Summary
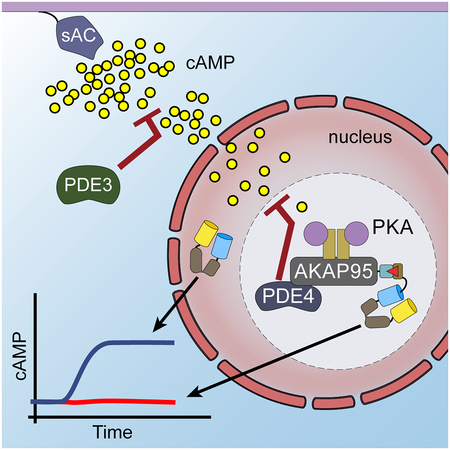
Contrary to the classic model of PKA residing outside of the nucleus, we identify a nuclear signaling complex that consists of AKAP95, PKA, and PDE4D5 and show that it forms a functional cAMP signaling microdomain. Locally generated cAMP can accumulate within the vicinity of this complex; but when cAMP is generated at the plasma membrane, PDE4 serves as a local sink and PDE3 as a barrier to prevent accumulation of cAMP within the microdomain as a means of controlling activation of tethered nuclear PKA.
Keywords: imaging, FRET biosensors, compartmentalization, spatiotemporal
Graphical Abstract
eTOC Blurb
Clister et al. identified that AKAP95 anchors PDE4 and PKA to create a distinct microdomain in the nucleus. This microdomain tightly regulates the accumulation of cAMP available to activate anchored PKA. The regulation of cAMP here depends on the anchored PDE working in concert with more distally located PDE isoforms.
Introduction
Protein kinase A (PKA) is a ubiquitous serine/threonine kinase involved in regulating multiple cellular processes. The PKA holoenzyme is a tetramer composed of a specific regulatory subunit isoform (RIα, RIβ, RIIα, or RIIβ) homodimer and two catalytic subunits, where each catalytic subunit is bound to a regulatory subunit in the dimer. Activation of PKA occurs when cAMP binds the regulatory subunits, unleashing the active catalytic subunits. In the classic understanding of the pathway, PKA signaling in the nucleus was thought to occur by activation of extranuclear PKA and diffusion of the catalytic subunit into the nucleus. Recent studies raise some questions as to whether or not the catalytic subunit of the PKA holoenzyme completely dissociates under physiological conditions (Smith et al., 2013, 2017). In the absence of translocated catalytic subunit, nuclear PKA signaling would need to be activated and regulated through alternative mechanisms. Our previous work suggests that a pool of activatable PKA holoenzyme exists in the nucleus, which can be activated by local cAMP generated in the cytosol or nucleus (Sample et al., 2012). Consistent with our findings, a recent study showed that high cAMP production resulting from synergistic actions of two GPCRs at the endosome can lead to the activation of nuclear PKA (Jean-Alphonse et al., 2016). However, the molecular components that localize this pool of PKA to the nucleus and the mechanism by which nuclear PKA is controlled locally remain to be identified.
AKAP95, a PKA RII binding protein, has been shown to localize to the nucleus. It has been associated with multiple functions, including chromatin condensation, RNA processing, and gene expression, and recently its upregulation has been correlated with increased cyclin D1 and E1 expression in multiple types of cancer (Akileswaran et al., 2001; Arsenijevic et al., 2004, 2006; Coghlan et al., 1994; Collas et al., 1999; Eide et al., 2002, 2003; Jiang et al., 2013; Marstad et al., 2016; Zhao et al., 2015). We hypothesized that AKAP95 is a nuclear AKAP that anchors a specific pool of PKA holoenzyme within the nucleus.
Results
AKAP95 forms an endogenous complex with PKA and PDE
To determine if AKAP95 forms a complex with PKA holoenzyme in the nucleus, we isolated nuclear proteins from HEK293T cells. These nuclear lysates endogenously contain AKAP95 (Figure 1a). The western blot results were further supported by immunofluorescence studies that detected AKAP95 in the nucleus (Figure 1b).
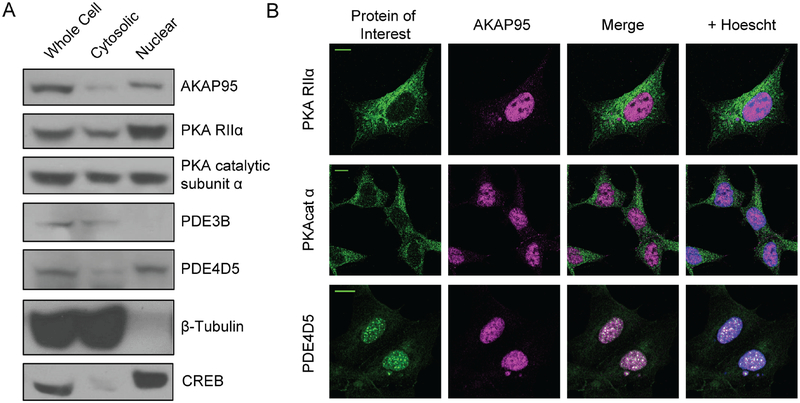
Figure 1: AKAP95, PKA, and PDE are present in the nucleus.
a) Western blot images of whole-cell, non-nuclear, and nuclear HEK293T lysates reveal localization of AKAP95, PKARIIα, and PKAcat within the nucleus. PDE4D5 is also detected in nuclear fractions, though PDE3B is not. β-tubulin antibody staining shows a lack of cytosolic protein in the nuclear fraction, and probing for CREB provides a positive control for nuclear isolation. b) Representative images showing immunofluorescence staining of endogenous protein similarly detects the candidate proteins in the nucleus. Scale bars, 10 μm (See Supplemental Figure 1 for quantification of nuclear localization of PKAcat and PKA RIIα).
To better characterize AKAP95 in the nucleus we performed fluorescence recovery after photobleaching (FRAP) experiments using GFP-tagged AKAP95. On average, bleached areas showed a half recovery time of 25.7 ± 5.7 s (Supplemental Figure 1a) (N=11 cells). Based on these experiments, the majority of AKAP95 is mobile in the nucleus, with an average mobile fraction of 65.8 ± 11.2%. Interestingly, the observed AKAP95 puncta do not easily recover after photobleaching (Supplemental Figure 1b and 1c), suggesting that AKAP95 within these puncta is less mobile.
