Abstract
Hemopexin (Hpx) is critical for hemin scavenging after the erythrocyte lysis that occurs following intracerebral hemorrhage (ICH). Low-density lipoprotein receptor-related protein-1 (LRP1, also called CD91) is an important receptor through which the hemin-Hpx complex can undergo endocytosis. This study investigated changes in the hemin-Hpx-CD91 axis in both hematoma and perihematomal tissue in a large animal ICH model. The effect of deferoxamine (DFX) on hemin-Hpx-CD91 was also examined.
The study consisted of two parts. First, piglets had an injection of autologous blood into the right frontal lobe of brain and were euthanized from day 1 to day 7. Hematoma and perihematomal tissue of brains were used for hemin assay, immunohistochemistry, and immunofluorescence. Second, piglets with ICH were treated with deferoxamine or vehicle, and were euthanized for hemin measurement and Hpx and CD91 immunohistochemistry.
We found that there was an increase of hemin levels within the hematoma and perihematomal brain tissue after ICH. Hpx and CD91-positive cells were present in the clot and perihematomal tissue from day 1. Hpx and CD91 positive cells were Iba1 positive. After DFX therapy, hemin dropped markedly in the hematoma and perihematomal brain tissue. Furthermore, DFX treatment decreased the number of Hpx and CD91 positive cells in and around the hematoma.
In conclusion, hemin accumulation occurs in and around the hematoma. Increases in Hpx and CD91 may be important in scavenging that hemin. DFX treatment decreased hemin release from the hematoma and reduced the expression of Hpx and CD91.
Keywords: Intracerebral hemorrhage, Deferoxamine, Hemin, Hemopexin, CD91, Swine
Introduction
Intracerebral hemorrhage (ICH) is a particularly devastating form of stroke which causes severe clinical disability and mortality (1-4). Clot resolution is an important pathophysiological process following ICH(5). However, it can release toxic molecules such as hemoglobin and its degradation products, including hemin (Fe3+ protoporphyrin IX) and iron (6). Hemin is a toxic alarmin that can initiate oxidative stress, inflammation, cytotoxicity and brain parenchyma damage (7-10). Thus, it is important to understand how extracellular hemin is removed after ICH. Hemopexin (Hpx) shows the highest known binding affinity for hemin (Kd <10−13M), making hemin oxidatively inert(11) and Hpx has protective effects in plasma and brain (11-13). Moreover, recent studies demonstrated that Hpx possesses a pivotal ability in neuroprotection after hemorrhagic and ischemic stroke (10, 14-16). However, the changes in brain hemin and hemopexin following ICH are poorly understood.
Low-density lipoprotein receptor-related protein-1 (LRP1, also called CD91) is a transmembrane protein. As well as being a signaling receptor, it has versatile functions including protein scavenging and lipid endocytosis (17, 18), and it has been identified as the receptor for hemin-Hpx complex endocytosis (10, 11, 19). After cell uptake, the hemin-Hpx complex is dissociated in lysosomes and the hemin catabolized by heme oxygenase into biliverdin, carbon monoxide, and iron (10, 11, 20, 21). Thus, CD91 has a key role in clearing free hemin to prevent toxicity.
Deferoxamine (DFX), an iron chelator, has been demonstrated to reduce hemorrhagic brain injury in animal models(2, 6). In a swine ICH model, DFX significantly attenuated perihematomal iron accumulation, white matter injury and neuronal death (22, 23). Recently, we found that DFX also slows hematoma lysis after ICH in aged rats (24). This was confirmed in another study, which showed that DFX reduced the formation of membrane attack complex and heme oxygenase-1 (HO-1) positive cells in hematoma after ICH in piglets (25).
The current study was to examine the expression of hemin, Hpx and CD91 in a piglet ICH model. The effect of DFX on those changes was also investigated.
Materials and Methods
Animal preparation
All the animal studies were approved by the University of Michigan Committee on the Use and Care of Animals and performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male piglets weighing between 8 and 10 kg (Michigan State University, East Lansing) were used in the present study. Piglets were allowed free access to food and water before and after the surgical procedures. This study complies with the ARRIVE guidelines for reporting in vivo experiments. Randomization was carried out using odd/even numbers.
Surgical procedures
The ICH models were performed based on the technique as previously described (23, 25, 26). Briefly, ketamine (25 mg/kg, i.m.) were used for sedation and 2% isoflurane were used through nose cone for anesthetization. The maintenance concentration of isoflurane was at 1.0 to 1.5% during the surgical operations. Core body temperature was kept at 37.5 ± 0.5°C using a heating pad. A polyethylene catheter (PE-160) was inserted into the right femoral artery to obtain blood for brain injection and monitoring of arterial blood pressure, blood gases, and glucose during the surgery. A cranial burr hole (1.5 mm) located at the point 11 mm right of the sagittal suture and 11 mm anterior to the coronal suture was drilled. An 18-mm-long 20-gauge sterile plastic catheter was inserted stereotaxically into the right frontal cerebral white matter. Then, 1.0 ml of autologous arterial blood was injected with an infusion pump at a rate of 100 μl/min. After a 5-minute break, another 1.5 ml blood was infused. The catheter was then slowly withdrawn. The burr hole was filled with bone wax and the skin incision was closed with sutures.
