Abstract
The acute toxicity of organophosphorus-based compounds is primarily a result of acetylcholinesterase inhibition in the central and peripheral nervous systems. The resulting cholinergic crisis manifests as seizure, paralysis, respiratory failure and neurotoxicity. Though overstimulation of muscarinic receptors is the mechanistic basis of central organophosphorus (OP) toxicities, short-term changes in synapse physiology that precede OP-induced seizures have not been investigated in detail. To study acute effects of OP exposure on synaptic function, field excitatory postsynaptic potentials (fEPSPs) were recorded from Schaffer collateral synapses in the mouse hippocampus CA1 stratum radiatum during perfusion with various OP compounds. Administration of the OPs paraoxon, soman or VX rapidly and stably depressed fEPSPs via a presynaptic mechanism, while the non-OP proconvulsant tetramethylenedisulfotetramine had no effect on fEPSP amplitudes. OP-induced presynaptic long-term depression manifested prior to interictal spiking, occurred independent of recurrent firing, and did not require NMDA receptor currents, suggesting that it was not mediated by activity-dependent calcium uptake. Pharmacological dissection revealed that the presynaptic endocannabinoid type 1 receptor (CB1R) as well as postsynaptic M1 and M3 muscarinic acetylcholine receptors were necessary for OP-LTD. Administration of CB1R antagonists significantly reduced survival in mice after a soman challenge, revealing an acute protective role for endogenous CB1R signaling during OP exposure. Collectively these data demonstrate that the endocannabinoid system alters glutamatergic synaptic function during the acute response to OP acetylcholinesterase inhibitors.
Keywords: Organophosphorus nerve agents, endocannabinoid system, cholinergic receptors, muscarinic receptors, cannabinoid type 1 receptor, cholinesterase inhibitors
Introduction.
Inhibition of acetylcholinesterase (AChE) by organophosphorus (OP) compounds prevents fast termination of cholinergic neurotransmission, causing hyperstimulation of muscarinic and nicotinic receptors in the peripheral and central nervous systems (Bajgar, 2004; Colovic et al., 2013). Systemic exposure to lethal doses of OPs elicits convulsion, seizure and death within minutes, whereas chronic exposure to sublethal doses can result in neurological syndromes, delayed neuropathies and persistent neuropsychiatric ailments (Jamal, 1997). Despite their widespread use as insecticides in developing countries, OPs can be highly toxic to mammals, and OP exposures are responsible for nearly 3,000,000 poisonings and 200,000 deaths annually worldwide (Rastogi et al., 2010). Because of their extreme toxicities, highly potent OPs such as soman, sarin and VX have been weaponized for use against military and civilian targets (Holstege et al., 1997; Rosman et al., 2014).
Many OPs readily cross the blood brain barrier and inhibit AChE in the central nervous system, eliciting proconvulsive seizures at lethal doses (Grob and Harvey, 1953). OP-induced seizures rapidly transition to refractory status epilepticus (SE), characterized by sustained seizure activity that is resistant to anticonvulsant drugs (McDonough and Shih, 1997; McDonough et al., 2010). Animal models of OP-induced SE reveal exaggerated synchronicity of excitatory firing and profound neurotoxicity in limbic and cortical brain regions (Filliat et al., 2007; McDonough and Shih, 1997), mediated in part by M1 muscarinic receptor overstimulation (Miller et al., 2017). Human survivors of severe OP exposures exhibit a range of impairments consistent with neurological damage, including amnesia, persistent epileptiform discharges and prolonged memory difficulties (Hoffman et al., 2007; Sidell, 1974; Yanagisawa et al., 2006). Currently, aggressive administration of benzodiazepines at early stages of seizure is the only treatment strategy to prevent permanent neurological damage following onset of SE (McDonough et al., 1999; Todorovic et al., 2012). Although timely administration of oxime-based AChE reactivators can reverse the peripheral symptoms of OP exposure, currently fielded oximes have poor central penetration and therefore are largely ineffective in treating central OP toxicities (Bajgar et al., 2007). Similarly, muscarinic antagonists such as atropine can mitigate peripheral symptoms of OP exposure, however a dosage regimen that provides central protection without eliciting adverse physiological consequences has not been identified (Eddleston and Chowdhury, 2016).
The rapid emergence of irreversible neurological injury following OP exposure has created a critical need for centrally acting countermeasures that are effective in preventing or terminating cholinergic seizure at early stages. Although nerve agent-induced seizures are initiated by hyperstimulation of muscarinic acetylcholine receptors (mAChR), seizures are sustained and reinforced primarily by glutamatergic activity (McDonough and Shih, 1997). However, the precise mechanisms by which cholinergic overstimulation modulates excitatory glutamatergic neurotransmission prior to this transition are unknown. Changes in synaptic physiology that precede spontaneous neuronal discharges are of particular interest, since these changes may promote seizurogenesis or alternatively serve as compensatory responses to increased excitatory drive. In either case, identification of synaptic modifications acutely associated with OP exposure would potentially enable novel therapies, as well as improve understanding of the synaptic response to cholinergic overstimulation.
Hippocampal function is extensively regulated by cholinergic signaling, and consequently the hippocampus is highly susceptible to AChE inhibition (Teles-Grilo Ruivo and Mellor, 2013). Treatment of acute brain slices with OPs causes interictal spiking in hippocampal cornus ammonis subfield 3 (CA3) and CA1 pyramidal neurons (Apland et al., 2009; Aroniadou-Anderjaska et al., 2016; Lewis et al., 1967), while in vivo OP exposure results in frank CA3 and CA1 neuropathology and chronic alteration of excitatory hippocampal circuits (Alexandrova et al., 2014; Apland et al., 2010; Munirathinam and Bahr, 2004). We took advantage of this sensitivity to study the effects of OP compounds on excitatory synaptic physiology at hippocampal Schaeffer collateral (SC) synapses in acute mouse brain slices. We found that OP perfusion evokes a chemically induced form of long-term depression (LTD) in presynaptic nerve terminals that requires activation of the cannabinoid type 1 receptor (CB1R) as well as M1 and M3 muscarinic acetylcholine receptors (mAChRs). Administration of a CB1R antagonist significantly increased mortality in an in vivo soman challenge model, revealing an endogenous role for CB1R activation in protecting against OP toxicity. Collectively these studies demonstrate a novel mechanism by which AChE inhibition acutely depresses excitatory neurotransmission through mAChR-dependent activation of CB1R.
Methods
Drugs.
The following chemicals were purchased from Sigma-Aldrich (St Louis, MO): NaCl, MgCl2, CaCl2, NaH2PO4, glucose, NaHCO3, atropine, tetramethylenedisulfotetramine (TETS; 2,6-dithia-1,3,5,7- tetraazaadamantane, 2,2,6,6-tetraoxide) and paraoxon (diethyl 4-nitrophenyl phosphate). VU 0255035 (N-[3-Oxo-3-[4-(4-pyridinyl)-1-piperazinyl]propyl]-2,1,3-benzothiadiazole-4-sulfonamide), AM-2510 (N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide, 4-DAMP (1,1-Dimethyl-4-diphenylacetoxypiperidinium iodide), DL-APV (DL-2-Amino-5-phosphonopentanoic acid), AQ-RA (11-[[4-[4-(Diethylamino)butyl]-1-piperidinyl]acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one), picrotoxin, ACEA (N-(2-chloroethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide) and PD 102807 (3,6a,11,14-Tetrahydro-9-methoxy-2-methyl-(12H)-isoquino[1,2-b]pyrrolo[3,2-f][1,3]benzoxazine-1-carboxylic acid, ethyl ester) were purchased from Tocris Biosciences (Bristol, United Kingdom). Soman (3,3-dimethyl-2-butanyl methylphosphonofluoridate) and VX (S-[2-(diisopropylamino)ethyl] O-ethyl methylphosphonothioate) were produced by the U.S. Army, validated by tandem mass spectrometry and stored in exempt chemical surety quantities at the U.S. Army Medical Research Institute of Chemical Defense (Gunpowder, MD).
