Abstract
In addition to protecting body from infections and diseases, the immune system produces auto-antibodies that can cause complex autoimmune disorders, such as Type I diabetes, primary biliary cirrhosis, rheumatoid arthritis, and multiple sclerosis, to name a few. In such cases, the immune system fails to recognize between foreign agents and its own body cells. Different factors, such as genetic factors (CD25, STAT4), epigenetic factors (DNA methylation, histone modifications) and environmental factors (xenobiotics, drugs, hormones) trigger autoimmunity. Glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressive and biological agents are currently used to manage autoimmune diseases of different origins. However, complete cure remains elusive. Many dietary and natural products including polyphenols have been widely studied as possible alternative treatment strategies for the management of autoimmune disorders. Polyphenols possess a wide-range of pharmacological and therapeutic properties, including antioxidant and anti-inflammatory activities. As immunomodulatory agents, polyphenols are emerging pharmaceutical tools for management of various autoimmune disorders including vitiligo, ulcerative colitis and multiple sclerosis (MS). Polyphenols activate intracellular pathways such as arachidonic acid dependent pathway, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling pathway, mitogen-activated protein kinases (MAPKs) pathway, phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway and epigenetic modulation, which regulate the host’s immune response. This timely review discusses putative points of action of polyphenols in autoimmune diseases, characterizing their efficacy and safety as therapeutic agents in managing autoimmune disorders.
Keywords: Polyphenols, autoimmunity, NF-κB, MAPKs, PI3K/Akt, epigenetic modulation
1. Introduction
The immune system protects from infections and diseases resulting from bacterial, viral and other causative agents. However, under certain circumstances, the immune system may produce auto-antibodies against its own cells, leading to autoimmune diseases. In such cases, the immune system fails to recognize between foreign agents and own body cells [1]. Complex systems of innate and adaptive immunity and their interaction with genes and environmental factors trigger the development of systemic autoimmune diseases [2]. Although thought to be rare, more than 80 autoimmune diseases have been identified to date, including autoimmune hepatitis, Type I diabetes, Primary biliary cirrhosis, Rheumatoid arthritis, Multiple sclerosis etc. [3]. Autoimmune diseases are thought to affect 8–10% of the population [4]. The age of onset of symptoms may differ from one autoimmune disease to another. There are also many underlying genetic factors for the development of these diseases.
Conventional and common treatment options for autoimmune diseases include analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids [5]. However, in recent years, therapeutic immunosuppression and biological agents have also been shown beneficial in the management of autoimmune disorders [4]. Yet, while attenuating the inflammatory symptoms or progression of the diseases, overall cure remains elusive. Dietary natural products and herbal medicines are also studied widely as possible treatment strategies for autoimmune diseases [6–9].
Dietary natural products play important roles in the maintenance of human health [10]. Polyphenols are secondary metabolites in plants and to date more than 8,000 polyphenols have been identified [11]. They are commonly found in fruits, leafy vegetables, tea, coffee, and legumes, to name a few [11, 12]. Polyphenols can be divided into chemical classes of phenolic acids, flavonoids, tannins, lignans, stilbenes, etc, affording protection in plants against pathogenic organisms [13, 14], predominantly given their antioxidant and anti-inflammatory activities [11, 15].
Many polyphenols, especially flavonoids possess potent anti-inflammatory properties [16–19]. and regulate immunity [6, 7, 17, 20–22]. Several natural products such as epigallocatechin gallate (EGCG) [23], resveratrol [24], curcumin and capsaicin [25] have been well studied for their beneficial effect in autoimmune diseases, and will be the subject of this review, summarizing the clinical data on their efficacy in the treatment of autoimmune diseases.
2. Pathophysiology of autoimmune diseases
The immune system affords a defense mechanism, providing self-tolerance to harmless interactions, while protecting the organism against dangerous predators such as pathogens (bacteria, viruses etc.) and environmental threats. Dysfunction of this system, such as loss of immune tolerance and improper rearrangement of homeostasis may lead to autoimmune diseases (AIDs).
