Abstract
Background:
Radiation therapy (RT) confers local tumor control and survival advantages in some patients with osteosarcoma, yet pediatric and adolescent and young adult (AYA) population studies are limited.
Methods:
Twenty-eight patients treated with curative-intent RT (median dose, 59.4 Gy; range, 40–76 Gy) at our institution from 1990 to 2017 were retrospectively identified. Cumulative incidence (CIN) of local failure (LF) was estimated by Gray’s method and overall survival (OS) by the Kaplan-Meier method. Competing-risk regression and Cox proportional hazards models determined predictors of outcome. Toxicity was reported according to CTCAE v4.0.
Results.
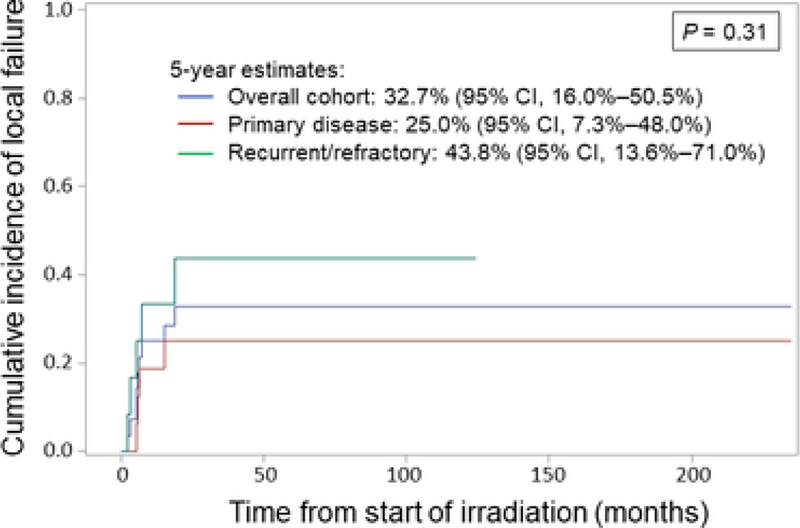
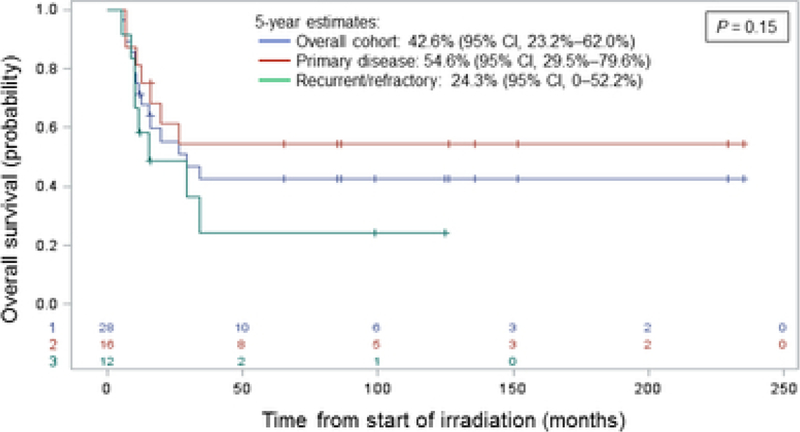
With a median follow-up of 99.1 months in living patients, nine patients (32.1%) developed LF. Estimated CINs of LF with competing risk of death at 5 years for the entire cohort, patients at initial diagnosis (n=16), and recurrent/refractory patients (n=12) were 32.7% (95% CI, 16.0%–50.5%), 25.0% (95% CI, 7.3%–48.0%), and 43.8% (95% CI, 13.6%–71.0%), respectively (P = 0.31). Estimated 5-year OS was 42.6% (95% CI, 23.2%–62.0%), 54.6% (95% CI, 29.5% –79.6%), and 24.3% (95% CI, 0–52.2%), respectively (P= 0.15). No clinicopathologic features were significantly associated with LF, yet lack of chemotherapy or metastasis at the time of RT were independent significant prognostic factors of decreased OS. Eleven patients experienced RT-related morbidity, with two grade 3 toxicities and no grade 4/5 events.
Conclusions.
Curative-intent RT in pediatric and AYA patients was well tolerated and achieved a local tumor control rate of 75% in primary patients. Local control rates were similar to those in primarily adult studies, with similar or lower doses.