Animals.
All animal studies were reviewed and approved by an Institutional Animal Care and Use Committee, and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), and the Animal Welfare Act of 1966 (P.L. 89–544), as amended. C57BL/6J male mice (8–10 weeks, https://www.jax.org/strain/000664) were maintained on a 12 h light/dark cycle and provided food and water ad libitum in accordance with IACUC regulations.
For ex vivo studies, mice were anesthetized with isoflurane and decapitated by guillotine. The brain was rapidly dissected and placed in ice-cold solution containing (in mM): 80 NaCl, 24 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5 MgCl2 (pH 7.3) and bubbled with 95% O2 and 5% CO2. Coronal hippocampal slices (350 μm thick) were cut using a vibratome (VT1200S Leica, Germany), bisected and placed in artificial cerebral spinal fluid (ACSF) containing (in mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 11.4 glucose, and 21.4 NaHCO3 (pH 7.3), bubbled with 95% O2 and 5% CO2 and maintained at 30°C. Slices were allowed to recover for at least 1 hour before being placed in a submersion recording chamber perfused with oxygenated ACSF at 1.6–2.0 mL/min. Slices were visualized on an upright fixed-stage microscope (Olympus BX51WI, Waltham, MA) equipped with IR-DIC optics. Recording pipettes (3–6 MΩ resistance) were filled with ACSF and placed in the CA1 stratum radiatum to record excitatory postsynaptic potentials. Recordings were made using HEKA EPC 10 amplifiers (Lambrecht, Germany) at 20 kHz, filtered at 5–10 kHz using Patchmaster software (HEKA, Lambrecht, Germany) and analyzed using Axograph (version 1.1.6). Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded in the CA1 dendritic arbor with a HEKA headstage using glass electrodes filled with ACSF. fEPSPs were induced with two 100 μs current pulses at 50 ms interpulse interval administered through monopolar ACSF-filled stimulating pipettes placed in the CA3 stratum radiatum. Stimulation intensity was set at 30% of the maximum intensity current required to elicit a population spike at each recording site and ranged from 0.2 to 0.7 mA. In all cases, stable baselines were recorded for at least 20 minutes prior to superfusion of drug or vehicle. Recordings were terminated if > 10% variation in fEPSP was observed during the baseline period. Recordings continued for at least 40 min after perfusion of soman. As indicated, SC fibers were cut using a scalpel under a dissecting microscope.
For in vivo studies, mice were randomized and treated with HI-6 dimethanesulfonate (50 mg/kg in saline) and either the CB1R antagonist AM-251 (10 mg/kg in DMSO) or vehicle (DMSO) via intraperitoneal injection. Five minutes later, soman was administered (147 μg/kg in saline) by subcutaneous injection. Mortality was evaluated at 30 min intervals based on the lack of a heartbeat and failure to respond to strong toe-pinch, starting 15 min after soman administration.
Immunohistochemistry.
Brains were extracted following transcardial perfusion with saline followed by 10% formalin at room temperature and embedded in paraffin. Hippocampal lesions were generated in 5 μm coronal sections by transverse incision of SC fibers. Deparaffinized sections were permeabilized with 0.1% Triton X-100 in PBS and blocked in 0.1% Saponin and 3% bovine serum albumin in PBS (PBSS). Sections were incubated in Anti-NeuN Alexa Fluor®488 conjugated antibody (EMD Millipore MAB377X 1:250) in PBSS, washed and mounted with ProLong™ Diamond containing DAPI (ThermoFisher Scientific). Tile-scanned 20× images were collected using a Zeiss LSM 700 confocal microscope (Carl Zeiss Inc., Thornwood, NY).
Statistical analyses.
For each slice, fEPSP amplitudes, paired-pulse ratios and fiber volley amplitudes were averaged over the final five minutes of treatment and normalized to average values from the final 5 min of baseline recordings. When appropriate, the effects of treatment conditions on fEPSPs were compared between average values from the final 5 min of baseline recordings and graphed separately. Representative fEPSP traces were generated by averaging among at least five traces. Paired-pulse ratios were calculated by dividing the amplitude of the second fEPSP by the first fEPSP, averaged over the last 5 min of baseline or treatment. Each data point in figures represents average values binned per min. For kinetic analyses, the second fEPSP in each paired pulse was normalized to baseline amplitudes, and areas under the curve were determined over the 200 ms period after peak amplitude using Axograph. All statistical analyses were conducted with Graphpad Prism v 6.05 (Graphpad Software, La Jolla, CA). Statistical comparisons between baseline values to treated values were made using paired Student’s t-test; comparison between different treatment conditions were made using unpaired Student’s t-test with Welch’s correction; comparisons among multiple samples were made using one-way ANOVA followed by Dunnett’s test for significance; and survival data were compared using Fisher’s exact test. Grouped data are presented as mean ± SEM and graphically annotated with the following levels of statistical significance: ns indicates not significant; * indicates p < 0.05; ** indicates p < 0.01; and *** indicates p < 0.001.
Results
OP perfusion rapidly elicits long-term fEPSP depression at the Schaffer collateral (SC) synapse.
The effects of OP perfusion on synaptic physiology were monitored at the Schaffer collateral (SC) synapse in mouse acute brain slices. Field excitatory post-synaptic potentials (fEPSPs) were recorded at the CA1 dendritic arbor in response to paired-pulse stimulation of afferent CA3 nerve fibers (100 μs current pulses with 50 ms interpulse intervals, administered at 0.05 Hz). We initially evaluated SC synapse function following perfusion with the OP nerve agent soman. Soman irreversibly alkylates AChE within minutes, thus mitigating the potentially confounding effects of spontaneous AChE reactivation on SC synapse activity (Leikin et al., 2002). After establishing stable baseline fEPSP amplitudes during perfusion with vehicle, fEPSPs were measured in response to perfusion with soman (10 μM). The soman concentration was chosen during pilot studies, which revealed no significant differences in ictogenic responses between 1 and 10 μM soman, whereas perfusion with 0.1 μM soman did not have an apparent neurophysiological effect within 20 min (not shown). Use of 10 μM soman is consistent with previous hippocampal soman perfusion studies (Apland et al., 2009; Wang et al., 2011) and corresponds to the estimated maximum plasma concentration (Cmax) following subcutaneous administration of 1 LD50 soman to mice.
