Abstract
This study investigated associations between changes in the total joint moment (TJM) at the knee and changes in cartilage thickness after anterior cruciate ligament reconstruction (ACLR). Seventeen subjects (5 males; age: 29.6±7.3 yrs) with unilateral ACLR underwent gait analysis and MRI at baseline (2.2±0.3 yrs post-ACLR) and at long-term follow-up (7.7±0.7 yrs post-ACLR). Knee loading was assessed using the TJM, and differences in loading were analyzed using repeated measures ANOVA. Pearson correlation coefficients assessed associations between changes in TJM and changes in (medial-to-lateral) M/L femoral cartilage thickness ratios in the ACLR limb. Bilaterally there was no significant change in the magnitude of the TJM first peak (TJM1), however, there was a significant increase in percent contribution of the knee flexion moment (KFM) (p<0.001) and decrease in percent contribution of the knee adduction moment (KAM) to TJM1 (p<0.001). The change in percent contributions of KFM and KAM to TJM1 were associated with changes in M/L femoral cartilage thickness in the ACLR limb. Specifically, subjects with smaller increases in KFM contribution (R=0.521, p=0.032) and smaller decreases in KAM contribution (R=−0.521, p=0.032) had a reduction in M/L ratio in the central femoral sub-region over the follow-up period, with similar trends in the external femoral sub-region. The study results provide new insight into changes in the loading environment at the knee joint prospectively following ACL reconstruction and give evidence that there are modifiable gait metrics that are associated with cartilage changes after ACLR.
Keywords: ACL, Reconstruction, Total Joint Moment, Cartilage Thickness, Gait Analysis
Introduction
Despite the success of anterior cruciate ligament reconstruction (ACLR) surgery in restoring knee stability, nearly 70% of individuals under the age of 30 show signs of cartilage degeneration on MRI at an average of less than 3 years after reconstruction1. Further, a large percentage of individuals develop rapidly progressive premature knee osteoarthritis (OA) after anterior cruciate ligament (ACL) injury2. The ACLR limb is five times as likely to develop moderate-to-severe post-traumatic OA (PTOA) as compared to the contralateral knee3, with 50–70% of individuals developing radiographic knee OA within 10–15 years of their injury2; 4; 5. The young age of sustaining ACL tears6 taken together with the risk of developing symptomatic PTOA as early as 30–50 years old4; 5 calls for early interventions to modify the risk of PTOA in this population. Thus, identification of modifiable risk factors for PTOA, such as knee mechanics during gait, is an important consideration when exploring methods to reduce the risk of premature joint degeneration after ACLR.
Figure 4:
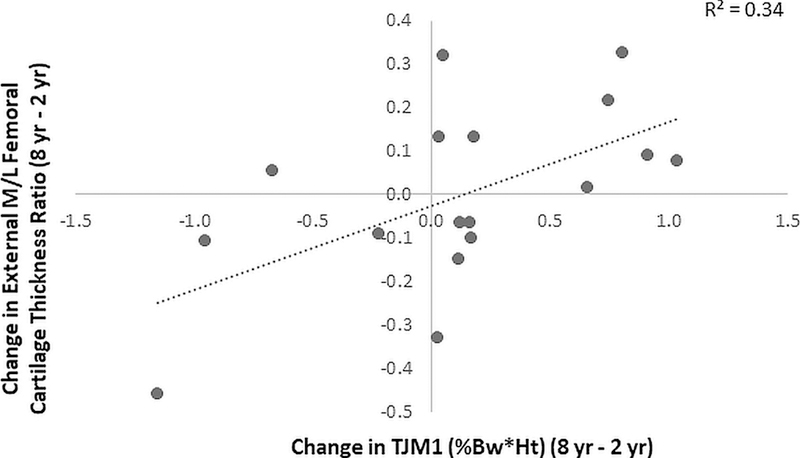
Association between change in TJM1 in the ACLR limb from 2 to 8 years post-ACLR and change in M/L cartilage thickness ratio in the external femur.
Alterations in gait mechanics that occur after ACL injury, which are not restored by ACLR surgery7; 8, suggest specific gait metrics as a target for modifying the risk of PTOA. As cartilage is known to adapt to its loading environment9, altered loading during gait is believed to contribute to the development of PTOA in this population10. In particular, kinetic changes to the ACLR limb have been described in all 3 planes of motion7; 8; 11. Reductions in both the sagittal plane knee flexion moment (KFM)7 and transverse plane internal rotation moment (KRM)11 have been reported, while reports of changes in the frontal plane knee adduction moment (KAM) have varied, with studies finding increases12, no change13, and decreases in KAM11; 14. Recent work has demonstrated that the kinetics of the ACLR limb change over time15, and it is unknown if such changes may be related to the development of PTOA after ACLR.
Several recent studies have investigated associations between gait mechanics and cartilage health after ACLR, finding significant associations between both sagittal and frontal plane gait mechanics and cartilage health as assessed by compositional magnetic resonance imaging (MRI) techniques16–18. In both cross-sectional and prospective studies, Kumar et al.17; 18 found ACLR subjects with higher KAM had increased medial cartilage T1ρ and T2 relaxation times, indicative of a greater deterioration of cartilage health19–21. Teng et al.16 also found higher peak KFM 6 months after ACLR was associated with a greater increase in medial cartilage T1ρ and T2 relaxation times up to 2 years after ACLR. All studies were conducted at a relatively early time point after ACLR surgery, and the authors noted the need for future studies to examine the influence of these gait parameters on cartilage health over a longer time period. In patients with radiographic knee OA, both the peak KAM and KFM have been shown to be associated with the ratio of medial-to-lateral cartilage thickness22, as well as cartilage thickness ratio changes over time23. Given the nature of the changes in gait mechanics following ACLR it is useful to consider a surrogate metric that captures the influence of both KFM and KAM in the assessment of PTOA risk.
