching, while animals in the CON group served as injured controls and were not stretched. Gentling procedures (daily handling for 10–15 min) and baseline assessments began when the animals were 3 months old.
Pre-injury sensory assessments.
To assess the functional effectiveness of TRPV1+ C-fiber depletion by capsaicin, animals were tested for withdrawal thresholds to painful stimuli using electro-von Frey (mechanical) and Hargreaves’ (thermal) sensory tests, as described in detail previously (Caudle et al., 2011). Briefly, for von Frey testing, the animals were placed on a metal grid and an electro-von Frey rigid filament was applied to the plantar portion of the foot. The force recorded was that registered at the point of paw withdrawal when followed by a stereotypic behavioral response of attending to the stimulated foot. For the Hargreaves’ test, the animals were placed on a warmed glass surface (32°C) and an infrared light was shone onto the plantar surface of the foot. The latency of paw withdrawal from the stimulus was recorded.
C-fiber depletion was also assessed using the cutaneous trunci muscle reflex (CTMR), which is purely nociceptive in rats (Petruska et al., 2014). CTMR testing was performed following administration of a sub-surgical dose of sodium pentobarbital (35mg/kg). A five by three centimeter grid of black dots was drawn on the skin of the back starting from the midline (3 cm from the base of the tail). The midline dots were 1 cm apart (rostrocaudally) and 5 mm apart mediolaterally (a schematic of the grid is shown in Fig. 1C). Mechanical (forceps pinch) and thermal (metal probe heated to 65°C) stimuli were applied at two sites bilaterally. Digital video recordings of the skin were made with a camera placed directly above the animal. MaxTraq software (Innovision Systems) was used to digitize the dots and to quantify contraction distance and time to minimum distance using two dots rostral to the stimulation sites. Measurements were made using a custom-designed excel macro. Assessments were done by individuals blinded to the experimental groups.
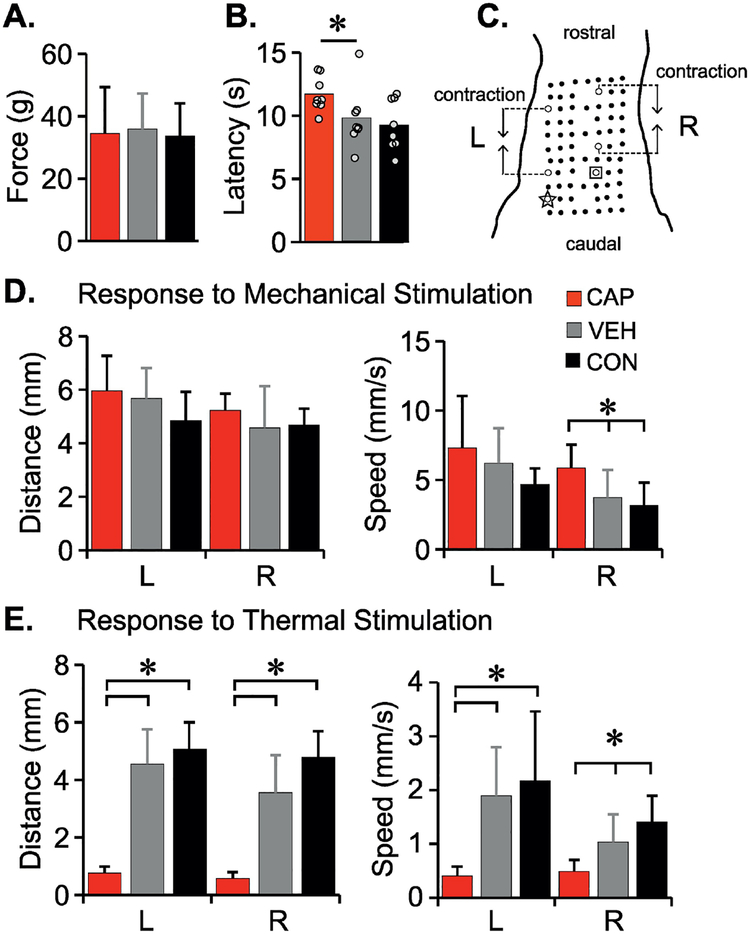
Figure 1. Neonatal capsaicin treatment reduces response to cutaneous thermal stimulation only.
To assess the effectiveness of TRPV1+ nociceptor depletion by neonatal capsacicin treatment, sensory function was assessed using von Frey (A), Hargreaves’ (B) and Cutaneous Trunci Muscle Reflex (CTMR, D-G). C. shows a schematic of CTMR markings on the dorsal skin for quantitative analysis of the reflex in response to mechanical (forceps pinch) or thermal (heated metal probe) stimulus. Dot A (star) and B (square) are two sites for stimulus application on either side of the midline. The data for contraction distance and speed to minimum distance (mechanical – D, thermal – E) between the two hollow dots rostral to the stimulation sites are shown. Data reported as means + SD for A and D-E (One-way ANOVA, Tukey HSD post hoc, p<.05), for B, bars represent means, dots each individual animal (n=8 in each group) (Mann Whitney U test, p<.05).
Spinal cord injury.
Spinal cord injuries were delivered as previously described (Magnuson et al., 2009). Animals were anesthetized with ketamine/xylazine and a midline incision was made over the lower thoracic spine, followed by a laminectomy at T9 to expose the spinal cord. Moderately-severe spinal cord contusions (25g/cm) were delivered using the NYU “MASCIS” Impactor (W. Young, Rutgers University, Piscataway, NJ).
Locomotor assessment.
Locomotor function was assessed using the BBB Open Field Locomotor Scale (Basso et al., 1995) pre-injury and then weekly for the first 6 weeks to demonstrate a functional plateau. During the weeks of stretching, BBB scores were assessed 3 times a week, Monday am (pre- stretch), Monday pm (after one stretching session) and Friday pm (after 5 days of stretching), as previously described (Caudle et al., 2015; Keller et al., 2016). These assessments included determining the proportion of animals that could receive a BBB subscore, which indicated that they achieved consistent weight-bearing stepping (Basso, 2004).
Stretching protocol.
Our standard hindlimb stretching protocol (Caudle et al., 2015) was initiated at 6 weeks post SCI when BBB scores had plateaued and locomotor function was stable for 3 weeks. Normal or sham injured control groups were not included because uninjured animals will not tolerate the stretching protocol. The protocol consists of two 12 minute sessions of 6 stretches (each held at the end range of motion for 1 minute) applied to major hindlimb muscle groups bilaterally (ankle, knee, hip flexors/extensors and hip adductors and abductors). For the stretching sessions, animals were gently wrapped in a towel, leaving their hindlimbs exposed, and placed on their backs. Seven rat PTs, who were blinded to experimental groups, participated in the daily stretching sessions and animals were rotated through the PTs so that they were not stretched by the same PT more than twice per week. During each stretch the PTs closely monitored the animals and noted any responses displayed. Some of the most common responses were kicking (vigorous movement of either or both hindlimbs), vibrations (high frequency, low range of motion movement mostly around the ankle) (Keller et al., 2017a) of either limb, withdrawal or pull-back (hindlimb resistance to stretch) and air-stepping (slow rhythmic step-like movement of either or both of the hindlimbs). The responses were given a score of 1, 2 or 3 based on intensity and frequency during each stretch: 1= mild/infrequent (5–20% of the stretch time), 2=moderate, frequent (20–70%), 3=severe/very frequent (70–100%). Control animals were handled daily: they were wrapped in a towel and placed on their backs, but not stretched. Animals were stretched 5 days a week for 3 weeks and then were allowed to recover for 2 weeks. The rats were then stretched for an additional week (5 days) plus 2, 3 or 4 days on the following week, and were sacrificed 2 hours after the last stretching session. Two to three animals from each stretch group (CAP and VEH) were sacrificed each day. Control animals were all sacrificed 2 hours after being wrapped in a towel and handled. All the animals were assessed using the BBB Open Field Locomotor Scale between the final stretching session and the time of sacrifice.
Euthanasia and tissue processing.