Keywords: osteosarcoma, pediatric, radiation therapy, curative-intent, toxicity
1. INTRODUCTION
Osteosarcomas are tumors originating from the bony mesenchyme, which produces osteoid and immature bone growth from the outer cortex of the bone.1 Osteosarcomas are the most common primary malignant neoplasm of bone in children, adolescents, and young adults, and they also have a high prevalence among older patients (≥65 years of age). In children and adolescents, the incidence rate of osteosarcoma is 2.4%, making it the eighth most prevalent pediatric cancer.2 Chemotherapy and surgical resection are the mainstays of treatment for osteosarcomas, resulting in 5-year overall survival of 68%.2 Multi-agent chemotherapeutic agents, including doxorubicin, high-dose methotrexate with leukovorin rescue, cisplatin, and ifosfamide, have been used to faciliate complete resection and to enhance postoperative local control.3 However, several factors limit the applicability of surgical resection, including restricted anatomic primary sites (head and neck, shoulder, pelvis, or vertebrae), which may result in resection with positive margins or gross residual disease or in excessively morbid surgery, poor tumor response to chemotherapy, or the patient declining surgery. Additionally, in some patients with widely metastatic disease or recurrent/refractory disease, the disease burden may limit the indication for surgical resection. Some cases of multiple locally recurrent or metastatic lesions do not warrant surgery because of the overall low benefit-to-complications ratio. In these cases, radiotherapy (RT) may be considered, often in combination with chemotherapy, for local tumor control.
Picci et al. suggested that the risk of local recurrence of osteosarcoma was associated with limited surgical margins after resection and poor response to chemotherapy as assessed by tumor necrosis, suggesting that an enhanced tumor response and wide-margin resections are ideal for patients with osteosarcoma.4 The Cooperative Osteosarcoma Study Group (COSS) has shown that definitive RT improves outcomes for unresectable tumors in the pelvis and spine, improving 5-year overall survival from zero to 29% (P = 0.003).5,6 This suggests that osteosarcoma does respond moderately to RT, which has better, albeit still poor, applicability as the primary treatment for patients with unresectable disease. Delaney et al. noted that unresectable or positive-margin osteosarcomas treated with RT had a local control rate of 68% ± 8.3% at 5 years.7 A similar study by Ciernik et al. showed a local control rate after 5 years of 72%, 5-year disease-free survival of 65%, and 5-year overall survival of 67%.8 The same study showed that late grade 3 or 4 treatment-related toxicities were observed in 30.1% of patients. In a study by Mahajan et al., RT was shown to improve symptom palliation, resulting in improvements in pain related to osteosarcomas in 76% of patients and suggesting that local control is not the only indication for RT in patients with osteosarcoma.9 Collectively, these studies highlight the potential utility of RT with standard-of-care therapy for osteosarcoma and begin to address potential complications associated with this intervention.
Despite the prevailing notion that osteosarcomas as a group are uniformly radioresistant, the results of the above-mentioned studies and others suggest that RT can play an important role in the management of osteosarcoma in some patients. However, prospective data are lacking, and reports are particularly limited regarding the indications and outcomes in children or adolescents and young adults (AYAs) with osteosarcoma treated with RT. With the goal of better understanding the role of RT in the management of osteosarcoma and the treatment consequences of this modality in this vulnerable population, we reviewed patients with osteosarcoma treated with curative-intent RT at our institution. Here, we provide details of the disease outcomes, patterns of disease failure, predictors of local failure and survival, and RT-associated toxicities.