The total joint moment (TJM)24 appears to provide a meaningful metric that captures the influence of each moment component acting at the knee joint by incorporating contributions of the KAM, KFM, and KRM. While the TJM acts externally to the joint, it appears to reflect temporal patterns similar to direct measures of internal joint contact force25, with two peaks occurring in early and late stance during walking. In a prospective study of patients with radiographic knee OA, it was shown that the contribution of KFM to the first peak of the TJM decreased and the contribution of KAM increased, while the magnitude of the first peak of the TJM did not change over a five-year follow-up24, suggesting that compensatory changes between the KFM and KAM occurs over time in OA patients. In patients following ACLR at 2 years post-surgery11, it was reported that the second TJM peak was lower in the ACLR knee versus the contralateral knee during walking. Given that the knee loading assessed by the TJM and the relative contribution of the KFM and KAM may change over time, as well as the potential for these metrics to influence cartilage changes, it is useful to examine if changes in TJM are associated with cartilage changes over time following ACLR.
Thus, the purpose of this study was to investigate associations between the loading condition at the knee after ACLR and cartilage changes over time. We hypothesized (1) the TJM at the knee following ACLR changes with time past surgery with an increase in the first peak of the TJM and a decrease in second peak of the TJM; (2) the relative contributions of KFM and KAM to the TJM peaks change over time following ACLR; and (3) the changes in TJM over time in the ACLR limb are associated with changes in cartilage thickness in the ACLR knee.
Methods
Study Design and Level of Evidence.
Prognostic Study, Level II
Human Subjects.
Seventeen subjects with unilateral primary ACLR and no subsequent injuries or operations to either knee were tested at baseline at approximately 2 years post-ACLR (2.2±0.3 yrs post-ACLR) and returned for follow-up testing approximately 8 years post-ACLR (7.7±0.7 yrs post-ACLR). The cohort was a convenience sample of a larger group of 42 subjects who underwent baseline testing approximately 2 years post ACLR and agreed to further contact by researchers. As long-term subject follow-up was not part of the original study design, we received Institutional Review Board (IRB) approval to re-contact subjects from the 2 year study for additional follow-up. Of the subjects who did not return for full follow-up testing, 9 did not respond, 5 were unable to participate due to time constraints, 3 had moved away from the area, 3 had additional arthroscopic surgery on their affected limb, 2 had re-torn their ACL, 2 were not interested, and 1 had injured their contralateral limb.
The study was approved by the Stanford University IRB, and all subjects provided written informed consent prior to participation. Initial inclusion criteria at baseline11 included 1) successful single-bundle unilateral ACLR based on clinical exam (KT-1000 side-to-side difference < 5 mm), 2) no other history of serious lower limb injury, 3) self-reported history of knee stability, and 4) knee MRI to confirm intact graft. Exclusion criteria included: 1) removal of more than 25% of the meniscus, 2) a history of other serious ligamentous injury to either lower limb, 3) clinical instability of the reconstructed knee, 4) BMI > 30 kg/m2, 5) significant observable chondral defects by MRI, and 6) a history of surgical procedures performed on either lower limb or revision. Further inclusion criteria for the 8-year follow-up visit included 1) no additional serious injuries or surgeries to either limb and 2) a willingness to attend an in-person follow-up visit.
Gait Analysis:
At both the baseline and follow-up visits, all subjects underwent bilateral gait analysis in the same motion capture laboratory. Subjects performed three successful walking trials at self-selected normal walking speed separately for each limb. The order of testing of the limbs was randomly selected. A trial was considered successful if the foot of the leg being tested fully struck the force plate. Kinematic data were collected using a multi-camera motion capture system (Qualisys Medical, Gothenburg, SE) and the point cluster technique, which uses a redundant set of 21 reflective markers26. A force plate embedded in the ground (Bertec Corporation, Columbus, OH) was used to capture ground reaction forces. The systems were synchronized and recorded data at 120 Hz. Lower-limb kinetics were calculated using the software application BioMove (Stanford University, CA) following previously described methods26–28. Briefly, external knee moments were calculated using an inverse dynamics approach29, with the foot, lower leg, and thigh segments idealized as rigid bodies and their scaled inertial properties taken from the literature30. The external moments about the knee joint center are created by the ground reaction force and inertial forces. The external moment is equal and opposite to an internal moment created by muscles and soft tissues, as previously described.29 The knee moments were expressed relative to the tibial anatomical frame based upon the position of anatomical landmarks identified by palpation27, and were normalized to bodyweight and height (%Bw*Ht) to allow for comparison between subjects.
The knee adduction moment (KAM), knee flexion moment (KFM), and the knee rotational moment (KRM) were extracted during stance phase for all trials. A vertical ground reaction force threshold of 10N was used to detect the beginning and the end of the stance phase (heel-strike and toe-off). The total joint moment (TJM)24 was calculated during each time point of stance phase by taking the square root of the sum of the squares of the KFM, KAM, and KRM:
The TJM first peak (TJM1) and second peak (TJM2) were defined as the maximum TJM during the first and second half of stance, respectively. The percent contributions of the KFM, KAM, and KRM to the TJM at the time of the first and second TJM peaks were calculated as the square of the planar moment at the time of the peak over the square of the total moment at the time of the peak, multiplied by 100:
The amplitudes of each variable for the three successful walking trials were averaged for each knee.
