Abstract
A number of natural products with medicinal properties increase DNA cleavage mediated by type II topoisomerases. In an effort to identify additional natural compounds that affect the activity of human type II topoisomerases, a blind screen of a library of 341 Mediterranean plant extracts was conducted. Extracts from Nuphcir lutea, the yellow water lily, were identified in this screen. N. lutea has been used in traditional medicine by a variety of indigenous populations. The active compound in N. lutea, 6,6’-dihydroxythiobinupharidine, was found to enhance DNA cleavage mediated by human topoisomerase IIα and IIβ ~8-fold and ~3-fold, respectively. Mechanistic studies with topoisomerase IIα indicate that 6,6’-dihydroxythiobinupharidine is a “covalent poison” that acts by adducting the enzyme outside of the DNA cleavage-ligation active site and requires the N-terminal domain of the protein for its activity. Results suggest that some of the medicinal properties of N. lutea may result from the interactions between 6,6’-dihydroxythiobinupharidine and the human type II enzymes.
Keywords: 6,6’-Dihydroxythiobinupharidine; Covalent poison; DNA cleavage; Topoisomerase IIα; Topoisomerase IIβ
Graphical Abstract:
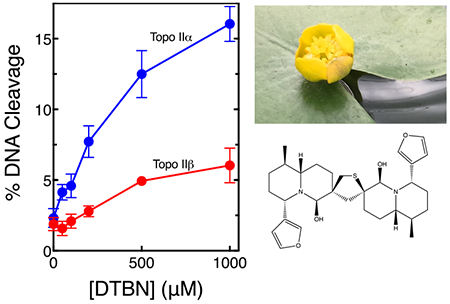
Type II topoisomerases are essential enzymes that remove knots and tangles from the genetic material and modulate torsional stress in DNA.1–6 These enzymes regulate the topological state of DNA by passing an intact double helix through a transient double-stranded break that they generate in a separate segment of DNA.1–6 Humans encode two isoforms of topoisomerase II, α and β1, 2, 7–9 Topoisomerase IIα is essential for cell survival and is the isoform required for the termination of DNA replication and for untangling daughter chromosomes prior to mitosis.2, 3, 6, 9, 10 The roles of topoisomerase IIβ are less well defined, but the enzyme appears to play important roles in the transcription of hormonally regulated genes.2, 3, 6, 7, 9, 10 Although the β isoform is non-essential at the cellular level, it is required during development.2, 3, 6, 7, 9–12
Beyond their critical physiological functions, topoisomerase IIα and IIβ are the targets for some of the most widely prescribed anti-cancer agents worldwide.2, 10, 13–16 Drugs such as etoposide and doxorubicin are used to treat a variety of hematological and solid tumors and mitoxantrone is used in the treatment of breast cancer and autoimmune diseases such as multiple sclerosis.16, 17 Topoisomerase II-targeted drugs act by stabilizing covalent enzyme-cleaved DNA complexes (cleavage complexes) that are requisite intermediates in the catalytic cycle of topoisomerase IIα and IIβ.2, 10, 13–16 Thus, these agents convert type II topoisom erases from essential enzymes to toxic proteins that fragment the genome. Drugs that act by this mechanism are referred to as topoisomerase II “poisons” to distinguish them from “catalytic inhibitors” that act by robbing the cell of the catalytic functions of these enzymes.2, 10, 13–16
Despite the wide clinical use of topoisomerase II-targeted anti-cancer drugs, treatment with these agents is associated with a number of serious side effects, including cardiomyopathy and the induction of secondary leukemias.13, 16, 18 Circumstantial evidence suggests that the β isoform is primarily responsible for these detrimental outcomes.16, 19–22
A variety of phytochemicals with anti-cancer, chemopreventative, or other health-promoting properties also act as topoisomerase II poisons.10 Among these compounds are bioflavonoids23–25, catechins26, 27, curcumin28, 29, isothiocyanates30, and antioxidants such as hydroxytyrosol, oleuropein, and verbascoside.31 In an effort to identify additional natural products that act as poisons of human type II topoisomerases, we conducted a blind screen of a library of 341 Mediterranean plant extracts. Species in the library were collected primarily from the Tel Aviv University Botanical Garden or arid lands. Previous work with this library determined that extracts from Phillyrea latifolia L. enhanced DNA cleavage mediated by human topoisomerase IIα, which led to the identification of hydroxytyrosol, oleuropein, and verbascoside as topoisomerase II poisons.31
In the present study, an extract (50 μg/mL) from Nuphar lutea, the yellow water lily, increased levels of DNA cleavage mediated by human topoisomerase IIα ~2.4 ± 0.1-fold. This flower is found in ponds and marshes nearly worldwide.32 N. lutea extracts have been used for the treatment of inflammation in the traditional medicines of Lebanon, Japan, and the Gitskan people of British Columbia, Canada.33–35 They also have been reported to possess anti-leishmanial, anti-bacterial, and potentially anti-cancer properties.32, 36–40 6,6’-Dihydroxythiobinupharidine (DTBN, Fig. 1) is the active compound in N. lutea.32 Therefore, we determined the effects of DTBN on the DNA cleavage activities of human topoisomerase IIα and IIβ.