Experimental protocol
This study was in two parts. In the first part, animals were subjected to ICH in the right frontal lobe. Then animals were euthanized at day 1 (n=8), day 3 (n=8), and day 7 (n=8) and brains were harvested for immunohistochemistry, immunofluorescence and hemin assay. Three sham animals only had a needle insertion. In the second part, a total of 32 piglets with ICH model in the frontal lobe were treated with vehicle (saline, n=16) or DFX (n=16) with a dose of 50 mg/kg via intramuscular injection at 2 hours after ICH and then every 12 hours for up to 7 days. Brains were harvested at day 3 (n=8) and 7 (n=8) for analysis of brain histology (n=4 each group) and hemin assay (n=4 each group).
Brain in situ freezing and brain histology
The piglets were anesthetized and the brains were frozen in situ by decanting liquid nitrogen into a bottomless foam cup adhered to the head with Dow Corning high vacuum grease (Dow Corning, Midland, MI)(27). The piglets were decapitated and brains were harvested following the injection of potassium chloride to stop the heart beating. Then the frozen head was cut into 5-mm-thick coronal sections by a band saw. Tissues were sampled from the points of interest. For brain histology, the piglets were re-anesthetized and the brains were perfused with 10% formalin. Brains were then sectioned coronally. Paraffin-embedded brain was cut into 10-μm-thick sections and examined with immunohistochemistry and immunofluorescence.
Hemin assay
For free hemin assay, the brains were in situ frozen. The hematoma and perihematomal white matter tissue samples were collected, weighed, and homogenized as previously described (28). The amount of free hemin was determined by using Hemin Colorimetric Assay kit (Biovision, K672) according to the manufacturer’s instructions.
Immunohistochemistry and Cells Counting
The 10-μm-thick brain sections were deparaffinized in xylene and rehydrated in a graded series of alcohol dilutions. The microwave boiling method was conducted to retrieve antigen using citrate buffer (10 mmol/L, pH 6.0). Immunohistochemistry examination was performed by avidin-biotin complex techniques(29). The primary antibodies were rabbit monoclonal anti-CD91 (Abcam, ab92544, 1:200 dilution) and rabbit polyclonal anti-Hpx (Abcam, ab133415, 1:400 dilution). Phosphate-buffered saline (0.1M, pH 7.4) was employed as a negative control. Counting of CD91and Hpx-positive cells was performed on high-power images (×40 magnification) taken using a digital camera. Counts were performed on 4 areas in each brain section.
Immunofluorescence
For immunofluorescence triple labeling, paraffin-embedded sections were dealt with the same method as described above to retrieve antigen. Then they were treated with 0.3% Triton X-100 in PBS and blocked using normal donkey serum for 30 min at room temperature. The primary antibody incubation was applied for 1.5 hr or overnight at 4°C. Sections were then rinsed with PBS for 10 min 3 times and incubated with secondary antibodies at room temperature for 2 hr. The primary antibodies were rabbit monoclonal anti-CD91 (Abcam, ab92544, 1:100 dilution), rabbit polyclonal anti-Hpx (Abcam, ab133415, 1:300 dilution), goat polyclonal anti-Iba1 (1:300, ab107159, Abcam). DAPI was used for nuclear labeling.
Statistical analysis
Measurements were performed by personnel blinded to each group. All the data are expressed as mean ± SD. Data were compared by using one- or two-way ANOVA with a Bonferroni multiple comparisons test. A probability value of less than 0.05 was considered statistically significant. Because these are novel endpoints for a porcine ICH model, we were not able to perform a power analysis to determine sample size. However, in a prior study(22), four animals per group was able to detect a 50% reduction in ferritin-positive cells with DFX at the p<0.05 level.
Results
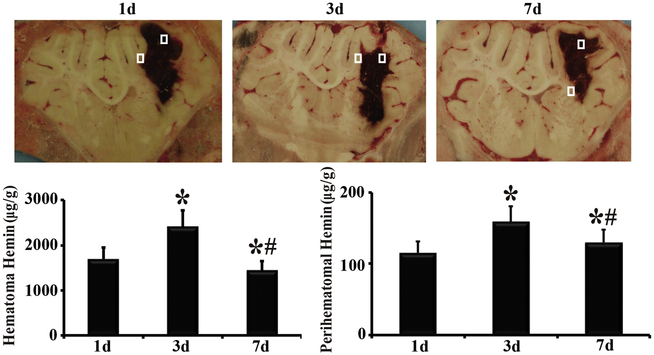
Hemin content in the hematoma and perihematomal brain tissue was measured to assess erythrolysis. Hemin concentrations in the contralateral hemisphere were low (3.3 ± 0.3 μg/g). In contrast, hemin levels in the hematoma were ~500-700 folds higher (1,693±339 and 2,408± 474 μg/g at day 1 and 3, respectively) although there was some reduction by day 7 (p<0.05, Figure 1). Hemin content in the perihematomal brain tissue was also much higher (35-50 fold) than in the contralateral tissue (e.g. 115 ± 17 μg/g at day 1). Like, hematomal hemin, perihematomal hemin peaked at day 3 (Figure. 1).