2. METHODS
2.1. Study description and patient population
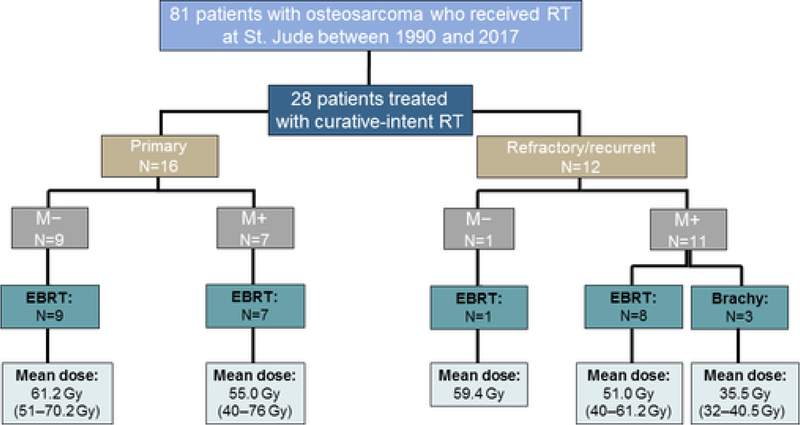
This institutional review board-approved retrospective study reviewed 28 patients aged between 7 and 19 years at initial diagnosis who had a histologically confirmed osteosarcoma and were treated with curative-intent RT at St. Jude Children’s Research Hospital between 1990 and 2017 (Figure 1). Curative-intent RT was defined as the use of RT in patients with primary disease and patients with recurrent/refractory disease who may, upon multidisciplinary review, achieve enhanced survival outcomes from definitive RT (≥40 Gy) as a component of therapy. Patients with newly diagnosed osteosarcoma (with or without metastasis) and those with refractory/recurrent tumors (with or without metastasis) were included. Indications for curative-intent RT included close/positive tumor margins after surgical resection, unresectable tumors, poor response to neoadjuvant chemotherapy (determined via the percentage of tumor necrosis in excised specimens), and/or consolidation of metastatic site disease. All patients received chemotherapy as part of their primary therapy. Tumor staging was performed according to the AJCC Cancer Staging Manual (7th edition).10 The definitions of the extent of surgical resection were based on descriptions by Enneking,11 with margin status being identified via pathologic assessment. The histologic response to neoadjuvant chemotherapy was recorded as tumor necrosis less than or greater than or equal to 90% upon pathologic examination of the excised tumor.12 The location of the primary tumor and the irradiated site were also recorded. Departmental and hospital charts and records were reviewed to assess local control, overall survival, and treatment-related morbidity.
Figure 1.
Consort diagram and radiation therapy management of the study cohort. RT, radiation therapy; M, metastasis; EBRT, external beam radiation therapy; Brachy, brachytherapy.
2.2. Study therapy and follow-up
Radiotherapy was delivered to all patients after they received chemotherapy with or without surgical resection. With the exception of doxorubicin, chemotherapy was delivered concurrently with RT in patients with newly diagnosed disease. External beam radiation therapy (EBRT) was delivered to patients as 3D conformal radiotherapy (3D CRT), intensity-modulated radiation therapy (IMRT), or intensity-modulated proton therapy (IMPT). The EBRT doses ranged from 40 to 76 Gy and were delivered in daily fractions of between 1.5 and 8.0 Gy. The duration of EBRT ranged from 6 to 64 days. Interstitial high dose-rate brachytherapy was used in three patients, with doses ranging from 34 to 40.5 Gy delivered in 3.4 to 4.5 Gy fractions twice daily.
Radiotherapy target volumes were delineated based on co-registration of computed tomography (CT) with magnetic resonance imaging (MRI) treatment planning data sets obtained with the patient in the treatment position. Generally, the gross tumor volume (GTV) encompassed the postoperative surgical bed or gross tumor. An anatomically constrained, 2.0-cm clinical target volume (CTV) margin was added to the GTV and/or resection cavity without specific targeting of incisions, drain sites, or adjacent nodal sites. A patient-specific planning target volume (PTV) margin that ranged from 0.4 to 1.0 cm was added to the CTV for photon irradiation, whereas robust optimization with 5-mm/3% setup/range uncertainty was employed for proton irradiation.
Treatment-related toxicities were assessed weekly during RT and at each follow-up visit. Toxicities were defined as acute (occurring within 3 months of starting RT) or late (occurring more than 3 months after starting RT). The Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4), was used to classify and grade RT-related toxicities. Local failure (LF) and distant failure were defined relative to the high-dose RT field employed at the treatment site. Biopsy or, alternatively, CT and/or MRI demonstrating tumor growth or new sites of disease that resulted in a change in therapeutic management was used to assess the date of local and/or distant progression of disease. The development of any subsequent malignant neoplasm after the initiation of RT was noted.
2.3. Statistical analysis
The cumulative incidence (CIN) of LF was estimated using the competing-risks method and was compared using Gray’s test. Competing risk included death of any cause, and the duration of the CIN was defined as the time from the start of RT to progression at the irradiated site, or death if occurring prior to LF, or last follow-up for patients without LF or death. Overall survival was defined as the time from the start of RT to death of any cause or date of last follow-up for survivors. Probability estimates of overall survival with 95% confidence intervals (CIs) were calculated using the Kaplan-Meier method and were compared using the log-rank test. A regression model for competing risks proposed by Fine and Gray was used to identify independent predictors of LF, and a Cox proportional hazards model was used to identify predictors of overall survival. Risk estimates, expressed as hazards ratios (HRs) and 95% CIs, were reported. Patients who had recurrent disease or who died within 3 months of the end of RT were not included in the analysis of late toxicity. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A two-sided significance level of P < 0.05 was considered to indicate statistical significance.