In addition to AKAP95, endogenous RIIα and the catalytic subunit of the PKA holoenzyme (PKAcat) were also detected in the nucleus with nuclear fractionation followed by western blotting (Figure 1a) as well as immunofluorescence (Figure 1b). For RIIα and PKAcat, the nuclear staining is significantly less than the cytosolic staining, but is clearly above background (Supplemental Figure 1d, e). Previous studies suggest PDEs, specifically PDE4D isoforms, are directly involved in regulating nuclear PKA activity (Sample et al., 2012). We found evidence of PDE4D, specifically isoform PDE4D5, in the nucleus (Figure 1a) where PDE4D5 appears to form puncta (Figure 1b).
To determine if the different components can form a complex in the nucleus, co-immunoprecipitation studies on the nuclear lysates were performed. We first pulled down endogenous AKAP95 and found that it associates with endogenous PKA RIIα, PKAcat, and PDE4D5 (Figure 2a), which was further confirmed via reciprocal co-immunoprecipitation experiments in which we isolated RIIα or PDE4D5 and probed for the remaining components (Figure 2a). The signals were detected above the background from normal IgG controls, indicating the specificity of these interactions.
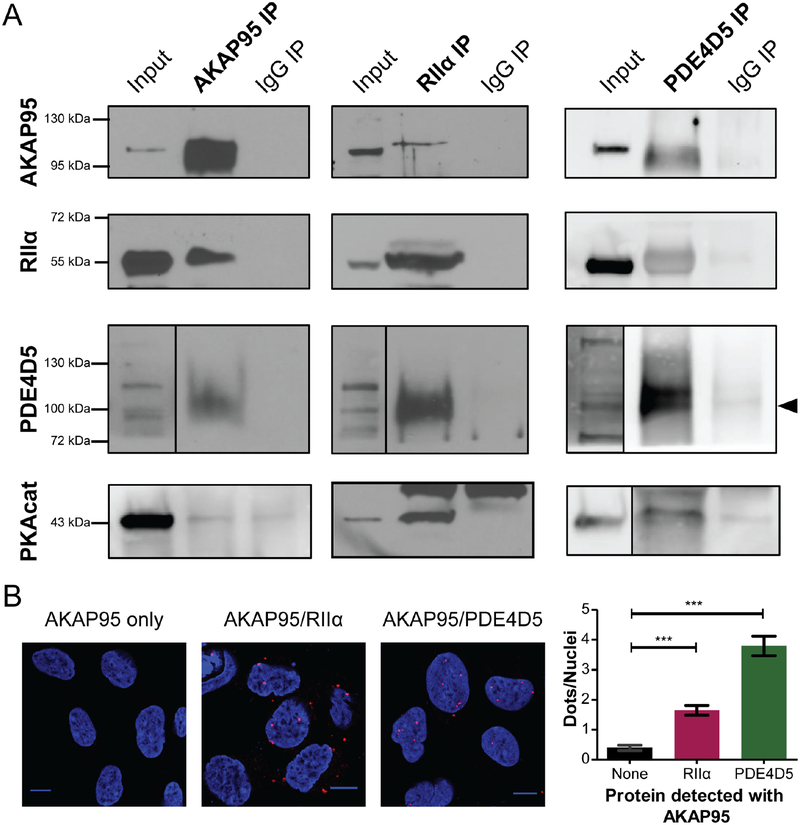
Figure 2: AKAP95 forms an endogenous complex with PKA and PDE.
a) Co-immunoprecipitation of nuclear fractions of HEK293T cells show interactions between AKAP95, RIIα and PDE4D5. The left column shows the AKAP95 IP, including lanes for the nuclear lysate input, AKAP95 IP, and the control IP using normal IgG. PDE4D5 (indicated by arrow), RIIα, and PKAcat are detected in the AKAP95 pulldown. The other components are also detected in immunprecipitates of RIIα or PDE4D5, shown in the right columns (western blot images representative of 2 or 3 repeated experiments). b) Proximity Ligation Assay (PLA) was performed with AKAP95 (N = 53 cells) and either RIIα (N = 66 cells) or PDE4D5 (N = 79 cells). Representative images are shown with quantification of nuclear PLA signal (average ± SEM). *** signifies p<0.0001. (See also Supplemental Figure 1). The co-IP and PLA experiments indicate that RIIα, AKAP95, PDE4D5 form a complex within the nucleus of HEK293T cells.
Further support for the endogenous nuclear AKAP95 complex containing PKA and PDE4D comes from proximity ligation assay (PLA) experiments. In these experiments complexes containing AKAP95 and RIIα were detected in intact, fixed cells (Figure 2b). On average 1.65 ± 0.17 dots per nuclei (N=66 cells) were observed when measuring the AKAP95 and RIIα interaction, which is significantly (p<0.0001) above the background signal observed with AKAP95 (Figure 2b) or RIIα (Supplementary Figure 1f–g) antibody alone (0.40 ± 0.09 dots/nuclei, N=53 cells). Interestingly, although by immunofluorescence AKAP95 and RIIα are mainly detected in the nucleus and cytoplasm, respectively, PLA signal was observed in both the nucleus and cytoplasm (Supplemental Figure 1f–g). Therefore, although there are limited amounts of AKAP95 and RIIα in specific subcellular compartments, they are still interacting in a significant way. PDE4D5 and AKAP95 were also observed to interact in the PLA experiments with an average of 3.80 ± 0.33 dots per nuclei (N=79 cells), which is significantly above the background signal produced by AKAP95 antibody alone (p<0.0001). These results suggest that AKAP95 forms a complex with a resident pool of nuclear PKA, as well as PDE4D5.