Fig. 1.
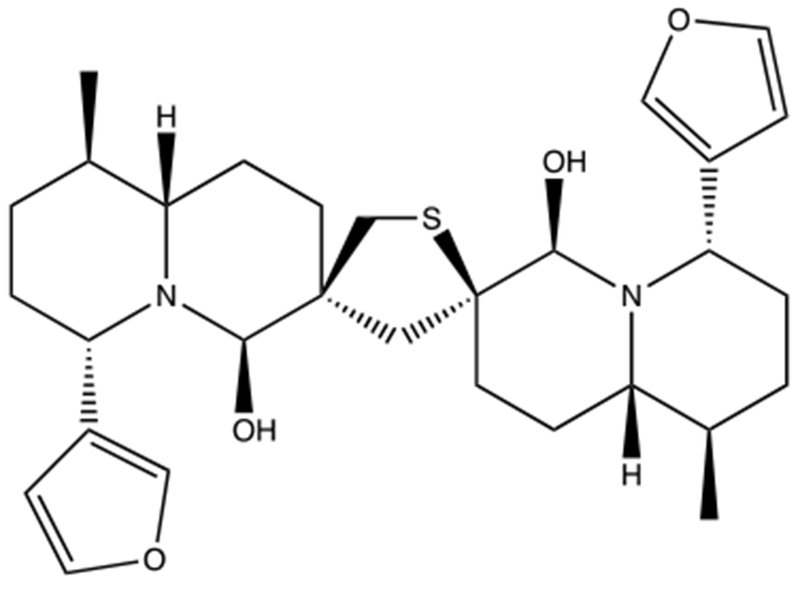
Structure of 6,6’-dihydroxythiobinupharidine (DTBN). DTBN is the active compound in N. lutea.
As seen in Fig. 2, DTBN enhanced DNA cleavage mediated by both enzyme isoforms, but had a considerably larger effect on topoisomerase IIα. Whereas the compound increased levels of double-stranded DNA breaks generated by the α isoform ~8-fold (from ~2% to −16% of the DNA substrate), it increased cleavage by the β isoform ~3-fold (from −2% to −6% of the DNA substrate). Because of the enhanced activity of DTBN against topoisomerase IIα, this isoform was used for all of the mechanistic studies that follow.
Fig. 2.
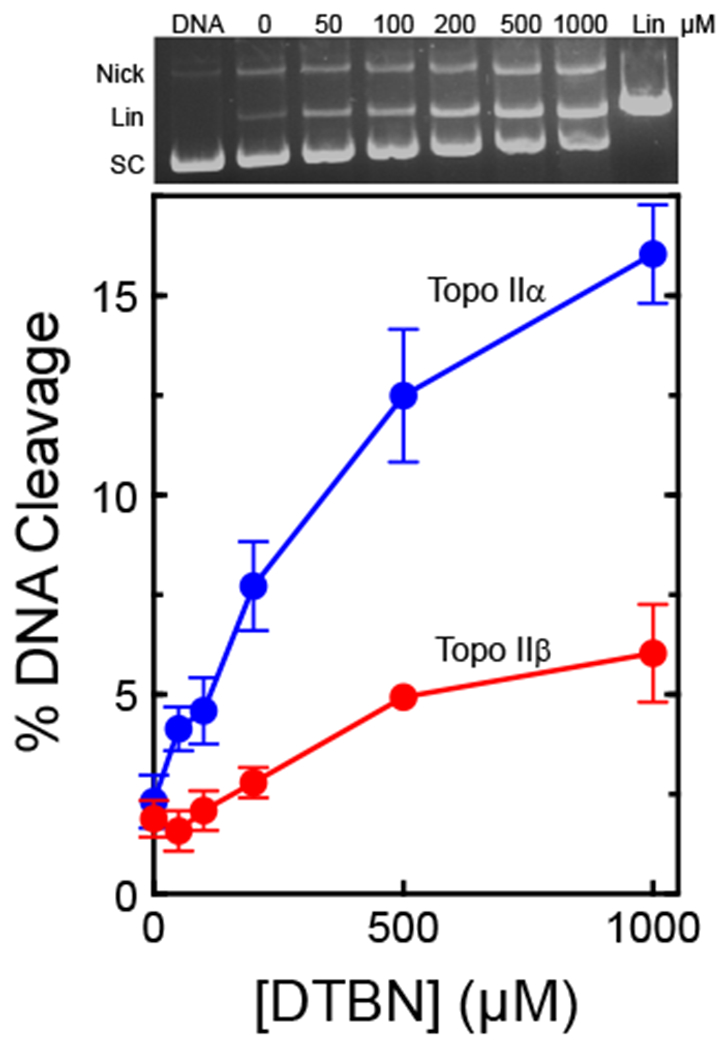
DTBN increases levels of DNA double-stranded cleavage mediated by human type II topoisomerases. The effects of DTBN on double-stranded DNA cleavage mediated by human topoisomerase IIα (Topo IIα, blue) and topoisomerase IIβ (Topo IIβ, red) are shown. DNA cleavage levels were normalized by comparison to linear standards, which were set to 100%. Error bars represent standard deviations of three independent experiments. A representative ethidium bromide-stained agarose gel containing an experiment with topoisomerase IIα is shown at the top. The positions of negatively supercoiled (SC), nicked (Nick), and linear (Lin) plasmid are shown.