3. RESULTS
3.1. Patient and treatment characteristics
Table 1 summarizes the overall clinicopathologic and treatment characteristics of the study cohort at primary diagnosis. The median age of the 28 patients was 13.4 years, with a range from 7 to 19 years. The male-to-female ratio was 1.5:1. Most patients (42.9%) presented with primary tumors of the extremities. Head and neck sites and pelvic sites each made up approximately 20% of the cases. Only one patient presented with stage I disease, whereas 10 patients (35.7%) had metastatic disease at diagnosis. Twenty-two patients (78.6%) underwent surgical resection at the primary tumor site, and positive margins were found in two patients at the time of primary disease management and two at the time of local tumor recurrence. At diagnosis, fifteen patients (53.6%) were treated with individualized systemic therapy treatment plans with standard cisplatin, high-dose methotrexate, and doxorubicin (MAP) chemotherapy. The other 13 patients (46.4%) were enrolled on prospective clinical trials investigating carboplatin in lieu of cisplatin (n=6),13 omission of methotrexate (n=3),14 addition of bevacizumab to MAP chemotherapy (n=3),15 and neoadjuvant vincristine, ifosfamide, and doxorubicin (n=1 with extraosseous osteosarcoma).16 A histologic response of at least 90% necrosis after neoadjuvant chemotherapy was observed in half of the patients who underwent tumor resection.
TABLE 1.
Clinicopathologic features at diagnosis and radiotherapy characteristics.
| Characteristic | N | % or Median (Range) | |
|---|---|---|---|
| Age | 28 | 13.4 (7–19) years | |
| Gender | Male | 17 | 61% |
| Female | 11 | 39% | |
| Site of primary tumor | Head/neck | 6 | 21% |
| Spine/trunk | 4 | 14% | |
| Extremity | 12 | 43% | |
| Pelvis | 6 | 22% | |
| Primary tumor size | 28 | 5.2 (1.1–24.0) cm | |
| AJCC stage | IA | 1 | 4% |
| IIA | 10 | 36% | |
| IIB | 7 | 25% | |
| IVA | 10 | 35% | |
| Surgery for primary tumor | Yes | 22 | 79% |
| No | 6 | 21% | |
| Primary surgical margins | Positive | 2 | 9% |
| Negative | 20 | 91% | |
| Histologic response | <90% | 8 | 36% |
| ≥90% | 11 | 50% | |
| N/A | 3 | 14% | |
| Disease course @ RT | Primary | 16 | 57% |
| Recurrent | 12 | 43% | |
| Indications for RT* | Unresectable primary | 6 | 21% |
| Positive/close margins | 12 | 43% | |
| Poor histologic response | 5 | 14% | |
| Metastatic consolidation | 9 | 32% | |
| Irradiated site | Head/neck | 5 | 18% |
| Spine/trunk | 6 | 21% | |
| Extremity | 7 | 25% | |
| Pelvis | 8 | 29% | |
| Lung | 2 | 7% | |
| Radiotherapy | Dose/fraction | 28 | 2.7 (1.1–8.0) Gy |
| Total EBRT dose | 25 | 59.4 (40.0–76.0) Gy | |
| Total brachy dose | 3 | 34.0 (32.0–40.5) Gy | |
| RT technique | 3D CRT | 14 | 50% |
| IMRT (SBRT) | 10 (4) | 36% (14%) | |
| Brachy | 3 | 11% | |
| IMPT | 1 | 3% |
RT, radiation therapy; EBRT, external beam radiation therapy; 3D CRT, 3D conformal radiotherapy; Brachy, brachytherapy; IMRT, intensity-modulated radiotherapy; IMPT, intensity-modulated proton therapy; N/A, not available;
Indications for RT for 5 patients included both poor histologic response and positive/close margins.