The AKAP95 microdomain tightly regulates cAMP
The identification of AKAP95 as a specific nuclear scaffold protein that binds to PKA and PDE allows us to directly test the hypothesis that PDEs co-anchored to the same AKAP complex help regulate the activation of this PKA pool by reducing local cAMP levels. First, we employed our previously developed mathematical model (Sample et al., 2012) to generate predictions of the localized cAMP dynamics within the nuclear AKAP compartment. This ordinary differential equation model was developed to test hypotheses for differences in cAMP dynamics between the cytosol and nucleus. We extended the model to include a FRET-based cAMP biosensor, ICUE3 (DiPilato and Zhang, 2009), in the nuclear AKAP compartment and used the previously developed parameters and initial conditions to predict how cAMP dynamics will differ between the general nucleus and AKAP95 compartments (Figure 3a, b). This model predicts that when a modest amount of cAMP is generated at the plasma membrane, the nucleus experiences increased cAMP accumulation, but the nuclear AKAP compartment does not. Furthermore, the model predicts that higher cAMP production can overcome PDE-mediated inhibition in the AKAP compartment, such that cAMP accumulates in both the general nucleus and nuclear AKAP compartments.
Figure 3: The AKAP95 microdomain tightly regulates cAMP.
a) Based on a previous computational model for nuclear PKA activity, a new model was developed to predict cAMP levels in the AKAP microdomain and nucleus when cAMP is generated at the plasma membrane in a dose-dependent manner using PM-sAC. b) The model suggests different cAMP responses in the nucleus (orange) and AKAP microdomain (black) upon low-dose (LD, 2.5 mM) and high-dose (HD, 15 mM) NaHCO3 stimulation of cAMP production by PM-sAC. c) Using the FKBP/FRB dimerization system to localize ICUE3 to AKAP95, the model predictions were directly tested under three different experimental conditions: cAMP generated by PM-sAC and detected by the cAMP biosensor ICUE3 targeted to either i.) the nucleus or ii.) the AKAP95 microdomain of HEK293T cells, or iii.) cAMP generated in the nucleus by sAC-NLS and detected by AKAP95-targeted ICUE3. (See Supplemental Figure 3 for representative images). d) ICUE3 emission ratio (cyan/yellow) responses in the nucleus (orange, N=39 cells) and in the AKAP95 microdomain (black, N=35 cells) stimulated by LD NaHCO3 (2.5 mM) followed by HD (15 mM) in HEK293T cells expressing PM-sAC. Shown is the average trace ± SEM. Comparison of the maximum responses to LD NaHCO3 shows a significant difference between the microdomain and nucleus (p<0.0001). e) ICUE3 emission ratio (cyan/yellow) responses in the AKAP95 microdomain stimulated by LD NaHCO3 (2.5 mM) followed by HD (15 mM) in HEK293T cells expressing PM-sAC (black, N=18 cells) or sAC-NLS (red, N=18 cells). The maximum response to LD NaHCO3 in the microdomain when cAMP is generated at the plasma membrane is significantly different compared with cAMP generated in the nucleus (inset, p<0.0001).
To test these predictions experimentally, we targeted the FRET-based cAMP biosensor ICUE3 to AKAP95 to measure cAMP levels around AKAP95. This biosensor contains a cAMP binding domain sandwiched between a FRET pair and reports cAMP dynamics via changes in FRET. Targeting of this biosensor to AKAP95 was achieved using a chemically induced dimerization approach based on FK506 binding protein (FKBP) and FKBP-rapamycin binding domain (FRB), two proteins that bind specifically and tightly in the presence of rapamycin (Banaszynski et al., 2005). This chemically inducible targeting system was chosen to avoid concerns of expressing a large fusion protein in the nuclear compartment. Although not utilized here, this system could offer some flexibility for experimental designs to evaluate nuclear and AKAP95 compartments in the same cells before and after rapamycin. FKBP was fused to the N-terminus of nuclear-localized ICUE3 (FKBP-ICUE3-NLS), and FRB was fused to the C-terminus of human AKAP95 (AKAP95-FRB) (Figure 3c and Supplemental Figure 2a). When HEK293T cells co-expressing FKBP-ICUE3-NLS and AKAP95-FRB were treated with rapamycin (100 nM), the diffuse pattern of reporter fluorescence became more punctate within the nucleus within minutes, indicating the translocation and recruitment of ICUE3 to AKAP95 (Supplemental Figure 2b). HEK293T cells co-expressing these same two constructs were treated with rapamycin or DMSO (control) and harvested for co-immunoprecipitation experiments. Immunoprecipitation using anti-AKAP95 antibody followed by western blotting for GFP revealed that ICUE3 interacted with AKAP95 in cells treated with rapamycin, confirming the localization of the reporter to AKAP95 (Supplemental Figure 4c). To ensure that targeting ICUE3-NLS to AKAP95 did not affect the dynamic range of the reporter, the maximum responses of cells expressing either FKBP-ICUE3-NLS alone (N=39 cells) or both FKBPICUE3-NLS and AKAP95-FRB (N=35 cells) were compared (Supplemental Figure 4d). In both cases, cells were treated with rapamycin, and the maximum response was stimulated by the addition of forskolin and IBMX to activate adenylyl cyclases and inhibit PDEs, respectively. After rapamycin treatment, the biosensor responded similarly to maximal cAMP stimulation regardless of AKAP95-FRB co-expression, indicating that the dynamic range of the biosensor is not affected by its localization to AKAP95.
To determine what effect the site of cAMP generation has on the cAMP levels within the AKAP95 compartment, we combined live-cell imaging using the localized cAMP biosensor with spatiotemporal manipulation of cAMP using soluble adenylyl cyclase (sAC), or SMICUS, which is a method previously developed to generate local pools of cAMP using subcellularly targeted sAC (Sample et al., 2012). sAC is pharmacologically distinct from the transmembrane adenylyl cyclase (tmAC). Instead of being activated by GPCRs, it is activated by bicarbonate, ATP, or calcium (Zippin et al., 2013). We can therefore use exogenously expressed mCherry-tagged sAC as a tool to generate cAMP in specific subcellular locations in a dose-dependent manner in HEK293T cells by the addition of sodium bicarbonate to the culture medium (Sample et al., 2012). To this end, mCherry-tagged sAC was localized to either the plasma membrane (PM-sAC) or the nucleus (sAC-NLS) (Figure 3c). PM-sAC generates cAMP at the plasma membrane, mimicking the production of cAMP by tmACs. HEK293T cells expressing PM-sAC and either FKBP-ICUE3-NLS alone for general nuclear targeting, or FKBP-ICUE3-NLS plus AKAP95-FRB for AKAP95 targeting, were first treated with rapamycin (100 nM) (Figure 3d). Cells were then treated with a low dose (2.5 mM) of sodium bicarbonate to activate targeted sAC. Although cAMP production at the plasma membrane by sub-maximal sAC activation led to a clear response in the general nuclear compartment within 15 min, with an average emission ratio change of 11.3 ± 1.5% (N=39 cells), cAMP was not detected within the AKAP95 microdomain (mean emission ratio change −1.9 ± 0.8%, N=35 cells), suggesting that cAMP accumulation is tightly regulated in the area surrounding the AKAP95 complex, which represents a functional microdomain with distinct cAMP dynamics. Subsequent treatment with a saturating dose (15 mM) of bicarbonate to maximally activate sAC resulted in increased FRET responses from both nuclear-localized and AKAP95-targeted ICUE3 (Figure 3d). Hence, although some cAMP generated at the plasma membrane is able to diffuse into the nucleus, an added level of regulation limits the accumulation of cAMP within the AKAP95 microdomain. While there are differences in kinetics, these data qualitatively agree with the model predictions of an ablated cAMP response to the low stimulation condition in the AKAP95 compartment but a response to the high stimulation condition.