Prior to further analysis, two experiments were carried out to ensure that the DNA cleavage observed in the presence of DTBN was mediated by topoisomerase IIα (Fig. 3). In the first, 500 μM DTBN was incubated with DNA in the absence of enzyme under the conditions of the DNA cleavage assay. No DNA cleavage was seen. In the second, topoisomerase IIα was included in assays, however, reactions were incubated with 25 mM EDTA prior to the addition of SDS (which traps the cleavage complex). The addition of this chelating agent removes the active site Mg2+ ions that are required for DNA scission, but only when the DNA is in the ligated form.41 Therefore, EDTA reverses DNA cleavage mediated by the type II enzyme.41 This is seen in Fig. 3; the addition of EDTA substantially diminished levels of double-stranded DNA scission (p = 0.056). This reversal of cleavage is inconsistent with a non-enzymatic reaction and provides strong evidence that the DNA cleavage enhancement observed in the presence of DTBN is mediated by the human type II enzyme.
Fig. 3.
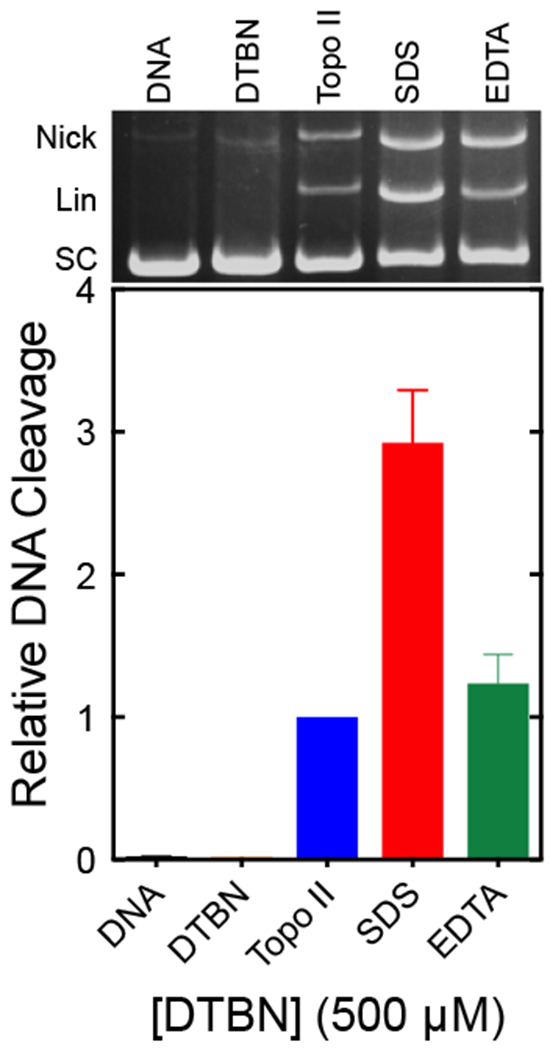
DNA cleavage induced by DTBN is mediated by human topoisomerase IIα. Assay mixtures contained DNA alone (DNA, black), DNA with DTBN in the absence of enzyme (DTBN, orange), topoisomerase IIα with DNA in the absence of DTBN (Topo II, blue), complete reactions stopped with SDS prior to the addition of EDTA (SDS, red), or complete reactions treated with EDTA prior to the addition of SDS (EDTA, green). Error bars represent the standard error of the mean of two independent experiments. A representative ethidium bromide-stained agarose gel is shown at the top and DNA positions are as indicated in Fig. 2.
A previous study found that compounds that formed longer lasting cleavage complexes displayed greater cytotoxicity.42 Therefore, we determined the stability of cleavage complexes formed in the presence of DTBN (Fig. 4). This was accomplished by establishing DNA cleavage-ligation equilibria and diluting samples 20-fold with buffer that included no divalent cation. Thus, once the DNA dissociates from topoisomerase IIα, it is unlikely that the enzyme will form new cleavage complexes. Whereas the T1/2 of the cleavage complex was less than 30 s when formed in the absence of DTBN, it was stable over a 4 h time course in the presence of the compound.
Fig. 4.