Two hours after their last stretching session the animals were overdosed with a ketamine (50 mg/kg)/xylazine (0.024 mg/kg)/acepromazine (0.005 mg/kg) cocktail and transcardially perfused with phosphate buffered saline (PBS) followed by 4% PFA as previously described (Jonkers et al., 1984). The spinal cord including the injury epicenter and lumbar enlargement was dissected out and post-fixed in 4% PFA for one hour and transferred to 30% sucrose for cryoprotection for at least 4 days. The spinal cords were examined under the dissecting microscope confirming that all of the lesions were at the T10 level. A 12 mm length of each cord containing the injury epicenter was isolated, placed in a block with tissue freezing medium completely covering the sample and rapidly frozen on dry ice. Transverse 30 μm sections were cut and stained with eriochrome cyanine and were photographed at 4X as described previously (Kuerzi et al., 2010). In order to provide a quantitative measure of injury severity, spared white matter (SWM) was assessed using the cross-sectional area (CSA) of darkly stained, compact tissue, traced using ImageJ (NIH) and compared to uninjured (control) sections. The section with the least SWM was assigned as the injury epicenter. The lumbar spinal cord (L1-L5) was cryoprotected and sectioned at 20 μm for immunohistochemical (IHC) analysis of c-Fos and CGRP. Three hindlimb muscles (Tibialis Anterior, Medial Gastrocnemius and Biceps Femoris) were also dissected out for histological analysis. Muscle length (origin to insertion) was measured and a 5 mm length, standardized from the middle one-third, proximal to origin, was post-fixed in 4% PFA and cryoprotected in 30% sucrose. Each muscle sample was sectioned at 10 μm and stained with hematoxylin and eosin (H&E). The muscle was analyzed for the presence of centralized nuclei and fiber cross sectional area as previously described (Keller et al., 2017b). Photomicrographs of four muscle areas standardized to dorsal, ventral, medial and lateral within each cross section were acquired at 20X. The number of muscle fibers (MF) containing centralized nuclei (CN) were counted and expressed as a percentage of the total number of MFs analyzed. In addition, the perimeters of over 150 individual muscle fibers were traced from each muscle and CSA area determined using ImageJ software.
c-Fos immunohistochemistry and analysis.
Nuclei positive for c-Fos were immunolabeled using avidin-biotin peroxidase (Hsu et al., 1981). Slides with sections of lumbar spinal cord were warmed and washed with PBS. Endogenous peroxidase activity was quenched using 3% hydrogen peroxide (15 min). The sections were then blocked with 10% normal donkey serum (NDS) and 10% bovine serum albumin (BSA) in 0.3% PBS-Triton (PBST) for 1 hour. The sections were incubated with c-Fos primary antibody (mouse monoclonal anti-c-Fos, 1:1000, ab208942, lot #GR269424–3 Abcam) overnight at 4°C. Sections were then rinsed with PBS/PBST, incubated with biotinylated antibody (donkey- antimouse, 1:1000, Immunostar, 715-065-151, lot# 125258) for 1 hour, rinsed with PBS/PBST, incubated with horseradish peroxidase-streptavidin (1:1000 in PBS, Vector Laboratories SA- 5004, lot#ZB1113) for 1 hour and rinsed with PBS/PBST. Sections were then incubated with DAB solution (SigmaFast DAB D0426, lot# SLBJ7196V) for 10 minutes to visualize the immuno- labelled complexes. The reaction was stopped in distilled water and the slides were air dried for at least 10 minutes, followed by 3 minutes in Xylene. The slides were coverslipped using paramount. Images of each section were acquired on an inverted microscope with a 10x objective. Using ImageJ the gray matter was divided into three regions of interest (bilaterally): Substantia Gelatinosa (SG, border was clearly visible in stained tissue), dorsal horn (DH) and intermediate gray matter/ventral horn (IGM&VH). Darkly-stained, c-Fos positive nuclei were counted manually in ImageJ by an individual blinded to experimental groups. In addition, random, de-identified sections were analyzed by other investigators and counts were compared to ensure consistent identification of stained (c-Fos+) nuclei. Three images for each lumbar level (L1-L5, 15 sections total) spaced a minimum of 200 μm apart, were acquired and the average number of nuclei from the sections for each animal was used for group analysis.
CGRP immunohistochemistry and analysis.
CGRP IHC was performed on sections of L3 spinal cord. Slides were warmed, rinsed and blocked (10% NDS, 10% BSA in 0.3% PBST) for 1 hour. Sections were incubated with CGRP primary antibody (rabbit polyclonal anti-CGRP 1:2000, AB15360, lot# 2475751 Abcam) overnight at 4°C, rinsed and incubated with secondary antibody (donkey-anti-rabbit with Alexa 647, Cat# 711-606-152, lot #118661 Abcam). The sections were rinsed then coverslipped with fluoromount. The dorsal horns from three different sections (around 200 μm apart) for each animal were imaged at 20X. The area of CGRP-positive puncta within the dorsal horn was identified and differentiated using the threshold tool within Elements (Nikon Instruments Software). The same threshold range was applied to all the images from all the animals. The area of CGRP was quantified for each dorsal horn excluding the dorsal root entry zones that were removed manually from each image. The average CGRP area from 3 sections represented the per-animal mean, and these values were used to generate group averages. All these analyses were performed by an individual blinded to the experimental groups.
Experimental Design and Statistical Analysis.
This behavioral study included 24 animals that were randomly assigned to treatment, vehicle or control groups. Control, non-SCI animals are not amenable to the stretching procedures due to their resistance of hindlimb manipulation that could potentially result in injury to their muscle and/or joint and thus were no included in the study. Based on our previous studies, post-injury motor assessment (BBB) scores of rats with NYU injury severities ranging from mild-moderate to moderate-severe result in average group differences from 1.5–2.5 points with standard deviations of approximately 1.0–2.0. Based on power analysis calculations, detection of significant differences between the groups with similar expected results can be achieved at a power of 88.7–96.0% with equal sample sizes of 8–10 per group. Researchers were blinded to experimental groups during stretching and for all the behavioral and histological assessments.
One-way ANOVA followed by Tukey HSD post hoc t-tests for multiple comparisons was used to compare differences among the groups (baseline von Frey; terminal CTMR, kinematic excursions, CGRP & muscle fibers) at one time-point. Repeated measurements (BBB locomotor tests assessed over time and c-Fos+ nuclei counts across lumbar regions) were compared using Repeated Measures ANOVA (within factor) with the Group factor (between groups factor) followed by Bonferroni post hoc t-tests for multiple comparisons. The proportion of animals within the groups who achieved a BBB subscore were compared with the Binomial Proportion Test. Hindlimb responses of the CAP & VEH groups during stretching were compared using Independent t-tests between means with equal variance. Hargreaves’ responses at baseline were analyzed using the nonparametric Mann Whitney U test to compare rankings of the groups due to high and unequal variance among the groups. The relationship between the hindlimb responses of kicking and vibrations during stretching and the number of c-Fos+ neurons was compared using nonparametric Spearman Rank correlations.
Data were analyzed using IBM SPSS v22, 24. Results and figure legends indicate which test was used for the represented set of data. Display of data means (ANOVA, t-tests) are reported as mean±standard deviation (SD) as indicated.
Results.
Baseline sensory and locomotor function.
Primary afferent neurons expressing the TRPV1 receptor are known as thermal and chemical nociceptors. To assess the effectiveness of depletion by capsaicin treatment, and any secondary consequences, we utilized three standard assessments, von Frey filaments to assess mechanical nociception, the Hargreaves’ test to assess thermal nociception and the cutaneous trunci muscle reflex that allowed us to directly compare responses to mechanical and thermal nociceptive stimuli. Neonatal capsaicin treatment had no observable effect on the responses to mechanical nociceptive stimuli in adults. Paw withdrawal thresholds to von Frey filament stimulation were similar for the CAP, VEH and CON groups (F=.065, df=2,21, p=.937) (Fig. 1A). There were also no significant differences in contraction distance of the CTMR in response to forceps pinch at either site of stimulation, A (F=1.86, df=2,20, p=.181) or B (F=.798, df=2,20, p=.464; Fig 1D). Unexpectedly, the CAP group had an increased speed to minimum distance in response to the mechanical stimulus applied to site B (p=.013) as compared to CON (Fig. 1E). However, as expected, CAP animals had increased latency to paw withdrawal in response to the heat stimulus of the Hargreaves’ test as compared to VEH animals (p=.021) (Fig. 1B). Also as expected, in the CAP group the CTMR in response to the thermal probe (65°C) was practically abolished (p<.001) (Fig. 1F, G).