Next, the cAMP responses within the AKAP95 compartment were compared when cAMP was generated at the plasma membrane or within the nucleus. When cAMP was generated in the nuclei of cells expressing sAC-NLS, even a low dose of sodium bicarbonate resulted in clear cAMP responses of 4.9 ± 0.8% within the AKAP95 microdomain (N=18 cells) compared to the lack of a response within the microdomain when cAMP was generated at the plasma membrane (−0.9 ± 0.8%, N=18 cells) (Figure 3e). Maximal activation of sAC in the nucleus or at the plasma membrane further increased the cAMP levels in the AKAP95 microdomain.
These findings further support the existence of a functional microdomain that is distinct from the general nuclear environment in the immediate vicinity of AKAP95, where cAMP accumulation is limited unless cAMP is generated more locally or in excess levels from the plasma membrane. These results support the hypothesis that AKAP95 is able to create a distinct microdomain that limits cAMP concentrations, thereby providing a way to tune the activation threshold of the AKAP-anchored pool of PKA.
PDE4 and PDE3 have distinct roles in regulating cAMP around AKAP95
To test the role of different PDE classes in regulating the microdomain, we used inhibitors specific to either PDE4 or PDE3. HEK293T cells expressing PM-sAC and rapamycin-induced AKAP95-targeted ICUE3 were treated with rolipram (PDE4 inhibitor), milrinone (PDE3 inhibitor) or vehicle control. Cells were subsequently treated with a low dose (2.5 mM), followed by a high dose (15 mM), of sodium bicarbonate to activate PM-sAC.
The initial response after the addition of PDE inhibitor indicates the basal level of PDE activity at the microdomain. As shown in Figure 4a and b, cells treated with the PDE4 inhibitor rolipram (1 μM) exhibited an immediate increase in cAMP levels in the AKAP95 microdomain (9.4 ± 1.9% emission ratio change, N=29 cells), suggesting that co-anchored PDE4 is basally active in restricting cAMP levels within the AKAP95 microdomain. Subsequent addition of low-dose sodium bicarbonate following PDE4 inhibition further increased cAMP levels in the microdomain (10.0 ± 0.8%, N=29 cells), in sharp contrast to the limited response (4.9 ± 0.8%, N=39 cells) in the absence of any inhibitor (Figure 4c). Furthermore, a dominant-negative mutant of PDE4D5 (dnPDE4D5) was used as an alternative to global PDE4 inhibition by rolipram to more specifically investigate the role of PDE4D isoforms at the microdomain. This dominant-negative mutant is inactive and does not directly inhibit endogenous PDEs, but instead competes with and displaces them from binding to scaffolding proteins such as AKAP95 (Terrin et al., 2006). We can therefore specifically interrogate the role of the scaffolded PDE4D5 in regulating the AKAP95 microdomain. HEK293T cells expressing AKAP95-targeted ICUE3, PM-sAC, and a dominant-negative mutant of PDE4D5 were treated with a low dose followed by a high dose of sodium bicarbonate. Cells expressing the mutant PDE4D5 exhibited a robust cAMP response to submaximal activation of PM-sAC (16.5 ± 1.9%, N=24 cells) compared with control cells that were not transfected with dominant-negative PDE4D5 (−1.9 ± 0.8%, N=35 cells) (Figure 4d). These data suggest that basally active PDE4 restricts cAMP accumulation in the AKAP95 microdomain under both basal state and stimulated conditions.
Figure 4: PDE4 and PDE3 have distinct roles in regulating cAMP around AKAP95.
The effect of isoform-specific PDE inhibition was tested in the AKAP95 microdomain. Cells expressing AKAP95-targeted ICUE3 and PM-sAC were co-treated with PDE inhibitor and rapamycin (100 nM) and subsequently with NaHCO3 (LD, 2.5 mM; HD, 15 mM) to generate cAMP at the plasma membrane via PM-sAC. a) AKAP95-targeted ICUE3 emission ratio (cyan/yellow) responses when cells were treated with the PDE4 inhibitor rolipram (1 μM) (purple, N=29 cells), the PDE3 inhibitor milrinone (10 μM) (blue, N=19 cells), or without inhibitor treatment (black, N=39 cells). Cells were additionally treated with a low dose (LD, 2.5 mM) and subsequently a high dose (HD, 15 mM) of NaHCO3. b) The initial FRET response after inhibitor addition indicates basal PDE activity in the AKAP95 microdomain. The change in normalized ICUE3 emission ratio (cyan/yellow) is shown. There is a statistically significant response in cells treated with rolipram (p<0.0001) but not in cells treated with milrinone (p=0.1293). c) After low-dose NaHCO3 stimulation, a statistically significant response is observed in PDE4-inhibited cells (p<0.0001) and PDE3-inhibited cells (p=0.0002) compared to cells lacking inhibitor treatment. d) AKAP95-targeted ICUE3 emission ratio (cyan/yellow) responses in cells expressing dnPDE4D5 (purple, N=24 cells) or cells not expressing dominant negative PDE isoforms (black, N=35 cells). Cells were treated with LD (2.5 mM) followed by HD (15 mM) NaHCO3 to stimulate PM-sAC. e) LD PM-sAC stimulation induced statistically significant responses in cells expressing the dominant negative PDE4D5 compared to control cells (inset, p<0.0001).