Magnetic Resonance Imaging (MRI):
At both baseline and follow-up, the ACLR knee was scanned on the same 1.5T MRI (GE Signa; GE Medical Systems, Milwaukee, WI) using the same sagittal-plane fat-saturated three-dimensional spoiled gradient recalled echo sequence (TR 60 ms; TE 5 ms; FOV 140×140mm; in-plane pixel size 0.547×0.547mm; slice thickness 1.5mm; 60 slices). The femoral cartilage was manually segmented by a single user from each slice using custom software based on B-spline contour definition allowing subpixel resolution31 and three-dimensional computer cartilage models were created. Cartilage thickness maps were calculated for the total subchondral femoral bones. The coefficients of variation for this method have been previously evaluated to be 1–3%31.
The total weight-bearing areas of the medial and lateral femoral surfaces were defined as the regions between the intercondylar notch and 60% of the distance to the posterior end of the femoral condyles32. The medial and lateral areas were divided into 3 sub-regions (central, internal, and external) using a standard method (Figure 1)33. Average cartilage thickness in the medial and lateral sub-regions were calculated, and the medial-to-lateral (M/L) cartilage thickness ratios were calculated (average medial thickness divided by average lateral thickness for each sub-region).
Figure 1:
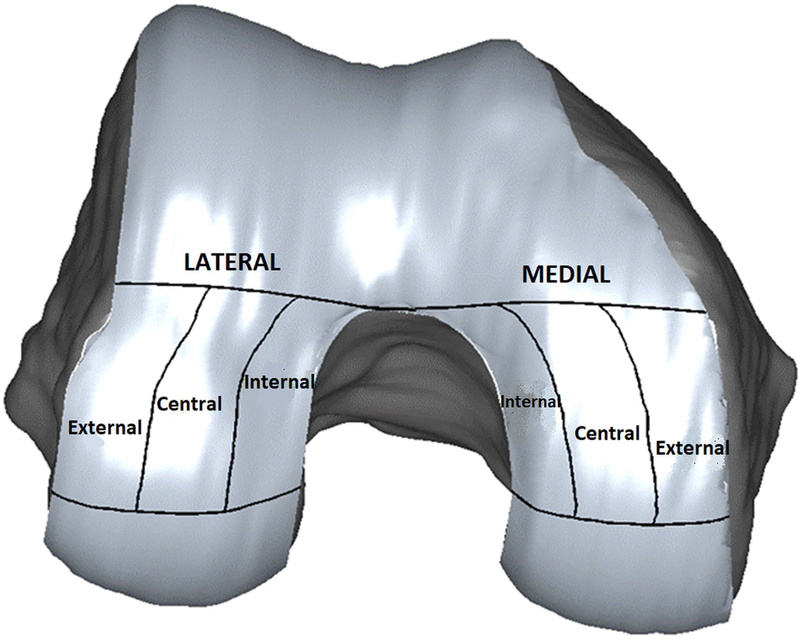
Analyzed medial and lateral femoral sub-regions.
Statistical Analyses:
Differences in TJM peaks and differences in contributions of the individual moments to the TJM peaks, between the ACLR limb and contralateral limb, and between baseline and follow-up were analyzed using 2 (limb) x 2 (time) repeated measures ANOVA with time and limb as within-subject factors. Upon a significant result of the ANOVA, post hoc paired Student’s t-tests were used for analyses. Associations between changes in TJM peaks and individual moment contributions in the ACLR limb and changes in M/L cartilage thickness ratios in the ACLR limb were analyzed with Pearson correlation coefficients. The changes in kinetic and thickness measures were calculated as the difference between the 8-year follow-up and baseline values. For all analyses, P-values <0.05 were considered significant. All statistical calculations were performed with SPSS version 23.0 (SPSS Inc., Chicago, IL).
Results
Participant Characteristics:
The study population included twelve females and 5 males. Nine subjects had ACLR on the right knee and 8 subjects had ACLR on the left knee. The mean (± standard deviation) time to surgery from injury was 2.0±1.8 months. Fifteen subjects had reconstructions with Achilles allografts, 1 subject had a reconstruction with bone-patellar tendon-bone autograft, and 1 subject had a reconstruction with a bone-patellar tendon-bone allograft. All surgeries were performed with a transtibial technique and single-bundle ACLR. Demographic data of the study population are presented in Table 1.
Table 1:
Demographics of the 17 study participants.
| Number | 17 |
| Gender | 5 males / 12 females |
| Injured Limb | 8 left / 9 right |
| Mean age at baseline (years; SD, range) | 29.6 (7.3, 21 to 41) |
| Mean age at follow-up (years; SD, range) | 35.1 (7.3, 27 to 45) |
| Mean BMI at baseline (kg/m2; SD, range) | 23.5 (2.7, 19.6 to 29.8) |
| Mean BMI at follow-up (kg/m2; SD, range) | 25.0 (2.2, 29.0 to 28.6) |
| Mean time from injury to operation (days; SD, range) | 61.3 (53.7, 14 to 238) |
| Mean time to baseline testing postoperatively (years; SD, range) | 2.2 (0.3, 1.9 to 2.9) |
| Mean time to follow-up testing postoperatively (years; SD, range) | 7.7 (0.7, 6.5 to 8.7) |
SD: standard deviation; BMI: body mass index
TJM changes over time:
The temporal pattern of the TJM exhibited two major peaks (Figure 2), with one in early stance (TJM1) and one in late stance (TJM2) in both the ACLR and contralateral knee. There was no significant change in the magnitude of TJM1 (Table 2, p=0.69) from 2 to 8 years post-ACLR, while there was a significant reduction in TJM2 from baseline to follow-up (p<0.001). The TJM1 and TJM2 changed similarly over time for both the ACLR and contralateral limbs (no significant interaction effects were observed). However, there was a significant difference between limbs at the 8-year time point with a higher TJM2 in the contralateral limb as compared to the ACLR limb (p=0.02).