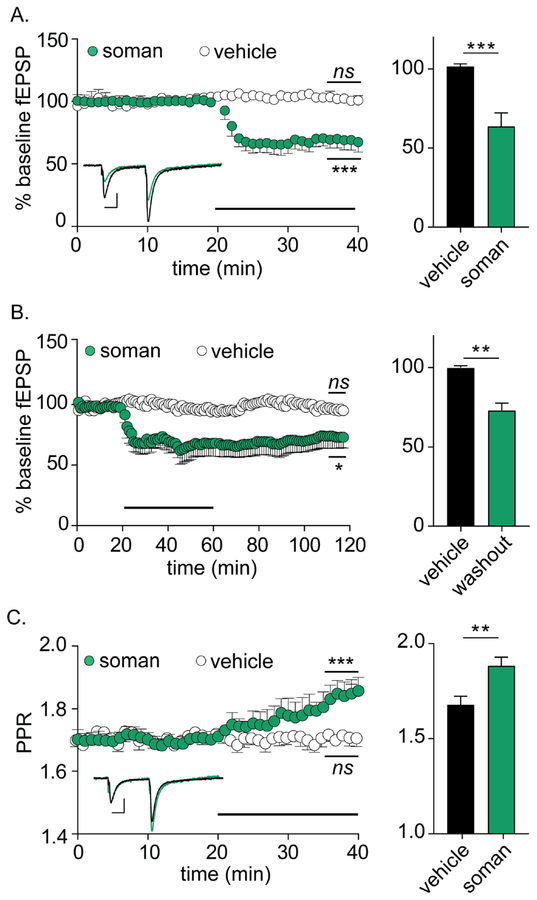
Soman elicited a 36.0 ± 7.2% reduction in fEPSP amplitudes, with a median time-to-maximal depression (t50) of 3.42 ± 1.67 min (n = 12; Figure 1A). fEPSP depression persisted for at least 60 min after soman washout, suggesting that soman was inducing long-term depression of synaptic function (Figure 1B). To evaluate the mechanism(s) involved in fEPSP depression, we evaluated paired pulse ratio (PPR) and fEPSP kinetics. PPR increased coincident with fEPSP depression, indicating a presynaptic mechanism (Figure 1C) (Creager et al., 1980). Soman had no effect on fEPSP decay (baseline: −6.54 ± 0.14 ms, soman: −6.41 ± 0.28 ms, p = 0.69, n = 12 each), excluding changes in postsynaptic receptor kinetics as a mechanism for fEPSP depression. Finally, fiber volley amplitudes remained constant following soman perfusion, confirming that fEPSP depression was not a result of direct injury to SC nerve fibers (baseline: −0.16 ± 0.01 mV, soman: −0.15 ± 0.01 mV, p = 0.91, n = 12 each).
Figure 1.
Soman perfusion induces presynaptic LTD at mouse SC synapses. For all panels, statistical significances were determined between the final 5 min of baseline versus the final 5 min of treatment (indicated on plot), as well as between the final 5 min of each treatment condition (bar graphs on right). Horizontal bars represent perfusion with respective treatments. (A) Soman (10 μM) significantly depresses normalized fEPSP amplitudes at the CA1:SC synapse (n = 12 each p = 0.004 versus baseline; p = 0.002 versus vehicle). Inset depicts paired-pulse traces from baseline (black) and 20 min after addition of soman (green) demonstrating reduced fEPSP amplitudes in response to soman treatment. (B) Soman stably depresses fEPSP amplitudes for at least 30 min after wash-out (n = 5 each; p = 0.04 versus baseline; p = 0.010 versus vehicle). (C) Soman perfusion increases paired-pulse ratios (n = 6 soman, n = 5 control, p = 0.008 versus baseline; p = 0.012 versus vehicle). Inset depicts normalized paired-pulse traces from baseline (black) and 20 min after soman addition (green) demonstrating increased PPR after soman treatment. Scale bars = 0.20 mV by 100 ms.
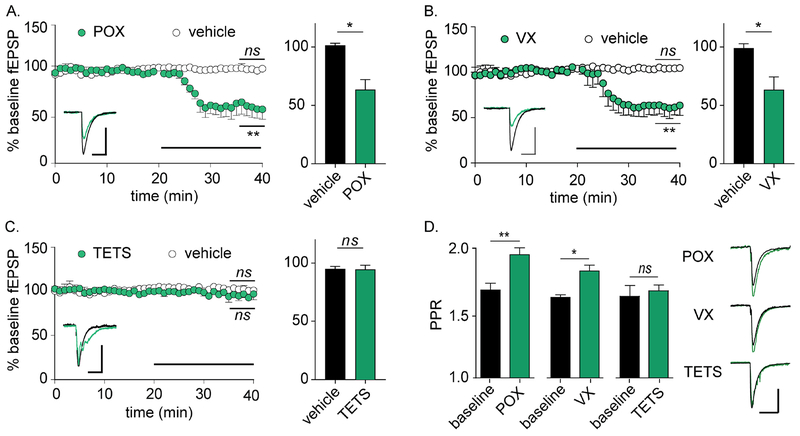
To evaluate whether soman-induced depression of SC synapses represented a class effect, synaptic function was monitored following perfusion with two additional OPs: paraoxon, which is the active metabolite of the pesticide parathion, and the second-generation nerve agent VX. Paroxon (8 μM) was applied based on previous reports of altered neurophysiological responses between 1–10 μM (Miller et al., 2017; Narimatsu et al., 2010), while VX (10 μM) was used at a concentration that matched soman based on similar inhibitory rate constants against AChE (Wille et al., 2011). Similar to soman, perfusion with either paraoxon or VX significantly reduced fEPSP amplitudes and increased PPR (Figure 2A–B). Both OPs exhibited longer latencies to fEPSP depression than soman, with t50 values of 8.62 ± 2.71 and 10.37 ± 0.86 min, respectively (n = 5 each; p < 0.01 for each versus soman, one-way ANOVA with Tukey’s multiple comparisons test). In contrast, the non-OP GABAA receptor antagonist tetramethylenedisulfotetramine (TETS; 3 μM) had no effect on fEPSP amplitude (Figure 2C). However, TETS did cause recurrent firing that altered fEPSP decay kinetics (Figure 2C), confirming target engagement and indicating that fEPSP depression was not a generic response to seizurogenic agents. Similar to soman, paraoxon and VX increased PPR, whereas TETS had no effect on PPR (Figure 2D). Since depression of SC synapse function appeared to be a class effect in response to chemically diverse OP compounds, we termed this phenomenon OP-induced long-term depression (OP-LTD).
Figure 2.
Reduced synaptic release is common to OP AChE antagonists, but not non-OP seizurogenic compounds. For panels A-C, statistical significances are determined between the final 5 min of baseline versus the final 5 min of treatment (indicated on plot), as well as between the final 5 min of each treatment condition (bar graphs on right). Horizontal bars represents perfusion with respective treatments. (A) Perfusion with paraoxon (POX; 8 μM) depresses fEPSP amplitudes (n = 5 each; p = 0.008 versus baseline; p = 0.016 versus vehicle). (B) Perfusion with VX (10 μM) depresses fEPSP amplitudes (n = 5 each; p = 0.003 versus baseline; p = 0.031 versus vehicle). (C) Perfusion with TETS (3 μM) has no effect on fEPSP amplitudes (n = 4 each; p = 0.59 versus baseline; p = 0.91 versus vehicle). Representative fEPSPs for baseline (black) and following treatment (green) are shown as insets in each plot. Note confirmation of TETS activity on synaptic function as demonstrated by altered fEPSP kinetics, which are consistent with GABAA receptor antagonism. (D) Baseline PPRs increase following perfusion with POX (p = 0.002) and VX (p = 0.012), but not TETS (p = 0.78). Representative paired pulses are presented to the right. Scale bars = 0.20 mV by 100 ms.