Regarding the timing of RT, 16 patients (57.1%) were treated during the primary disease course (i.e. RT was employed as a component of upfront therapy), whereas 12 (42.9%) received RT at the time of recurrent or refractory disease. Of the 12 patients with recurrent/refractory disease, the median number of treatment regimens prior to definitive management with RT was 1 (1 regimen: seven patients, 2 regimens: three patients, 5 regimens: two patients). Fourteen of the 16 patients with newly diagnosed disease received RT to the primary tumor site, while two patients underwent metastatic disease site RT. Treatment was delivered to the primary tumor site in 5 of 12 patients with recurrent/refractory disease and to sites of metastatic lesions in the other 7 patients. The pelvis was the most commonly irradiated site, and although seven patients underwent irradiation of the extremities, only one of these patients underwent RT during the primary disease course. All but three patients were treated with EBRT, with 3D CRT being the most commonly employed technique. The median EBRT dose was 59.4 Gy (range, 40–76 Gy) for the total cohort, with a median dose of 61.9 Gy, 54.1 Gy, and 49.0 Gy for patients treated with definitive, adjuvant, and metastatic site consolidative RT, respectively.
3.2. Local tumor failure and associated prognostic factors
With a median follow-up time of 17.8 months for the entire cohort and 99.1 months for living patients, nine patients (32.1%) developed local failure at the irradiated site. Eight of these nine patients also presented with stage IVA disease. Four of the nine patients received RT as a component of primary therapy, and five patients were treated at the time of recurrent/refractory disease. Eight of the nine patients ultimately developed distant failure after RT, and all eight eventually died of disease. The estimated CIN of LF with the competing risk of death for the entire cohort at 5 years was 32.7% (95% CI, 16.0%–50.5%) (Figure 2). The CIN at 5 years was 25.0% (95% CI, 7.3%–48.0%) for patients with primary disease and 43.8% (95% CI, 13.6%–71.0%) for patients with recurrent/refractory disease (P= 0.31). Local tumor progression was observed in 3/13 patients (23.1%) treated with adjuvant RT after resection, while 3/6 patients (50%) treated with definitive RT experienced local progression. On regression analysis with competing risk, none of the following clinicopathologic variables were significantly associated with LF: age at primary diagnosis, patient sex or race, irradiated tumor size, site, or stage, receipt of chemotherapy or surgery at the time of RT, or cumulative RT dose or modality.
Figure 2.
Cumulative incidence of local tumor failure for the overall cohort (blue), for patients with primary disease (red), and for patients with recurrent/refractory disease (green). CI, confidence interval.
3.3. Survival outcomes and associated prognostic factors
The Kaplan-Meier estimates for overall survival at 5 years were 42.6% (95% CI, 23.2%– 62.0%) for the total cohort, 54.6% (95% CI, 29.5% –79.6%) for the patients with primary disease, and 24.3% (95% CI, 0–52.2%) for the patients with recurrent/refractory disease (P= 0.15) (Figure 3). Fifteen patients have died, 14 as a result of progression of osteosarcoma and one of a subsequent glioblastoma that developed outside the RT field. Of these fourteen patients who died of progressive disease, seven patients were treated with RT for primary osteosarcoma and seven for recurrent/refractory disease. All 14 of these patients developed metastatic disease or experienced progression of existing metastatic disease. Cox regression analysis found the lack of receipt of chemotherapy at the time of RT and metastasis at the time of RT to be significant independent predictors of inferior OS, whereas irradiated tumor size (in cm) was marginally associated with overall survival (Table 2).
Figure 3.
Overall survival estimates for the overall cohort (blue), for patients with primary disease (red), and for patients with recurrent/refractory disease (green). CI, confidence interval; +, censored patients.
TABLE 2.
Cox proportional hazards regression model of overall survival
| Variable | Parameter estimate | Standard error | P-value | Hazard ratio |
|---|---|---|---|---|
| Chemotherapy at time of RT | −1.19 | 0.53 | 0.03 | 0.31 |
| Irradiated tumor size (cm) | 0.07 | 0.04 | 0.07 | 1.07 |
| Metastasis at time of RT | 1.28 | 0.65 | 0.05 | 3.59 |
3.4. Treatment-related toxicities
Eleven different patients experienced complications potentially attributable to RT (Table 3; described in detail in Supplemental Table S1). There were 16 RT-associated toxicities in total (with three patients having two different events each and one patient having three events). Thirteen of the events occurred in the acute setting (during RT treatment or within 3 months of starting RT), and three events occurred in the late setting (≥3 months after starting RT). Only two significant, grade 3 toxicities occurred, both in the late setting. One patient, a 9-year old female, experienced growth arrest of the right breast that required reconstruction after irradiation to a cumulative dose of 59.4 Gy of the T12 vertebral body region of the spine. The other patient, a 20-year old male, experienced confirmed bilateral sensorineural hearing loss after irradiation to 59.4 Gy of the right maxilla. The same patient also received neoadjuvant and adjuvant cisplatin. No patients had grade 4 toxicity. The remaining 14 cases of toxicity were assessed as grade 1 or 2 toxicities. As expected, most of the events were related to radiation dermatitis, mucositis, and/or pain, accounting for 10 (62.5%) of the 16 cases. The single case of late grade 1 radiation necrosis in the central nervous system (brain) was observed radiographically and was without associated symptoms. RT-related toxicity was observed in only those patients who received EBRT. Five of the 11 individual patients underwent irradiation for unresectable tumors, and their disease was managed with chemotherapy and RT alone. No patient developed a subsequent malignant neoplasm within the initial RT field.