Figure 2:
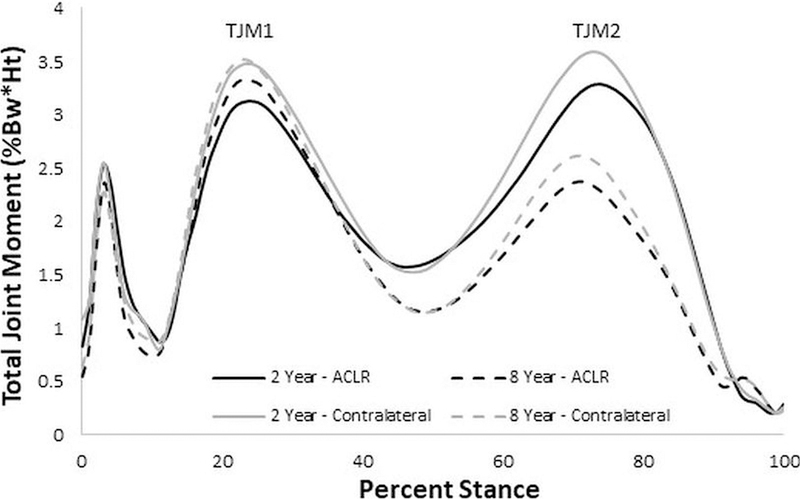
TJM ensemble curves at baseline (solid line) and follow-up (dashed line) for the ACLR (black) and contralateral (gray) limbs. A significant reduction in TJM2 was observed from baseline to follow-up.
Table 2:
Means ± standard deviation for TJM peaks and individual moment contributions for both the ACLR and contralateral limbs at baseline and follow-up.
| 2 years post-ACLR | 8 years post-ACLR | ANOVA Main Effect P-value | ANOVA Interaction P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACLR | Contralateral | ACLR | Contralateral | Time | Limb | Time × Limb | ||||
| TJM1 (%Bw*Ht) | 3.24 ± 0.72 | 3.59 ± 0.61 | 3.36 ± 0.66 | 3.58 ± 0.84 | 0.69 | 0.16 | 0.60 | |||
| KFM % at TJM1 | 24.4 ± 22.8 | 32.3 ± 15.1 | 49.2 ± 22.7 | 52.5 ± 17.3 | <0.001 | 0.23 | 0.54 | |||
| KAM % at TJM1 | 75.4 ± 22.8 | 67.4 ± 15.1 | 50.5 ± 22.6 | 47.3 ± 17.3 | <0.001 | 0.23 | 0.53 | |||
| KRM % at TJM1 | 0.2 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.99 | 0.75 | 0.11 | |||
| TJM2 (%Bw*Ht) | 3.34 ± 0.88 | 3.65 ± 0.90 | 2.44 ± 0.82 | 2.68 ± 0.83 | <0.001 | 0.02 | 0.66 | |||
| KFM % at TJM2 | 69.0 ± 20.8 | 74.2 ± 13.7 | 60.1 ± 27.1 | 65.1 ± 22.9 | 0.06 | 0.35 | 0.97 | |||
| KAM % at TJM2 | 21.5 ± 18.1 | 16.4 ± 11.8 | 28.2 ± 25.7 | 23.9 ± 21.1 | 0.11 | 0.34 | 0.90 | |||
| KRM % at TJM2 | 9.5 ± 4.0 | 9.4 ± 3.3 | 11.7 ± 4.9 | 11.0 ± 4.0 | 0.001 | 0.67 | 0.70 | |||
| Speed (m/s) | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.2 | 0.577 | 0.177 | 0.496 | |||
P-values shown are for results of repeated measures ANOVA. Bold indicates significance.
While there was no significant change in the magnitude of TJM1 from 2 to 8 years, there were substantial changes in the percentage contribution of the KAM and KFM to TJM1 in the ACLR limb, with an increase from 24.4% to 49.2% in %KFM (p<0.001, Table 2) and a decrease from 75.4% to 50.5% in %KAM (p<0.001, Figure 3). The changes in %KAM and %KFM were consistent among most subjects where 14 of 17 subjects had a reduced KAM percent contribution and an increased KFM contribution from baseline to follow-up. For TJM2, only the percent contribution of the smaller magnitude KRM significantly changed (increased) over follow-up (p=0.001, Table 2 and Figure 3). Similar results were seen for the contralateral limb, with no significant limb x time interaction effects or main effects of limb observed. Importantly, there were no differences in walking speed between baseline and follow-up testing or between legs (Table 2).