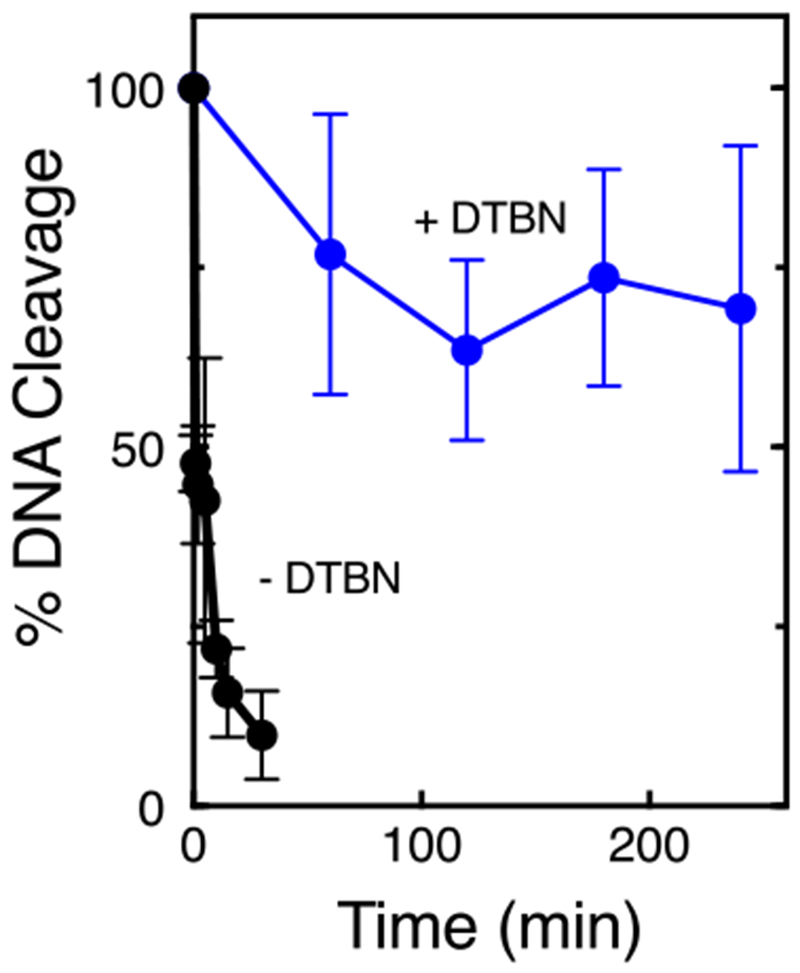
DTBN induces stable topoisomerase IIα-DNA cleavage complexes. Reactions were allowed to reach cleavage-ligation equilibrium, diluted with reaction buffer that lacked Mg2+, and persistence was measured by assessing the loss of double-stranded breaks. DNA cleavage at time zero was set to 100%. Reactions included no compound (black) or 500 μM DTBN (blue). Error bars represent standard deviations of three independent experiments.
Topoisomerase II poisons can be grouped into two different classes. Anti-cancer drugs such as etoposide interact noncovalently at the interface between the enzyme and its DNA substrate, contacting both.43 Once DNA scission has taken place, the drug intercalates between the bases of the cleaved scissile bond, blocking ligation.43, 44 Compounds that act in this non-covalent fashion are referred to as interfacial topoisomerase II poisons.43
In contrast, compounds such as benzoquinone interact covalently with topoisomerase II outside of the DNA cleavage active site.2, 10, 45 These compounds form adducts with cysteine (and potentially other) residues and are believed to enhance cleavage by stabilizing the dimerization of the N-terminal domain of the enzyme.30, 46–49 Compounds that act by adducting the enzyme are referred to as covalent topoisomerase II poisons.2, 10, 46 In contrast to interfacial poisons, covalent poisons can have variable effects on enzyme-mediated DNA ligation. Whereas compounds such as benzoquinone strongly inhibit ligation, others such as hydroxytyrosol have relatively little effect.31, 45
As a first step towards characterizing the mechanism by which DTBN enhances DNA cleavage, we examined the effects of the compound on the rate of DNA ligation mediated by human topoisomerase IIα. Unlike etoposide and other anti-cancer drugs, DTBN had very little effect on the rate of ligation (T1/2 = 11 s versus 12.5 s in the presence or absence of DTBN, respectively). This finding suggests that the compound may act as a covalent poison of topoisomerase IIα.
To further elucidate the mechanistic basis for the actions of DTBN, we took advantage of several hallmark characteristics of covalent poisons that they do not share with interfacial poisons. First, because covalent poisons often form adducts with cysteine residues and require redox cycling, their activity against the type II enzyme can be diminished in the presence of thiol-containing compounds or reducing agents.2, 29, 45, 49 Therefore, we examined the effects of these compounds on the activity of DTBN. As seen in Fig. 5, the thiol reagents dithiothreitol and glutathione (1 mM) decreased the ability of DTBN to induce DNA cleavage by ~50%. In addition, the reducing agent ascorbic acid (2.5 mM) abrogated the effects of DTBN on DNA cleavage mediated by human topoisomerase IIα. These results are consistent with the covalent mechanism of poisoning.
Fig. 5.