TABLE 3.
Adverse events associated with radiation therapy by grade according to Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE, v4.0)
| Acute (<3 months) | Grade I | Grade II | Grade III |
|---|---|---|---|
| Dermatitis | 1 | 3 | 0 |
| Mucositis | 0 | 2 | 0 |
| Pain | 1 | 3 | 0 |
| Diarrhea | 1 | 0 | 0 |
| Dysphagia | 1 | 0 | 0 |
| Procitis | 0 | 1 | 0 |
| Acute total | 4 | 9 | 0 |
| Late (≥3 months) | Grade I | Grade II | Grade III |
| Radiation necrosis | 1 | 0 | 0 |
| Breast hypoplasia | 0 | 0 | 1 |
| Hearing loss | 0 | 0 | 1 |
| Late total | 1 | 0 | 2 |
4. DISCUSSION
This study documents the local control outcomes of pediatric patients with osteosarcoma in both the upfront and recurrent settings and at sites of both primary and metastatic disease when combined with chemotherapy and/or surgery, with comparable results to limited studies in this population.6,9 This study also recapitulates data from several prior studies demonstrating that surgery is a critical component of local control of osteosarcoma, with local tumor progression observed in one-half of the patients treated with definitive RT. The LF rate for our cohorts was comparable to that in the study by Delaney et al., which demonstrated an actuarial LF rate of 32% at 5 years after treatment in patients with marginally resected or unresected osteosarcomas treated with RT.7 Importantly, the patients in that seminal study were primarily adults, with a median age of 29 years (compared to the median age of 13 years in our study), and that study included fewer patients with metastatic or recurrent/refractory disease. Additionally, the doses employed in that study were generally higher (median, 66 Gy) than those used in our study (median, 59.4 Gy). Thus, our findings suggest that curative-intent RT results in local tumor-control benefits for some pediatric and AYA patients and that these benefits are of a similar extent to those seen in older patients. Although perhaps clinically intuitive, this finding stands in distinction to some reports of RT treatment for related sarcomas, including pediatric desmoid tumors and adult Ewing sarcoma.17,18
Although local tumor control of osteosarcoma after surgical resection without supplemental RT is excellent, generally exceeding 90%,4,8 factors that limit the applicability of this approach are known to negatively influence tumor control. These factors include anatomic sites that prevent complete resection and disease extent that precludes limb/organ-salvage surgery.6,19 In such cases, RT is often variably employed, frequently with reservations arising from perceived radioresistance and/or associated morbidity. Thus, several reports have attempted to identify prognostic factors that influence the response of osteosarcoma to RT. In the report by Schwarz et al., in which 100 patients with osteosarcoma who received RT were identified within the COSS registry, RT modality (RT + surgery vs. RT alone) and RT indication (primary, locally recurrent, or metastatic disease) were significantly associated with local tumor control.14 The impact of surgical resection on local control outcomes in patients who received RT was also observed in the study by DeLaney et al.,7 as well as in the recent large study of patients with extremity osteosarcoma managed with neoadjuvant chemotherapy, resection, and adjuvant RT.20 In our study of 28 patients, which was restricted to the pediatric and AYA population, we observed no significant prognostic factors associated with local tumor control on regression analysis for competing risk. However, some numerical differences in LF by disease course were clearly noted, with a 1-year LF rate of 19.6% for patients with primary disease, compared to 33.5% for those with recurrent/refractory disease. Clearly, the small sample size was a limitation of our study, as was the case with several other reports exploring the role of RT in the management of osteosarcoma, and this precluded an analysis of LF with respect to the extent of resection. An additional acknowledged limitation of this descriptive cohort study includes selection bias, particularly related to the selection of a potentially curative patient population.