Animals were assessed using the BBB Open Field Locomotor Scale and all received a normal score and subscore of 21 and 13 respectively (Basso, 2004; Basso et al., 1995), showing that neonatal CAP treatment did not overtly alter overground locomotion in otherwise intact adult female rats. Pre-injury BBB scores are shown in Figure 2.
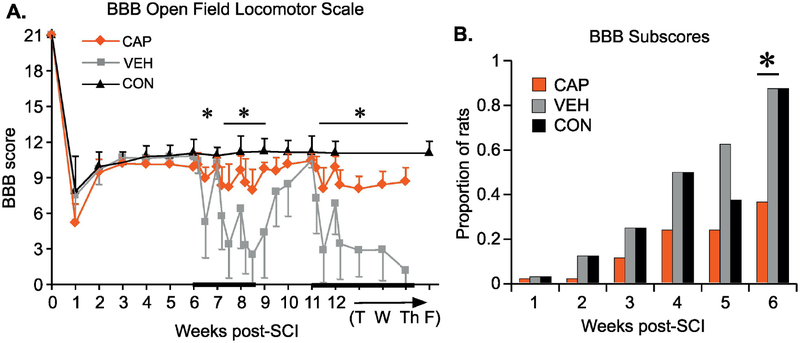
Figure 2. Locomotor performance following contusion injury and hindlimb stretching.
BBB Open Field Locomotor assessment was performed weekly for the first 6 weeks after SCI (A). During the 5 weeks of the stretching protocol (beginning at 6 weeks post-SCI), BBBs were assessed three times a week (Monday am/pm and Friday pm). During the week 12 the sacrificing procedures began after the post-stretch BBB assessment: T-Tuesday, n=8 in each group; W-Wednesday, n=5, Th-Thursday, n=2. Data shown as means +/− SD (RM ANOVA, Bonferroni post hoc, p<.05). B) Proportion of animals achieving a BBB subscore of at least 0 (for consistent weight support). Analyzed using Binomial Proportions Test (p<.05).
Post-injury spinal cord histology.
The CAP, VEH and CON groups had 9.4 ± 5.3%, 8.4 ± 4.2% and 15.5 ± 8.69% spared white matter at the epicenter, respectively. One-way ANOVA analysis showed no significant group differences (F=2.82, df=2,20, p=.083).
Post-injury bladder function.
As is usual, all the nociceptor-intact animals (VEH and CON) developed spontaneous bladder emptying by 10–14 days post-injury and no longer required manual bladder expression. To our surprise, none of the nociceptor-depleted animals (CAP) developed spontaneous bladder emptying and required manual bladder expression for the duration of the experiment. This anectdotal observation suggests that TRPV+ nociceptors play an important role in this process after a mid-thoracic contusion injury.
Locomotor function after SCI and during stretching.
Locomotor function, as assessed by BBB scores, plateaued at 3 weeks post-SCI in all groups (Fig. 2A). The proportion of animals achieving sufficient function to receive a BBB subscore continued to increase slightly over the 6 weeks after injury but prior to stretching (Fig. 2B). By week 6, a significantly larger proportion of VEH and CON (p=.016) animals showed consistent weight supported stepping (a BBB subscore of at least 0) as compared to the CAP group (Fig. 2B). After the first 5 days of stretching the BBB scores of VEH animals decreased significantly compared to the pre-stretch plateau (p=.001) and were significantly lower than those of CAP animals (p=.005). Stretching had only minor negative effects on the locomotor function of CAP animals; their BBB scores were not significantly different after 5 days of stretching. Although the locomotor function of VEH animals recovered back to pre-stretch levels over the weekend, just one stretching session on Monday of the following week negated that recovery (p=.021, as compared to Monday pre-stretch values) and the next 4 days of stretching resulted in further detriments. Some recovery was achieved over the second weekend without stretching, but this was not as robust as after the first weekend, with the BBB scores of VEH animals remaining significantly lower than those of the CAP (p=.022) and CON (p=.002) groups. The same pattern of BBB score change persisted in the 3rd week of stretching. By the end of the 3rd week VEH animals were able to achieve BBB scores of only 2.5 corresponding to extensive movement of one of the three joints. This is significantly different (p<.001) from their pre-stretch locomotor function when animals achieved consistent weight-supported stepping (BBB score of 11 or 10 in case of dorsal stepping). On the contrary, BBB scores of the CAP rats fluctuated only slightly during the weeks of stretching (ranged from 8 to 10) and never dropped significantly below the scores of CON rats. Two weeks without stretching (weeks 9 and 10 post-SCI) allowed VEH animals to recover back to pre-stretch levels, but once stretching resumed at week 11, locomotor function again dropped to its lowest level.
CGRP analysis.
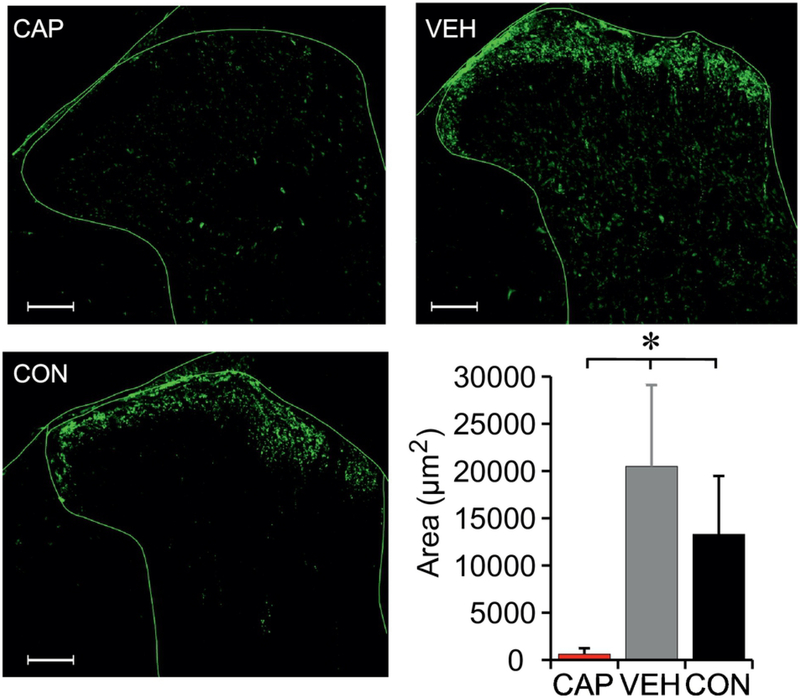
Representative images of CGRP IHC are shown in Fig. 3. As expected, CAP animals had very low area with CGRP positive puncta, significantly less area than the VEH (p<.001) and CON groups (p=.001). In addition, VEH animals (SCI plus stretch) had significantly greater area of CGRP within the dorsal horn compared to CON animals (SCI without stretch)(p=.047.), implying an effect of stretch.
Figure 3. Immunohistochemistry for CGRP following contusion injury and hindlimb stretching.
Effectiveness of neonatal capsaicin treatment was histologically confirmed with immunohistochemistry of CGRP in the dorsal horn of the spinal cord (L3 segment). Data shown as means + SD (One-way ANOVA, Tukey HSD post hoc, p<.05). The scale bar = 100 μm.
Activation of neurons in response to stretching.