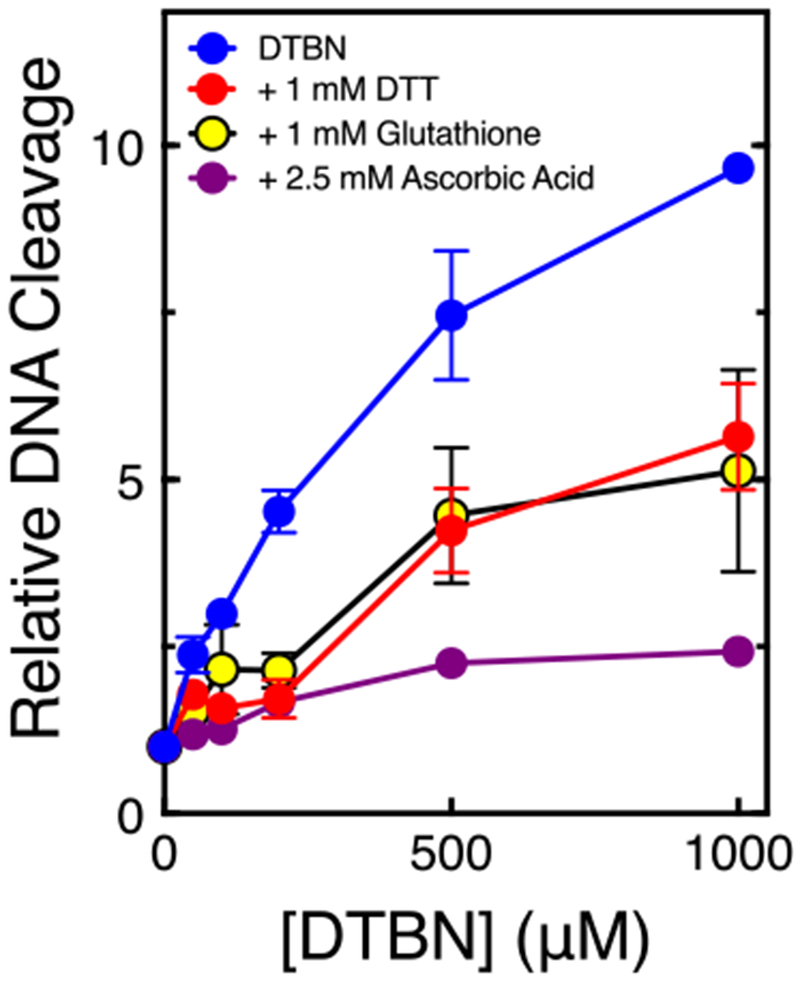
The ability of DTBN to poison topoisomerase IIα is diminished by thiol containing compounds and reducing agents. DNA cleavage reactions were carried out in the presence of 1 mM dithiothreitol (DTT) (red), 1 mM glutathione (yellow), or 2.5 mM ascorbic acid (purple). Error bars represent standard deviations of three independent experiments.
Second, when covalent poisons are incubated with topoisomerase II prior to the addition of DNA, they inactivate the enzyme.2, 29, 45, 49 This is likely due (in part) to the fact that they close the N-terminal protein gate which prevents plasmid DNA from entering the active site of the enzyme.46, 47, 50 There is also evidence that in the absence of DNA, covalent poisons may adduct a critical residue in the active site of the enzyme.46 When incubated with topoisomerase IIα prior to the addition of DNA, DTBN completely inactivated the enzyme within 120 s (T1/2 = 30 s) (Fig. 6). Once again, this finding indicates that DTBN acts as a covalent poison.
Fig. 6.

DTBN inhibits the activity of human topoisomerase IIα when incubated with the enzyme prior to the addition of DNA. Data represent incubation times for 500 μM DTBN and topoisomerase IIα prior to the addition of DNA. The effects on double-stranded breaks are shown. DNA cleavage levels were calculated relative to that at time zero which was set to 1.0. Error bars represent standard deviations of three independent experiments.
Third, because of their mechanism of action, covalent poisons require the presence of the N-terminal domain of topoisomerase II.46–48, 50 Therefore, we examined the ability of DTBN to induce DNA cleavage with the catalytic core of human topoisomerase IIα. The catalytic core (residues 431 to 1193) includes the active site tyrosine residues that act as the nucleophiles for DNA scission as well as the TOPRIM domain that contains the residues necessary to bind the active site divalent metal ions.48
Although the catalytic core of the enzyme is competent to cleave and ligate DNA substrates, it lacks both the N-terminal and C-terminal domains of the enzyme and cannot carry out the DNA strand passage reaction.48 As seen in Fig. 7, DTBN concentrations as high as 1 mM were unable to induce enzyme-mediated DNA cleavage with the catalytic core. This result is in contrast to that with etoposide, which acts at the DNA cleavage active site2, 10, 43, 44 and induced DNA scission at 50 and 100 μM.
Fig. 7.