Figure 1.
Hematomal and perihematomal hemin levels at days 1, 3, and 7 following ICH. Sample areas are shown in brain sections. *, p<0.05, compared with day 1; #,p<0.05, compared with day 3.
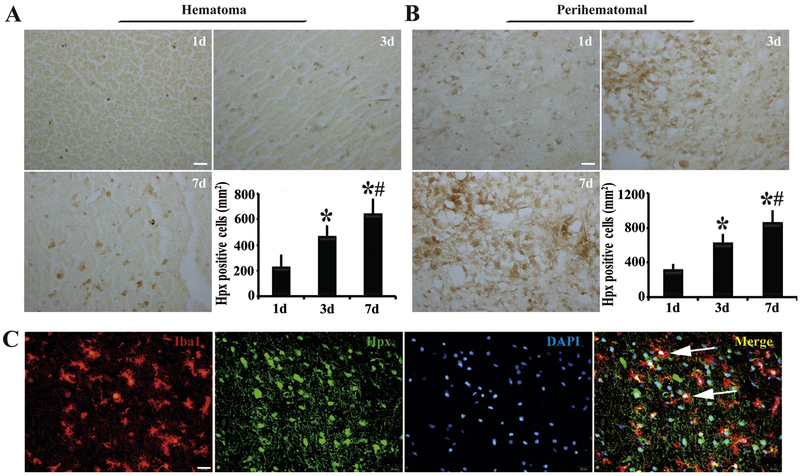
Expression of Hpx, the major hemin scavenger, in brain hematoma and perihematomal areas was determined using immunohistochemistry. We found that some Hpx expression in the clot at day 1 (233 ± 86 cells/mm2) after ICH, then it increased at day 3 and at day 7 (e.g. 645 ± 109 cells/mm2 at day 7, Figure 2A). Hpx positive cells in the perihematomal tissue showed a similar time course (Figure 2B). To further determine what cells expressed Hpx, we performed triple immunofluorescence staining. We found that Hpx was co-localized with Iba1 (a microglia/macrophage marker, Figure 2C).
Figure 2.
Presence of hemopexin (Hpx)-positive cells in (A) hematoma and (B) perihematomal tissue at days 1, 3 and 7 after ICH with quantification. Scale bar=20μm. n=4 for each group, *, p<0.05, compared with day 1; #, p<0.05, compared with day 3. (C) At day 1 after ICH, Hpx immunoreactivity colocalized with Iba1 in the perihematomal area. Arrows indicate double-stained cells. Scale bar=20 μm.
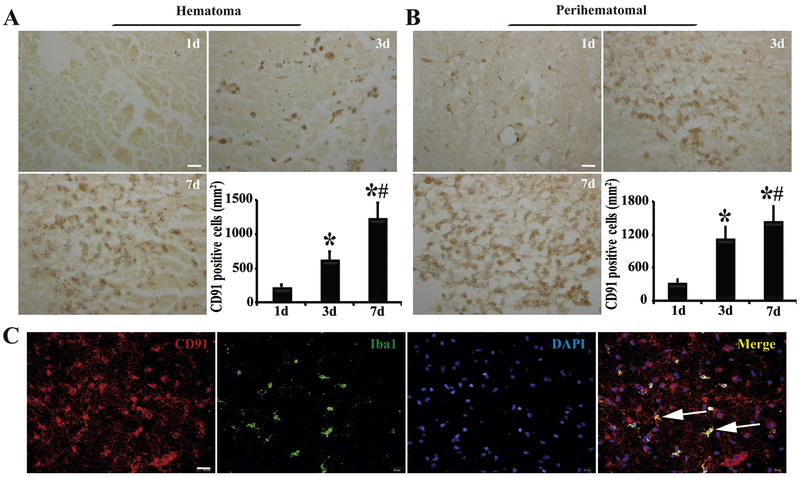
The levels of CD91 in the hematoma and perihematomal zone were also determined. Immunohistochemistry showed that CD91-positive cells infiltrated into the hematoma from the edge to center after ICH, with a marked upregulation at day 3 and stayed at high level at day 7 (1224 ± 191 cells/mm2, Figure 3A). CD91 expression in the perihematomal zone increased with time (p<0.05, Figure 3B). The morphological features of the CD91-positive cells in and around the clot were microglia/macrophage like, which was further confirmed by its co-localization with Iba1 using immunofluorescence (Figure 3C).
Figure 3.
Presence of CD91-positive cells in (A) clot and (B) perihematomal tissue 1, 3 and 7 days after ICH with quantification. Scale bar=20μm. n=4 for each group, *, p<0.05, compared with day 1; #, p<0.05, compared with day 3. (C) CD91-immunoreactivity colocalized with Iba1 in the perihematomal area at day 1. Scale bar=20μm. Arrows indicate double-stained cells.