The prevalence of AIDs is around 5% worldwide, and 80 different types of autoimmune diseases have been recognized to date [26]. Genetic, epigenetic and environmental factors (drugs, hormones, nutrition, microbiota, apoptosis, xenobiotics and others) are predisposing factors for autoimmunity [27]. Systemic autoimmune diseases such as systemic lupus erythematosus (SLE), psoriasis and rheumatoid arthritis (RA) are caused as a result of the interaction of many autoantigens, including cell surface molecules and intracellular matrix proteins with reactive autoantibodies. Furthermore, some specific disorders, including type-I diabetes (T1D), multiple sclerosis (MS) and Hashimoto, inflammatory bowel disease (IBD) can also occur due to an immune response to autoantigens localized within a particular organ. Furthermore, the presence of AIDs may affect the development of other chronic diseases. For example, patients with chronic inflammatory diseases (such as SLE, RA) are at greater risk than the general population for developing specific types of lymphoma [28].
The effector mechanisms involved in the production of pro-inflammatory cytokines and the self-reactive T helper cells (Th) are commonly responsible for the initiation of autoimmune pathogenesis. T helper cells present the T cells to autoreactive B cells and as a consequence, the autoreactive B cells elaborate autoantibodies that cause tissue inflammation. The cells grow after interaction with an antigen and proceed to differentiate into effector (Th1, Th2, Th9 and Th17) and regulator (Treg) subsets [29]. To maintain an effective immunological homeostasis, a balance between Th cells activation and Treg cells mediated suppression is required. The disruption of this balance causes development of an immune response by lymphocyte and/or antibody production against its own cells and tissues [30]. Moreover, the aforementioned disbalance in favor of Th17 cells causes a significant increase in the severity of AID. Treg cells modulate the activity of Th cells and secrete immunomodulatory cytokines (interleukin (IL)-35 and 10) and transforming growth factor (TGF)-β), thus inducing tolerance to antigens and leading to the release of cytotoxic molecules such as granzyme and perforin. In addition, IL-6 and IL-21 have an active role in maintaining the balance between Th17 cells and Treg cells [31].
T cell subgroups involved in inflammatory responses are mainly Th1 and Th17. Th1 cells provide protection against intracellular infections by secreting tumor necrosis factor (TNF)-α and interferon (IFN)-γ. Th2 cells secrete cytokines, including IL-4, IL-5, IL-10, IL-13, which in turn target parasitic organisms and cause allergic diseases. Th17 cells, one of the major pathogenic Th cell population, require a number of transcription factors (such as NF-κB, STAT3) and specific cytokines (such as TGF-β, IL-6, IL-23) for activation and proliferation. In addition, they secrete TNF-α and interleukins including IL-17A, IL-17F, IL-21, IL-22. IL-17A and IL-17F, which play a key role in the pathophysiology of AIDs, such as RA, SLE and IBD, by inhibiting extracellular matrix production in chondrocytes and osteoblasts and by activating the production of matrix metalloproteinases [32, 33].
IL-17 and TNF-α-induced increase in intestinal barrier permeability facilitate the pathophysiology of Crohn’s disease (CD), IBD (ulcerative colitis (UC) and MS [34, 35]. Similarly, in intestinal barrier-microbiome and brain connectivity, it was found that increased plasma LPS levels in MS patients were related to IL-6 production, proliferation of Th17 cells in the intestine and differences in expression in genes associated with NF-kB signaling [36, 37].
IBD and SLE are autoimmune diseases characterized by increased levels of the pro-inflammatory cytokine IL-1 and LTB4 [38]. Furthermore, overexpression of IL-6 leads to the development of AIDs by stimulating B-cell differentiation. Although serum IL-6 concentration is higher in CD compared to UC, the production of macrophage-derived cytokines (IL-1, IL-6 and TNF-α) in both diseases is increased. Similarly, IL-6 levels also elevated in patients with T1D, RA and psoriasis [39].
In addition, heterodimer structure IL-23 that regulates the development of IL-17 and IL-22 producing T cells is also associated with the etiology of the autoimmune diseases. For example, psoriasis is known to result from the activation of the IL-23/Th17 cytokine axis [40, 41]. Recent studies have also corroborated a role for IL-27p28 in diseases associated with CNS (central nervous system) autoimmunity, such as MS, where it suppresses the functions of Th1 and Th17 effector cells [42].
AIDs result from a combination of genetic predisposition and environmental factors. Indeed, the genetic background of AIDs is closely related to the major histocompatibility complex (MHC) referred to as human leukocyte antigen (HLA). The relationship between MHC class II molecules (HLA-DR2b and HLA-DR4) and increased susceptibility to diseases, such as MS and RA has been documented [43]. Similarly, common AIDs, such as T1D, SLE, psoriasis, are associated with specific HLA alleles [44]. Moreover, AIRE (autoimmune regulator), TNFRSF6 (TNF receptor super family member 6), Foxp3, CD25, PTPN2, IRF5, STAT4, ICAM3, BANK1 gene mutations were shown to be strongly related with AIDs [45, 46].