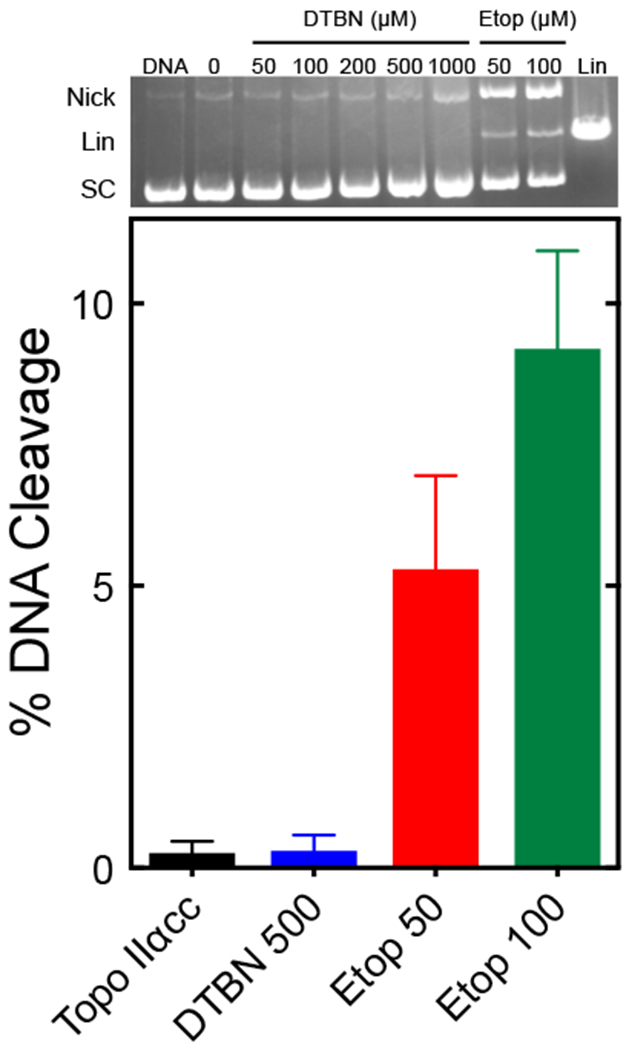
DTBN acts outside the active site of topoisomerase IIα. DNA cleavage assays utilized a human topoisomerase IIα deletion construct that was lacking both the N- and C-terminal domains (catalytic core; Topo IIαcc). The bar graph shows results for reactions that contained no compound (Topo IIαcc, black), 500 μM DTBN (DTBN 500, blue), or 50 or 100 μM etoposide (Etop 50, red; Etop 100, green). Error bars represent standard deviations of three independent experiments. A representative ethidium bromide-stained gel is shown at the top. The gel shows an assay with DNA alone (DNA), and assays containing Topo IIαcc and 0-1000 μM DTBN or 50 or 100 μM etoposide. A linear standard (Lin) is shown. DNA positions are as indicated in Fig. 2.
To ensure that results with the catalytic core were due to the loss of the N-terminal domain, we examined the effects of DTBN on a topoisomerase IIα deletion construct that contained the N-terminal portion of the protein but lacked the C-terminal domain.51 The compound still retained the ability to induce cleavage with the C-terminal deletion construct (Fig. 8). As a final control, we determined whether DTBN could interfere with the actions of etonoside in the catalytic core. As discussed above, etoposide interacts at the DNA cleavage-ligation active site of topoisomerase II2, 10, 43, 44 and covalent poisons act at residues outside of the active site and enhance DNA cleavage by impacting the actions of the N-terminal domain. Therefore, if DTBN (which does not induce DNA cleavage with the catalytic core, Fig. 7) is acting as a covalent poison, it should not interfere with the actions of etoposide in the catalytic core of topoisomerase IIα.48 As seen in Fig. 9, this was the case. DTBN had no effect on the ability of etoposide to induce enzyme-mediated DNA cleavage.
Fig. 8.
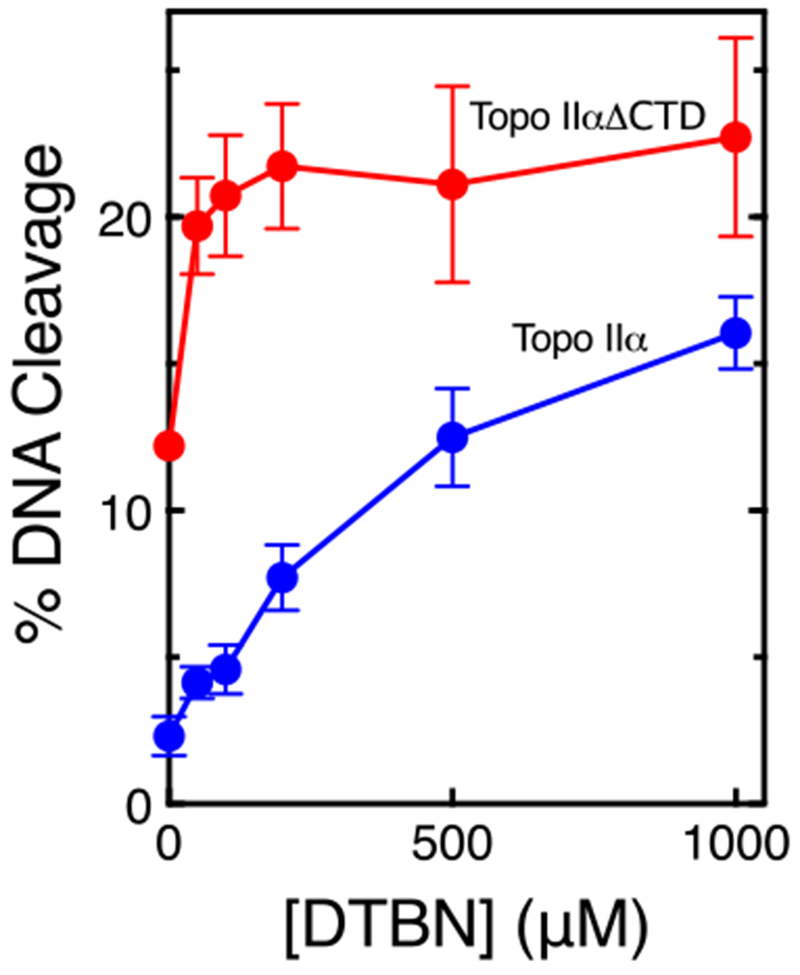
DTBN enhances DNA cleavage with a deletion construct of topoisomerase IIα lacks the C-terminal domain. Assays utilized either the deletion construct (Topo IIαΔCTD, red) or full-length topoisomerase IIα (Topo IIα, blue). DNA cleavage levels were compared to linear standards, which were set to 100%. Error bars represent standard deviations of three independent experiments.