The concept that osteosarcoma is a uniformly radioresistant tumor has been challenged by in vitro studies. For example, Larsen et al. demonstrated that the α and β values, derived from clonogenic cell survival curves of three osteosarcoma cell lines, were similar to values obtained for cell lines derived from human tumors that are frequently cured with RT.21 Similarly, a separate study showed that the dose necessary to achieve 50% survival (D50%) in osteosarcoma cell lines was, in fact, much lower than the D50% of cell lines derived from human melanomas, which are also frequently viewed as radioresistant.22 Furthermore, a clear dose-response relation with local tumor control was not established in our study or in the studies by DeLaney et al.7 and Schwarz et al.19, yet this relationship may be more apparent with proton therapy.8 Although the clinical outcomes in patients with osteosarcomas managed with definitive chemoradiation are poor and the LF rates of tumors managed with surgery and RT are inferior to those for tumors managed with surgical resection alone, it is likely that this result is, at least in part, influenced by the more aggressive tumor biology manifested in tumors for which RT is ultimately employed. Thus, it is conceivable that potential heterogeneity of the RT response may be predicated by the heterogeneous genomic landscape of osteosarcoma.23,24 Moving forward, it may be important to take into account not only clinical factors but also the evolving molecular subgroups of osteosarcoma when assessing the utility of RT in this population.
With the addition of multi-agent, dose-intensive chemotherapy to surgical resection, improvements in 5-year survival of up to 70% have been achieved for localized disease, but patients with metastatic or recurrent disease fair much more poorly.25 Beyond the disease burden and treatment course, additional prognostic factors consistently associated with survival in patients with localized osteosarcoma include the histologic response to chemotherapy and the extent of primary site surgical resection.4 Similar to these findings and those of related studies, the disease burden and treatment course of our patient population influenced their survival outcomes. Eighteen (64.2%) of the 28 patients presented with metastasis to the lungs, other bone sites, or both at the time of RT, whereas 12 patients (42.9%) presented with recurrent/refractory disease. Although not reaching statistical significance, patients with primary disease were found to have a 5-year overall survival estimate of 55.6%, compared to 24.3% for those with recurrent/refractory disease (P= 0.15). Furthermore, Cox proportional hazards modeling revealed a significant association between metastatic disease at the time of RT and overall survival, with a hazard ratio of 3.6 (P =0.051). Additionally, the receipt of chemotherapy at the time of RT was significantly associated with survival (HR, 0.31; P= 0.03), and irradiated tumor size (cm) was marginally associated with survival (P= 0.07). Although the 5-year overall survival rate of our cohort is inferior to that reported by DeLaney et al.7 (43.7% vs 65.5%, respectively), this probably reflects the patient selection for the two studies; as discussed above, our study included a higher proportion of high-risk patients.
Finally, any assessment of the role of RT in the management of osteosarcoma in the pediatric and AYA population must include treatment-related toxicities. Although the inherent limitations of retrospective chart review regarding toxicity assessment are acknowledged, most (87.5%) of the toxicities attributed to RT in our study were of grade 1 or 2. There were only two cases of grade 3 toxicity, one of which included bilateral hearing loss, which was probably influenced by concurrent cisplatin administration. Importantly, there were no cases of grade 4 toxicity. Overall, although our patient cohort tolerated RT relatively well, requiring little or no medical intervention for treatment complications, the morbidity of RT in this study and others was not insignificant.7,20,26 With the advances in RT techniques20 and the incorporation of charged-particle therapy,8,27 it is hoped that continued progress in enhancing the therapeutic ratio through the reduction in treatment morbidity will improve outcomes for those patients with osteosarcoma who may benefit from RT.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript.
SUPPORT:
This work was supported in part by the American Lebanese Syrian Associated Charities (ALSAC), the National Cancer Institute grant P30 CA021765 (St. Jude Cancer Center Support Grant), and the National Cancer Institute grant R25CA23944 (JL).
Abbreviations
- 3D CRT
Three-dimensional conformal radiation therapy
- AYA
Adolescent and young adult
- Brachy
Brachytherapy
- CI
Confidence interval
- CIN
Cumulative incidence
- CT
Computed tomography
- CTV
Clinical target volume
- EBRT
External beam radiation therapy
- GTV
Gross tumor volume
- HR
Hazard ratio
- IMPT
Intensity-modulated proton therapy
- IMRT
Intensity-modulated radiation therapy
- LF
Local failure
- LF
Local failure
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PTV
Planning target volume
- RT
Radiation therapy
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
DATA SHARING STATEMENT:
Data sharing is not applicable to this article as no new data were created in this study.