In contrast, addition of the PDE3 inhibitor milrinone (10 μM) induced no response within the microdomain (2.0 ± 1.3%, N=19 cells) (Figure 4a–b), suggesting PDE3 does not play a role in controlling the basal level of cAMP within the AKAP95 microdomain. Interestingly, when PDE3 is inhibited with milrinone, submaximal activation of PM-sAC led to increased cAMP levels in the microdomain (13.4 ± 1.8%, N=19 cells) (Figure 4c), indicating that PDE3 plays a role in preventing low levels of plasma membrane-generated cAMP from accessing the microdomain.
Discussion
Here we presented the identification of a nuclear signaling complex consisting of AKAP95, PKA, and PDE4D5. AKAP95 has previously been found to bind RIIα during mitosis after the nuclear envelope has dissolved (Collas et al., 1999). Using both nuclear fractionation studies and whole-cell immunofluorescence experiments we demonstrate that nuclear AKAP95 interacts with the regulatory subunit of PKA during interphase. These findings suggest that AKAP95 can anchor PKA in the nucleus irrespective of cell cycle stages. Previous studies have found that AKAP95 can associate with PDE4A in T-lymphocytes, but the functional role of anchored PDE4A was not tested (Asirvatham et al., 2004). Here we show that anchored PDE4D5 plays a critical role in the functional cAMP signaling microdomain assembled by AKAP95.
The roles played by different PDE isoforms are complex. Although it is widely accepted that cAMP diffusion is controlled and limited, the mechanisms underlying this compartmentalization are debated. One model for cAMP regulation suggests the existence of a barrier to prevent cAMP diffusion away from a particular location (e.g., the plasma membrane). An alternative model posits that PDEs concentrate in certain locations to prevent cAMP accumulation at those locations. Our results suggest these two mechanisms could co-exist in a PDE isoform-specific manner. PDE4 isoforms are part of a complex with AKAP95 and create a local sink to limit the accumulation of cAMP within the AKAP95 microdomain. Our data suggest that PDE3 is not a part of the AKAP95 complex but could act as a gate to prevent free diffusion of cAMP from the plasma membrane, the specific mechanisms and functional roles of which will be further investigated. The intricate interplay between these two different PDE isoforms and their effects on local signaling expands the concept of localized cAMP compartmentalization control to include proximate and distal PDE isoforms working in concert. cAMP is thus tightly regulated within this microdomain to prevent accidental nuclear PKA activation and reserve this pool of scaffolded PKA holoenzyme for transducing signals that generate specific, local sources of cAMP.
What are the specific signals that can lead to the activation of this pool of nuclear PKA holoenzymes? Endogenous sAC has been shown to localize to subcellular locations such as the cytosol, nucleus, and mitochondria and respond to changes in bicarbonate, calcium, and ATP. sAC has been shown to act as a sensor for changes in pH and metabolism in different cell types (Tresguerres et al., 2010, 2011, Zippin et al., 2003, 2010, 2013) and could generate cAMP proximal to this pool of nuclear PKA. In addition, it has been demonstrated that endocytosed GPCRs continue signaling at the endosome and that this endosomal signaling elicits distinct transcriptional responses (Irannejad et al., 2013; Tsvetanova and von Zastrow, 2014). cAMP generated at the endosome could provide a local source of cAMP that activates the nuclear PKA pool. Supporting this hypothesis, Jean-Alphonse et al (Jean-Alphonse et al., 2016) recently showed that a synergistic effect between PTHR and β2-AR signaling induces pronounced endosomal cAMP production that corresponds with activation of nuclear PKA and increased levels of phosphorylated CREB, a PKA substrate. Given that GPCR internalization and endosomal cAMP production vary by receptor and ligand, the strength and extent of nuclear PKA activation by internalized GPCRs may be receptor specific. The tight regulation of cAMP, and by extension nuclear PKA, within the AKAP95 microdomain shown here provides a new example of signaling specificity arising from precise spatiotemporal control.
Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jin Zhang (jzhang32@ucsd.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Culture
HEK293T cells (human embryonic kidney, female) were maintained in DMEM (10% (v/v) FBS and 1% (v/v) penicillin-streptomycin at 37°C with 5% CO2.
METHOD DETAIL
Nuclear Fractionation
Sucrose-gradient centrifugation was used to isolate and collect nuclei. All steps were performed on ice. HEK293T cells were collected in DPBS and pelleted at 470 × g for 10 min. Gentle vortexing was used to resuspend the cell pellet in ice-cold Sucrose Buffer I (0.32 M), and cells were lysed by 15 strokes of a Dounce homogenizer B pestle. Lysates were gently mixed with Sucrose Buffer II (2.0 M) in a 50 mL tube. The lysate-sucrose mix was layered on top of Sucrose Buffer II in a polyallomer SW 40.1 ultracentrifuge tube. Any space remaining in the tube was filled with Sucrose Buffer I. A Beckman centrifuge with a SW41 Ti rotor was used to separate the nuclei at 30,000 rpm for 45 min, at 4°C. Whole-cell, non-nuclear, and nuclear fractions were probed with antibodies against CREB (Cell Signaling 9197S) and β-tubulin (Cell Signaling 2146S) to ensure clean fractionation. Antibodies used on fractionation samples: AKAP95 (R-146) (Santa Cruz, sc-10766), RIIα (H-12) (Santa Cruz, sc-137220), PKAcat (A-2) (Santa Cruz, sc-28315), PDE3B (H-300) (Santa Cruz, sc-20793), and PDE4D5 (gift from Dr. George Baillie).
Co-Immunoprecipitation
Protein A/G PLUS-Agarose beads (Santa Cruz sc-2003) or Protein A/G Magnetic Beads (bimake.com B23202) were equilibrated in Tris buffer. Pre-clearing was achieved by incubation of agarose beads with 1 mg of nuclear protein and 5 μg rabbit IgG (Santa Cruz sc-2027) for 1 h at 4°C to remove proteins that nonspecifically bind the beads or IgG. Pre-cleared lysate was incubated with the immunoprecipitating antibody for 1 h at 4°C. The lysate-antibody mix was incubated with agarose beads overnight. After 4 washes with ice-cold DPBS, bound protein was eluted by boiling for 5 min in SDS sample buffer. Antibodies used for Co-IP: PKA IIα reg (C-20) (sc-908), PDE4D5 (gift from Dr. George Baillie’s Lab), AKAP95 (R-146) (sc-10766), Clean Blot (Thermo Fisher Scientific 21230).