OP-LTD is mediated through activation of M1 and M3 muscarinic acetylcholine receptors.
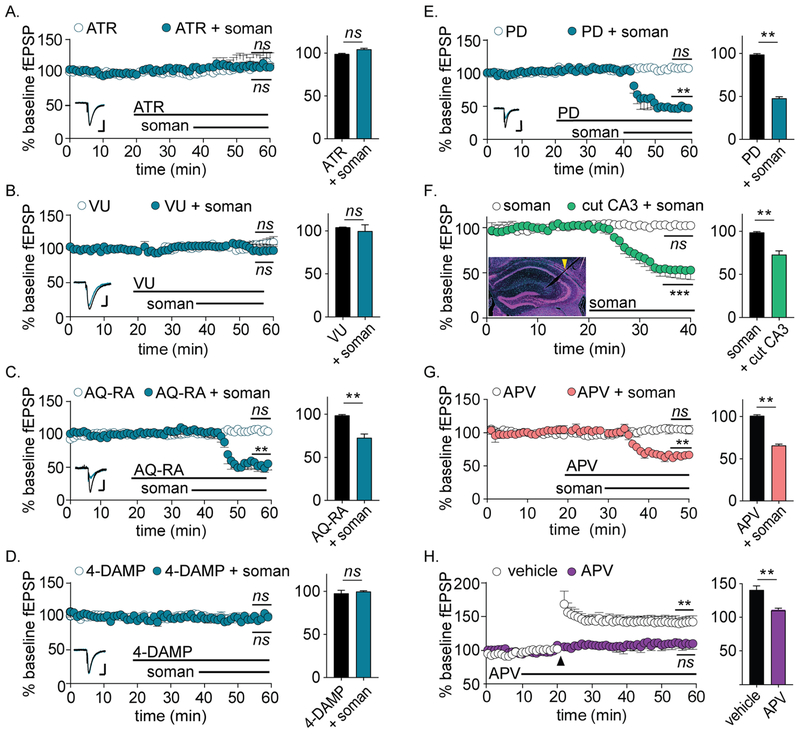
We next sought to understand the signaling mechanisms involved in OP-LTD caused by soman perfusion. Because overstimulation of metabotropic muscarinic acetylcholine receptors (mAChRs) is the initiating event of central OP toxicity (McDonough and Shih, 1997), we first evaluated the effects of mAChR blockade on OP-LTD. Co-administration of the non-selective mAChR antagonist atropine (5 μM) blocked OP-LTD, demonstrating a functional requirement for mAChR activation (Figure 3A) (Lebois et al., 2010). Four mAChRs subtypes are robustly expressed in the hippocampus: M1 and M3, which are coupled to Gq/11 proteins, and M2 and M4, which are coupled to inhibitory Gi/o proteins (Levey, 1996). To discern which mAChR were involved in OP-LTD, we applied selective antagonists for each receptor subtype at concentrations reported to cause selective inhibition (Augelli-Szafran et al., 1998; Dorje et al., 1991; Liu et al., 1998; Sheffler et al., 2009) (Figure 3B–E). Whereas selective antagonists of M2 (AQ-RA, 1 μM) and M4 (PD 102807, 500 nM) had no effect on fEPSPs, antagonists of either M1 (VU 0255035, 1 μM) or M3 (4-DAMP, 100 nM) completely blocked OP-LTD. In control slices perfused with vehicle, neither atropine nor selective antagonists of M1-M4 mAChRs affected baseline fEPSP amplitudes (Figure 3A–E).
Figure 3.
OP-LTD requires M1 and M3 mAChR signaling, but does not require spontaneous neuronal activity or NMDAR currents. For all panels, statistical significances are determined between average fEPSP amplitudes during the final 5 min of baseline versus the final 5 min of treatment (indicated on plot) as well as between the final 5 min of treatment between vehicle and soman (bar graphs on right). (A) Non-specific mAChR antagonist atropine (ATR; 5 μM) blocks OP-LTD (n = 4 each; p = 0.36 versus baseline; p = 0.43 versus vehicle). (B) M1 mAChR antagonist VU 0255035 (VU; 1 μM) blocks OP-LTD (n = 4 each; p = 0.52 versus baseline; p = 0.60 versus vehicle). (C) M2 mAChR antagonist AQ-RA (1 μM) does not prevent OP-LTD (n = 4 each; p = 0.003 versus baseline; p = 0.003 versus vehicle). (D) M3 mAChR antagonist 4-DAMP (100 nM) blocks OP-LTD (n = 4 each; p = 0.71 versus baseline; p = 0.62 versus vehicle). (E) M4 mAChR antagonist PD 102807 (PD; 500 nM) does not prevent OP-LTD (n = 3 each; p < 0.001 versus baseline; p < 0.001 versus vehicle). For A-E, perfusion with mAChR antagonists alone had no effect on fEPSP amplitudes (white circles, n ≥ 4 for each). Representative fEPSPs for baseline (black) and following treatment (blue) are shown as insets in each plot. Scale bars = 0.20 mV by 50 ms. (F) OP-LTD occurs despite incision of CA3 afferent fibers (n = 3 each; p = 0.011 versus baseline; p = 0.003 versus vehicle). (Inset) Coronal hippocampal slice with representative incision at the Schaffer collateral fibers. (G) APV does not prevent OP-LTD (n = 4 each; p = 0.007 versus baseline; p = 0.004 versus vehicle), even though (H) APV blocks high frequency-induced long-term potentiation (n = 3 each; p < 0.001 versus baseline; p = 0.003 versus vehicle).
OP-LTD is dependent on CB1R activation, but not on spontaneous neuronal activity or NMDA receptor activation.
Although increased PPR values suggested that OP-LTD is mediated by a presynaptic mechanism, M1 and M3 mAChR subtypes are predominantly expressed at postsynaptic membranes in the hippocampus (Levey, 1996). Thus, we sought to identify a retrograde signaling mechanism that linked post-synaptic mAChR activation with reduced presynaptic release. We first evaluated whether spontaneous CA1 or CA3 action potential firing contributed to soman-mediated OP-LTD (Bonansco and Fuenzalida, 2016; Lopantsev et al., 2009). Transection of CA3 nerve fibers had no effect on OP-LTD, eliminating a role for recurrent or spontaneous presynaptic firing in fEPSP depression (Figure 3F). Similarly, in continuous recordings spontaneous CA1 firing emerged more than 6.5 min after fEPSP depression (3.42 ± 1.67 min versus 10.19 ± 4.19 min; n = 6 each, p = 0.034), indicating that spontaneous postsynaptic firing is not required for OP-LTD emergence. Because Ca2+ influx through presynaptic NMDA receptors (NMDAR) is a well-described mechanism of LTD at SC synapses, we next evaluated a potential role for NMDARs in OP-LTD (Rodriguez-Moreno and Paulsen, 2008). Co-administration of the competitive NMDAR antagonist APV (50 μM) had no effect on soman-induced fEPSP depression (Figure 3G), despite control experiments demonstrating that APV treatment blocked NMDAR-mediated long- term potentiation of SC synapses in response to high frequency stimulation (Figure 3H).
