Abstract
Little is known about whether a mother’s psychological state during pregnancy influences her offspring’s microbiome. This study examined whether maternal anxiety, depression, and stress during pregnancy is associated with the diversity of meconium microbiome, the first internal discharge, in 75 newborns from an existing birth cohort study. The meconium microbiome was profiled using multi-barcode16S rRNA sequencing at V3-V4 hyper-variable region followed by taxonomic assignment to the green gene 16S references at 97% similarity and diversity analysis at the genus level. Results showed that the meconium contained diversified microbiota, and greater pregnancy-related anxiety was significantly associated with a less diverse meconium microbiota community (p-value=0.001). At the specific taxa level, greater pregnancy-related anxiety was correlated with a lower level of the Enterococcaceae family (p-value=2e-4, Spearman rho=−0.43). These findings support a significant role of prenatal maternal mood in the early life bacteria colonization of their offspring.
Keywords: meconium microbiome, maternal antenatal mood and anxiety, pregnancy-related anxiety, gut-brain axis, in-utero programming
Pregnancy is very often stressful, and some women experience stress and symptoms of depression and anxiety, including pregnancy-related anxiety (Glover, 2015). It has been reported that 15–28% of women show signs of anxiety or depression during pregnancy (Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, et al., 2005; Melville, Gavin, Guo, Fan, & Katon, 2010; Verreault, Da Costa, Marchand, Ireland, Dritsa, & Khalifé, 2014). Furthermore, considerable evidence shows that if the mother is stressed, anxious, or depressed during pregnancy, her child is more likely to have a suboptimal developmental trajectory with a range of physical and mental health problems (Entringer, Buss, & Wadhwa, 2011; Field, 2011; van den Bergh, Mulder, Mennes, & Glover, 2005). However, the underlying mechanisms that explain the link are still unclear. A review of recent literature shows that maternal psychosocial stress during pregnancy is a complex phenomenon that affects mothers’ emotions, behaviors, and physiology in many different ways that may influence the neurodevelopment of the fetus through a network of pathways (Glover, 2015; Jašarević, Howerton, Howard, Bale, & Howerton, 2015; Jašarević, Rodgers, & Bale, 2015). One emerging area of study is through the transmission of bacteria or their metabolites from the maternal environment to the fetus via the bloodstream, placenta, umbilical cord, and maternal intestinal microbiota (Walker, Clemente, Peter, & Loos, 2017; Gomez de Agüero, Ganal-Vonarburg, Fuhrer, Rupp, Uchimura, Li et al., 2016).
Recent animal models suggest that newborn gut microbiota might contribute to the link between maternal stress in pregnancy and infant health and neurodevelopment (Jašarević et al., 2015a, 2015b; Mackos, Maltz, & Bailey, 2017), which have challenged the “sterile womb paradigm”. Those studies contributed to a growing consensus that commensal bacteria are present in the intrauterine environment and may influence the bacterial colonization of babies before birth (DiGiulio, Romero, Amogan, Kusanovic, Bik, Gotsch, et al., 2008; Jiménez, Fernández, Marín, Martín, Odriozola, Nueno-Palop, et al., 2008; Rautava, Collado, Salminen, & Isolauri, 2012; Satokari, Grönroos, Laitinen, Salminen, & Isolauri, 2012) or during the delivery when the baby goes through the birth canal (Jašarević, Rodgers, et al., 2015). Although this in-utero colonization hypothesis has recently been questioned for its inconsistency, in part, due to the generally extreme low bacterial load in the intrauterine environment and to possible artifacts from environmental contaminations (Lauder et al., 2016; Perez-Muñoz, Arrieta, Ramer-Tait, & Walter, 2017), a growing number of studies have supported the existence of meconium microbiota and their association to health outcomes (Chu et al., 2016; Dong et al., 2018; Durack et al., 2018). It has been hypothesized that the central nervous system (CNS) and intestinal microbiota develop in parallel during the prenatal and early postnatal periods and interact during critical phases of development (Borre, O’Keeffe, Clarke, Stanton, Dinan, & Cryan, 2014). Intestinal microbiota have an essential role in the development of the gastrointestinal tract, as well as the immune, metabolic and hypothalamic-pituitary-adrenal (HPA) systems. During this time, environmental and maternal factors can have long-term effects on neural functioning and also the signaling between the gut microbiota and the brain.
Animal studies have shown that maternal prenatal stress can lead to changes in the microbiome of the mother and offspring (Jašarević, Howerton, et al., 2015). Rhesus monkey infants born to mothers exposed to prenatal stress had lower levels of Bifidobacteria and Lactobacilli than monkey infants born to non-exposed mothers (Bailey, Lubach, & Coe, 2004). Moreover, while mice born to mothers exposed to maternal stress had normal microbiome, when stressed or given immune challenges, the exposed pups’ microbiome and metabolic profiles differed from the non-exposed animals (Gur, Worly, & Bailey, 2015; Jašarević et al., 2015a; 2015b).
In a study with humans, Zijlmans et al. (2015) were the first to evaluate a relationship between maternal prenatal stress and infant intestinal microbiota development between 6 days and 110 days postnatally. Results showed that self-reported maternal stress and cortisol concentration were associated with differences in newborns’ microbiome that persisted through the first months of life. However, their study lacked data on the effect of maternal psychosocial health during pregnancy on the initial bacterial composition. The accumulating evidence suggests an important role for early microbiome colonization on the priming of the immune system, with long lasting consequences on disease risk later in life (Abrahamsson et al., 2012; Azad et al., 2015; Dong et al., 2018; Wilczyńska, Skarżyńska, & Lisowska-Myjak, 2018), and emphasizes the need to have a better understanding of the predictors of the initial microbiota composition in human offspring. Such knowledge could help develop new strategies directed to modify aberrant or altered bacterial communities.