Immunofluorescence Imaging
Cells were rinsed in HBSS and then fixed in 4% PFA for 30 min, followed by permeabilization for 1 h at room temperature. Cells were then incubated overnight at 4°C or for 30 min at 37°C with primary antibody. Alexa Fluor 488-conjugated and Alexa Fluor 568-conjugated secondary antibodies (Thermo Fisher Scientific A-11034, A-11001, A-11011, or A-11004) were incubated at room temperature for 1 h or 30 min at 37°C in the dark. Cells were incubated with Hoescht 33342 stain at room temperature for 30 min before imaging. Cells were imaged on a Zeiss LSM 880 Confocal with Airyscan processing using a 40x/1.2NA objective. Images were acquired with the suggested settings using 405 nm, 488 nm, and 561 nm lasers. Antibodies used for IF: AKAP95 (F-11) (Santa Cruz, sc-390335) or AKAP95 (R-146) (Santa Cruz, sc-10766), RIIα (C-20) (Santa Cruz, sc-908), PKAcat (BDBiosciences Clone 5B cat#610980), PDE4D3 (gift from Dr. George Baillie), and PDE4D5 (gift from Dr. George Baillie).
Proximity Ligation Assay
PLA experiments were performed using the Duolink® in situ red starter kit for proximity ligation assays (Sigma Aldrich, DU092101) according to the provided protocol. The only protocol modification was to extend the amplification time by 50 min. Briefly, cells were fixed and permeabilized as in the immunofluorescence experiments before incubation with primary antibody (same used for IF experiments), then with the provided secondary antibody (conjugated with nucleotides) for 30 min at 37°C each with washes after each step. Ligation of the nucleotides and amplification of the strand occurred sequentially by incubating cells with first ligase then polymerase and detection solution. PLA experiments with AKAP95 antibodies from different species were used as positive controls, and experiments withjust one primary antibody (AKAP95) provided our negative control. Images were acquired on a Zeiss LSM 880 Airyscan confocal as described above. A cross section of the nucleus (3.6–5 μm) was acquired and the number of dots within that cross section counted both inside and outside the nucleus.
Computational Modeling
A previously developed computational model of compartmentalized cAMP and PKA dynamics in HEK293 cells (Sample et al., 2012) was modified to include ICUE in the nuclear AKAP compartment and evaluated in MATLAB (Mathworks). Briefly, the “nucAKAP Model” is a compartmental ordinary differential equation (ODE) model that consists of plasma membrane, cytosol, nucleus and nuclear AKAP compartments. This model consists of equations describing cAMP production by adenylyl cyclases (both endogenous and over-expressed sAC), cAMP degradation by PDEs, and cAMP activation of PKA. Additionally, the cAMP biosensor ICUE and PKA biosensor AKAR were included in all the compartments except for the nuclear AKAP compartment. We extended this “nucAKAP Model” to include ICUE in the nuclear AKAP compartment by making the following modifications.
A new second-order reaction of cAMP binding to ICUE in the nuclear AKAP compartment:
And an additional conservation of mass equation for total ICUE in the nuclear AKAP compartment:
And fmally, the conservation of mass for free cAMP in the nuclear AKAP compartment was updated to account for the cAMP that is bound to ICUE.
No new kinetic parameters were introduced, as the rates of cAMP association and dissociation with ICUE are assumed to be consistent across compartments. Total ICUE in the AKAP compartment, ICUEtotAKAP, was assumed to be equal to the total amount ofPKA in the AKAP compartment, which assumes that each AKAP has both PKA and ICUE bound to it. All other equations and parameters are as described in the supplement of Sample et al 2012 (Sample et al., 2012).
This model was run using the initial conditions from the published model and allowed to equilibrate by running for 109 s. Using the simulated plasma membrane-targeted sAC built into the model (EsAC pm basal = 1.663 · 10−1 mM/s), cAMP production was stimulated with a low dose ofNaHCO3 (increase EsAC,pm,basal by 1×1.726 · 10−1) for 15 min and then subjected to a high dose ofNaHCO3 (increase EsAC,pm,basal by 6×1.726 · 10−1) for another 15 min.
Cloning
Full-length human AKAP95 was kindly provided by Dr. John D. Scott (University of Washington). FKBP and FRB constructs were kindly provided by Dr. Takanari Inoue (Johns Hopkins University). FRB or GFP were tagged to the C-terminus of AKAP95 using BamHI and EcoRI or NotI restriction sites. FKBP with a flexible linker was added to the N-terminus of ICUE3 (DiPilato and Zhang, 2009) using HindIII and BamHI restriction sites.
Live-cell Imaging
HEK293T cells were transfected with Lipofectamine 2000 (Invitrogen) when cells were 50–60% confluent and imaged 16–24 h later. Cells were washed twice with and maintained in Hank’s balanced salt solution buffer (HBSS). Cells were imaged at room temperature.
FRAP Imaging
FRAP experiments were performed on a Leica TCS SP8 confocal microscope using its associated FRAP software, a 63x/1.4NA objective, and a DD488/552 beamsplitter. A total of 5 images were acquired before bleaching a rectangular cross-section or circular area of the nucleus with 25% laser power. Post-bleaching images were acquired every 5 or 10 s for at least 5 min. Images were analyzed using the Jython script found at: http://imagej.net/Analyze_FRAP_movies_with_a_Jython_script.