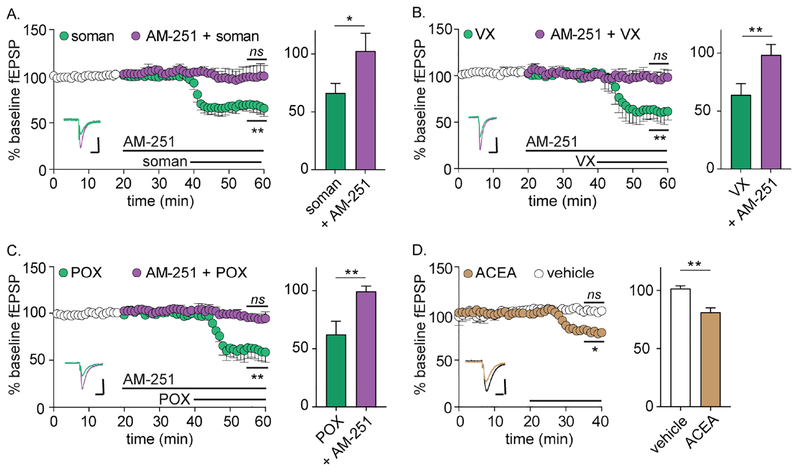
We next evaluated whether the G protein-coupled cannabinoid type-I receptor (CB1R) was involved in OP-LTD (Vaughan and Christie, 2005). CB1R is expressed predominantly in the presynaptic membrane of neurons, including at SC synapses, and is typically associated with inhibitory Gi/o signaling (Herkenham et al., 1990; Mechoulam and Parker, 2013). The release of eCBs such as anandamide and 2- arachidonoylglycerol from postsynaptic membranes activates presynaptic CB1R-mediated signaling cascades which, in turn, decrease release probabilities (Kano et al., 2009). Notably, selective M1 and M3 agonists have been found to activate retrograde CB1R signaling in the hippocampus, directly linking muscarinic receptor activity with presynaptic inhibition (Alger et al., 2014; Kim et al., 2002; Ohno-Shosaku et al., 2003). Co-administration with the selective CB1R reverse inhibitor AM-251 prevented fEPSP depression (Figure 4A) and PPR increases in comparison to baseline values (1.64 ± 0.05 versus 1.67 ± 0.04 respectively, n = 6, p = 0.55), indicating that CB1R activation was necessary for OP-LTD. AM-251 treatment similarly blocked OP-LTD in response to perfusion with VX or paraoxon (Figure 4B–C). We next evaluated whether direct activation of CB1R could elicit changes in synaptic transmission associated with OP-LTD. Perfusion of naïve slices with the CB1R-selective agonist ACEA (50 nM) depressed fEPSP amplitudes (Figure 4D) and increased PPR (1.60 ± 0.01 versus 1.76 ± 0.03, n = 5, p = 0.01) to levels similar to soman perfusion, indicating that activation of CB1R was sufficient to elicit changes in synaptic physiology consistent with OP-LTD (Figure 4D).
Figure 4.
mAChR-dependent LTD is mediated by the endogenous cannabinoid type 1 receptor. For all panels, statistical significances are determined between average fEPSP amplitudes during the final 5 min of baseline versus the final 5 min of treatment (indicated on plot) as well as between the final 5 min of treatment between vehicle and soman (bar graphs on right). (A) Selective CB1R antagonist AM-251 (AM; 8 μM) blocks OP-LTD during perfusion of soman (n = 5; p = 0.73 versus baseline; p = 0.001 versus soman). (B) AM-251 blocks OP-LTD during perfusion of VX (n = 3, p = 0.70 versus baseline; p = 0.001 versus soman). (C) AM blocks OP-LTD during perfusion of paraoxon (POX; n = 3; p = 0.22 versus baseline; p = 0.010 versus soman. For A-C, representative fEPSPs for OPs (green) and following AM-251 treatment (purple) are shown as insets in each plot. White circles represent average baseline fEPSPs among all groups prior to perfusion with AM or AM plus OP. (D) Perfusion with selective CB1R agonist ACEA causes fEPSP depression (n = 5; p = 0.042 versus baseline; p = 0.010 versus vehicle). Representative fEPSPs for vehicle (black) and following ACEA treatment (tan) are shown as inset. Scale bars = 0.20 mV by 50 ms.
Endogenous CB1R signaling protects against soman toxicity.
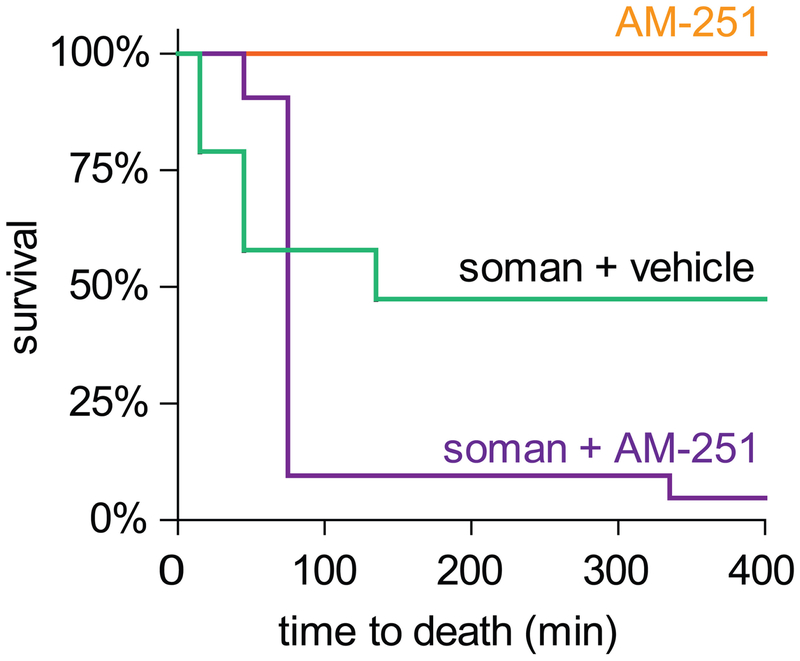
The above data demonstrated that CB1R activation is necessary for OP-induced fEPSP depression at SC synapses. CB1R is the most abundant G protein-coupled receptor in the central nervous system (Freund et al., 2003; Howlett et al., 2010), and CB1R signaling acts as a principal regulator of neuronal function, with diverse and context-specific effects on neurological responses (Hebert-Chatelain et al., 2014; Herkenham et al., 1990; Lu and Mackie, 2016; Moldrich and Wenger, 2000). To determine whether CB1R signaling mediated a globally protective physiological response to cholinergic overstimulation, we evaluated the effects of in vivo CB1R antagonism on survival in a mouse soman challenge model (Figure 5). Mice were given HI-6 oxime (50 mg/kg) to mitigate peripheral soman toxicity and either AM-251 (10 mg/kg; n = 20) or vehicle (n = 19), followed 5 min later by subcutaneous administration of 147 μg/kg soman, which represented a medial lethal dose under these conditions. Treatment with AM-251 significantly reduced 24 h survival rates from 47.4% in the vehicle-treated group to 5.0% in the AM-251-treated group, with similar results in two experimental cohorts (p = 0.003; Fisher’s exact test). Naïve mice did not appear to be affected by treatment with AM-251 through 24 h (n = 5). The significantly increased mortality of soman-exposed mice treated with AM-251 suggested that endogenous CB1R activation has a globally protective effect in response to central cholinergic overstimulation.
Figure 5.