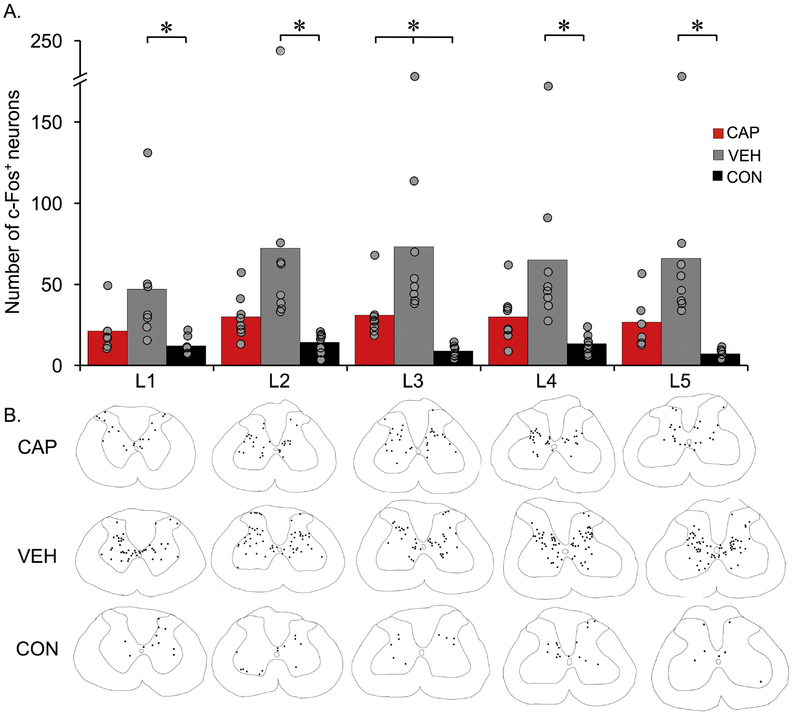
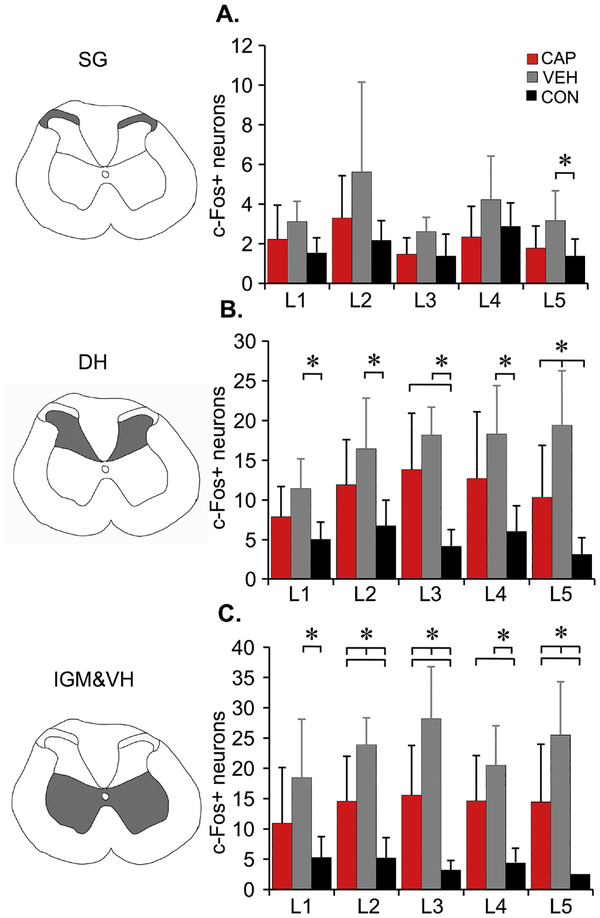
Hindlimb stretching resulted in significant increases in the number of c-Fos positive nuclei throughout the lumbar enlargement gray matter (Figures 4 and 5. F=31.5, df=2,94; L1, p=.05; L2, p=.019; L3, p=.001; L4, p=.005; L5, p=.003) in VEH (SCI plus stretch) animals as compared to the CON group (SCI but no stretching), and also compared to the CAP group at L3 (p=.029). There were significant group differences in the number of c-Fos+ neurons when analyzed by different region within the gray matter (Figure 6): Substantia Gelatinosa (SG,F=12.97, df=2, 76.207, p<.001), dorsal horn (DH, F=46.48, df=2, 82.139, p<.001) and intermediate gray matter and ventral horn (IGM&VH, F=71.73, df=2,79, p<.001). The number of animals for each level and region varied slightly after the removal of outliers (>2.5 SD) and/or the lack of tissue integrity at L1 and/or L5 segments: VEH n=6 for all regions and levels except at L2 (SG) n=5; CAP: n=8, except for L1 and L5 n=6; CON n=8, except for L1 n=7 and L4 (SG) n=7. C-Fos+ neuron distribution appeared to be less different in the SG than in the DH and IGM&VH (Figure 6), as post-hoc analysis revealed significant differences in the number of c-Fos+ neurons between VEH and CON in SG level L5 (p=0.032)(Figure 6A), VEH had significantly increased numbers of c-Fos+ neurons in DH compared to CON at L1 (p=.008), L2 (p=.007), L3 (p<.001), L4 (p=.006), L5 (p<.001) and at L5 when compared to CAP (p=.026)). CAP had significantly greater number of c-Fos+ neurons in DH as compared to CON at L3 (p=.002) (B). However, the greatest differences were found in the IGM&VH (Figure 6C). Similarly, the numbers of c-Fos+ neurons of VEH rats was significantly greater as compared to CON within IGM & VH at L1 (p=.022), L2-L5 (p<.001), and at L2 (p=.015), L3 (p=.007)) and L5 (p=.045) compared to CAP. CAP animals also had increased numbers of c-Fos+ neurons compared to CON at L2 (p=.008), L3 (p=.005) L4 (p=.007) and L5 (p=.018)) (Figure 6C).
Figure 4. Activation of spinal cord neurons by stretching estimated using immunohistochemistry for c-Fos.
c-Fos immunohistochemistry was performed on lumbar spinal cords (L1-L5 segments). The bars represent an average number of c-Fos positive neurons from 3 sections at each level, while each dot represents individual animals (A). n=8 per group for each level except at L1 and L5 CAP (n=6) and at L1 VEH & CON (n=7), each. Analyzed using RM ANOVA, Bonferroni post hoc t-test, p<.05. Traces of spinal cord sections representative of each group for each level with black dots corresponds to c-Fos+ neurons within the chosen sections (B).
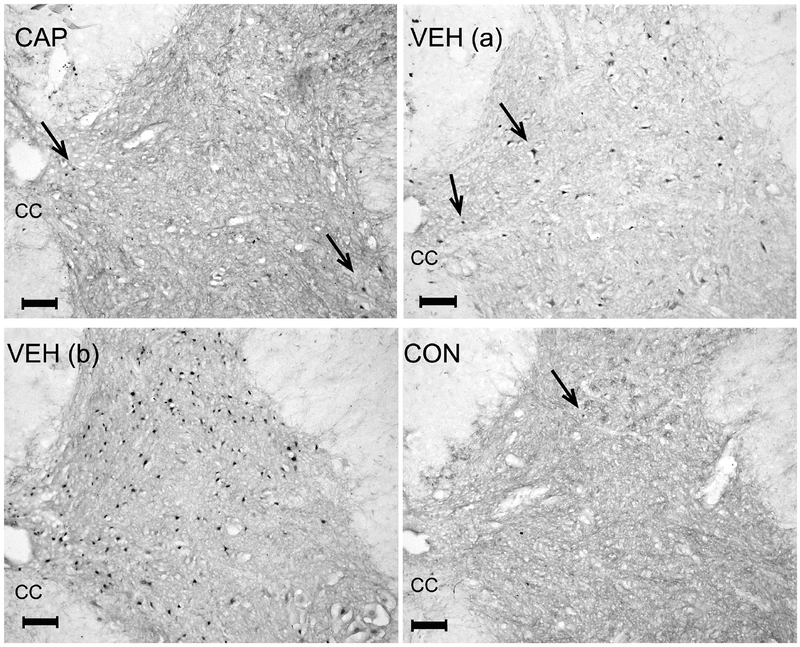
Figure 5. Example images of c-Fos+ nuclei using immunohistochemistry.