The overall goal of our study was to investigate the relationship between prenatal maternal stress and the composition of early life intestinal microbiota in offspring, as sampled in newborn meconium. We hypothesized that maternal antenatal stress would be associated with the diversity and the composition of infants’ initial microbiota.
METHODS
Subjects and samples
The study was approved by the Institutional Review Boards at the Icahn School of Medicine at Mount Sinai, New York Presbyterian Hospital, and Queens College, CUNY. The sample set came from 75 newborns whose mothers participated in a longitudinal birth cohort recruited at the prenatal obstetrics and gynecology clinics at Mount Sinai Hospital and New York Presbyterian/Queens Hospital. A detailed description of the population is published in Finik and Nomura (2017). Both sites serve predominantly low-income ethnic minority populations residing in New York City. Exclusion criteria for participation included HIV infection, maternal psychosis, maternal age <15 years, life-threatening medical complications of the mother, and congenital or chromosomal abnormalities of the fetus. All subjects were consented per protocol approved by the local IRBs. Participating mothers were followed from their 2nd trimester to delivery. The first neonate meconium passed within 48 hours after birth and was transferred by the research staff to sterile Falcon tubes using a sterile tongue depressor and stored at −80°C until processing. A detailed description can be found elsewhere (Hu et al., 2013).
Demographic and clinical data collection
Demographics:
Participating mothers reported age, education, parity, and marital status at the time of enrollment to the study.
Obstetric history and pregnancy outcomes:
Gestational age, birth weight, Ponderal Index (PI) calculated as a relationship between weight and height, and past and current birth complications including obstetric (e.g., forceps delivery or premature rapture of membrane) and/or neonatal problems (e.g., jaundice, admission to the neonatal intensive care unit, and shoulder dystocia) were obtained from the mother’s electronic medical records. The cumulative sum of obstetric and neonatal problems was used as the birth complication index.
Maternal stress during the 2nd trimester:
Using a battery of well-validated questionnaires, the mother’s level of stress was measured by i) symptoms of depression, ii) symptoms of general anxiety, iii) pregnancy-related anxiety, iv) perceived prenatal stress, and v) self-reported stressful life events experienced during the 2nd trimester. A dimensional measure for each scale for stress was used to evaluate the relative abundance and a dichotomous (median split) measure was used to evaluate the overall microbiota dissimilarities (beta diversity) analysis.
(i). Symptoms of depression:
Maternal depression symptomatology was measured during the 2nd trimester by the Edinburgh Postnatal Depression Scale (EPDS) (Murray & Carothers, 1990), a well-utilized self-report inventory that measures depression symptomatology. The participants were asked to report how they had felt in the past 7 days on a four-point Likert scale. Response options include “yes, all the time,” “yes most of the time,” “no not very often,” and “no, not at all.” Some questions were reverse coded, and the sum score constituted the “maternal depression” scale. The sum was split at the median score of 7, which constituted the dichotomous low and high depression score. The inventory is well-validated in different languages and has acceptable reliability ranging from 0.79 to 0.86 (Kheirabadi, et al., 2012; Mazhari & Nakhaee, 2007; Montazeri, Torkan, & Omidvari, 2007; Small, Lumley, & Toomey 2006), and satisfactory sensitivity (79%) and specificity (85%) (Kheirabadi, Maracy, Akbaripour, & Masaeli, 2012).
(ii). Symptoms of general anxiety:
The State-Trait Anxiety Inventory (STAI) (Spielberger, 1989) measures the mother’s temporary condition of “state anxiety” and long-standing condition of “trait anxiety.” Each type (trait and state) is assessed by 20 statements that may or may not describe the participant, who responded to the statements on a 4-point Likert scale ranging from 1 “not at all” to 4 “very much so.” The sum was split at the median score of 35 for state and 37 for trait anxiety, which constituted the dichotomous low and high state and trait anxiety scores. A meta-analysis of 45 articles reporting Cronbach’s alpha for internal consistency reliability for this inventory determined the mean to be 0.92 (Barnes, Harp, & Jung, 2002).
(iii). Pregnancy-related anxiety:
The Pregnancy-Related Anxiety Questionnaire-revised (PRAQ-R) (Huizink, Mulder, Robles de Medina, Visser, & Buitelaar, 2004) measures specific fears and worries related to pregnancy. It comprised of three sub-scales of perinatal anxiety related specifically to pregnancy, including fear of giving birth, fear of bearing a mentally or physically handicapped child, and worries about changes in appearance due to pregnancy. The participants were asked to describe their feelings and thoughts using a five-point Likert scale (1= absolutely not relevant; 2=hardly ever relevant; 3=sometimes relevant; 4=reasonably relevant; 5=very relevant). The mean item scores for each of the three dimensions were first computed, with a minimum of 1 and maximum of 5. The sum of the three dimensional scores comprised of Pregnancy-Related Anxiety Questionnaire-total (PRAQ-total) score. PRAQ-total, with a theoretical range of 15, was split at the median score of 6, which constituted the dichotomous low and high pregnancy-related anxiety scores. A score of 6 or greater was defined as high pregnancy-related anxiety and a score of less than 6 was defined as low. PRAQ-total is generally independent of other general anxiety measures such as STAI (Huizink, Robles de Medina, Mulder, Visser, & Bultelaar, 2002).
(iv). Perceived prenatal stress:
Perceived Stress Scale (PSS-14) (Cohen, Kamarck, & Mermelstein, 1983) was used to ask about the mother’s feelings and thoughts within the last months as an indicator of perceived stress during pregnancy. The sum was split at the median score of 37, which constituted the dichotomous low and high perceived stress scores.
(v). Stressful life events:
The Psychiatric Epidemiology Research Interview Life Events Scale (PERI) (Dohrenwend, Yager, Egri, & Mendelsohn, 1978) assesses the occurrence of stressful events in five major areas of life: relationships, health, legal matters, work and financials, and friendships. Mothers reported their experiences during the second trimester of specific stressful life events in those five areas of life, and reported the valence associated with each. This measure is widely used, has been shown to have good validity with narrative reports of life events, and has low intra-category variability (see review Dohrenwend, 2006). We used the total number of negative life events reported by the mothers as our measure of stressful life events. The score was also split at the median score of 3, which constituted the dichotomous low and high stressful life events scores.