Figure 3:
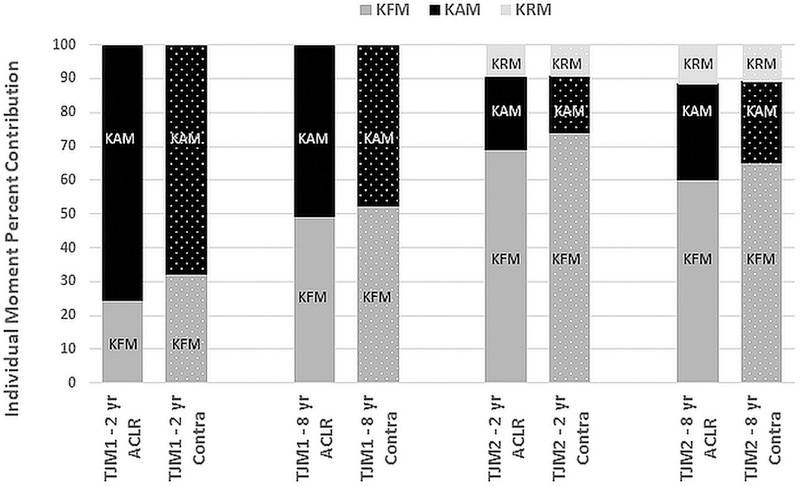
Individual moment percent contributions to TJM1 (left) and TJM2 (right) in the ACLR limb (solid bars) and contralateral limb (dotted bars) at baseline (2 year) and follow-up (8 year). A significant increase in KFM contribution (medium gray) and decrease in KAM contribution (black) to TJM1, and a significant increase in KRM contribution (light gray) to TJM2, were seen from baseline to follow-up.
Cartilage changes over time:
Changes in femoral cartilage thickness over the follow-up period are presented in Table 3. No significant changes, on average over all subjects, were observed in medial thickness, lateral thickness, or cartilage thickness ratios, though trends towards a reduction in central (p=0.09) and internal (p=0.07) medial femoral cartilage thickness were observed.
Table 3:
Means ± standard deviation for cartilage thickness in the medial and lateral femoral regions, and medial-to-lateral femoral cartilage thickness ratios, for the ACLR limb at baseline and follow-up.
| Cartilage region | Baseline | Follow-Up | P-value |
|---|---|---|---|
| Medial Femur | |||
| External | 1.46 ± 0.25 | 1.44 ± 0.22 | 0.71 |
| Central | 2.15 ± 0.30 | 2.05 ± 0.34 | 0.09 |
| Internal | 1.97 ± 0.24 | 1.89 ± 0.30 | 0.07 |
| Lateral Femur | |||
| External | 1.40 ± 0.24 | 1.37 ± 0.22 | 0.66 |
| Central | 1.93 ± 0.31 | 1.91 ± 0.35 | 0.59 |
| Internal | 1.55 ± 0.27 | 1.52 ± 0.30 | 0.56 |
| Medial-to-Lateral Femoral Thickness Ratio | |||
| External | 1.07 ± 0.24 | 1.07 ± 0.17 | 0.97 |
| Central | 1.12 ± 0.11 | 1.09 ± 0.13 | 0.16 |
| Internal | 1.29 ± 0.17 | 1.27 ± 0.20 | 0.55 |
P-values shown are for results of paired Student’s t-test.
TJM change associations to cartilage changes:
A significant positive correlation (R=0.580, p=0.015) was observed between change in TJM1 in the ACLR limb over the follow-up period and change in external M/L cartilage thickness ratio (Figure 4). Specifically, subjects who increased TJM1 over the follow-up period had an increase in the femoral M/L cartilage thickness ratio in the external region. There was also a positive correlation (Figure 5A) between the change in percent contribution of the KFM to TJM1 and change in central M/L femoral cartilage thickness ratio, where subjects with smaller increases in %KFM (R=0.521, p=0.032) had a reduction in M/L ratio in the central femoral sub-region over the follow-up period. Conversely, the change in M/L thickness ratio of the central femoral cartilage was negatively correlated (Figure 5B) with the change in contribution of KAM to TJM1 (R=−0.521, p=0.032), where subjects with smaller reductions in %KAM had reduced M/L thickness ratio. There were similar trends for the external femoral sub-region (%KFM: R=0.438, p=0.079, %KAM: R=−0.436, p=0.081). No associations were observed between changes in TJM2 or individual moment contributions to TJM2 and cartilage changes over time.
Figure 5:
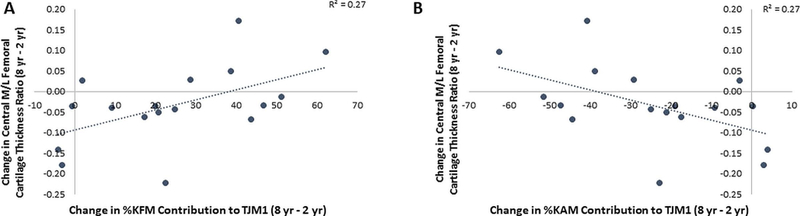
Associations between change in (A) KFM and (B) KAM percent contributions to TJM1 in the ACLR limb from 2 to 8 years post-ACLR and change in M/L cartilage thickness ratio in the central femur.
Discussion
The study results provide new insight into changes in the loading environment at the knee joint prospectively following ACL reconstruction. Significant changes from 2 to 8 years post-ACLR in the knee loading environment were observed that were associated with changes in femoral cartilage thickness ratios over time. Specifically, the finding that the relative contributions of the KFM and KAM to TJM1 changed over time post-ACLR, and that these changes were correlated with changes in cartilage thickness between 2 and 8 years following ACLR, gives evidence that there are modifiable gait metrics that can influence cartilage changes.