Several environmental factors stimulate epigenetic and intrinsic components that can alter gene function, and thus, are associated with immune cell expressions/functions. Epigenetic mechanisms (DNA methylation, histone acetylation, microRNAs), in the influence of environmental factors, affect the prevalence of many AIDs. These mechanisms play an important role in embryogenesis, cellular differentiation, X-chromosome inactivation, and genomic imprinting. In a recent methylation study of >1,200 genes, methylation patterns of osteoarthritis patients were found to be dissimilar to those in patients with RA [47].
Numerous AIDs are more common in women of reproductive age than men due to sex hormones and X chromosome inactivation [48]. SLE represents an AID of epigenetic origin characterized by the deterioration of T-cell DNA methylation. The reason for the higher incidence in women is demethylation of CD40LG on the inactive X chromosome and over-stimulated of the B cells to produce immunoglobulin G (IgG) [49].
The factors that influence the development of autoimmunity are summarized in Figure 1.
Figure 1.
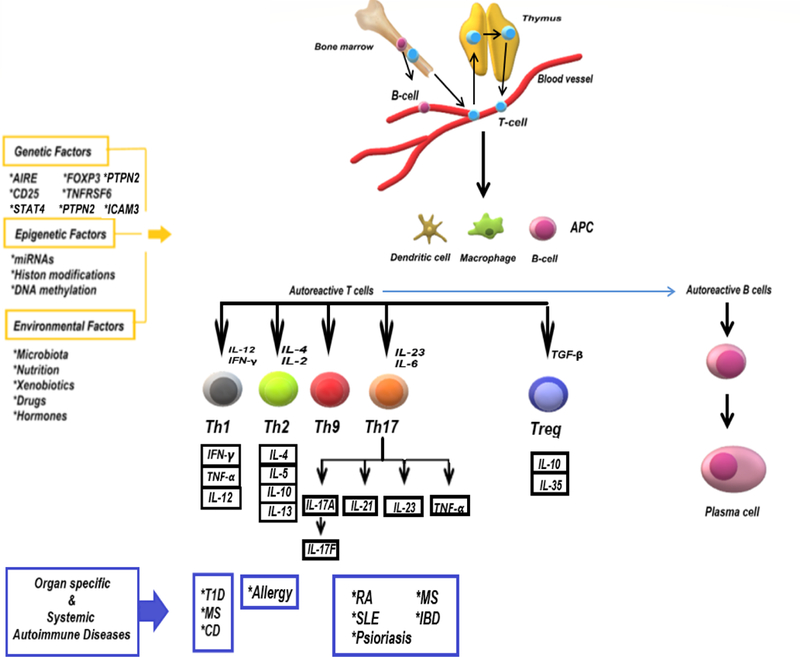
The factors that influence the development of autoimmunity.
3. Therapeutic significance of polyphenols
Polyphenols are the most abundant phytochemicals in human diet with antioxidant potential [50]. Polyphenols have dynamic role as antimicrobials (against viruses, bacteria and fungi) [51], cardioprotective [52], antiasthma [53], antidepressant and anxiolytic [54], antidiabetic [55], neuroprotective [56] anticarcinogenic [57], to name a few. Their antioxidant properties render then invulnerable in the management of age related diseases [58, 59].
Given their immunomodulatory activities, polyphenols have emerged as pharmaceutical tools for the treatment of various autoimmunity disorders [60]. Currently, a combination of drugs is used in the management of autoimmune disorders such as rheumatic arthritis [61]. Such combinations of drugs e.g. DMARDs in rheumatic arthritis provides synergistic effects and reduces side effects associated with individual drugs; yet, the results have been disappointing. Additionally, vaccination has been shown efficacious in the management of several autoimmune disorders [62], therefore, more effects are required in .
4. Clinical status of polyphenols in autoimmune disorders
Autoimmune disorders have been categorized into several types, and approximately 80 such diseases have been identified. Various clinical trials have been conducted on the beneficial effect of polyphenolics on autoimmune diseases and its associated complications (Table 1).