Sample processing
On average, meconium samples were collected after 50 minutes (ranging from 0 to 5.5 hours, SD=11) of passing. The meconium was transferred from the diaper to sterile 15 ml Falcon tubes using a sterile tongue depressor by the research staff and stored at −80°C until processing. During the sample collection and processing, extreme precaution was taken to avoid possible environmental contaminations. Bacterial DNA was extracted using Qiagen DNA stool mini kit (Qiagen, CA). Total DNA concentration was determined with Qubit 2.0 Fluorometer (Life Technologies, Norwalk, CT). The phylogenetically informative V3-V4 region of 16S ribosomal RNA (rRNA) gene was amplified using the universal primer set 347F/803R. The primers were synthesized by IDT (Integrated DNA Technology, Coralville, IA). A pair of 8-mer error-correcting Golay barcodes was added to both the reverse and forward PCR primers.
16S ribosomal RNA (rRNA) sequencing and quality control
We used dual-barcoding to label the 16S rRNA amplicons from each sample as described previously (Hu et al., 2013). The bacterial 16S rRNA gene in the sequence analysis is regarded as a preferred culture-independent approach to identify bacterial taxa. The 16S rRNA amplicons were further pooled with equal molarity and submitted for MiSeq 2×300 pair-end sequencing at high depth. The paired sequence reads were merged and filtered by size (>400bp) and quality score (>Q30) using PANDAseq. The processed reads were further split by dual barcodes for each sample and assigned taxonomic classification using QIIME pipeline 1.9.0 (Caporaso et al., 2010). Three duplicate samples were included to assess sequencing reproducibility. Finally, a total of 4,105,299 (mean 54,737; SD 38,942; min 9,134, max 197,210) sequences remained and each sample was normalized to 3000 sequences for further analysis. After processing, QIIME provided detailed operational taxa unit (OTU) tables containing the microbiota composition and relative abundance for each individual sample.
Data analysis
Firstly, using QIIME pipeline, we summarized the microbiota composition and measured the average relative abundances and the standard deviations for each bacteria taxon from phylum to genus level across all meconium samples. Secondly, we accessed the diversity of the overall microbiota communities within or across each sample. The alpha-diversity, or bacterial richness within each sample, was measured using the Shannon index. The beta-diversity, representing taxonomic relative abundance across samples, was measured using Bray-Curtis distance matrices computed from genus level OTU tables (Werner et al., 2012). The univariate and multivariate PerMANOVA tests with permutation 999 were performed to analyze the variance using the Bray-Curtis distance as the dependent variable and the clinical/psychological categorical or continuous variables as independent variables. A Spearman correlation between bacterial taxa and the maternal stress score was measured at phylum, family and genus level. Strongly correlated taxa were further examined while adjusting for potential confounders in a regression model, which included maternal ethnicity/race, age, education, marital status, time of sampling (time between birth and sample collection), the mode of delivery (Cesarean, or C-section vs. vaginal), and antibiotic use during pre- and peri-natal periods.
RESULTS
Demographic characteristics
Descriptive statistics for the demographic characteristics are presented in Table 1. The mean (±SD) age of the mothers was 28 (±6) years. Approximately 52% of the mothers were Hispanic/Latino, 27% Black, 5% White, 4% Asian, and 12% identified their race/ethnicity as other. A little over 29% had not completed high school. The majority (64%) reported being single. There was a general balance of the sex of their offspring with 53% male and 47% female. The mean (±SD) gestational age at birth and birthweight were 38.8 (±3.2) weeks and 3.1 (±0.6) kg, respectively. Approximately a quarter of the participants delivered via C-section and one-eighth of the babies were admitted to neonatal intensive care unit. Note that the C-section mode of delivery was 98.6% (p-value=7.4e-5) overlapped with antibiotic usage prior to birth in our studied population.
Table 1.
Demographic and Obstetric Outcomes (N=75)
| Maternal Characteristics | |
| Mean (SD) | |
| Mother’s age (years) | 27.85 (6.21) |
| Mother’s ethnicity | N (%) |
| Black | 20 (26.7%) |
| Hispanic/Latino | 39 (52.0%) |
| White | 4 (5.3%) |
| Asian | 3 (4.0%) |
| Others | 9 (12.0%) |
| Mother’s educational attainment | N (%) |
| Elementary school education | 5 (6.7%) |
| Some high school | 17 (22.7%) |
| High school diploma/GED | 16 (21.3%) |
| Some college | 16 (21.3%) |
| Associate degree | 10 (13.3%) |
| Bachelor’s degree | 4 (5.3%) |
| Graduate degree | 7 (9.3%) |
| Mother’s marital status | N (%) |
| Married | 24 (32.0%) |
| Common Law | 3 (4.0%) |
| Single | 48 (64.0%) |
| Divorced/Separated | 0 (0%) |
| Mother’s medical condition | N (%) |
| Obesity | 23 (30.7%) |
| Gestational Diabetes | 4 (5.3%) |
| Diabetes | 3 (4.0%) |
| Gestational Hypertension | 1 (1.3%) |
| Preeclampsia | 7 (9.3%) |
| Offspring Characteristics | |
| Gender | N (%) |
| Male | 40 (53.3%) |
| Female | 35 (46.7%) |
| Gestational age (weeks), Mean (SD) | 38.76 (3.24) |
| Birthweight (kg), Mean (SD) | 3.06 (0.62) |
| Preterm | 8 (10.7%) |
| C-section | 19 (25.3%) |
| NICU admission | 9 (12.0%) |
| Number of birth complications, Mean (SD) | 1.43 (.10) |
| Antenatal Stress | |
| Mean (SD), Medium | |
| (i) Prenatal depression | 8.26 (6.16), 7 |
| (ii) State & trait anxiety | |
| State anxiety | 38.07 (12.33), 35 |
| Trait anxiety | 38.74 (10.58), 37 |
| (iii) Pregnancy-related anxiety (total) | 5.89 (2.34), 6 |
| Fear of having handicapped child | 1.79 (0.92), 2 |
| Fear of appearance change | 1.84 (1.01), 2 |
| Fear of birth/delivery | 2.25 (1.15), 2 |
| (iv) Prenatal perceived stress | 36.36 (6.88), 37 |
| (v) Stressful life events (total) | 2.68 (2.65), 3 |
| Time took before sampling meconium (min) | 50.33 (10.91), 15 |
Survey of meconium microbiota
From pooled barcoded PCR amplicons from 75 meconium samples, we obtained 4.5 million high-quality reads. After splitting by barcodes, we obtained an average of 60,362 reads (min=10,499, max=208,918) per sample.