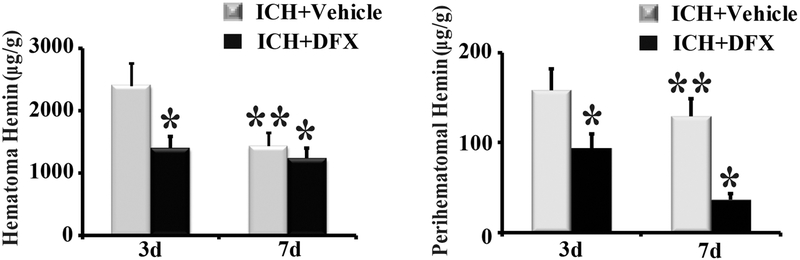
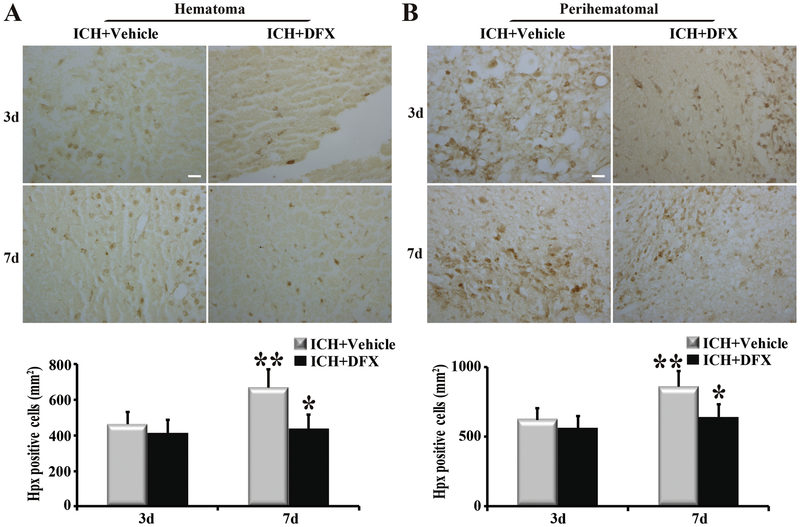
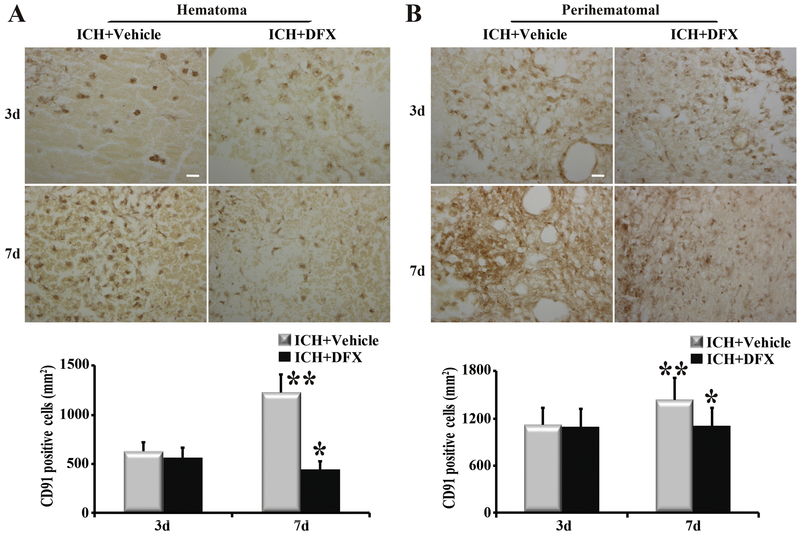
To determine whether DFX affects hemin release and clearance, ICH rats were treated with vehicle or DFX for up to 7 days. Our results showed that hemin content in the hematoma was significantly lower in the DFX-treated group than in the vehicle-treated group at both day 3 and day 7 (p<0.05, Figure 4). The same phenomena happened in the perihematomal brain (p<0.05, Figure 4). Whether DFX produces an effect on Hpx and CD91 expression was further investigated. Hpx levels in the hematoma and perihematomal zone declined significantly after DFX therapy at day 7 (p<0.01, Figure 5). Additionally, DFX treatment reduced CD91-positive cells in the clot and perihematomal tissue at day 7 (p<0.01, Figure 6).
Figure 4.
Deferoxamine (DFX) treatment reduced hemin levels in (A) hematoma and (B) perihematomal tissue at day 3 and 7 following ICH. n=4 for each group. * p<0.05, compared with the vehicle-treated group; ** p<0.05 compared with day 3.
Figure 5.
Deferoxamine (DFX) treatment down regulated the expression of hemopexin (Hpx) in (A) clot and (B) perihematomal tissue at day 7 following ICH. Examples of Hpx immunohistochemistry at days 3 and 7 after ICH. Scale bar=20μm. Quantification of the numbers of Hpx immunopositive cells with and without DFX treatment. n=4 for each group. * p<0.01, compared to vehicle-treated group; ** p<0.05 compared with day 3.
Figure 6.