Images of spinal cord sections (L3) stained for c-Fos. Arrows point to c-Fos+ cell nuclei. VEH (a) is an image from an animal representative of the average, sacrificed after 2 days of stretching in the last week of the stretching protocol. VEH (b) is an image from an animal that was a statistical outlier; it was sacrificed after 4 days of stretching that same week and had the highest number of c-Fos+ nuclei. CC – central canal. The scale bar = 100µm.
Figure 6. Distribution of c-Fos+ nuclei using immunohistochemistry.
c-Fos+ nuclei count separated into three general regions of gray matter bilaterally, schematically shown next to the graphs (shaded areas) SG- Substantia Gelatinosa (A), DH – dorsal horn (B), IGM&VH – intermediate gray matter and ventral horn (C). Data shown as means + SD (RM ANOVA, Bonferroni post hoc t-test, p<.05). Two outliers (defined as ≥ 2.5 standard deviations) from VEH group were removed, so VEH n=6 for all regions and levels except at L2 (SG) n=5; CAP: n=8, except for L1 and L5 n=6; CON n=8, except for L1 n=7 and L4 (SG) n=7.
Hindlimb responses to stretch and correlation with the number of c-Fos+ neurons.
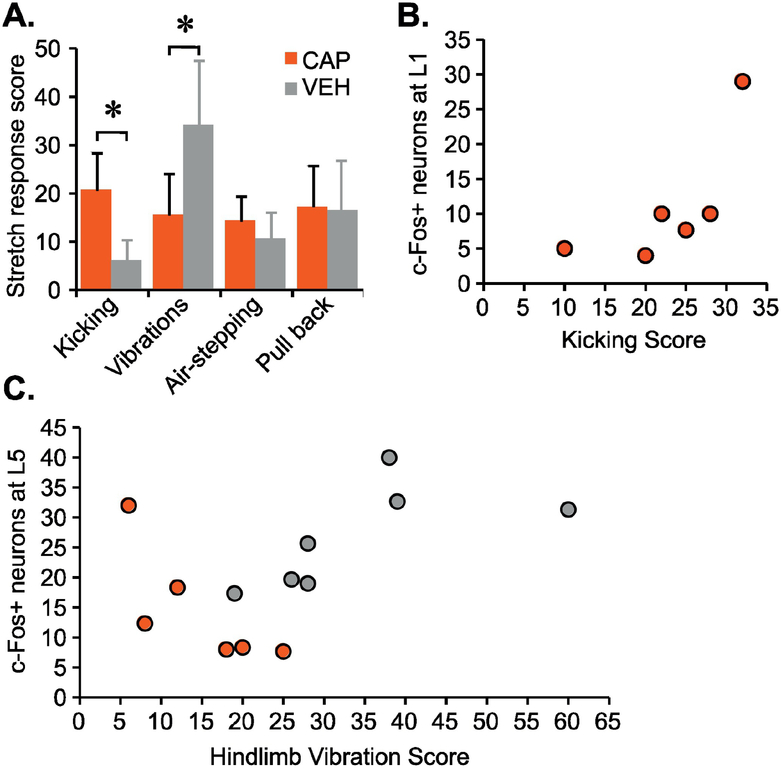
To determine if there was a difference between groups in the observed hindlimb responses, an overall score for the four most common hindlimb responses (kicking, vibration, air-stepping and pull back) was generated. This was done by summing the grades of intensity/severity of the response recorded during each stretch of the last stretching session for each animal and comparing the means using Independent t-test. First, we found that CAP animals had significantly higher number of kicking responses as compared to VEH (t=4.4, df=13, p=.001), whereas VEH rats had significantly higher number of vibration responses as compared to CAP rats (t=4.4, df=13, p=.006) (Fig. 7A). There were no significant differences in air-stepping or pull back responses between the groups.
Figure 7. Hindlimb responses to stretch and counts of c-Fos+ nuclei.
Four major hindlimb responses observed and quantified during stretching were kicking, vibrations, air-stepping and pull back (A); data shown as means + SD, (Independent t-test between means with equal variances, p<.05). The CAP group shows a significant positive correlation (Spearman Rank Test) for the kicking score and the number of c-Fos+ neurons at L1 (rs =.841, p=.036) (B). The VEH group vibration score has a significant positive correlation with the number of c-Fos+ neurons at L5 (rs =.793, p=.033) and the CAP group vibration score has a significant negative correlation with the number of c-Fos+ neurons at L5 (rs =−.886, p=.019) (C). Other lumbar segments showed no significant correlations.
Based on the finding that the majority of significant differences in the number of c-Fos+ neurons came from the IGM and VH region of the spinal cord which processes motor related information, we hypothesized that this upregulation was related to the activation of stereotypical hindlimb movements in response to stretch. We found a significant correlation between the vibration hindlimb response, that involves primarily the ankle, and the number of c-Fos+ neurons at L5 for both VEH (rs=.793, p=.033, n=7) and CAP group (rs=−.886, p=.019, n=6) (Fig. 7C). Interestingly, for the VEH group it was a positive correlation whereas CAP showed a negative correlation. Furthermore, the number of c-Fos+ neurons at L1 strongly correlated with the kicking response scores, that involves primarily the hip and knee, in the CAP group (rs=.841, p=.036, n=6) (Fig. 7B), whereas there were no significant correlations between kicking and number of c-Fos+ neurons at any level in the VEH group. Significant correlations were not observed at other lumbar levels.
Muscle histology.
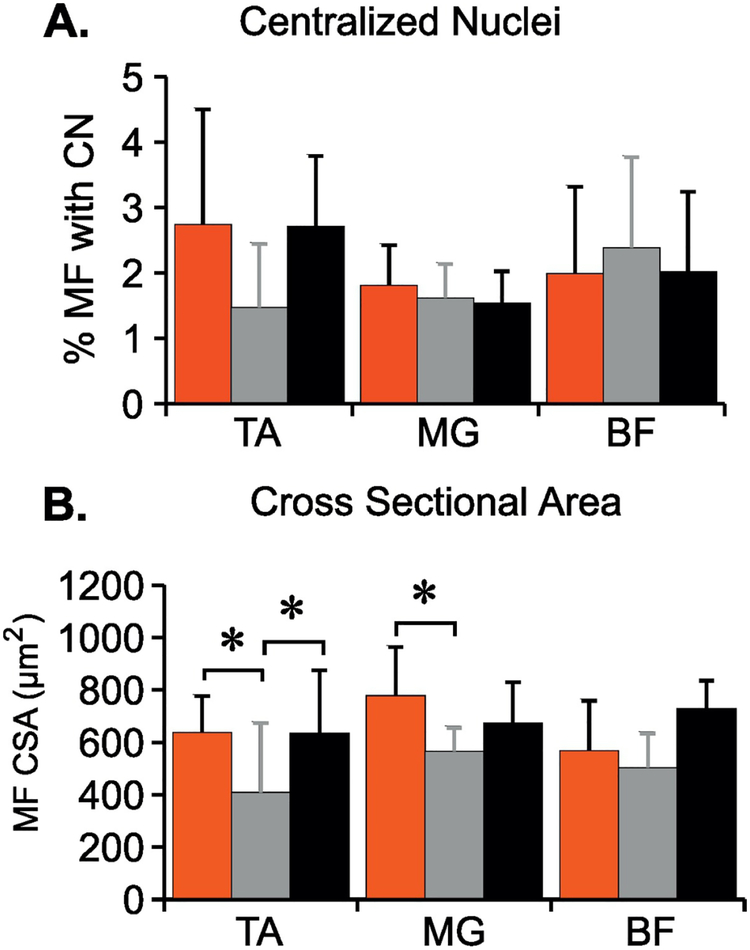
Muscles involved in the stretching protocol were examined to assess the possibility that overt damage was being done to the muscles themselves. The proportion of muscle fibers (MF) with centralized nuclei, an indicator of muscle repair following damage (Snow, 1977; Bodine-Fowler, 1994) were not different between the groups and were very close to 3%, the percentage present in normal adult rat hindlimb muscles (Figure 8A). VEH rats had significantly reduced MF cross sectional area (CSA) in the Tibialis Anterior muscle as compared to CON (p=.016) and CAP (p=.014) rats, and CAP rats had larger MF CSA in the Medial Gastrocnemius as compared to VEH (p=.026) (Figure 8B).