Fig. 9.

DTBN does not compete with etoposide at the DNA cleavage-ligation active site of the enzyme. DNA cleavage assays utilized the human topoisomerase IIα deletion construct that was lacking both the N- and C-terminal domains (Topo IIαcc). Assays were carried out in the absence of compound (Topo IIαcc, black), or in the presence of 50 μM etoposide (Etop 50, red), 500 μM DTBN (DTBN 500, blue), or both 50 μM etoposide and 500 μM DTBN simultaneously (Both, purple). DNA cleavage levels were compared to linear standards, which were set to 100%. Error bars represent standard deviations of three independent experiments. A representative ethidium bromide-stained gel is shown at the top. DNA positions are as indicated in Fig. 2.
Taken together, the findings described above provide strong evidence that DTBN is a covalent poison of human type II topoisomerases. They further suggest that at least some of the biological/medicinal effects of the compound may result from this activity. Finally, DTBN is unusual in that it has a greater effect on topoisomerase IIα than topoisomerase IIβ, which may mitigate some of the leukemogenic potential of the β isoform.
Supplementary Material
Acknowledgements
The authors declare no competing financial interests. This research was supported by National Institutes of Health grant GM126363 (N.O.), and funding from ICA in Israel, the Deutsche Forschungsgemeinschaft, and the Richard H. Holzer Foundation (A.G.-G. and J.G.). We are grateful to Dr. Heather J. McCartney for her work on the initial screen, to Dr. Joseph E. Deweese for generously providing the catalytic core of human topoisomerase IIα, to Dr. Wolfgang Eisenreich for confirming the identity and purity of DTBN by NMR analyses, and to Dr. Elizabeth G. Gibson, Alexandria A. Oviatt, and Justin W. Lopez for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Experimental details for the sources of materials and the biochemical methods are available in the accompanying Supplementary Data.
References
- 1.Deweese JE, Osheroff MA, Osheroff N. Biochem. Mol. Biol. Educ 2008;37:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deweese JE, Osheroff N. Nucleic Acids Res. 2009;37:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitiss JL. Nat. Rev. Cancer. 2009;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos SM, Tretter EM, Schmidt BH, Berger JM. Nat. Rev. Mol. Cell. Biol 2011;12:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen SH, Chan N-L, Hsieh T-S. Ann. Rev. Biochem 2013;82:139. [DOI] [PubMed] [Google Scholar]
- 6.Pommier Y, Sun Y, Huang S-yN, Nitiss JL. Nat. Rev. Mol. Cell Biol 2016;17:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin CA, Marsh KL. Bioessays. 1998;20:215. [DOI] [PubMed] [Google Scholar]
- 8.Gentry AC, Osheroff N, DNA topoisomerases: Type II, in: a.L.M.D. Lennarz WJ (Ed.) Encyc. Biol. Chem, Academic Press, Waltham, MA, 2013, pp. 163. [Google Scholar]
- 9.Chen SH, Chan NL, Hsieh TS. Annu. Rev. Biochem 2013;82:139. [DOI] [PubMed] [Google Scholar]
- 10.Ketron AC, Osheroff N. Phytochem. Rev 2014;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heng X, Le WD. Neurosci. Bull 2010;26:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Li W, Prescott ED, Burden SJ, Wang JC. Science. 2000;287:131. [DOI] [PubMed] [Google Scholar]
- 13.Pommier Y, Leo E, Zhang H, Marchand C. Chem. Biol 2010;17:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pommier Y ACS Chem. Biol. 2013;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitiss JL. Nat. Rev. Cancer. 2009;9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendleton M, Lindsey RH Jr., Felix CA, Grimwade D, Osheroff N. Ann. N.Y. Acad. Sci 2014;1310:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan SK, Mays AN, Ottone T, Ledda A, La Nasa G, Cattaneo C, Borlenghi E, Melillo L, Montefusco E, Cervera J, Stephen C, Satchi G, Lennard A, Libura M, Byl JA, Osheroff N, Amadori S, Felix CA, Voso MT, Sperr WR, Esteve J, Sanz MA, Grimwade D, Lo-Coco F. Blood. 2008;112:3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldwin EL, Osheroff N. Curr. Med. Chem. Anticancer Agents. 2005;5:363. [DOI] [PubMed] [Google Scholar]