FRET Imaging
Prior to imaging, cells were equilibrated in HBSS for 10 min in a CO2-independent incubator. Cells were treated with NaHCO3 (J.T. Baker, purity=100%), which induced a small change in FRET for the FKBP-tagged biosensor with or without co-expression of AKAP95-FRB (seen in Figure 3), forskolin (Calbiochem, purity ≥99% by HPLC), IBMX (Sigma, purity ≥98% by TLC), rolipram (Alexis, purity ≥98% by TLC), or milrinone (Alexis, purity ≥97% by TLC) as indicated. Cells were imaged on a Zeiss Axio Observer Z1 microscope equipped with a 40x/1.3 NA objective and Photometrics Evolve 512 EMCCD, using METAFLUOR 7.7 software (Molecular Devices) to control dual-emission-ratio imaging acquisition every 30 s. A 420DF20 excitation filter, a 450DRLP dichroic mirror, and two emission filters (475DF40 for CFP and 535DF25 for YFP) alternated by a Lambda 10–2 filter-changer (Sutter instruments) were used for the FRET measurements. The microscope settings for RFP (sAC) fluorescence acquisition were as follows: 555DF25 excitation filter, 568rdc dichroic mirror, and 650DF100 emission filter. Exposure times for all channels ranged from 50–500 ms. Emission intensities of individual cells were background-subtracted, and the ratio between the CFP and FRET channel was normalized to the timepoint just before addition of low-dose NaHCO3 (t = 0 min). Because overexpressed sAC has some basal activity prior to stimulation, the starting FRET ratio for AKAP95-localized ICUE3 was higher in cells expressing sAC-NLS compared to PM-sAC. Therefore, a moderate range of overlap in starting ratios between these two experiments was determined, and cells were selected for comparison based on starting ratio within this range to control for basal activity differences. On the other hand, the expression of PM-sAC was controlled to be within a defined range, and this range was held constant between different experimental conditions; cells outside this range were excluded from analysis.
QUANTIFICATION AND STATISTICAL ANALYSIS
2–3 biological replicates were done for Co-IP and PLA experiments, and at least 3 biological replicates performed for all other experiments. Unpaired two-tailed t-tests with Welch’s correction were used for statistical analyses, done in Graphpad Prism. *** indicates a p-value<0.0001. N values, as indicated in figure legends and the main text, represent number of cells. All error bars indicate standard error.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti-mouse Alexa Fluor 488-conjugate | Thermo Fisher Scientific | Cat# A-11001, RRID:AB 253406 9 |
| Goat anti-rabbit Alexa Fluor 568-conjugate | Thermo Fisher Scientific | Cat# A-11011, RRID:AB 143157 |
| Goat anti-mouse Alexa Fluor 568-conjugate | Thermo Fisher Scientific | Cat# A-11004, RRID:AB 253407 2 |
| Goat anti-rabbit Alexa Fluor 488-conjugate | Thermo Fisher Scientific | Cat# A-11034, RRID:AB 257621 7 |
| PKA RIIa (C-20) monoclonal rabbit | Santa Cruz Biotechnology | Cat# sc-908, RRID:AB 632214 |
| PKA RIIa (H-12) monoclonal mouse | Santa Cruz Biotechnology | Cat# sc-137220, RRID:AB 226860 8 |
| AKAP95 (R-146) polyclonal rabbit | Santa Cruz Biotechnology | Cat# sc-10766, RRID:AB 222606 0 |
| AKAP95 (F-11) monoclonal mouse | Santa Cruz Biotechnology | Cat# sc-390335 |
| PKAcat (A-2) monoclonal rabbit | Santa Cruz Biotechnology | Cat# sc-28315, RRID:AB 628136 |
| PKAcat (Clone 5B) monoclonal mouse | BD Biosciences | Cat# 610980, RRID:AB 398293 |
| CREB monoclonal rabbit | Cell Signaling Technology | Cat# 9197, RRID:AB 331277 |
| β-Tubulin polyclonal rabbit | Cell Signaling Technology | Cat# 2146, RRID:AB 221054 5 |
| PDE3B polyclonal rabbit | Santa Cruz Biotechnology | Cat# sc-20793, RRID:AB 228357 9 |
| PDE4D3 rabbit | Dr. George Baillie | n/a |
| PDE4D5 rabbit | Dr. George Baillie | n/a |
| Clean Blot | Thermo Fisher Scientific | Cat# 21230 |
| Bacterial and Virus Strains | ||
| E. coli DH5α | Thermo Fisher Scientific | Cat# 18265017 |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| BamHI | Thermo Fisher Scientific | Cat# FD0054 |
| EcoRI | Thermo Fisher Scientific | Cat# FD0274 |
| Notl | Thermo Fisher Scientific | Cat# FD0594 |
| HindIII | Thermo Fisher Scientific | Cat# FD0504 |
| NaHCOs | J.T. Baker | Cat# 3506–01 |
| forskolin | Calbiochem | Cat# 344270 |
| milrinone | Alexis | Cat# ALX-270-083-M005 |
| IBMX | Sigma | Cat# I5879 |
| rolipram | Alexis | Cat# ALX-270-119 |
| Critical Commercial Assays | ||
| Duolink® in situ red starter kit | Sigma | DUO92101 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| HEK293T | ATCC | CRL-11268 |
| Experimental Models: Organisms/Strains | ||
| Oligonucleotides | ||
| Recombinant DNA | ||
| Full-length human AKAP95 | Dr. John D. Scott (University of Washington) | n/a |
| FRB | Dr. Takanari Inoue (Johns Hopkins University) | n/a |
| FKBP | Dr. Takanari Inoue (Johns Hopkins University) | n/a |
| Software and Algorithms | ||
| FRAP Analysis Jython Script | http://imagej.net/AnalyzeFRAPmovies_with_a_Jython_script | |
| ImageJ | NIH | imagej.nih.gov/ij/ |
| MATLAB | Mathworks | https://www.mathworks.com/products/matlab.html |
| Prism 5 | GraphPad Software | www.graphpad.com |
| METAFLUOR 7.7 | Molecular Devices | www.moleculardevices.com |
| Leica Application Suite, Advanced Fluorescence FRAP wizard | Leica | www.leica-microsystems.com |
| Other | ||
| Protein A/G Magnetic Beads for IP | Bimake.com | Cat# B23202 |
| Protein A/G PLUS-agarose beads | Santa Cruz Biotechnology | Cat# sc-2003, RRID:AB 102014 00 |
Significance.