While on average across all subjects from 2 to 8 years post-ACLR the magnitude of first peak of the TJM did not change, the relative contributions of the KFM and KAM to TJM1 did change. In particular, a significant increase in %KFM to TJM1 was observed. Reduced peak KFM7, which is an indication34; 35 of the well-documented quadriceps weakness following ACL injury that is not restored by reconstruction36, is common to patients following ACL injury and reconstruction. Given the growing evidence that quadriceps weakness plays a role in the development of OA after ACL injury37; 38, the increasing contribution of the KFM to the first peak of the TJM over time may suggest an improvement in gait mechanics and restoration of quadriceps function from baseline to follow-up. The finding that increased %KFM (reflecting net quadriceps function) to TJM1 was associated with a smaller decrease in the M/L cartilage thickness ratio suggests that restoring ambulatory quadriceps function following ACL may help to resist detrimental cartilage changes.
The negative correlation (Figure 5B) between changes in M/L thickness ratio and changes in KAM contribution to TJM1 is important since a higher KAM is an indication of greater medial joint loading39 and has been associated with reduced medial compartment thickness23; 40 in patients with established medial compartment knee OA. While nearly all the ACLR subjects reduced %KAM contribution to TJM1 over time, the patients with lowest reduction had greatest reduction in M/L thickness ratio. Consistent with the results of the current study, recent work by Kumar et al.17; 18 demonstrated that ACLR patients with higher KAMs had compositional MRI metrics (T1ρ and T2 relaxation times) indicative of a greater deterioration of cartilage18. Further, subjects with an increase in KAM from pre-ACLR to 6 months post-ACLR had a worsening of MRI metrics (T1ρ and T2 relaxation times) prospectively17. Thus, our data also suggests that interventions to reduce the KAM should be considered following ACLR. It is likely that interventions that address both the KFM and KAM might be optimal since the M/L femoral cartilage thickness ratio changes were associated with contributions of both the KFM and KAM to TJM1 over time.
The finding that there was no change in the magnitude of the TJM1 on average over all subjects from 2 to 8 years post-ACLR suggests there was a balance between the increase in KFM contribution and decrease in KAM contribution in this population. However, inter-subject variations in the change in TJM1 were correlated with the change in M/L femoral cartilage thickness. The positive relationship between the change in TJM1 and M/L cartilage thickness ratio indicated that subjects having a reduction or smaller increase in TJM1 from 2 to 8 years post-ACLR also had a reduction in M/L cartilage thickness ratio (Figure 4). Increasing the TJM1 to maintain cartilage health in ACLR patients might seem counterintuitive given the body of literature suggesting higher loads at the knee lead to more rapid cartilage degeneration in patient with knee OA23. However, this observation is consistent with a study by Wellsandt et al.41 who reported that ACLR patients with radiographic knee OA 5 years after reconstruction had lower medial compartment joint contact forces than patients without OA.
It appears that the nature of the gait changes over time reflected in KFM and KAM contributions to the TJM1 in this ACLR population are different than a population with symptomatic medial knee OA24. In contrast to our ACLR subjects, knee OA patients24 had a decrease in %KFM and an increase in %KAM over time. The difference in gait changes between the ACLR subjects and knee OA population appear to reflect clinical characteristics that change from a population at risk for OA (ACLR) to a population with established knee OA. Specifically, the reduction in the %KFM contribution in the knee OA population appears to suggest increasing pain over time as KFM has been associated with pain,42 while the %KAM increase might be indicative of increasing varus alignment and progressing disease severity43. In contrast, the ACLR population, with mild or no pain, had an increase in the KFM contribution and decrease in the KAM contribution to the TJM1 over time, where patients with the greatest %KFM increase and %KAM decrease had an increase (or smaller reductions) in the M/L thickness ratio. Future interventions to modify KFM and KAM could benefit from considering these gait differences between ACLR and knee OA patients.
There was a reduction observed in the magnitude of the TJM2 from 2 to 8 years that was due to a reduction in the knee extension moment (net knee flexor muscle) in late stance which has been previously described15. The only change observed in individual moment contributions to the second peak of the TJM was for the smaller magnitude internal KRM. However, the lack of associations between changes in the second peak of the TJM and cartilage changes over time questions the clinical relevance of this change with respect to development of knee OA. The lack of significant findings between the total loading condition in the second half of stance is consistent with prior literature suggesting that the first peak KAM and peak KFM, both occurring in early stance around the timing of the TJM1, had the strongest influence on disease progression in an established OA cohort23; 40. Thus, similar to primary OA population studies, it may be that the critical loading time point following ACLR is in early stance23.
It should be noted that no significant differences were seen in changes over time in the loading condition at the knee between the ACLR and contralateral limbs (no interaction effects were observed), suggesting subjects changed their gait bilaterally. The bilateral changes observed are consistent with prior work finding bilateral alterations in quadriceps function44 and gait45 after ACL injury and reconstruction. The impact of contralateral gait changes over time on contralateral cartilage changes should be investigated in future work.