Antagonism of endogenous CB1R signaling reduces survival in vivo following a median lethal soman challenge in mice. Mice were injected with HI-6 (50 mg/kg) and the CB1R antagonist AM-251 (10 mg/kg) at 5 mins before i.p. challenge with soman (147 μg/kg), and survival was monitored at 30 min intervals for up to 24 h. Although AM-251 had no effects on survival of naïve mice, AM-251 reduced survival in soman-exposed mice, with nearly all deaths occurring prior to 100 min after exposure.
Discussion.
The toxic effects of OPs are caused primarily by accumulation of synaptic acetylcholine in the central and peripheral nervous systems secondary to cholinesterase inhibition (Bajgar, 2004; Colovic et al., 2013). However, the mechanisms by which cholinergic overstimulation affects excitatory neurotransmission prior to seizure onset are unknown. Here we investigated acute changes in glutamatergic SC synaptic physiology during perfusion of mouse coronal slices with OP and non-OP seizurogenic compounds. OP perfusion caused a robust presynaptic chemical LTD that was dependent on activation of CB1R and M1 and M3 mAChRs. The suppressive effects of OPs on glutamatergic neurotransmission were entirely reversed by a CB1R antagonist, suggesting that activation of M1 and M3 mAChRs mobilizes eCBs to activate presynaptic CB1R signaling pathways. Antagonism of CB1R activation increased lethality in a mouse soman challenge model, demonstrating that endogenous CB1R signaling protects against central OP toxicity. Collectively, these data suggest that CB1R activation represents an endogenous compensatory response to muscarinic overstimulation following AChE inhibition and furthermore provide a mechanistic basis for previous reports that pharmacological modulation of eCB signaling can enhance or mitigate central OP toxicity (Liu and Pope, 2015; Nallapaneni et al., 2006, 2008; Wright et al., 2010).
A major function of the eCB system is suppression of neurotransmission through the retrograde activation of CB1R (Kano et al., 2009). According to this model, overstimulation of postsynaptic Gq/11-coupled mAChRs (e.g., M1, M3 and/or M5) elicits release of eCB signaling lipids from dendritic membranes via the phospholipase C signaling pathway. eCBs undergo retrograde transport to bind receptors on presynaptic membranes, including the Gi/o-coupled CB1R. CB1R activation reduces presynaptic release probability through protein kinase A-dependent mechanisms, including inhibition of adenylyl cyclase activity, inhibition of voltage-gated Ca2+ channels and potentiation of rectifying K+ channels (Mechoulam and Parker, 2013). Although eCB signaling is most commonly associated with reduction in inhibitory synaptic activity, eCBs also act at excitatory synapses to alter presynaptic release (Gerdeman et al., 2002; Robbe et al., 2002). While our pharmacological and electrophysiology data indicate that OP-LTD is mediated by presynaptic suppression of excitatory SC synapses, we cannot exclude the possibility that changes in inhibitory neurotransmission also contribute to reduced fEPSP amplitudes. Indeed, it has been shown that paraoxon transiently increases inhibitory postsynaptic currents followed by increased excitatory currents in rat basolateral amygdala, suggesting that OPs can have dynamic temporal effects on synaptic function (Miller et al., 2017). Furthermore, although mGluR agonism has been directly linked to CB1R-mediated suppression of inhibitory synapses (Varma et al., 2001), we found that stimulation of mAChRs resulted in CB1R-mediated suppression of excitatory synapses. This difference raises the possibility that metabotropic neurotransmitter receptors may have subtype-specific effects on eCB signaling that differentially regulate network activity. Notably, it was recently reported that synaptic activation of mGlu5 metabotropic glutamate receptors is involved in M1 mAChR receptor-mediated presynaptic LTD in the prefrontal cortex, potentially illustrating a coordinated response to glutamateric and cholinergic overstimulation (Ghoshal et al., 2017).
The finding that OPs activate CB1R-mediated presynaptic LTD through M1/M3 mAChR-dependent signaling provides a single mechanism to explain previous reports of depressed synaptic release in response to cholinergic overstimulation. Interestingly, suppression of field potentials coincident with development of epileptiform activity was observed in rat basolateral amygdala following soman perfusion, suggesting that depression of excitatory synaptic activity is a common response to cholinergic overstimulation in multiple brain regions (Apland et al., 2009). Chemical activation of M1 or M3 mAChRs has been shown to initiate the release of eCBs in diverse brain regions, including SC synapses (Fukudome et al., 2004; Hashimotodani et al., 2005; Kim et al., 2002; Martin et al., 2015). OP exposure was also shown to elevate eCB levels in brain, which was initially attributed to direct inhibition of eCB hydrolases by OPs (see below) (Liu et al., 2013; Nomura et al., 2008). Decreased excitatory signaling in hippocampal circuits has been demonstrated in rat and guinea pig brain slices at delayed times after in vivo soman challenge (Alexandrova et al., 2014; Munirathinam and Bahr, 2004); in cultured hippocampal neurons exposed to VX (Rocha et al., 1999); in hippocampal slices exposed to paraoxon (Narimatsu et al., 2010); and at rat SC synapses exposed to the reversible AChE antagonist physostigmine (an effect that was originally attributed to tonic activation of presynaptic mAChRs) (Mans et al., 2014). Separately it was found that physostigmine-induced presynaptic depression could be blocked by treatment with a CB1R antagonist, but not a M2 antagonist (Colgin et al., 2003). Intrastrial administration of a muscarinic agonist resulted in rapid decrease in glutamatergic tone (Smolders et al., 1997). Here we unify these diverse findings to demonstrate that AChE antagonism causes presynaptic LTD through activation of CB1R and M1/M3 mAChRs.
Our finding that antagonists of either M1 or M3 were suffiicent to block OP-LTD suggests that activation of both M1 and M3 mAChRs are required for OP-LTD. Although M1 and M3 mAChRs are often co-localized in neuronal cells, the two receptors exhibit distinct functional properties that may differentially affect neuronal responses to cholinergic overstimulation (Buck and Fraser, 1990; Burford et al., 1995; Leaderbrand et al., 2016). Thus the requirement for concurrent activation of M1 and M3 mAChRs in OP-LTD could indicate a coincidence detector function in the context of cholinergic overstimulation (Hashimotodani et al., 2005). For example, eCB release and CB1R activation may result from convergence of signaling pathways that are differentially regulated by or coupled to M1 and M3 signaling. Alternatively, the need for concurrent activation of M1 and M3 may represent a nonlinear or volume response, in which activation of both mAChR subtypes is required for sufficient release of eCBs to elicit OP-LTD. However, it should be noted that while 4-DAMP was applied at a concentration reported to cause specific inhibition (Liu et al., 1998), other studies suggest that 4-DAMP is only moderately selective for M3 versus M1 (Dorje et al., 1991). Therefore the apparent requirement for both M1 and M3 mAChR may simply reflect non-selective antagonism of the M1 receptor by 4-DAMP. Further studies are required to discriminate whether concurrent activation of M1 and M3 mAChR are truly required for OP-LTD, or whether activation of M1 mAChR is sufficient to elicit OP-LTD.