Figure 1 shows the relative abundance of bacterial taxa at the phylum, family, and genus levels in the meconium. We found that the dominant phylum included Actinobacteria (mean=17%), Bacteroidetes (mean=9.3%), Firmicutes (mean=20%), and Proteobacteria (mean=71%). At the family and genus level, we found that most of the assigned taxa were from Firmicutes and Proteobacteria. The most abundant taxa were Enterobacteriaceae family with a non-identified genus in Enterobacteriaceae family (mean=46%). After further blasting the 16S reads of this Enterobacteriacea genus, we found that its nearest neighbors included 3 strains from Escherichia fergusonii and 1 strain from each Shigella sonnei, Brenneria alni, Escherichia coli, Shigella flexneri or Escherichia vulneris.
Figure 1. Relative abundances of dominant bacterial taxa in meconium samples.
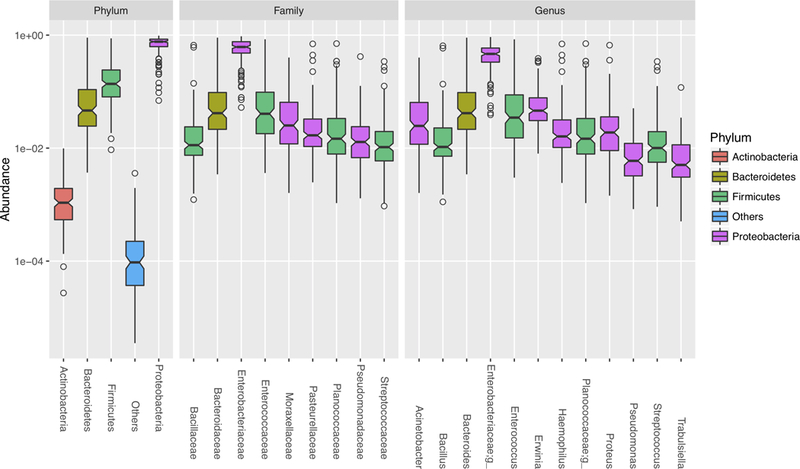
Box plots showed the mean and variance of relative abundance of bacterial taxa at Phylum, Family and Genus levels. Color represents the classification at phylum level.
The meconium microbiome composition by maternal pregnancy-related anxiety (PRAQ-total)
We observed significant differences in the meconium microbiota beta-diversity by high versus low PRAQ-total scores (Figure 2A). The univariate PerMANOVA test using the beta-diversity distance matrix showed that the overall meconium microbiota at genus level were significantly associated with maternal PRAQ-total as a continuous variable (p=0.001), delivery mode (p=0.033), and two subscales of maternal PRAQ (i.e., fear of appearance changes and fear of childbirth; p=0.003 and p=0.005, respectively; Table 2). However, when adjusted in multivariate analysis, only PRAQ-total and the delivery mode remained significant. In addition, a trend for association between the increased microbial alpha-diversity and the increased PRAQ-total was detected (p-value=0.07; Supplementary Figure 1).
Figure 2. Association of meconium microbiota and the pregnancy-related anxiety.
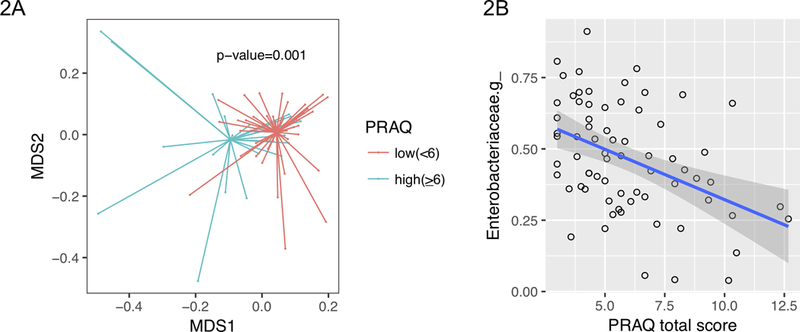
[2A] Overall meconium microbiota dissimilarities presented by NMDS plots. The level of total pregnancy-related anxiety was defined as low (PRAQ-total score <6) or high (PRAQ-total score ≥6). P-value was obtained from PERMANOVA test using Bray-Curtis distance matrices as dependent variable and the continuous PRAQ-total score as independent variable. [2B] The inverse correlation between the level of total pregnancy-related anxiety and the relative abundance of Enterobacteriaceae genus. The linear fit line (blue) with 95% confidence interval (gray area) was taken from the linear regression model.
Table 2.