Figure 8. Histological assessment of muscle to determine the effect of stretching.
Three hindlimb muscles: Tibialis Anterior (TA), Medial Gastrocnemius (MG), Biceps Femoris (BF) were analyzed for number of muscle fibers (MF) with centralized nuclei (CN, a marker of regeneration) (A) and muscle fiber cross sectional area (CSA) (B). Data shown as means + SD (One-way ANOVA, Tukey HSD post hoc t-test, * p<.05).
Discussion.
Functional aspects of capsaicin treated animals pre- and post-SCI.
In order to confirm the success of C-fiber depletion we assessed responses to thermal and mechanical nociceptive stimuli prior to SCI. Consistent with previous findings (Jancso et al., 1987), CAP animals had significantly reduced thermal nociceptive responses but intact mechanical nociception (Fig. 1). Further, CAP and VEH animals had normal locomotor function as assessed by the BBB Open Field Locomotor Scale (Fig. 2). We also confirmed that CAP animals had dramatically reduced CGRP signal in the dorsal horn of the L3 segment (Fig. 3).
Depletion of TRPV1+ nociceptive afferents had a modest negative influence on locomotor recovery after SCI (Fig. 2). This suggests that global depletion of TRPV1+ fibers in some way hampered the normal course of spontaneous functional recovery without grossly altering white matter loss/sparing at the injury site. At this time we have no explanation for this observation and these issues will need further investigation. An additional observation of interest is that nociceptor-depleted animals never established spontaneous bladder-emptying after SCI and required daily manual expression for the duration of the experiment. Zinck et al. showed that sprouting of lumbosacral CGRP positive primary afferents precedes the emergence of spontaneous bladder emptying after complete spinal cord transection (Zinck et al., 2007). Thus, CGRP afferent spouting appears necessary for the emergence of spontaneous bladder activity that normally occurs 6–10 days post-SCI. Taken together, these observations show that TRPV1+ fibers play a complex role in recovery after SCI, appearing necessary for bladder emptying and some aspects of recovered stepping, despite their obvious involvement in the negative impact of muscle stretch on locomotor function.
Stretching and locomotor function.
The pattern of stretch-induced functional loss in nociceptor-intact (VEH) rats is complex, and is suggestive of a priming phenomenon. As we observed previously, the very first stretching session had a negligible impact, but there was significant functional decline after 5 consecutive days of stretching. In contrast, the first stretching session of the second week (i.e., after 2 days without stretching) dramatically reduced locomotor function and by Friday of the second week the BBB scores dropped to below 5 (Keller et al., 2017b). Two weeks without stretching allowed the animals to return to pre-stretch BBB scores (Monday morning of week 11 post-SCI, Fig. 2), indicating that stretch-induced locomotor deficits are temporary. However, the first stretching session after the two-week break again induced a dramatic drop in locomotor function (~3 points) showing a persistent sensitizing effect of the initial 3 weeks of stretching. Overall, the pattern of locomotor disruption and recovery suggests that stretching sensitizes or primes the system leading to the large functional declines and a persistent underlying sensitivity to stretching.
In stark contrast to the VEH animals, stretching had only a minor effect on the locomotor function of nociceptor depleted (CAP) rats. The pattern of functional loss, that never reached statistical significance, nonetheless retained the complex profile of the VEH animals suggesting that it may reflect the influence of the small proportion of fibers that escaped the neonatal capsaicin treatment. We conclude that TRPV1+ afferents are necessary for the stretching- induced reduction in locomotor function after SCI and that the standard clinical intervention of muscle stretching may carry unintended and unrecognized negative effects. These may be largely covert in the majority of clinical situations where individuals have little function to lose, but could have a major impact on those SCI individuals with significant spared function, and/or the potential to benefit from other therapies such as spinal cord stimulation. It is not yet clear if the stretching initiates action potential signaling in these neurons, but this is likely. Even if the stretch does not damage muscle, or if it is of insufficient force to induce C-fiber signaling in spinal-intact animals, post-SCI sensory sensitization will lower their threshold for activation, thus enabling normally non-noxious stretch to now induce signaling in nociceptors.
Other aspects of the stretching procedure-response may help to reveal potential mechanisms. We observed robust motor responses which could result in eccentric muscle contractions during limb restraint and stretching. Eccentric contractions are very effective at inducing delayed onset muscle soreness (DOMS)(Armstrong, 1984), a phenomenon dependent on, and that results in, the sensitization of TRPV1+ neurons (Kubo et al., 2012; Ota et al., 2013). Thus, it is possible that the nociceptor-intact VEH rats experienced nociceptor activation due to stretch, and additional nociceptor activity due to eccentric contractions and conditions equivalent to DOMS if they were sensate. Whether or not eccentric contractions/DOMS are/is necessary and sufficient for stretch-induced locomotor deficits after SCI is currently unknown, but it remains a feasible mechanism.
It may appear counter-intuitive that afferents expressing TRPV1 (thus largely heat-responsive), are involved in the effects of mechanical muscle stretch. First of all, there are many TRPV1- expressing neurons innervating tissues other than muscle, such as skin, that could be involved in the stretching effect (Kniffki et al., 1981). In addition, few small sensory neurons are as narrowly-tuned as their larger counterparts, and nearly all are polymodal to some degree. For example, half of the recorded single Group III and IV muscle afferents were heat responsive, and of those, all were responsive to at least one other stimulus type (metabolites or mechanical), with over half also demonstrating mechanical-sensitivity (Jankowski et al., 2013). In spinal-intact conditions, these sensory neurons act to inhibit motor output (Amann et al., 2011; Amann, 2012; Amann et al., 2013; Jankowski et al., 2013), indicating that they already have access to motor-suppressive circuitry, even without intraspinal sprouting. It has yet to be directly demonstrated that clinically-modelled stretch activates muscle (or other) nociceptors after SCI. Nonetheless it is clear that they can become sensitized, including a reduction in their mechanical threshold (e.g., Ross et al., 2014), which suggests that they might be activated by forces that would not be considered painful in the sensate condition. The involvement of TRPV1+ afferents in the stretching-induced drop in locomotor function implies that they are activated by at least one of their adequate stimuli to a degree that affects spinal cord circuitry even in the absence of frank muscle damage.
Histological findings.
Sparse CGRP immunoreactivity in CAP animals confirmed the effective depletion of TRPV1+ C- fibers by capsaicin. However, VEH rats showed increases in CGRP area in the dorsal horn compared to the CON group, and these increases were particularly visible outside of the substantia gelatinosa and into the deep dorsal horn (Fig.3). This is particularly interesting given that CGRP+ axons are known to spontaneously sprout in the spinal cord caudal to an injury (Ondarza et al., 2003; Detloff et al., 2016) and that the sprouting is inversely related to training or exercise (Detloff et al., 2016; Nees et al., 2016)). The increased CGRP seen in the VEH animals might represent an exacerbation of the spontaneous sprouting or the induction of sprouting in additional CGRP+ populations. The sprouting may be due to stretching-induced nociceptor activation and/or may be due to the drop in hindlimb movement and weight support during in-cage activity.