Table 1:
List of clinical trials (completed/ongoing) on the effect of polyphenolic on autoimmune diseases
| S.No. | Plant/polyphenols | Autoimmune disorders | Study design (age, sex, doses) | Status | Reference (clinical trial number) |
|---|---|---|---|---|---|
| 1 | MEMOREX 60 mg BID (Gingko biloba) and narrow band UVB | Vitiligo | Randomized double-bling placebo controlled trial (160 participants, 12–65 years) | RECRUITING | NCT01006421 |
| 2 | 60 mg Ginkgo biloba BID for 12 weeks | Vitiligo vulgaris | Non-randomized single group assignment (12 participants, 12–18 years) | completed | NCT00907062 |
| 3 | 5 aminosalicylicacid +curcumin (50–100 mg BID) | ULCERATIV E COLITIS | Randomized parallel assignment (50 participants, 18–70 years) | RECRUITING | NCT02683759 |
| 4 | 5 aminosalicylicacid +curcumin (500 mg BID) | ULCERATIV E COLITIS | Randomized parallel assignment (50 participants, 18–70 years) | completed | NCT01320436 |
| 5 | Aloe barbadnsis (200 ml per day for three months) | Inflammation in patients with mild ulcerative colitis | Randomized parallel assignment (60 participants, 18–59 years) | recruiting | NCT01783119 |
| 6 | CURCUMIN (1–2 g BID) | Pediatric ulcerative colitis | Randomized double-blind parallel assignment (60 participants, 6–18 years) | Not yet recruiting | NCT02277223 |
| 7 | Curcumin (3g per day) | ULCERATIVE COLITIS IN REMISSION | Randomized double-blind parallel assignment (172 participants, 18 years and older) | RECRUITING | NCT03122613 |
| 8 | Quercetin (1000 mg per day) | Sarcoidosis | Randomized double-blind parallel assignment (18 participants) | Completed (reduced markers of oxidative stress and inflammati on in sarcoidosis patients) | NCT00402623 |
| 9 | Ashwagandha (3 ml for 5 days) | Inflammation, cancer and autoimmune disease | Non-randomized single group assignment (25 participants) | Completed | NCT00817752 |
| 10 | Red Yeast Rice and Tea | Rheumatoid Arthritis | Randomized parallel assignment (120 participants, 18–75 years) | Recruiting | NCT02257047 |
| 11 | Resveratrol (80 mg per day for 2 months) | Spastic Paraplegia | Randomized crossover assignment (12 participants, 18 years and older) | Completed | NCT02314208 |
| 12 | High Flavanol Cocoa drink (450 mg cocoa flavanols BID for 7 days) | Peripheral and cerebral blood flow in diabetes type −2 | Randomized crossover assignment (18 participants, 35–70 years) | Completed | NCT01654172 |
| 13 | Green tea extract (capsule containing 160 mg teavigo (94% epigallocatechin-3-gallate) DAILY FOR 3 MONTHS | Multiple sclerosis | Randomized crossover assignment (20 participants, 20–60 years) | completed | NCT01417312 |
| 14 | Green Tea (1000 mg aqueous green tea extract per day for 3 months) | Lupus | Randomized double-blind parallel assignment (68 participants, 15 years and older) | Completed | NCT02875691 |
| 15 | Antioxidant cocktail (vitamin C, E, alpha lipoic acid) and Resveratrol (270mg) | Type 1 diabetes | Randomized double-blind parallel assignment (198 participants, 18–40 years) | Recruiting | NCT03436992 |
| 16 | Curcumin | Pelvic inflammatory disease, Endometritis, wound infection | Randomized parallel assignment (180 participants, 18–52 years) | NCT03016039 | |
| 17 | Liquorice (150 g glycyrrhizinic acid) and Grapefruit juice (200 ml three times a day) | Addison Disease | Randomized crossover assignment (17 participants, 18–80 years) | Completed | NCT01271296 |
| 18 | Epigallocatechinga llate (600mg per day) | Multiple Sclerosis | Randomized parallel assignment (60 participants, 18–52 years) | Recruiting |
NCT03740295 |
| 19 | Migh Tea flow (green tea, jaborandi extract, xylitol) 4–6 times daily for 8 weeks | Sjogren syndrome | Randomized double-blind parallel assignment (60 participants, 18–75 years) | Completed |
NCT01647737 |
(NCT: numbers refer to the source of www.clinicalTrails.gov).
Vitiligo is one of the common skin disorders characterized by hypopigmentation with significant psychiatric issues and problems if occurring before adulthood. Recently, an open label pilot clinical trial with 12 adolescents (age 12 to 18 years) was conducted using Ginkgo biloba powder [63]. Ginkgo biloba is known to be a rich source of polyphenolics [64]. G. biloba was administered in dose of 60 mg BID, i.e. two times per day for 12 weeks. The Vitiligo European Task Force (VETF) and Vitiligo Area Scoring Index (VASI) were used to access the effectiveness of the G. biloba extract whereas serum coagulation factors (platelets, PTT, INR) at baseline and week 12 were used to access other parameters including. Treatment results in completely depigmentation, 0.4% decrease in VETF total vitiligo lesion area and improvement of total VASI score, VETF staging score, VETF spreading score by 0.5, 0.7 and 3.9 respectively. No significant changes in coagulation factors were reported.