- 19.Azarova AM, Lin RK, Tsai YC, Liu LF, Lin CP, Lyu YL. Biochem. Biophys. Res. Commun 2010;399:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Nat. Med 2012;18:1639. [DOI] [PubMed] [Google Scholar]
- 21.Cowell IG, Austin CA. Int. J. Environ. Res. Public Health. 2012;9:2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowell IG, Sondka Z, Smith K, Lee KC, Manville CM, Sidorczuk-Lesthuruge M, Rance HA, Padget K, Jackson GH, Adachi N, Austin CA. Proc. Natl. Acad. Sci. USA 2012;109:8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin CA, Patel S, Ono K, Nakane H, Fisher LM. Biochem. J 1992;282 ( Pt 3):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandele OJ, Osheroff N. Biochemistry. 2007;46:6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandele OJ, Clawson SJ, Osheroff N. Chem. Res. Toxicol 2008;21:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandele OJ, Osheroff N. Chem. Res. Toxicol 2008;21:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Lazaro M, Calderon-Montano JM, Burgos-Moron E, Austin CA. Mutagenesis. 2011;26:489. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Lazaro M, Willmore E, Jobson A, Gilroy KL, Curtis H, Padget K, Austin CA. J. Nat. Prod 2007;70:1884. [DOI] [PubMed] [Google Scholar]
- 29.Ketron AC, Gordon ON, Schneider C, Osheroff N. Biochemistry. 2013;52:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin RK, Zhou N, Lyu YL, Tsai YC, Lu CH, Kerrigan J, Chen YT, Guan Z, Hsieh TS, Liu LF. J. Biol. Chem 2011;286:33591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vann KR, Sedgeman CA, Gopas J, Golan-Goldhirsh A, Osheroff N. Biochemistry. 2015;54:4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozer J, Fishman D, Eilam B, Golan-Goldhirsh A, Gopas J. J. Cancer. 2017;8:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson LM. J. Ethnobiol. Ethnomed 2006;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Beyrouthy M, Arnold N, Delelis-Dusollier A, Dupont F. J. Ethnopharm 2008;120:315. [DOI] [PubMed] [Google Scholar]
- 35.Nakae H, Yokoi A, Kodama H, Horikawa A. Evid. Based. Complement. Alternat. Med 2012;2012:837958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-On J, Ozer L, Gopas J, Sneir R, Golan-Goldhirsh A. Phytomedicine. 2009;16:788. [DOI] [PubMed] [Google Scholar]
- 37.El-On J, Ozer L, Gopas J, Sneir R, Enav H, Luft N, Davidov G, Golan-Goldhirsh A. Ann. Trop. Med. Parasitol 2009;103:297. [DOI] [PubMed] [Google Scholar]
- 38.Ozer L, El-On J, Golan-Goldhirsh A, Gopas J. Exp. Parasitol 2010;126:510. [DOI] [PubMed] [Google Scholar]
- 39.Ozer J, Levi T, Golan-Goldhirsh A, Gopas J. J. Ethnopharmacol 2015;161:86. [DOI] [PubMed] [Google Scholar]
- 40.Levy DH, Chapple ILC, Shapira L, Golan-Goldhirsh A, Gopas J, Polak D. J. Clin. Periodontol 2019;46:62. [DOI] [PubMed] [Google Scholar]
- 41.Osheroff N. Biochemistry. 1987;26:6402. [DOI] [PubMed] [Google Scholar]
- 42.Bandele OJ, Osheroff N. Biochemistry. 2008;47:11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pommier Y, Marchand C. Nat. Rev. Drug. Discov 2012;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CC, Li TK, Farh L, Lin LY, Lin TS, Yu YJ, Yen TJ, Chiang CW, Chan NL. Science. 2011;333:459. [DOI] [PubMed] [Google Scholar]
- 45.Lindsey RH Jr., Bromberg KD, Felix CA, Osheroff N. Biochemistry. 2004;43:7563. [DOI] [PubMed] [Google Scholar]
- 46.Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Biochemistry. 2006;45:10140. [DOI] [PubMed] [Google Scholar]
- 47.Bender RP, Osheroff N. Chem. Res. Toxicol 2007;20:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsey RH, Pendleton M, Ashley RE, Mercer SL, Deweese JE, Osheroff N. Biochemistry. 2014;53:6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Mao Y, Chen AY, Zhou N, LaVoie EJ, Liu LF. Biochemistry. 2001;40:3316. [DOI] [PubMed] [Google Scholar]
- 50.Mondrala S, Eastmond DA. Chem. Biol. Interact 2010;184:259. [DOI] [PubMed] [Google Scholar]
- 51.Dickey JS, Osheroff N. Biochemistry. 2005;44:11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