Overall, it appears reasonable to infer that the pattern of, and increase in c-Fos+ nuclei in the VEH animals is a cellular representation of the mechanism(s) by which stretching reduces locomotor activity post-SCI (Figs. 4–6), potentially involving CGRP+ fibers in the deep dorsal horn. These animals displayed clonic-like co-contractions (van Gorp et al., 2014; Keller et al., 2018) involving the ankle and knee, and we found a positive correlation between c-Fos+ nuclei at L5 and “vibration” scores (Fig. 7). In contrast, CAP animals displayed more kicking (large and organized flexor-extensor locomotor-like movements involving primarily hip and knee) that correlated positively with the number of c-Fos+ nuclei at L2. Notably, the largest increases in the numbers of c-Fos+ nuclei in VEH over CAP animals occurred in the IGM&VH, including L2, important because lamina VII in particular, is the home for neurons that participate in locomotor rhythm and pattern generation (Kiehn and Kjaerulff, 1998; Pocratsky et al., 2017). We propose that stretching activates both nociceptors and other afferents, and therefore that VEH animals have afferent barrages from both nociceptors and non-nociceptors leading to significantly higher numbers of c-Fos+ nuclei, including populations of locomotor-related interneurons that in-turn limit the recruitment of circuitry necessary for locomotion. While the precise anatomical relationship between CGRP+ fibers in the deep dorsal horn and locomotor-related interneurons is unknown at present, they are certainly in the same vicinity and could interact directly and/or indirectly during stretching resulting in c-Fos expression and the observed hindlimb responses of clonus-like vibrations that were more prevalent in the VEH group (Fig. 7). When stretching activates a preponderance of non-nociceptive input in the CAP animals the consequences to locomotor function are minimal and the hindlimb responses are more likely robust kicking (Figs. 2 & 7). Clinically, it is known that severe myoclonus after SCI can be dependent on existing peripheral nociceptive inputs, and once the peripheral source is alleviated, clonus also declines (Calancie, 2006). It is intriguing that the number of c-Fos+ neurons in the substantia gelatinosa is not increased significantly in the VEH animals. This unexpected observation may be related to c-Fos expression generally being induced by a novel stimulus and that the SG neurons are responsive to the stretching-induced input in a way that is not really “new” in the long-term SCI condition assessed here. This possibility is supported by the increase in expression of the “sustained activity indicator” deltaFosB in the superficial DH over 6 weeks post-SCI (Watson et al., 2014), possibly because of spontaneous activity in the small neurons (Bedi et al., 2010) or other factors. Clearly much of the spinal cord neuronal circuitry does interpret the stretch- induced activity as “novel”, as there is a significant increase elsewhere in the cord.
Finally, although we determined that stretch-induced locomotor deficits can occur in the absence of concurrent muscle tissue damage, some changes in muscles across groups were noted. Some atrophy occurred in the Tibialis Anterior muscles of VEH rats as compared to CAP and CON animals (Fig. 8), presumably due to disuse. Interestingly, CAP rats had significantly greater CSA of the Gastrocnemius MF as compared to VEH animals. This observation might represent a stretch-induced hypertrophy (Tatsumi, 2010) unencumbered by disuse (Spector et al., 2009).
Conclusion and Clinical Relevance:
This study indicates that TRPV1+ (capsaicin-sensitive) afferents mediate a temporary disruption of locomotor function when a clinically-modeled daily hindlimb muscle stretching protocol is employed after incomplete SCI in adult rats. The specific role played by TRPV1+ nociceptors is not clear; our results suggest that their activation during stretch in-turn activates numerous spinal cord interneurons that reside in the intermediate gray matter of the lumbar enlargement, resulting in temporarily dysfunctional locomotor circuitry. While we currently are uncertain as to the clinical relevance of the stretching phenomenon, it appears to be both robust and to involve fundamental characteristics that would be common to all mammalian species including human. Searching the clinical literature reveals no published work where the impact of stretching on factors other than joint and muscle health, joint range-of-motion or spasticity have been examined. As reported by Katalinic and colleagues (Katalinic et al., 2010) stretching after SCI is generally ineffective for its intended purpose of preventing contractures, reducing spasticity and increasing joint range-of-motion. Furthermore, Harvey et al. demonstrated that some physical therapists apply torques when stretching “sensory complete” SCI patients of sufficient magnitude to activate nociceptive afferents and thus would not be tolerated by sensate individuals (Harvey et al., 2003a). Taken together, these observations and our results showing a disruption of locomotor function after SCI in rats (Keller et al., 2017a; Keller et al., 2017c; Keller et al., 2018), suggest that the use of stretching as a routine therapy in rehabilitation after SCI should be reevaluated.
Highlights.
C-fibers are necessary for stretch-induced locomotor deficits after rat SCI
Dysfunction involves C-fiber-dependent activation of lumbar interneurons
Stretching as a routine therapy in rehabilitation after SCI should be reevaluated.
Acknowledgments:
The authors acknowledge the assistance of Christine Yarberry, Johnny Morehouse and Dr. Kris Rau.
Funding: This work was supported by the DOD CDMRP SC110169, by the Kentucky Spinal Cord and Head Injury Research Trust and by the NIH P30 GM103507.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Amann M (2012) Significance of Group III and IV muscle afferents for the endurance exercising human. Clinical and experimental pharmacology & physiology 39:831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2011) Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. The Journal of physiology 589:5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, Richardson RS (2013) Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. Journal of applied physiology (Bethesda, Md: 1985) 115:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB (1984) Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Medicine and science in sports and exercise 16:529–538. [PubMed] [Google Scholar]
- Bandy WD, Irion JM (1994) The effect of time on static stretch on the flexibility of the hamstring muscles. Physical therapy 74:845–850; discussion 850–842. [DOI] [PubMed] [Google Scholar]
- Bandy WD, Irion JM, Briggler M (1997) The effect of time and frequency of static stretching on flexibility of the hamstring muscles. Physical therapy 77:1090–1096. [DOI] [PubMed] [Google Scholar]
- Bandy WD, Irion JM, Briggler M (1998) The effect of static stretch and dynamic range of motion training on the flexibility of the hamstring muscles. The Journal of orthopaedic and sports physical therapy 27:295–300. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of neurotrauma 12:1–21. [DOI] [PubMed] [Google Scholar]
- Bedi SS, Yang Q, Crook RJ, Du J, Wu Z, Fishman HM, Carlton SM, Walters ET (2010) Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 30(44): 14870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm DG, Blazevich AJ, Kay AD, McHugh M (2016) Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: a systematic review. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 41:1–11. [DOI] [PubMed] [Google Scholar]
- Bodine-Fowler S (1994) Skeletal muscle regeneration after injury: an overview. Journal of voice: official journal of the Voice Foundation 8:53–62. [DOI] [PubMed] [Google Scholar]
- Calancie B (2006) Spinal myoclonus after spinal cord injury. The journal of spinal cord medicine 29:413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle KL, Brown EH, Shum-Siu A, Burke DA, Magnuson TS, Voor MJ, Magnuson DS (2011) Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabilitation and neural repair 25:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle KL, Atkinson DA, Brown EH, Donaldson K, Seibt E, Chea T, Smith E, Chung K, Shum-Siu A, Cron CC, Magnuson DS (2015) Hindlimb stretching alters locomotor function after spinal cord injury in the adult rat. Neurorehabilitation and neural repair 29:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland CL, Hayward L, Rymer WZ (1990) Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. Journal of neurophysiology 64:1319–1330. [DOI] [PubMed] [Google Scholar]
- D’Amour FEAS, DONN L. (1941) A METHOD FOR DETERMINING LOSS OF PAIN SENSATION. Journal of Pharmacology and Experimental Therapeutics 72:74–79. [Google Scholar]