The univariate and multivariate PerMANOVA analysis of the association between overall microbiome diversity and demographic and clinical variables
| PerMANOVA test* |
||
|---|---|---|
| Variables | univariate | multivariate |
| Maternal variables | ||
| Ethnicity | 0.065 | |
| Maternal age | 0.57 | |
| Education1 | 0.25 | |
| Marital status | 0.10 | |
| Obesity | 0.17 | |
| Preeclampsia | 0.99 | |
| Offspring variables | ||
| Gender | 0.485 | |
| Preterm | 0.65 | |
| Birth weight | 0.64 | |
| Meconium collection time | 0.33 | |
| Mode of delivery (C-section vs. vaginal) | 0.033 | 0.009 |
| NICU admission | 0.72 | |
| Antenatal Stress | ||
| Prenatal depression | 0.30 | |
| State anxiety | 0.55 | |
| Trait anxiety | 0.14 | |
| Pregnancy-related anxiety (total) | 0.001 | 0.001 |
| Fear of having handicapped child | 0.24 | |
| Fear of appearance change | 0.003 | 0.45 |
| Fear of birth/delivery | 0.005 | 0.39 |
| Perceived stress | 0.44 | |
| Stressful Life events-total | 0.45 | |
| Life experiences-partner | 0.72 | |
| Life experiences-health | 0.24 | |
| Life experiences-legal | 0.56 | |
| Life experiences-work/finance | 0.45 | |
| Life experiences-friend | 0.41 | |
PerMANOVA test was performed at genus level.
Association between PRAQ-total and the relative abundance of particular taxa in the meconium
We further examined the association between PRAQ-total and the individual taxa from phylum to genus level (Figure 2B, Table 3). We found that the Proteobacteria phyla, its Enterobacteriaceae family and an unidentified genus from Enterobacteriacea family showed inverse correlations with PRAQ-total after adjustment for potential confounders (Spearman’s r=−0.40, −0.43 and −0.54; p-value=0.002, 2e-4, and 1.5e-4, respectively), suggesting that PRAQ-total was associated with lower relative abundance in those taxa from phylum to genus level.
Table 3.
Bacteria associated to the pregnancy-related anxiety accessed by PRAQ total score
| Taxa1 | Correlations2 | p-value 13 | p-value 24 |
|---|---|---|---|
| Phylum level | |||
| Proteobacteria | −0.40 | 0.0022 | 0.00028 |
| Family level | |||
| Enterobacteriaceae | −0.43 | 2.0e-4 | 6.8e-5 |
| Planococcaceae | 0.25 | 0.016 | 0.0023 |
| Pasteurellaceae | 0.16 | 0.0048 | 0.27 |
| Genus level | |||
| Enterobacteriaceae;g_ | −0.54 | 1.5e-4 | 8.2e-6 |
| Planococcaceae;g_ | 0.25 | 0.016 | 0.0022 |
| Dialister | −0.30 | 0.019 | 0.28 |
| Haemophilus | 0.19 | 0.0022 | 0.26 |
Taxa with less than 5% relative abundance was removed from the analysis.
Spearman’s correlation rho by R command cor().
p-value 1 was obtained from R command cor.test() to test for association between paired samples, using Spearman’s rho.
p-value 2 was obtained from linear regression model using relative abundance of selected taxa as dependent variable and PRAQ-total score and mode of delivery as independent variables.
DISCUSSION
In this study, we demonstrated that the low biomass first neonatal stool discharge, meconium, possessed diversified microbiota and that higher pregnancy-related anxiety was associated with the higher microbiota diversity in the offspring. Moreover, certain taxa from the Enterococcaceae family were inversely correlated with PRAQ-total. These findings contribute to the ongoing discussion on the potential role of maternal exposures during pregnancy in the initial bacterial colonization in the gut. Early life microbiome has been shown to significantly impact the priming of the immune system and determine immediate and long-lasting health outcomes (Romano-Keeler & Welkamp, 2015) with altered early life microbiota reportedly linked to the risk of developing asthma, eczema (Hong, Lee, & Aw, 2010), allergy (Johansson, Sjogren & Sverremark-Ekstrom, 2011), autism (Wang, Christophersen, Sorich, Gerber, Angley, & Conton, 2011) and other immune-mediated diseases (Sjogren, Tomicic, Lundberg, Bottcher, Bjoksten, Sverrmark-Skstorm, & Jenmalm, 2009).
We confirm prior findings that the meconium is already colonized with Enterococcaceae, Bacteroidetes, Firmicutes, and Proteobacteria. The relative abundances of these dominant phyla resembled that reported in our earlier study (Hu, et al., 2013) and showed a significantly higher relative abundance of Proteobacteria and lower relative abundance of Bacteroidetes and Firmicutes compared to adult stool. While some studies have shown the presence of bacteria in the newborn’s microbiome, suggesting a possible transfer of microbiota between mothers and children (Chu, Ma, Prince, Antony, Seferovic, & Aagaard, 2017; Hu et al., 2013; Valles, Gosalbes, de Vries, Abelián, & Francino, 2012), to date, the possibility that colonization of microbiome happens in utero remains controversial (Perez-Muñoz, Arrieta, Ramer-Tait, & Walter, 2017). A few recent studies that support the “sterile womb” hypothesis (Lauder, Roche, Sherrill-Mix, Bailey, Laughlin, Bittinger et al., 2016; Lim, Rodriguez, & Lori 2016) argue that the positive bacterial sequencing results in pregnancy-related samples, including the meconium, may be partially or fully due to environmental contamination, pointing to the establishment of germ-free animals as a proof of the sterility of the fetal environment in mammals (Perez-Muñoz, et al., 2017). On the other hand, accumulating evidence has shown the presence of viable bacterial cells in the fetal environment in the amniotic fluid (Bearfield, Davenport, Sivapathasundaram, & Allaker, 2002; Jiménez et al., 2008; Rautava et al., 2012), placental tissues (Aagaard, Ma, Antony, Ganu, Petrosino, & Versalovic, 2014), umbilical cord blood (Jiménez et al., 2005), and fetal membranes (Rautava et al., 2012; Steel, Malatos, Kennea, Edwards, Miles, Duggan, et al., 2005), without any indication of infection and inflammation. Even though the exact timing of establishment and the initial source of the microbes in the infant microbiome remain unknown, our findings support the notion that the initial colonization of the gut may start in utero (Rodríguez, Murphy, Stanton, Ross, Kober, Juge, et al., 2015).