Although OP toxicity is principally attributed to direct inhibition of AChE, interactions between OPs and other macromolecular targets may also contribute to symptoms of exposure (Pope et al., 2005; Terry, 2012). In vivo chemical tagging studies suggest that OPs can antagonize diverse serine hydrolases, including monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH). MAGL and FAAH terminate eCB signaling by degrading eCB signaling lipids, raising the possibility that OP modulation of eCB metabolism contributes to CB1R activation (Nallapaneni et al., 2008; Nomura et al., 2008; Quistad et al., 2001; Quistad et al., 2002). Many of the interactions between OPs and serine hydrolases are selective and concentration-dependent, and therefore disruption of eCB hydrolases may cause distinctive manifestations depending on the specific OP involved (Liu et al., 2013; Nomura et al., 2008). While the requirement for M1/M3 mAChR signaling indicates that direct modulation of eCB metabolism by OPs is not sufficient to elicit OP-LTD, it also remains possible that these types of non-cholinergic interactions contribute to the magnitude or diversity of eCB signaling in response to OP exposure.
In conclusion, pharmacological and electrophysiological approaches were used to characterize a muscarinic-CB1R signaling axis at excitatory SC synapses in response to OP exposure. This signaling pathway suppresses presynaptic release in response to OP exposure and is dependent on activation of CB1R as well as group I mAChRs. Notably, presynaptic depression manifested before emergence of interictal bursting, suggesting that suppression of excitatory release represents an early compensatory change in synapse function in response to excessive cholinergic signaling. The physiological relevance of these findings was confirmed in vivo using a mouse soman challenge model, in which pharmacological blockade of CB1R was found to enhance lethality. Collectively, these studies demonstrate that CB1R-mediated presynaptic depression is part of the acute response to cholinergic overstimulation following exposure to OP nerve agents, and raises the possibility that exogenous potentiation of CB1R activity may provide a novel therapeutic approach to mitigate central OP toxicity.
Highlights.
Organophosphorus nerve agents activate long-term depression (OP-LTD) at SC synapses
OP-LTD requires group 1 muscarinic signaling
OP-LTD is mediated through retrograde activation of presynaptic CB1R
Treatment with CB1R antagonists enhances soman toxicity in vivo
Retrograde CB1R activation protects against OPs by downregulating excitatory drive
CB1R activation represents a novel therapy to mitigate acute OP toxicity
Acknowledgments and disclaimers.
The authors would like to thank Philip Beske for technical assistance and Cindy Kronman for editorial and administrative assistance. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government. This research was supported by an interagency agreement between NIH/NIAID and USAMRICD (IAA number AOD12059-0001-00). This research was performed while KH held a Defense Threat Reduction Agency-National Research Council Research Associateship Award and ME held an Oak Ridge Institute for Science and Education fellowship. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Katie M. Hoffman, Biological Sciences, Lehigh University, 27 Memorial Drive West, Bethlehem, PA 18015 USA
Margaret R. Eisen, Department of Neuroscience, United States Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Gunpowder, MD, 21010, USA
Jessica K. Chandler, Department of Neuroscience, United States Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Gunpowder, MD, 21010, USA
Marian R. Nelson, Department of Neuroscience, United States Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Gunpowder, MD, 21010, USA
Erik A. Johnson, Department of Neuroscience, United States Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Gunpowder, MD, 21010, USA
Patrick M. McNutt, Department of Neuroscience, United States Army Medical Research Institute of Chemical Defense, 2900 Ricketts Point Road, Gunpowder, MD, 21010, USA
References
- Alexandrova EA, Alkondon M, Aracava Y, Pereira EF, Albuquerque EX, 2014. Galantamine prevents long-lasting suppression of excitatory synaptic transmission in CA1 pyramidal neurons of soman-challenged guinea pigs. Neurotoxicology 44, 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger BE, Nagode DA, Tang AH, 2014. Muscarinic cholinergic receptors modulate inhibitory synaptic rhythms in hippocampus and neocortex. Front Synaptic Neurosci 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Braga MF, 2009. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience 159, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MF, 2010. Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology 31, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, Prager EM, Pidoplichko VI, Miller SL, Braga MF, 2016. Long-term neuropathological and behavioral impairments after exposure to nerve agents. Ann N Y Acad Sci 1374, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augelli-Szafran CE, Jaen JC, Moreland DW, Nelson CB, Penvose-Yi JR, Schwarz RD, 1998. Identification and characterization of m4 selective muscarinic antagonists. Bioorg Med Chem Lett 8, 1991–1996. [DOI] [PubMed] [Google Scholar]
- Bajgar J, 2004. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 38, 151–216. [DOI] [PubMed] [Google Scholar]
- Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D, 2007. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini Rev Med Chem 7, 461–466. [DOI] [PubMed] [Google Scholar]
- Bonansco C, Fuenzalida M, 2016. Plasticity of Hippocampal Excitatory-Inhibitory Balance: Missing the Synaptic Control in the Epileptic Brain. Neural Plast 2016, 8607038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MA, Fraser CM, 1990. Muscarinic acetylcholine receptor subtypes which selectively couple to phospholipase C: pharmacological and biochemical properties. Biochem Biophys Res Commun 173, 666–672. [DOI] [PubMed] [Google Scholar]
- Burford NT, Tobin AB, Nahorski SR, 1995. Differential coupling of m1, m2 and m3 muscarinic receptor subtypes to inositol 1,4,5-trisphosphate and adenosine 3’,5’-cyclic monophosphate accumulation in Chinese hamster ovary cells. J Pharmacol Exp Ther 274, 134–142. [PubMed] [Google Scholar]
- Colgin LL, Kramar EA, Gall CM, Lynch G, 2003. Septal modulation of excitatory transmission in hippocampus. J Neurophysiol 90, 2358–2366. [DOI] [PubMed] [Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM, 2013. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11, 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creager R, Dunwiddie T, Lynch G, 1980. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J Physiol 299, 409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR, 1991. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther 256, 727–733. [PubMed] [Google Scholar]
- Eddleston M, Chowdhury FR, 2016. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the (possible) new. Br J Clin Pharmacol 81, 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Coubard S, Pierard C, Liscia P, Beracochea D, Four E, Baubichon D, Masqueliez C, Lallement G, Collombet JM, 2007. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology 28, 508–519. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D, 2003. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83, 1017–1066. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M, 2004. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci 19, 2682–2692. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM, 2002. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5, 446–451. [DOI] [PubMed] [Google Scholar]
- Ghoshal A, Moran SP, Dickerson JW, Joffe ME, Grueter BA, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2017. Role of mGlu5 Receptors and Inhibitory Neurotransmission in M1 Dependent Muscarinic LTD in the Prefrontal Cortex: Implications in Schizophrenia. ACS Chem Neurosci 8, 2254–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob D, Harvey AM, 1953. The effects and treatment of nerve gas poisoning. Am J Med 14, 52–63. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M, 2005. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45, 257–268. [DOI] [PubMed] [Google Scholar]
- Hebert-Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R, Piazza PV, Benard G, Grandes P, Marsicano G, 2014. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Mol Metab 3, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC, 1990. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87, 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A, Eisenkraft A, Finkelstein A, Schein O, Rotman E, Dushnitsky T, 2007. A decade after the Tokyo sarin attack: a review of neurological follow-up of the victims. Mil Med 172, 607–610. [DOI] [PubMed] [Google Scholar]