Deferoxamine (DFX) treatment decreased the expression of CD91 in (A) the hematoma and (B) perihematomal tissue at day 7. Examples of CD91 immunohistochemistry at days 3 and 7 after ICH; scale bar=20μm. Quantification of the numbers of CD91 immunopositive cells with and without DFX treatment. n=4 for each group. * p<0.05 compared to vehicle-treated group; ** p<0.05 compared with day 3.
Discussion
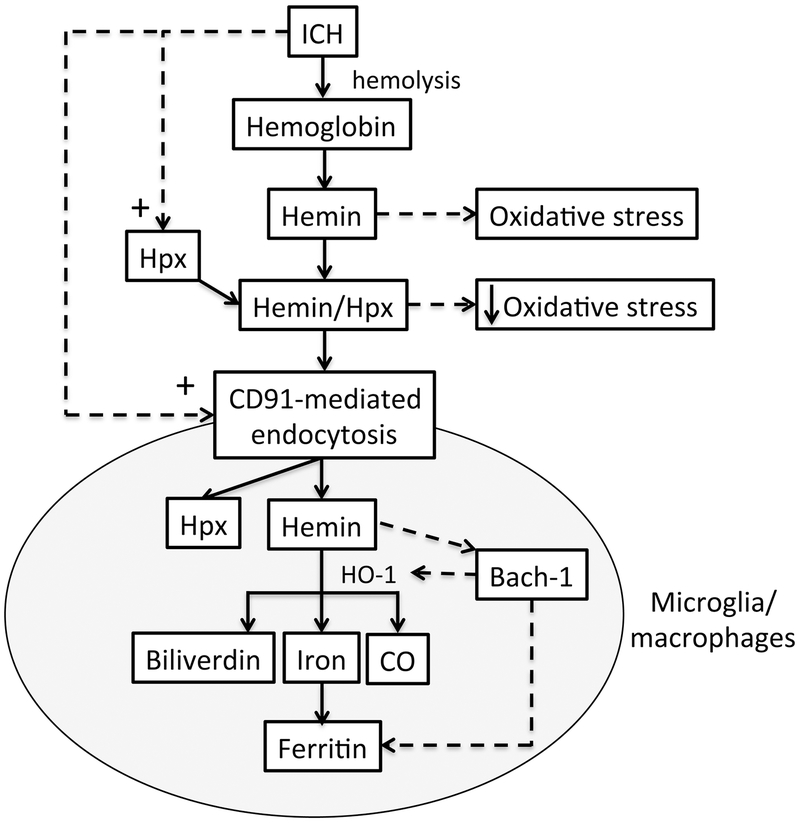
The major findings in the current study are: (1) hemin concentrations in hematoma and perihematomal tissue are markedly elevated compared to normal brain, (2) those hemin levels can be reduced by DFX treatment; (3) expression of Hpx in the clot and perihematomal tissue occurs at day 1 after ICH and increases with time; (4) there is a progressive increase of CD91-positive cells in hematoma and perihematomal brain tissue; (5) DFX down regulates Hpx and CD91 in clot and perihematomal brain tissue. These data suggest that hemin release hematoma occurs from the first day following ICH and that there is a compensatory activation of the hemin scavenger systems (Figure 7). DFX reduces hemin release and, thus, ICH-induced upregulation of Hpx and CD91.
Figure 7.
Schematic for hemin clearance by microglia/macrophages following ICH. Hemolysis after ICH causes the release of hemoglobin and hemin. The oxidative stress that can be caused by hemin is reduced by binding to hemopexin (Hpx) which is upregulated after ICH as shown by the current study. In addition, the hemin/Hpx complex is taken up into microglia/macrophages via CD91-mediated endocytosis. CD91 is also upregulated in brain after ICH as shown in the current study. Within microglia/macrophages, hemin is degraded into iron, carbon monoxide (CO) and bilverdin by heme oxygenase-1 (HO-1). Iron is chelated by ferritin to prevent iron-induced oxidative stress. Both HO-1 and ferritin are upregulated after ICH. Hemin increasing their expression by binding to Bach-1, a transcription factor that normally represses the expression of these proteins.
Hematoma resolution after ICH still remains a relatively understudied area. It may involve the phagocytosis of the hematoma by microglia/macrophages and erythrocyte lysis with the clearance of the products of such lysis, particularly hemoglobin and its degradation products(30-32). The latter include hemin and iron that are potentially neurotoxic (2, 6). To examine the progress of erythrocyte lysis after ICH, the current study measured hemin levels within the hematoma and in perihematomal brain. Very high hemin levels were recorded within the hematoma after ICH (~2,000 μg/g or approximately 3 mM) and in perihematomal tissue (~130 μg/g or approximately 0.2 mM). We have recently reported a progressive decrease in hematoma hemoglobin content (determined by ELISA) over the first three days in this piglet ICH model (25) that mirrors the increase in hemin. The increase in perihematomal hemin after ICH in the current study probably reflects diffusion of the hematomal hemin into the surrounding tissue. The hemin assay used in the current study uses the peroxidase activity of hemin. Such activity is reduced by hemopexin binding(11) and it is possible that tissue processing may have affected such binding. There is a need to determine free hemin concentrations in vivo, particularly in the extracellular space.