Our study also revealed that pregnancy-related anxiety, but not a general state and trait anxiety, was the significant predictor of the overall meconium microbiota composition, suggesting that the specific types of stress that mothers experience concerning their pregnancy, may have an impact on the initial bacterial colonization in the offspring. At taxa level, the higher maternal PRAQ-total was associated with a lower relative abundance of Proteobacteria phylum in the meconium. Several studies have demonstrated an increased relative abundance of bacterial members belonging to Proteobacteria phylum in diseases sustained by various degree of inflammation, including metabolic disorders and inflammatory bowel disease (reviewed in Rizzatti et al., 2017). Interestingly, prior research (Zijlmans et al. 2015) indicated that the cumulative stress mothers experienced during pregnancy was positively associated with the level of Proteobacterial groups in infants’ stool samples during the first 4 months of life. However, the significant correlation between a lower relative abundance of pro-inflammatory Proteobacteria in the meconium and higher pregnancy-related anxiety found in our study contradicts that of Zijlmans et al. (2015). The discrepancy in the direction of the association may be due to sampling differences (baby stools versus meconium), microbiome surveying technique (microarray versus 16S rRNA sequencing), or different measures of maternal stress during pregnancy. It may also be due to the fact that the total bacterial loading in the meconium is very low and the relative abundance of the Proteobacteria is high, with unknown microbiota viability. Future studies using culturing techniques are warranted to profile the live versus dead bacteria.
Other studies have also provided evidence that pregnancy-related anxiety, but not general anxiety, is associated with altered molecular programming in newborn relevant to long-term health as suggested by methylation of the promoter region of the stress-related glucocorticoid receptor gene (NR3C1) in cord blood (Hompes, Izzi, Gellens, Morreels, Fieuws, Pexsters, et al., 2013) and shorter telomere length (Shalev, Entringer, Wadhwa, Wolkowitz, Puterman, Lin, et al., 2013; Entringer, de Punder, Buss, & Wadhwa, 2017). Our findings add to this emerging evidence that maternal pregnancy-related anxiety may influence the health of the offspring through the effects on the initial colonization of gut microbiome.
Our data also showed the beta diversity of meconium microbiome is significantly associated with delivery mode. It is contradictory to the previous reports (Dong et al., 2018; Hu et al., 2013) that the mode of delivery is unlikely a major contributor to shape this initial bacterial community since the meconium microbiota is mostly derived from the in–utero environment. One possible reason for our result is the almost 100% overlapping between the delivery mode via C-section and antibiotic usage, in which the latter may affect the meconium microbiome composition significantly.
The strengths of this study include the longitudinal design that made it possible to collect information about the stress mothers experienced during pregnancy prospectively before the baby was born. Also, we profiled the bacterial 16S rRNA gene in the meconium, the first newborn fecal discharge, which is believed to be formed in utero. However, the study also has limitations. First, although maternal ethnicity/race, age, education, marital status, time of sampling, and mode of delivery, were included as potential confounders in the model, our sample size restricted the ability to consider a broader range of covariates. Second, this study lacks information on dietary intakes, while recently several studies show the important role of diet in shaping gut microbiome (Gohir et al., 2015; Lundgren et al., 2018; Makki, Deehan, Walter, & Bäckhed, 2018). Third, the current study lacks biological markers of HPA-axis functioning such as cortisol levels, which are known to correlate with stressful life events and psychopathology (Rubinow, Post, Savard, & Gold, 1984). Having a biological measure of maternal stress during pregnancy might help to better understand the underlying biological mechanisms of the observed associations. Fourth, evidence that tracks the newborn meconium microbiome to the maternal source (gut, oral, placenta or vaginal) is lacking since we do not have access to the matched maternal microbiome samples, preventing us from determining the maternal origin of the initial microbiome, if any. Future studies comparing the bacterial composition of maternal and neonatal microbiome are warranted to determine the major source of the newborn microbiota. Although the 16S sequencing-based taxonomy analysis is cost-efficient and allows us to compare the overall microbiome composition, it only profiles bacterial taxa to the genus level so that no conclusion can be made as to which particular strains are correlated with pregnancy-related anxiety. Readers should also be reminded that the microbiome analysis using 16S sequencing data in this study should be interpreted with caution since it only assessed the relative abundance of each taxa, not the absolute bacterial counts, which require additional qualification by real-time PCR (Nagpal et al., 2016) or flow cytometry (Vandeputte et al., 2017). Moreover, the time of passing the meconium ranged from 0 to 5.5 hours post delivery, raising the possibility of some bacteria being introduced from environmental influences, such as delivery mode, breast milk or formula feeding, etc. However, we adjusted for the time until meconium passing in regression models and found that it was not statistically significantly related to microbiome diversity and relative abundance.
In summary, while we acknowledge the limitations above, the current study adds to the growing body of literature supporting a link between maternal pregnancy-related stress and the microbiome in the offspring. Understanding the dynamics of the gut-brain axis during early life may help develop novel targets to promote a healthy microbiome and optimal neurobehavioral development.
Supplementary Material
ACKNOWLEDGEMENT
This research work was supported by the grants K01-080062, K01-080062S and R01-102729 from the National Institutes of Mental Health (to YN).
Footnotes
None of the authors has any conflict of interest.