This study has limitations which should be discussed. The sample size of this study is small, as long-term subject follow-up was not part of the original study design. Thus, while significant changes were observed in total knee joint loading parameters that were associated with cartilage changes over time, future work will be needed to confirm the results of this study in a larger cohort. Given the small sample size of the study, a multiple linear regression model incorporating percent contributions of both the KAM and KFM was not utilized for hypothesis 3. Thus, the dependency between the independent predictors of both the KAM and KFM was not accounted for in the correlation analyses performed. The inclusion of a healthy cohort for longitudinal follow-up was not within the scope of this project. Thus, expected longitudinal changes for a healthy population and correlations between gait changes and cartilage thickness changes in a healthy cohort remain unknown. Further, analysis of contralateral limb cartilage changes was not performed, and it is unknown if longitudinal gait changes in the contralateral limb are also associated with cartilage changes over time and should be investigated in future work. As is the nature with longitudinal studies, there is potential for error in regard to gait analysis techniques. However, all subjects were tested in the same laboratory at 2 and 8 years post-ACLR, by researchers trained by the same single individual with respect to gait analysis techniques. Lastly, this study used cartilage thickness ratio changes over the follow-up period to assess change in joint health after ACLR. Cartilage thickness in the load-bearing regions has been shown to be a sensitive measure of cartilage morphology46. Further, the M/L cartilage thickness ratio has been shown to be sensitive to knee OA disease state10; 47 and provides inter-subject normalization and information on the medial to lateral cartilage distribution. Future work, however, should incorporate other features of knee OA, such as bone marrow lesions and osteophytes.
In conclusion, this study demonstrated that there are changes in the loading environment at the knee joint from 2 to 8 years post-ACLR, which are associated with changes in M/L cartilage thickness ratios. The associations between changes in KFM and KAM contributions to the TJM and cartilage thickness ratio changes in the ACLR limb provide support for load modifying interventions to alter gait mechanics following ACLR. For example, gait retraining48; 49, bracing50, lateral wedging51, and specialized footwear52 can reduce the KAM, and could be explored in the ACLR population. Further, extended rehabilitation or interventions aiming to improve quadriceps function/KFM53; 54 after ACLR could be investigated in future work.
ACKNOWLEDGEMENTS
The authors thank Sean Scanlan, PhD, and Michael Zabala, PhD, for their assistance in baseline data collection. This study was funded by the National Institute of Health grants AR039421 and AR052784. The funding sources had no role in the study design, collection or analysis of data, or manuscript preparation or submission.
References
- 1.Weninger P, Zifko B, Liska M, et al. 2008. Anterior cruciate ligament reconstruction using autografts and double biodegradable femoral cross-pin fixation: functional, radiographic and MRI outcome after 2-year minimum follow-up. Knee Surg Sports Traumatol Arthrosc 16:988–995. [DOI] [PubMed] [Google Scholar]
- 2.Oiestad BE, Holm I, Aune AK, et al. 2010. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med 38:2201–2210. [DOI] [PubMed] [Google Scholar]
- 3.Ajuied A, Wong F, Smith C, et al. 2014. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med 42:2242–2252. [DOI] [PubMed] [Google Scholar]
- 4.von Porat A 2004. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Annals of the Rheumatic Diseases 63:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohmander LS, Ostenberg A, Englund M, et al. 2004. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum 50:3145–3152. [DOI] [PubMed] [Google Scholar]
- 6.Granan LP, Forssblad M, Lind M, et al. 2009. The Scandinavian ACL registries 2004–2007: baseline epidemiology. Acta Orthop 80:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur M, Ribeiro DC, Theis JC, et al. 2016. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sports Med 46:1869–1895. [DOI] [PubMed] [Google Scholar]
- 8.Hart HF, Culvenor AG, Collins NJ, et al. 2016. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med 50:597–612. [DOI] [PubMed] [Google Scholar]
- 9.Beaupre GS, Stevens SS, Carter DR. 2000. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev 37:145–151. [PubMed] [Google Scholar]
- 10.Andriacchi TP, Koo S, Scanlan SF. 2009. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am 91 Suppl 1:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabala ME, Favre J, Scanlan SF, et al. 2013. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech 46:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler RJ, Minick KI, Ferber R, et al. 2009. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med 43:366–370. [DOI] [PubMed] [Google Scholar]
- 13.Varma RK, Duffell LD, Nathwani D, et al. 2014. Knee moments of anterior cruciate ligament reconstructed and control participants during normal and inclined walking. BMJ Open 4:e004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster KE, Feller JA. 2012. The knee adduction moment in hamstring and patellar tendon anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc 20:2214–2219. [DOI] [PubMed] [Google Scholar]
- 15.Erhart-Hledik JC, Chu CR, Asay JL, et al. 2017. Longitudinal changes in knee gait mechanics between 2 and 8 years after anterior cruciate ligament reconstruction. J Orthop Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng HL, Wu D, Su F, et al. 2017. Gait Characteristics Associated With a Greater Increase in Medial Knee Cartilage T1rho and T2 Relaxation Times in Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am J Sports Med 45:3262–3271. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Su F, Wu D, et al. 2017. Frontal Plane Knee Mechanics and Early Cartilage Degeneration in People With Anterior Cruciate Ligament Reconstruction: A Longitudinal Study. Am J Sports Med:363546517739605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar D, Kothari A, Souza RB, et al. 2014. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: A pilot study. Knee 21:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosher TJ, Dardzinski BJ. 2004. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8:355–368. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Cheng J, Lin K, et al. 2011. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 29:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keenan KE, Besier TF, Pauly JM, et al. 2011. Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2 MRI. Osteoarthritis Cartilage 19:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erhart-Hledik JC, Favre J, Andriacchi TP. 2015. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. J Biomech 48:3868–3875. [DOI] [PubMed] [Google Scholar]