The release of hemin after erythrolysis may contribute to ICH-induced brain injury (10, 33-35). Thus, for example, direct intracaudate injection of hemin causes brain edema in the rat (36) and, in vitro, 50 μM hemin induces neuronal cell death (37). In this study, our measured brain tissue hemin concentrations adjacent to the hematoma (~200 μM) are greater than those required to cause neuronal death in vitro.
The current study showing increased hematomal and perihematomal hemin concentrations by as early as one day, supports growing evidence that some erythrocyte lysis occurs early after ICH. As noted above, we have found a progressive loss of hemoglobin in the hematoma over 3 days in the same piglet ICH model(25). T2* magnetic resonance imaging of the brain at day 1 also showed hyperintense signals in the center of hematoma which may represents the early erythrocyte lysis with the appearance of erythrocyte ghosts on histology(38). In line with these data, hemoglobin was present in the perihematomal zone at day 1 after ICH in a rabbit model (39). The time course of hemoglobin, hemin and iron release after ICH is important because there is an upregulation of various mechanisms in brain after ICH that may limit hemoglobin, hemin and iron toxicity (40). Hemoglobin, hemin and iron release prior to those protective changes may cause more brain injury.
The adverse effects of hemin may be limited by upregulation in Hpx and CD91. Hpx is a 60-kDa serum glycoprotein, which is recognized as the first line of defense system against free hemin. It binds hemin with very high affinity, serving a protective function through limiting the toxic effects of hemin (16) including limiting hemin peroxidase activity (10, 11). The hemin-Hpx complex can also be taken up by cells via CD91 receptor, and the hemin is then catabolized by the heme oxygenases (Figure 7) (20, 21). In present study, we found that Hpx was positive in the hematoma and perihematomal brain tissue at day 1 after ICH and Hpx expression progressively increased with time. Furthermore, our results showed that Hpx-positive cells were also Iba1 positive, which indicated that they were primarily microglia/macrophages. This might be endogenous protective mechanism in response to increased hemin content level with microglia/macrophages being activated and releasing Hpx after ICH to clear hemin. Multiple studies have shown that Hpx has a protective role in the central nervous system and systemic organs (14-16, 41-46).
CD91 is a transmembrane receptor that is expressed on several types of brain cell, including microglia/macrophages. Amongst several functions, it is involved in the endocytosis of the hemin/Hpx complex (10, 19). In our experiments, there was a progressive increase in CD91 expression after ICH both within the clot and in the perihematomal tissue, where CD91 colocalized with Iba-1, a microglial marker. While further studies are needed to explore whether CD91 upregulation on microglia/macrophages is beneficial in ICH, it may represent a method for clearing extracellular hemin.
Both Hpx and CD91 showed a progressive increase in expression after ICH. The mechanism involved in that upregulation is still uncertain and needs to be investigated. It is known that hemin is involved in the upregulation of HO-1 and ferritin by binding to Bach-1, a transcription factor that normally suppresses the transcription of those proteins (47) (Figure 7) but whether hemin, or some other clot-derived factor, induces the upregulation of Hpx and CD91 after ICH is uncertain. As with ferritin (40), the increase in Hpx and CD91 is delayed after ICH. Whether this means that early release (e.g. in the first day) of hemin after ICH causes more brain injury than later release needs to be investigated as does the role of heme carrier protein 1 (HCP1) that may also be involved in hemin clearance (10).
Our prior studies have shown that DFX reduces ICH-induced brain injury in mice, rats and pigs(2, 6) and DFX is currently in phase II clinical trial for ICH ( NCT02175225). DFX is an iron chelator and perihematomal iron overload occurs after ICH (40) and much evidence indicates iron is involved in ICH-induced brain injury (2, 6). The current study also indicates that DFX also reduces erythrolysis and the release of hemin within the hematoma and into perihematomal tissue. This work confirms a prior study demonstrating that DFX slowed erythrolysis and the release of hemoglobin from the hematoma (25). Thus, DFX may reduce ICH-induced brain injury by multiple mechanisms as well as iron chelation, including delaying the release of potential neurotoxic compounds (hemin and hemoglobin) from the hematoma by inhibiting erythrolysis. Interestingly, DFX also decreased the number of Hpx and CD91-positive cells in both clot and perihematomal area. This may be the result of the reduced erythrolysis and lower levels of the clot-derived factor that induces Hpx and CD91 expression.
Conclusions
In summary, this study presents data on the natural changes of hemin-hemopexin-CD91 axis in a large animal ICH model. DFX treatment reduces hemin levels in and around the hematoma. Enhancing hemin clearance may reduce ICH-induced brain injury.
Acknowledgments
Funding: YH, RFK and GX were supported by grants NS-091545, NS-090925, NS-096917 and NS106746 from the National Institutes of Health (NIH).
Footnotes
Conflict of Interests: Shengli Hu, Ya Hua, Richard F. Keep, Hua Feng and Guohua Xi declare no conflict of interests.