REFERENCES
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, & Versalovic J (2014). The Placenta Harbors a Unique Microbiome HHS Public Access. Sci Transl Med May, 21(6237), 237–65. 10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, & Jenmalm MC (2012). Low diversity of the gut microbiota in infants with atopic eczema. Journal of Allergy and Clinical Immunology, 129(2), 434–440.e2 10.1016/j.jaci.2011.10.025 [DOI] [PubMed] [Google Scholar]
- Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, … Kozyrskyj AL (2015). Infant gut microbiota and food sensitization: associations in the first year of life. Clinical & Experimental Allergy, 45(3), 632–643. 10.1111/cea.12487 [DOI] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, & Coe CL (2004). Prenatal stress alters bacterial colonization of the gut in infant monkeys. Journal of Pediatric Gastroenterology and Nutrition, 38(4), 414–421. 10.1097/00005176-200404000-00009 [DOI] [PubMed] [Google Scholar]
- Barnes LLLB, Harp D, & Jung WWS (2002). Reliability Generalization of Scores on the Spielberger State-Trait Anxiety Inventory. Educational and Psychological Measurement, 62(4), 603–618. 10.1177/0013164402062004005 [DOI] [Google Scholar]
- Bearfield C1, Davenport ES, Sivapathasundaram V, Allaker RP (2002). Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG, 109(5):527–33. [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, & Cryan JF (2014, September 1). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends in Molecular Medicine Elsevier Current Trends; 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, … Knight R (2010). QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5), 335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, & Aagaard KM (2016). The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Medicine, 8(1), 77 10.1186/s13073-016-0330-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, & Aagaard KM (2017). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med, advance on [DOI] [PMC free article] [PubMed]
- Cohen S, Kamarck T, & Mermelstein R (1983). A Global Measure of Perceived Stress. Journal of Health and Social Behavior, 24(4), 385 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, … Relman DA (2008). Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS ONE, 3(8), e3056 10.1371/journal.pone.0003056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP (2006). Inventorying Stressful Life Events as Risk Factors for Psychopathology: Toward Resolution of the Problem of Intracategory Variability. Psychological Bulletin, 132(3), 477–495. 10.1037/0033-2909.132.3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP, Yager TJ, Egri G, & Mendelsohn FS (1978). The Psychiatric Status Schedule as a measure of diensions of psychopathology in the general population. Archives of General Psychiatry, 35(6), 731–737. 10.1001/archpsyc.1978.01770300073007 [DOI] [PubMed] [Google Scholar]
- Dong T, Chen T, White RA, Wang X, Hu W, Liang Y, … Xia Y (2018). Meconium microbiome associates with the development of neonatal jaundice. Clinical and Translational Gastroenterology, 9(9), 182 10.1038/s41424-018-0048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack J, Kimes NE, Lin DL, Rauch M, McKean M, McCauley K, … Lynch SV (2018). Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nature Communications, 9(1), 707 10.1038/s41467-018-03157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, & Wadhwa PD (2011). Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Current Opinion in Endocrinology, Diabetes, and Obesity, 17(6), 1–18. 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Verner G, Wadhwa PD (2017) Fetal Programming of Telomere Biology: Role of Maternal Nutrition, Obstetric Risk Factors, and Suboptimal Birth Outcomes. pp. 569–593. In: Rajendram R, Preedy V, Patel V (eds) Diet, Nutrition, and Fetal Programming Nutrition and Health. Humana Press, Cham: 10.1007/978-3-319-60289-9_41 [DOI] [Google Scholar]
- Field T (2011). Prenatal depression effects on early development: A review. Infant Behavior and Development, 34(1), 1–14. 10.1016/j.infbeh.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Finik J, & Nomura Y (2017). Cohort Profile: Stress in Pregnancy (SIP) Study. International Journal of Epidemiology, dyw264 10.1093/ije/dyw264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, … Miller WC (2005). Perinatal depression: Prevalence, screening accuracy, and screening outcomes: Evidence report/technology assessment, Number 119. 10.1037/e439372005-001 [DOI] [PMC free article] [PubMed]
- Glover V (2015). Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms. In Advances in Neurobiology (Vol. 10, pp. 269–283). Springer New York; 10.1007/978-1-4939-1372-5_13 [DOI] [PubMed] [Google Scholar]
- Gohir W, Whelan FJ, Surette MG, Moore C, Schertzer JD, & Sloboda DM (2015). Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother’s periconceptional diet. Gut Microbes, 6(5), 310–320. 10.1080/19490976.2015.1086056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S1, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science 2016. March 18;351(6279):1296–302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- Gur TL, Worly BL, & Bailey MT (2015). Stress and the commensal microbiota: Importance in parturition and infant neurodevelopment. Frontiers in Psychiatry Frontiers Media SA; 10.3389/fpsyt.2015.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hompes T, Izzi B, Gellens E, Morreels M, Fieuws S, Pexsters A, Schops G, Dom M, Van Bree R,, Freson K, Verhaeghe J, Spitz B, Demyttenaere K, Glover V, Van den Bergh B, Allegaert K, Claes S (2013). Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J Psychiatr Res, 47(7):880–91. doi: 10.1016/j.jpsychires.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Hu J, Nomura Y, Bashir A, Fernandez-Hernandez H, Itzkowitz S, Pei Z, … Peter I (2013). Diversified microbiota of meconium is affected by maternal diabetes status. PloS One, 8(11), e78257 10.1371/journal.pone.0078257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK (2003). Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry, 44(6):810–8. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJHH, Robles De Medina PG, Visser GHAA, & Buitelaar JK (2004). Is pregnancy anxiety a distinctive syndrome? Early Human Development, 79(2), 81–91. 10.1016/j.earlhumdev.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL, Ja??arevi?? E, Howerton CL, … Bale TL (2015). Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology, 156(9), 3265–3276. 10.1210/en.2015-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Rodgers AB, & Bale TL (2015). A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiology of Stress, 1(1), 81–88. 10.1016/j.ynstr.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C … Rodríguez JM (2015). Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Current Microbiology, 51(4), 270–274. 10.1007/s00284-005-0020-3 [DOI] [PubMed] [Google Scholar]