- Holstege CP, Kirk M, Sidell FR, 1997. Chemical warfare. Nerve agent poisoning. Crit Care Clin 13, 923–942. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Blume LC, Dalton GD, 2010. CB(1) cannabinoid receptors and their associated proteins. Curr Med Chem 17, 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal GA, 1997. Neurological syndromes of organophosphorus compounds. Adverse Drug React Toxicol Rev 16, 133–170. [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M, 2009. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE, 2002. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22, 10182–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaderbrand K, Chen HJ, Corcoran KA, Guedea AL, Jovasevic V, Wess J, Radulovic J, 2016. Muscarinic acetylcholine receptors act in synergy to facilitate learning and memory. Learn Mem 23, 631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois EP, Bridges TM, Lewis LM, Dawson ES, Kane AS, Xiang Z, Jadhav SB, Yin H, Kennedy JP, Meiler J, Niswender CM, Jones CK, Conn PJ, Weaver CD, Lindsley CW, 2010. Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M(1) receptor function in the central nervous system. ACS Chem Neurosci 1, 104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikin JB, Thomas RG, Walter FG, Klein R, Meislin HW, 2002. A review of nerve agent exposure for the critical care physician. Crit Care Med 30, 2346–2354. [DOI] [PubMed] [Google Scholar]
- Levey AI, 1996. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci U S A 93, 13541–13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PR, Shute CC, Silver A, 1967. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol 191, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Parsons L, Pope C, 2013. Comparative effects of parathion and chlorpyrifos on extracellular endocannabinoid levels in rat hippocampus: influence on cholinergic toxicity. Toxicol Appl Pharmacol 272, 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pope C, 2015. The cannabinoid receptor antagonist AM251 increases paraoxon and chlorpyrifos oxon toxicity in rats. Neurotoxicology 46, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kumar A, Alreja M, 1998. Excitatory effects of muscarine on septohippocampal neurons: involvement of M3 receptors. Brain Res 805, 220–233. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Both M, Draguhn A, 2009. Rapid plasticity at inhibitory and excitatory synapses in the hippocampus induced by ictal epileptiform discharges. Eur J Neurosci 29, 1153–1164. [DOI] [PubMed] [Google Scholar]
- Lu HC, Mackie K, 2016. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry 79, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans RA, Warmus BA, Smith CC, McMahon LL, 2014. An acetylcholinesterase inhibitor, eserine, induces long-term depression at CA3-CA1 synapses in the hippocampus of adult rats. J Neurophysiol 112, 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HG, Bernabeu A, Lassalle O, Bouille C, Beurrier C, Pelissier-Alicot AL, Manzoni OJ, 2015. Endocannabinoids Mediate Muscarinic Acetylcholine Receptor-Dependent Long-Term Depression in the Adult Medial Prefrontal Cortex. Front Cell Neurosci 9, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough JH Jr., McMonagle J, Copeland T, Zoeffel D, Shih TM, 1999. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol 73, 473–478. [DOI] [PubMed] [Google Scholar]
- McDonough JH Jr., Shih TM, 1997. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev 21, 559–579. [DOI] [PubMed] [Google Scholar]
- McDonough JH, McMonagle JD, Shih TM, 2010. Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol 33, 279–283. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, 2013. The endocannabinoid system and the brain. Annu Rev Psychol 64, 21–47. [DOI] [PubMed] [Google Scholar]
- Miller SL, Aroniadou-Anderjaska V, Pidoplichko VI, Figueiredo TH, Apland JP, Krishnan JK, Braga MF, 2017. The M1 Muscarinic Receptor Antagonist VU0255035 Delays the Development of Status Epilepticus after Organophosphate Exposure and Prevents Hyperexcitability in the Basolateral Amygdala. J Pharmacol Exp Ther 360, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich G, Wenger T, 2000. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides 21, 1735–1742. [DOI] [PubMed] [Google Scholar]
- Munirathinam S, Bahr BA, 2004. Repeated contact with subtoxic soman leads to synaptic vulnerability in hippocampus. J Neurosci Res 77, 739–746. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C, 2006. Modulation of paraoxon toxicity by the cannabinoid receptor agonist WIN 55,212–2. Toxicology 227, 173–183. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C, 2008. Pharmacological enhancement of endocannabinoid signaling reduces the cholinergic toxicity of diisopropylfluorophosphate. Neurotoxicology 29, 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu E, Niiya T, Kawamata T, Kawamata M, Yamakage M, 2010. Effects of atropine and pralidoxime on neuronal actions of paraoxon in rat hippocampal slices. Neurosci Res 68, 276–284. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE, 2008. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat Chem Biol 4, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M, 2003. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci 18, 109–116. [DOI] [PubMed] [Google Scholar]
- Pope C, Karanth S, Liu J, 2005. Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Environ Toxicol Pharmacol 19, 433–446. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE, 2001. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol Appl Pharmacol 173, 48–55. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE, 2002. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol Appl Pharmacol 179, 57–63. [DOI] [PubMed] [Google Scholar]
- Rastogi SK, Tripathi S, Ravishanker D, 2010. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J Occup Environ Med 14, 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ, 2002. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A 99, 8384–8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha ES, Santos MD, Chebabo SR, Aracava Y, Albuquerque EX, 1999. Low concentrations of the organophosphate VX affect spontaneous and evoked transmitter release from hippocampal neurons: toxicological relevance of cholinesterase-independent actions. Toxicol Appl Pharmacol 159, 31–40. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Paulsen O, 2008. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci 11, 744–745. [DOI] [PubMed] [Google Scholar]
- Rosman Y, Eisenkraft A, Milk N, Shiyovich A, Ophir N, Shrot S, Kreiss Y, Kassirer M, 2014. Lessons learned from the Syrian sarin attack: evaluation of a clinical syndrome through social media. Ann Intern Med 160, 644–648. [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, Jones CK, Niswender CM, Weaver CD, Lindsley CW, Conn PJ, 2009. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol 76, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidell FR, 1974. Soman and sarin: clinical manifestations and treatment of accidental poisoning by organophosphates. Clin Toxicol 7, 1–17. [DOI] [PubMed] [Google Scholar]
- Smolders I, Bogaert L, Ebinger G, Michotte Y, 1997. Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J Neurochem 68, 1942–1948. [DOI] [PubMed] [Google Scholar]
- Teles-Grilo Ruivo LM, Mellor JR, 2013. Cholinergic modulation of hippocampal network function. Front Synaptic Neurosci 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV Jr., 2012. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther 134, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic MS, Cowan ML, Balint CA, Sun C, Kapur J, 2012. Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res 101, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE, 2001. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21, RC188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ, 2005. Retrograde signalling by endocannabinoids. Handb Exp Pharmacol, 367–383. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu L, Weiss T, Stewart C, Mikler J, 2011. Effect of acute soman exposure on GABA(A) receptors in rat hippocampal slices and cultured hippocampal neurons. Neurotox Res 20, 343–350. [DOI] [PubMed] [Google Scholar]
- Wille T, Thiermann H, Worek F, 2011. Effect of different buffers on kinetic properties of human acetylcholinesterase and the interaction with organophosphates and oximes. Arch Toxicol 85, 193–198. [DOI] [PubMed] [Google Scholar]
- Wright LK, Liu J, Nallapaneni A, Pope CN, 2010. Behavioral sequelae following acute diisopropylfluorophosphate intoxication in rats: comparative effects of atropine and cannabinomimetics. Neurotoxicol Teratol 32, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T, 2006. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci 249, 76–85. [DOI] [PubMed] [Google Scholar]