- Dalyan M, Sherman A, Cardenas DD (1998) Factors associated with contractures in acute spinal cord injury. Spinal cord 36:405–408. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Quiros-Molina D, Javia AS, Daggubati L, Nehlsen AD, Naqvi A, Ninan V, Vannix KN, McMullen MK, Amin S, Ganzer PD, Houle JD (2016) Delayed Exercise Is Ineffective at Reversing Aberrant Nociceptive Afferent Plasticity or Neuropathic Pain After Spinal Cord Injury in Rats. Neurorehabilitation and neural repair 30:685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huang Y-J, Turtle JD, Strain MM, Miranda RC, Garraway SM, Hook MA, 2017. When Pain Hurts: Nociceptive Stimulation Induces a State of Maladaptive Plasticity and Impairs Recovery after Spinal Cord Injury. Journal of neurotrauma 34: 1873–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC (2004) Uncontrollable stimulation undermines recovery after spinal cord injury. Journal of neurotrauma 21:1795–1817. [DOI] [PubMed] [Google Scholar]
- Harvey L, de Jong I, Goehl G, Mardwedel S (2006) Twelve weeks of nightly stretch does not reduce thumb web-space contractures in people with a neurological condition: a randomised controlled trial. The Australian journal of physiotherapy 52:251–258. [DOI] [PubMed] [Google Scholar]
- Harvey LA, McQuade L, Hawthorne S, Byak A (2003a) Quantifying the magnitude of torque physiotherapists apply when stretching the hamstring muscles of people with spinal cord injury. Archives of physical medicine and rehabilitation 84:1072–1075. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Glinsky JA, Katalinic OM, Ben M (2011) Contracture management for people with spinal cord injuries. NeuroRehabilitation 28:17–20. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Byak AJ, Ostrovskaya M, Glinsky J, Katte L, Herbert RD (2003b) Randomised trial of the effects of four weeks of daily stretch on extensibility of hamstring muscles in people with spinal cord injuries. The Australian journal of physiotherapy 49:176–181. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 29:577–580. [DOI] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Such G, Joo F, Nagy A (1987) Neurotoxic effect of capsaicin in mammals. Acta physiologica Hungarica 69:295–313. [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR (2013) Comprehensive phenotyping of group III and IV muscle afferents in mouse. Journal of neurophysiology 109:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers BW, Sterk JC, Wouterlood FG (1984) Transcardial perfusion fixation of the CNS by means of a compressed-air-driven device. Journal of neuroscience methods 12:141–149. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Ferguson AR, Crown ED, Patton BC, Grau JW (2003) Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behavioural brain research 141:159–170. [DOI] [PubMed] [Google Scholar]
- Katalinic OM, Harvey LA, Herbert RD (2011) Effectiveness of stretch for the treatment and prevention of contractures in people with neurological conditions: a systematic review. Physical therapy 91:11–24. [DOI] [PubMed] [Google Scholar]
- Katalinic OM, Harvey LA, Herbert RD, Moseley AM, Lannin NA, Schurr K (2010) Stretch for the treatment and prevention of contractures. The Cochrane database of systematic reviews:Cd007455. [DOI] [PubMed] [Google Scholar]
- Katz RT, Rymer WZ (1989) Spastic hypertonia: mechanisms and measurement. Archives of physical medicine and rehabilitation 70:144–155. [PubMed] [Google Scholar]
- Keller A, Rees K, Prince D, Morehouse J, Shum-Siu A, Magnuson D (2017a) Dynamic “Range of Motion” Hindlimb Stretching Disrupts Locomotor Function in Rats with Moderate Subacute Spinal Cord Injuries. Journal of Neurotrauma 34:2086–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AV, Wainwright GN, Shum-Siu A, Prince D, Hoeper A, Martin E, Magnuson DS (2017b) Disruption of locomotion in response to hindlimb muscle stretch at acute and chronic time points after a spinal cord injury in rats. Journal of Neurotrauma 34:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AV, Rees KM, Seibt EJ, Wood BD, Wade AD, Morehouse J, Shum-Siu A, Magnuson DSK (2018) Electromyographic patterns of the rat hindlimb in response to muscle stretch after spinal cord injury. Spinal Cord 56:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniffki KD, Schomburg ED, Steffens H (1981) Effects from fine muscle and cutaneous afferents on spinal locomotion in cats. The Journal of physiology 319:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Koyama M, Tamura R, Takagishi Y, Murase S, Mizumura K (2012) Absence of mechanical hyperalgesia after exercise (delayed onset muscle soreness) in neonatally capsaicin-treated rats. Neuroscience research 73:56–60. [DOI] [PubMed] [Google Scholar]
- Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, Smith RR, Magnuson DS (2010) Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Experimental neurology 224:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson DS, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, Burke D (2009) Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabilitation and neural repair 23:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Aagard P, Simonsen E, Bojsen-Moller F (1998) A biomechanical evaluation of cyclic and static stretch in human skeletal muscle. International journal of sports medicine 19:310–316. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D, Petersen NT, Nielsen JB (2001) Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain: a journal of neurology 124:826–837. [DOI] [PubMed] [Google Scholar]
- Moriyama H, Yoshimura O, Sunahori H, Tobimatsu Y (2006) Comparison of muscular and articular factors in the progression of contractures after spinal cord injury in rats. Spinal cord 44:174–181. [DOI] [PubMed] [Google Scholar]
- Nees TA, Tappe-Theodor A, Sliwinski C, Motsch M, Rupp R, Kuner R, Weidner N, Blesch A (2016) Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 157:687–697. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N, Crone C (1995) Changes in transmission across synapses of Ia afferents in spastic patients. Brain: a journal of neurology 118 (Pt 4):995–1004. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Aizawa J, Kanemura N, Takahashi T, Hosomi N, Maruyama H, Kimura H, Matsumoto M, Takayanagi K (2015) Immediate effect of passive and active stretching on hamstrings flexibility: a single-blinded randomized control trial. Journal of physical therapy science 27:3167–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE (2003) Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Experimental neurology 184:373–380. [DOI] [PubMed] [Google Scholar]
- Ota H, Katanosaka K, Murase S, Kashio M, Tominaga M, Mizumura K (2013) TRPV1 and TRPV4 play pivotal roles in delayed onset muscle soreness. PloS one 8:e65751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska JC, Barker DF, Garraway SM, Trainer R, Fransen JW, Seidman PA, Soto RG, Mendell LM, Johnson RD (2014) Organization of sensory input to the nociceptive-specific cutaneous trunk muscle reflex in rat, an effective experimental system for examining nociception and plasticity. The Journal of Comparative Neurology 522:1048–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy RR, Edgerton VR (2012) Neurobiological perspective of spasticity as occurs after a spinal cord injury. Experimental neurology 235:116–122. [DOI] [PubMed] [Google Scholar]
- Ross JL, Queme LF, Shank AT, Hudgins RC, Jankowski MP (2014) Sensitization of group III and IV muscle afferents in the mouse after ischemia and reperfusion injury. J Pain 15(12): 1257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H (1986) Fiber composition of the rat sciatic nerve. The Anatomical Record 215:71–81. [DOI] [PubMed] [Google Scholar]
- Snow MH (1977) Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. I. A fine structural study. The Anatomical record 188:181–199. [DOI] [PubMed] [Google Scholar]
- Spector ER, Smith SM, Sibonga JD (2009) Skeletal effects of long-duration head-down bed rest. Aviation, space, and environmental medicine 80:A23–28. [DOI] [PubMed] [Google Scholar]
- Stacey MJ (1969) Free nerve endings in skeletal muscle of the cat. Journal of Anatomy 105:231–254. [PMC free article] [PubMed] [Google Scholar]
- Strommen JA (2013) Management of spasticity from spinal cord dysfunction. Neurologic clinics 31:269–286. [DOI] [PubMed] [Google Scholar]
- Tatsumi R (2010) Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Animal science journal 81:11–20. [DOI] [PubMed] [Google Scholar]
- van Gorp S, Deumens R, Leerink M, Nguyen S, Joosten EA, Marsala M (2014) Translation of the rat thoracic contusion model; part 1-supraspinally versus spinally mediated pain-like responses and spasticity. Spinal Cord 52:524–528. [DOI] [PubMed] [Google Scholar]
- Watson JL, Hala TJ, Putatunda R, Sannie D, Lepore AC (2014) Persistent at-level thermal hyperalgesia and tactile allodynia accompany chronic neuronal and astrocyte activation in superficial dorsal horn following mouse cervical contusion spinal cord injury. PLoS One 9(9): e109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE (1988) Effect of intermittent stretch on immobilised muscle. Annals of the rheumatic diseases 47:1014–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE (1990) Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Annals of the rheumatic diseases 49:316–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PE, Catanese T, Lucey EG, Goldspink G (1988) The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. Journal of anatomy 158:109–114. [PMC free article] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW (2007) Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Experimental neurology 204:777–790. [DOI] [PubMed] [Google Scholar]