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, … Rodríguez JM (2008). Is meconium from healthy newborns actually sterile? Research in Microbiology, 159(3), 187–193. 10.1016/j.resmic.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Kheirabadi GR, Maracy MR, Akbaripour S, & Masaeli N (2012). Psychometric properties and diagnostic accuracy of the edinburgh postnatal depression scale in a sample of Iranian women. Iranian Journal of Medical Sciences, 37(1), 32–8. 10.1007/s00737-007-0204-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, … Bushman FD (2016). Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome, 4(1), 29 10.1186/s40168-016-0172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ES, Rodriguez C, Holtz LR. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome 2018. May 11;6(1):87. doi: 10.1186/s40168-018-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren SN, Madan JC, Emond JA, Morrison HG, Christensen BC, Karagas MR, & Hoen AG (2018). Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome, 6(1), 109 10.1186/s40168-018-0490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackos AR, Maltz R, & Bailey MT (2017, February 1). The role of the commensal microbiota in adaptive and maladaptive stressor-induced immunomodulation. Hormones and Behavior Academic Press; 10.1016/j.yhbeh.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki K, Deehan EC, Walter J, & Bäckhed F (2018). The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host & Microbe, 23(6), 705–715. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Mazhari S, & Nakhaee N (2007). Validation of the Edinburgh Postnatal Depression Scale in an Iranian sample. Archives of Women’s Mental Health, 10(6), 293–297. 10.1007/s00737-007-0204-x [DOI] [PubMed] [Google Scholar]
- Melville JL, Gavin A, Guo Y, Fan M-Y, & Katon WJ (2010). Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstetrics and Gynecology, 116(5), 1064–70. 10.1097/AOG.0b013e3181f60b0a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri A, Torkan B, & Omidvari S (2007). The Edinburgh Postnatal Depression Scale (EPDS): Translation and validation study of the Iranian version. BMC Psychiatry, 7(1), 11 10.1186/1471-244X-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, & Carothers AD (1990). The validation of the Edinburgh Post-natal Depression Scale on a community sample. British Journal of Psychiatry, 157(2), 288–90. 10.1192/bjp.157.2.288 [DOI] [PubMed] [Google Scholar]
- Nagpal R, Tsuji H, Takahashi T, Kawashima K, Nagata S, Nomoto K, & Yamashiro Y (2016). Sensitive quantitative analysis of the meconium bacterial microbiota in healthy term infants born vaginally or by cesarean section. Frontiers in Microbiology, 7(DEC), 1997 10.3389/fmicb.2016.01997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, & Walter J (2017, December 28). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome BioMed Central. 10.1186/s40168-017-0268-4 [DOI] [PMC free article] [PubMed]
- Rautava S, Collado MC, Salminen S, & Isolauri E (2012). Probiotics Modulate Host-Microbe Interaction in the Placenta and Fetal Gut: A Randomized, Double-Blind, Placebo-Controlled Trial. Neonatology, 102(3), 178–184. 10.1159/000339182 [DOI] [PubMed] [Google Scholar]
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A . Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int 2017;2017:9351507. doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, … Collado MC (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microbial Ecology in Health & Disease, 26(0). 10.3402/mehd.v26.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Post RM, Savard R, & Gold PW (1984). Cortisol Hypersecretion and Cognitive Impairment in Depression. Arch Gen Psychiatry, 41(3), 279–283. 10.1001/archpsyc.1984.01790140069008 [DOI] [PubMed] [Google Scholar]
- Satokari R, Grönroos T, Laitinen K, Salminen S, & Isolauri E (2009). Bifidobacterium and Lactobacillus DNA in the human placenta. Letters in Applied Microbiology, 48(1), 8–12. 10.1111/j.1472-765X.2008.02475.x [DOI] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 2013. September;38(9):1835–42. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small R, Lumley J, & Toomey L (2006). Midwife-led debriefing after operative birth: four to six year follow-up of a randomised trial [ISRCTN24648614]. BMC Medicine, 4(1), 3 10.1186/1741-7015-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1989). State-trait anxiety inventory: A comprehensive bibliography Consulting Psychologists Press. [Google Scholar]
- Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, … Sullivan MHF (2005). Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatric Research, 57(3), 404–411. 10.1203/01.PDR.0000153869.96337.90 [DOI] [PubMed] [Google Scholar]
- Vallès Y, Gosalbes MJ, de Vries LE, Abellán JJ, & Francino MP (2012). Metagenomics and development of the gut microbiota in infants. Clinical Microbiology and Infection, 18(SUPPL. 4), 21–26. 10.1111/j.1469-0691.2012.03876.x [DOI] [PubMed] [Google Scholar]
- Van Den Bergh BRHH, Mulder EJHH, Mennes M, & Glover V (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neuroscience and Biobehavioral Reviews, 29(2), 237–258. 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Vandeputte D, Kathagen G, D’Hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, … Raes J (2017). Quantitative microbiome profiling links gut community variation to microbial load. Nature, 551(7681), 507–511. 10.1038/nature24460 [DOI] [PubMed] [Google Scholar]
- Verreault N, Da Costa D, Marchand A, Ireland K, Dritsa M, & Khalifé S (2014). Rates and risk factors associated with depressive symptoms during pregnancy and with postpartum onset. Journal of Psychosomatic Obstetrics & Gynecology, 35(3), 84–91. 10.3109/0167482X.2014.947953 [DOI] [PubMed] [Google Scholar]
- Walker RW, Clemente JC, Peter I, Loos RJF (2017). The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes, Suppl 1:3–17. doi: 10.1111/ijpo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JJ, Koren O, Hugenholtz P, DeSantis TZ, Walters WA, Caporaso JG, … Ley RE (2012). Impact of training sets on classification of high-throughput bacterial 16s rRNA gene surveys. The ISME Journal, 6(1), 94–103. 10.1038/ismej.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczyńska P, Skarżyńska E, & Lisowska-Myjak B (2018). Meconium microbiome as a new source of information about long-term health and disease: questions and answers. The Journal of Maternal-Fetal & Neonatal Medicine, 0(0), 1–6. 10.1080/14767058.2017.1387888 [DOI] [PubMed] [Google Scholar]
- Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, & de Weerth C (2015). Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology, 53, 233–245. 10.1016/j.psyneuen.2015.01.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