Ethical approval: All institutional and national guidelines for the care and use of laboratory animals were followed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013. August 3;382(9890):397–408. PubMed PMID: 23726393. Pubmed Central PMCID: 3906609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012. August;11(8):720–31. PubMed PMID: 22698888. Pubmed Central PMCID: 3884550. Epub 2012/06/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selim M, Sheth KN. Perihematoma edema: a potential translational target in intracerebral hemorrhage? Transl Stroke Res. 2015. April;6(2):104–6. PubMed PMID: 25693976. Pubmed Central PMCID: 4359064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlunk F, Greenberg SM. The Pathophysiology of Intracerebral Hemorrhage Formation and Expansion. Transl Stroke Res. 2015. August;6(4):257–63. PubMed PMID: 26073700. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson DA, Keep RF, Hua Y, Xi G. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: From macro to micro. J Cereb Blood Flow Metab. 2018. April;38(4):741–5. PubMed PMID: 29350086. Pubmed Central PMCID: 5888862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006. January;5(1):53–63. PubMed PMID: 16361023. Epub 2005/12/20. eng. [DOI] [PubMed] [Google Scholar]
- 7.Soares MP, Bozza MT. Red alert: labile heme is an alarmin. Curr Opin Immunol. 2016. February;38:94–100. PubMed PMID: 26741528. Epub 2016/01/08. eng. [DOI] [PubMed] [Google Scholar]
- 8.Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001. September 15;98(6):1802–11. PubMed PMID: 11535514. Epub 2001/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Manaenko A, Shao A, Ou Y, Yang P, Budbazar E, et al. Low-density lipoprotein receptor-related protein-1 facilitates heme scavenging after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2016. June 17. PubMed PMID: 27317656. Epub 2016/06/19. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox report : communications in free radical research. 2009;14(6):228–35. PubMed PMID: 20003707. [DOI] [PubMed] [Google Scholar]
- 11.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013. February 21;121(8): 1276–84. PubMed PMID: 23264591. Pubmed Central PMCID: 3578950. Epub 2012/12/25. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011. June;42(6):1781–6. PubMed PMID: 21527759. Pubmed Central PMCID: 3123894. Epub 2011/04/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, et al. Hemoglobin and heme scavenging. IUBMB life. 2005. November;57(11):749–59. PubMed PMID: 16511968. [DOI] [PubMed] [Google Scholar]
- 14.Ma B, Day JP, Phillips H, Slootsky B, Tolosano E, Dore S. Deletion of the hemopexin or heme oxygenase-2 gene aggravates brain injury following stroma-free hemoglobin-induced intracerebral hemorrhage. J Neuroinflammation. 2016;13:26. PubMed PMID: 26831741. Pubmed Central PMCID: 4736638. Epub 2016/02/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong B, Cai M, Fang Z, Wei H, Zhu F, Li G, et al. Hemopexin induces neuroprotection in the rat subjected to focal cerebral ischemia. BMC Neurosci. 2013; 14:58. PubMed PMID: 23758755. Pubmed Central PMCID: 3694464. Epub 2013/06/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Zhang X, Chen-Roetling J, Regan RF. Increased striatal injury and behavioral deficits after intracerebral hemorrhage in hemopexin knockout mice. J Neurosurg. 2011. April;114(4):1159–67. PubMed PMID: 21128737. Pubmed Central PMCID: 3061252. Epub 2010/12/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer's disease: the role, regulation and restoration of LRP1. Front Aging Neurosci. 2015;7:136. PubMed PMID: 26236233. Pubmed Central PMCID: 4502358. Epub 2015/08/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008. July;88(3):887–918. PubMed PMID: 18626063. Pubmed Central PMCID: 2744109. Epub 2008/07/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hvidberg V Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005. October 1;106(7):2572–9. PubMed PMID: 15947085. Epub 2005/06/11. eng. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005. July 4; 157(3): 175–88. PubMed PMID: 15917143. Epub 2005/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 21.Piccard H, Van den Steen PE, Opdenakker G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J Leukoc Biol. 2007. April;81(4):870–92. PubMed PMID: 17185359. Epub 2006/12/23. eng. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009. June;40(6):2241–3. PubMed PMID: 19372448. Epub 2009/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Q, Gu Y, Hua Y, Liu W, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014. January;45(1):290–2. PubMed PMID: 24172580. Pubmed Central PMCID: 3886635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatakeyama T, Okauchi M, Hua Y, Keep RF, Xi G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res. 2013. October;4(5):546–53. PubMed PMID: 24187595. Pubmed Central PMCID: 3810989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao S, Zheng M, Hua Y, Chen G, Keep RF, Xi G. Hematoma Changes During Clot Resolution After Experimental Intracerebral Hemorrhage. Stroke. 2016. April 28;47:1626–31. PubMed PMID: 27125525. Epub 2016/04/30. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Xie Q, Xi G, Keep RF, Hua Y. Brain CD47 expression in a swine model of intracerebral hemorrhage. Brain Res. 2014. July 29;1574:70–6. PubMed PMID: 24931767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J Neurosurg. 1998;88(6):1058–65. [DOI] [PubMed] [Google Scholar]