- 23.Chehab EF, Favre J, Erhart-Hledik JC, et al. 2014. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage 22:1833–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asay JL, Erhart-Hledik JC, Andriacchi TP. 2018. Changes in the total knee joint moment in patients with medial compartment knee osteoarthritis over 5 years. J Orthop Res [DOI] [PubMed] [Google Scholar]
- 25.Bergmann G, Bender A, Graichen F, et al. 2014. Standardized loads acting in knee implants. PLoS One 9:e86035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andriacchi TP, Alexander EJ, Toney MK, et al. 1998. A point cluster method for in vivo motion analysis: applied to a study of knee kinematics. J Biomech Eng 120:743–749. [DOI] [PubMed] [Google Scholar]
- 27.Dyrby CO, Andriacchi TP. 2004. Secondary motions of the knee during weight bearing and non-weight bearing activities. J Orthop Res 22:794–800. [DOI] [PubMed] [Google Scholar]
- 28.Grood ES, Suntay WJ. 1983. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 105:136–144. [DOI] [PubMed] [Google Scholar]
- 29.Andriacchi TP JT, Hurwitz DE, Nataraja RN. 2005. Musculoskeletal dynamics, locomotion, and clinical applications. In: Mow VC HR editor. Basic Orthopaedic Biomechanics and Mechano-Biology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; pp. 91–122. [Google Scholar]
- 30.Dempster WT, Gaughran GRL. 1967. Properties of body segments based on size and weight. American Journal of Anatomy 120:33–54. [Google Scholar]
- 31.Koo S, Gold GE, Andriacchi TP. 2005. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage 13:782–789. [DOI] [PubMed] [Google Scholar]
- 32.Eckstein F, Hudelmaier M, Wirth W, et al. 2006. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis 65:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth W, Eckstein F. 2008. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging 27:737–744. [DOI] [PubMed] [Google Scholar]
- 34.Berchuck M, Andriacchi TP, Bach BR, et al. 1990. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am 72:871–877. [PubMed] [Google Scholar]
- 35.Lewek M, Rudolph K, Axe M, et al. 2002. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 17:56–63. [DOI] [PubMed] [Google Scholar]
- 36.Hart JM, Pietrosimone B, Hertel J, et al. 2010. Quadriceps activation following knee injuries: a systematic review. J Athl Train 45:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmieri-Smith RM, Thomas AC. 2009. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev 37:147–153. [DOI] [PubMed] [Google Scholar]
- 38.Oiestad BE, Juhl CB, Eitzen I, et al. 2015. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage 23:171–177. [DOI] [PubMed] [Google Scholar]
- 39.Zhao D, Banks SA, Mitchell KH, et al. 2007. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res 25:789–797. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki T 2002. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Annals of the Rheumatic Diseases 61:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellsandt E, Gardinier ES, Manal K, et al. 2016. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am J Sports Med 44:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyer KA, Angst MS, Asay J, et al. 2012. Sensitivity of gait parameters to the effects of anti-inflammatory and opioid treatments in knee osteoarthritis patients. J Orthop Res 30:1118–1124. [DOI] [PubMed] [Google Scholar]
- 43.Mundermann A, Dyrby CO, Andriacchi TP. 2005. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum 52:2835–2844. [DOI] [PubMed] [Google Scholar]
- 44.Urbach D, Nebelung W, Weiler HT, et al. 1999. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc 31:1691–1696. [DOI] [PubMed] [Google Scholar]
- 45.Noehren B, Wilson H, Miller C, et al. 2013. Long-term gait deviations in anterior cruciate ligament-reconstructed females. Med Sci Sports Exerc 45:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckstein F, Burstein D, Link TM. 2006. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed 19:822–854. [DOI] [PubMed] [Google Scholar]
- 47.Koo S, Andriacchi TP. 2007. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J Biomech 40:2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erhart-Hledik JC, Asay JL, Clancy C, et al. 2017. Effects of active feedback gait retraining to produce a medial weight transfer at the foot in subjects with symptomatic medial knee osteoarthritis. J Orthop Res 35:2251–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eddo O, Lindsey B, Caswell SV, et al. 2017. Current Evidence of Gait Modification with Real-time Biofeedback to Alter Kinetic, Temporospatial, and Function-Related Outcomes: A Review 2017 5:21. [Google Scholar]
- 50.Moyer RF, Birmingham TB, Bryant DM, et al. 2015. Biomechanical effects of valgus knee bracing: a systematic review and meta-analysis. Osteoarthritis Cartilage 23:178–188. [DOI] [PubMed] [Google Scholar]
- 51.Arnold JB, Wong DX, Jones RK, et al. 2016. Lateral Wedge Insoles for Reducing Biomechanical Risk Factors for Medial Knee Osteoarthritis Progression: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 68:936–951. [DOI] [PubMed] [Google Scholar]
- 52.Radzimski AO, Mundermann A, Sole G. 2012. Effect of footwear on the external knee adduction moment - A systematic review. Knee 19:163–175. [DOI] [PubMed] [Google Scholar]
- 53.Palmieri-Smith RM, Thomas AC, Wojtys EM. 2008. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med 27:405–424, vii-ix. [DOI] [PubMed] [Google Scholar]
- 54.Fischer AG, Erhart-Hledik JC, Asay JL, et al. 2019. Activating the somatosensory system enhances net quadriceps moment during gait. J Biomech 82:149–155. [DOI] [PubMed] [Google Scholar]