REFERENCES
- 1.Link M, Grier HE, Donaldson S. Sarcomas of bone. In: Fernbach D, Vietti T, eds. Clinical Pediatric Oncology St. Louis, MO: Mosby; 1991. [Google Scholar]
- 2.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009;152:3–13. [DOI] [PubMed] [Google Scholar]
- 3.Ritter J, Bielack SS. Osteosarcoma. Ann. Oncol 2010;21 Suppl 7:vii320–vii325. [DOI] [PubMed] [Google Scholar]
- 4.Picci P, Sangiorgi L, Bahamonde L, et al. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann Oncol 1997;8(9):899–903. [DOI] [PubMed] [Google Scholar]
- 5.Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer 2002;94(4):1069–1077. [PubMed] [Google Scholar]
- 6.Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol 2003;21(2):334–341. [DOI] [PubMed] [Google Scholar]
- 7.DeLaney TF, Park L, Goldberg SI, et al. Radiotherapy for local control of osteosarcoma. Int J Radiat Oncol Biol Phys 2005;61(2):492–498. [DOI] [PubMed] [Google Scholar]
- 8.Ciernik IF, Niemierko A, Harmon DC, et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 2011;117(19):4522–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahajan A, Woo SY, Kornguth DG, et al. Multimodality treatment of osteosarcoma: radiation in a high-risk cohort. Pediatr. Blood Cancer 2008;50(5):976–982. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 11.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986(204):9–24. [PubMed] [Google Scholar]
- 12.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 1977;101(1):14–18. [PubMed] [Google Scholar]
- 13.Meyer WH, Pratt CB, Poquette CA, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. J Clin Oncol 2001;19(1):171–182. [DOI] [PubMed] [Google Scholar]
- 14.Daw NC, Neel MD, Rao BN, et al. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children’s Research Hospital OS99 trial. Cancer 2011;117(12):2770–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navid F, Santana VM, Neel M, et al. A phase II trial evaluating the feasibility of adding bevacizumab to standard osteosarcoma therapy. Int J Cancer 2017;141(7):1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pappo AS, Devidas M, Jenkins J, et al. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol 2005;23(18):4031–4038. [DOI] [PubMed] [Google Scholar]
- 17.Merchant TE, Nguyen D, Walter AW, Pappo AS, Kun LE, Rao BN. Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys 2000;47(5):1267–1271. [DOI] [PubMed] [Google Scholar]
- 18.Sinkovics JG, Plager C, Ayala AG, Lindberg RD, Samuels ML. Ewing sarcoma: its course and treatment in 50 adult patients. Oncology 1980;37(2):114–119. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz R, Bruland O, Cassoni A, Schomberg P, Bielack S. The role of radiotherapy in oseosarcoma. Cancer Treat Res 2009;152:147–164. [DOI] [PubMed] [Google Scholar]
- 20.Sole CV, Calvo FA, Alvarez E, et al. Adjuvant radiation therapy in resected high-grade localized skeletal osteosarcomas treated with neoadjuvant chemotherapy: Long-term outcomes. Radiother Oncol 2016;119(1):30–34. [DOI] [PubMed] [Google Scholar]
- 21.Larsen RH, Bruland OS, Hoff P, Alstad J, Lindmo T, Rofstad EK. Inactivation of human osteosarcoma cells in vitro by 211At-TP-3 monoclonal antibody: comparison with astatine-211–labeled bovine serum albumin, free astatine-211 and external-beam X rays. Radiat Res 1994;139(2):178–184. [PubMed] [Google Scholar]
- 22.Larsen RH, Bruland OS, Hoff P, Alstad J, Rofstad EK. Analysis of the therapeutic gain in the treatment of human osteosarcoma microcolonies in vitro with 211At-labelled monoclonal antibody. Br J Cancer 1994;69(6):1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott MC, Sarver AL, Gavin KJ, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone 2011;49(3):356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bousquet M, Noirot C, Accadbled F, et al. Whole-exome sequencing in osteosarcoma reveals important heterogeneity of genetic alterations. Ann Oncol 2016;27(4):738–744. [DOI] [PubMed] [Google Scholar]
- 25.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 2018;18(1):39–50. [DOI] [PubMed] [Google Scholar]
- 26.Doi H, Oh RJ, Miura H, Masai N, Shiomi H, Inoue T. Outcomes and toxicity of radiotherapy for refractory bone and soft tissue sarcomas. Mol Clin Oncol 2016;4(1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsunobu A, Imai R, Kamada T, et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer 2012;118(18):4555–4563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.