- 28.Wagner KR, Xi G, Hua Y, Kleinholz M, de Courten-Myers GM, Myers RE, et al. Lobar intracerebral hemorrhage model in pigs: rapid edema development in perihematomal white matter. Stroke. 1996;27(3):490–7. [DOI] [PubMed] [Google Scholar]
- 29.Zheng M, Du H, Ni W, Koch LG, Britton SL, Keep RF, et al. Iron-induced necrotic brain cell death in rats with different aerobic capacity. Transl Stroke Res. 2015. June;6(3):215–23. PubMed PMID: 25649272. Pubmed Central PMCID: 4425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan S, Cheng Y, Jin H, Guo D, Hua Y, Keep RF, et al. Microglia Activation and Polarization After Intracerebral Hemorrhage in Mice: the Role of Protease-Activated Receptor-1. Transl Stroke Res. 2016. May 21;7:478–87. PubMed PMID: 27206851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong XY, Yang QW. Rethinking the roles of inflammation in the intracerebral hemorrhage. Transl Stroke Res. 2015. October;6(5):339–41. PubMed PMID: 25940771. [DOI] [PubMed] [Google Scholar]
- 32.Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/Macrophage Polarization After Experimental Intracerebral Hemorrhage. Transl Stroke Res. 2015. December;6(6):407–9. PubMed PMID: 26446073. Pubmed Central PMCID: 4628553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babu R, Bagley JH, Di C, Friedman AH, Adamson C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurgical focus. 2012. April;32(4):E8. PubMed PMID: 22463118. [DOI] [PubMed] [Google Scholar]
- 34.Garton T, Keep RF, Wilkinson DA, Strahle JM, Hua Y, Garton HJ, et al. Intraventricular Hemorrhage: the Role of Blood Components in Secondary Injury and Hydrocephalus. Transl Stroke Res. 2016. June 30;7:447–51. PubMed PMID: 27358176. [DOI] [PubMed] [Google Scholar]
- 35.Keep RF, Andjelkovic AV, Xiang J, Stamatovic SM, Antonetti DA, Hua Y, et al. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J Cereb Blood Flow Metab. 2018. August;38(8):1255–75. PubMed PMID: 29737222. Pubmed Central PMCID: 6092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002. February;96(2):287–93. PubMed PMID: 11838803. Epub 2002/02/13. eng. [DOI] [PubMed] [Google Scholar]
- 37.Karuppagounder SS, Alim I, Khim SJ, Bourassa MW, Sleiman SF, John R, et al. Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates ATF4-dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models. Science translational medicine. 2016. March 2;8(328):328ra29. PubMed PMID: 26936506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dang G, Yang Y, Wu G, Hua Y, Keep RF, Xi G. Early Erythrolysis in the Hematoma After Experimental Intracerebral Hemorrhage. Transl Stroke Res. 2017. October 25;8:174–82. PubMed PMID: 27783383. Epub 2016/10/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci. 1995;134:102–12. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34(12):2964–9. [DOI] [PubMed] [Google Scholar]
- 41.Li RC, Saleem S, Zhen G, Cao W, Zhuang H, Lee J, et al. Heme-hemopexin complex attenuates neuronal cell death and stroke damage. J Cereb Blood Flow Metab. 2009. May;29(5):953–64. PubMed PMID: 19277051. Epub 2009/03/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vinchi F, De Franceschi L, Ghigo A, Townes T, Cimino J, Silengo L, et al. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013. March 26;127(12): 1317–29. PubMed PMID: 23446829. Epub 2013/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 43.Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. 2016. January 28;127(4):473–86. PubMed PMID: 26675351. Pubmed Central PMCID: 4850229. Epub 2015/12/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin T, Liu J, Huang F, Van Engelen TS, Thundivalappil SR, Riley FE, et al. Purified and recombinant hemopexin: protease activity and effect on neutrophil chemotaxis. Mol Med. 2016. January 8. PubMed PMID: 26772775. Pubmed Central PMCID: 5004720. Epub 2016/01/17. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deuel JW, Vallelian F, Schaer CA, Puglia M, Buehler PW, Schaer DJ. Different target specificities of haptoglobin and hemopexin define a sequential protection systemagainst vascular hemoglobin toxicity. Free Radic Biol Med. 2015. December;89:931–43. PubMed PMID: 26475040. Epub 2015/10/18. eng. [DOI] [PubMed] [Google Scholar]
- 46.Hahl P, Davis T, Washburn C, Rogers JT, Smith A. Mechanisms of neuroprotection by hemopexin: modeling the control of heme and iron homeostasis in brain neurons in inflammatory states. J Neurochem 2013. April;125(1):89–101. PubMed PMID: 23350672. Pubmed Central PMCID: 3752430. Epub 2013/01/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hada H, Shiraki T, Watanabe-Matsui M, Igarashi K. Hemopexin-dependent heme uptake via endocytosis regulates the Bach1 transcription repressor and heme oxygenase gene activation. Biochim Biophys Acta. 2014. July;1840(7):2351–60. PubMed PMID: 24613679. Epub 2014/03/13. eng. [DOI] [PubMed] [Google Scholar]