PKA signaling is integral to numerous cellular processes, and the spatiotemporal control of PKA is necessary for the regulation of its diverse downstream effects. The discovery and characterization of a nuclear PKA signaling microdomain further complicates the classical understanding of PKA signaling. Instead of PKA activation occurring only in the cytosol, these findings suggest there exists a distinct nuclear pool of PKA that is reserved for sensing more “proximal” signals because the AKAP95 assembled nuclear microdomain specifically allows accumulation of locally generated cAMP. This study also demonstrates a clear example of synergistic effects of isoform-specific PDEs, where PDE4 is responsible for creating a local cAMP sink but also relies on more distal PDE3 to act as a gate and prevent the free diffusion of cAMP from overwhelming the local PDE4. The studies discussed in this article expand our understanding of PKA signaling and regulation. Furthermore, the knowledge gained by understanding the regulation of nuclear PKA, a prototypic kinase and signaling molecule, can aid in developing better models for the study of spatiotemporal regulation of other signaling molecules.
Highlights.
AKAP95 anchors PKA and PDEs in the nucleus.
AKAP95 creates a microdomain with tight control of cAMP levels.
PDE4 and PDE3 work in concert to regulate this microdomain.
Acknowledgements
We would like to thank J. Scott for the AKAP95 cDNA and T. Inoue for the FKBP/FRB cDNA. We thank L. Joosen for technical help with confocal and FRAP imaging. We thank S. Mehta for aid in preparing figures and text. This work was funded by R01 DK073368 (to J. Z.) and F32 GM120798–02 (to E.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial interests.
References
- Akileswaran L, Taraska JW, Sayer JA, Gettemy JM, and Coghlan VM (2001). A-kinase-anchoring Protein AKAP95 Is Targeted to the Nuclear Matrix and Associates with p68 RNA Helicase. J. Biol. Chem 276, 17448–17454. [DOI] [PubMed] [Google Scholar]
- Arsenijevic T, Degraef C, Dumont JE, Roger PP, and Pirson I (2004). A novel partner for D-type cyclins: protein kinase A-anchoring protein AKAP95. Biochem. J 378, 673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic T, Degraef C, Dumont JE, Roger PP, and Pirson I (2006). G1/S cyclins interact with regulatory subunit of PKA via A-kinase anchoring protein, AKAP95. Cell Cycle 5, 1217–1222. [DOI] [PubMed] [Google Scholar]
- Asirvatham AL, Galligan SG, Schillace RV, Davey MP, Vasta V, Beavo JA, and Carr DW (2004). A-Kinase Anchoring Proteins Interact with Phosphodiesterases in T Lymphocyte Cell Lines. J. Immunol [DOI] [PubMed] [Google Scholar]
- Banaszynski LA, Liu CW, and Wandless TJ (2005). Characterization of the FKBP-rapamycin-FRB ternary complex. J. Am. Chem. Soc 127, 4715–4721. [DOI] [PubMed] [Google Scholar]
- Coghlan VM, Langeberg LK, Fernandez A, Lamb NJC, and Scott JD (1994). Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J. Biol. Chem [PubMed] [Google Scholar]
- Collas P, Le Guellec K, and Taskén K (1999). The A-kinase-anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis. J. Cell Biol 147, 1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPilato LM, and Zhang J (2009). The role of membrane microdomains in shaping beta2-adrenergic receptor-mediated cAMP dynamics. Mol. Biosyst 5, 832–837. [DOI] [PubMed] [Google Scholar]
- Eide T, Carlson C, Taskén KA, Hirano T, Taskén K, and Collas P (2002). Distinct but overlapping domains of AKAP95 are implicated in chromosome condensation and condensin targeting. EMBO Rep 3, 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide T, Taskén KA, Carlson C, Williams G, Jahnsen T, Taskén K, and Collas P (2003). Protein kinase A-anchoring protein AKAP95 interacts with MCM2, a regulator of DNA replication. J. Biol. Chem 278, 26750–26756. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SGF, Sunahara RK, El-Samad H, Huang B, et al. (2013). Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Alphonse FG, Wehbi VL, Chen J, Noda M, Taboas JM, Xiao K, and Vilardaga J-P (2016). β2-adrenergic receptor control of endosomal PTH receptor signaling via Gβγ. Nat. Chem. Biol 13, 259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lu X, Shimada M, Dou Y, Tang Z, and Roeder RG (2013). Regulation of transcription by the MLL2 complex and MLL complex-associated AKAP95. Nat. Struct. Mol. Biol 20, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstad A, Landsverk OJB, Strømme O, Otterlei M, Collas P, Sundan A, and Brede G (2016). A-kinase anchoring protein AKAP95 is a novel regulator of ribosomal RNA synthesis. FEBS J 283, 757–770. [DOI] [PubMed] [Google Scholar]
- Sample V, DiPilato LM, Yang JH, Ni Q, Saucerman JJ, and Zhang J (2012). Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP. Nat. Chem. Biol 8, 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Reichow SL, Esseltine JL, Shi D, Langeberg LK, Scott JD, and Gonen T (2013). Intrinsic disorder within an AKAP-protein kinase A complex guides local substrate phosphorylation. Elife 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Esseltine JL, Nygren PJ, Veesler D, Byrne DP, Vonderach M, Strashnov I, Eyers CE, Eyers PA, Langeberg LK, et al. (2017). Local protein kinase A action proceeds through intact holoenzymes. Science (80-.). 356, 1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, et al. (2006). PGE1 stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: Role of compartmentalized phosphodiesterases. J. Cell Biol 175, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, and Buck J (2010). Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc. Natl. Acad. Sci. U. S. A 107, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Levin LR, and Buck J (2011). Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int 79, 1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetanova NG, and von Zastrow M (2014). Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat. Chem. Biol 10, 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Yi M, Yuan Y, Zhuang W, Zhang D, Yu X, Chen X, Teng B, Guan Z, and Zhang Y (2015). Expression of AKAP95, Cx43, CyclinE1 and CyclinD1 in esophageal cancer and their association with the clinical and pathological parameters. Int. J. Clin. Exp. Med 8, 7324–7332. [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, and Buck J (2003). Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17, 82–84. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Chadwick PA, Levin LR, Buck J, and Magro CM (2010). Soluble adenylyl cyclase defines a nuclear cAMP microdomain in keratinocyte hyperproliferative skin diseases. J. Invest. Dermatol 130, 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, Tso P, Holz GG, Sharp GWG, Levin LR, et al. (2013). CO2/HCO3-- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J. Biol. Chem 288, 33283–33291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.