The efficacy of G. biloba treatment was tested in slowly spreading vitiligo in double-blind placebo-controlled trial. A total number of 47 patients (including placebo) were given 40mg of G. biloba extract three times a day [65]. As compared to placebo, G. biloba extract was associated with disease arrest, noting it arrested the progression of vitiligo disease by reducing depigmentation and promoting repigmentation.
Curcumin a natural phenolic compound has multiple uses. Curcumin was examined for its effectiveness in maintaining remission of ulcerative colitis [66]. In a randomized double-blind placebo-controlled study, 50 patients with active mild-to-moderate ulcerative colitis already treated with mesalamine, were given curcumin capsule 3g/day for 1 month. It was concluded that 53.8% i.e. 14 patients achieved clinical remission of disease at week 4, as compared to none in placebo treatment. Clinical response was achieved by 65.3% (17 patients) in curcumin treated group as compared to 12.5% (3 patients) in the placebo group.
Quercetin, a flavonoid class of polyphenolic compound was tested for its beneficial effect to reduce oxidative stress and inflammation in sarcoidosis. A double-blind randomized placebo controlled clinical trial on 18 non-treated sarcoidosis patients was conducted. Quercetin at a dose of 4×500mg was administered within 24h. Quercetin treatment was increased total plasma antioxidant capacity. The oxidative and inflammatory markers (malondialdehyde, TNFα/IL-10 and IL-8/IL-10) were also downregulated in sarcoidosis patients after curcumin treatment.
Multiple sclerosis (MS) is one of the autoimmune diseases of neurologic origin characterized by fatigue and muscle weakness. In one of the study, metabolic response to EGCG and substrate utilization in patients with MS were examined [67]. A randomized, double-blind, placebo-controlled trial was conducted in 8 patients with relapsing-remitting multiple sclerosis. A dose of EGCG (600 mg/d) was given over 12 weeks. At rest, postprandial energy expenditure and carbohydrate oxidation, as well as glucose supply and adipose tissue perfusion were significantly lower in men, but higher in women receiving EGCG as compared with placebo. During exercise, postprandial energy expenditure was reported to be lower after intake of EGCG compared to placebo. After placebo, exercise EE was mainly fueled by fat oxidation in both men and women. After EGCG, there was a shift to a higher and more stable carbohydrate oxidation during exercise in men, but not in women.
5. Polyphenols targeting various signaling pathways
Polyphenols stand out for their antioxidant capacity, which allows them to reduce the negative effects of the reactive species produced by the over activation of the immune system when there is an autoimmune disease. In addition, polyphenols are pharmacologically active compounds with immunomodulatory activity [60]. However, one of the problems faced in evaluating their pharmacological activity is the great structural diversity of this group of compounds, and the high variability in their bioavailability and secondary metabolism to which they are subjected. Moreover, each type of polyphenol targets different immune cells and, therefore, triggers a plethora of diverse intracellular signaling pathways that ultimately regulate the host’s immune response. The modulation of several signaling pathways leads to alterations in the expression of proinflammatory genes, for instance those that code for multitude of cytokines, cyclooxygenase (COX), lipoxygenase (COX), phospholipase A2 (PLA2), inducible isoform of nitric oxide synthetase (iNOS)-, which combined with their ability to modulate the population and differentiation of specific immune cells and their direct antioxidant and anti-inflammatory activity allow the regulation of the inflammatory process (Table 2) [68, 69]. To date, the majority of the mechanistic studies were performed in cell cultures given their greater simplicity and reproducibility; however, they fail to recapitulate many of the problems that are inherent to oral administration, bioavailability and the intense secondary metabolism to which polyphenols are subject. Proposed points of action of polyphenols in autoimmune disorders are summarized in Figure 2. The structures of most widely studied polyphenols are given in Figure 3.
Table 2:
Polyphenols targeting signaling pathways
| Signaling Pathways | Polyphenols | Actions | References |
|---|---|---|---|
| Arachidonic acid dependent pathway | Quercetin, kaempferol, galangin, anthocyanidins1, Catechol (1,2-dihydroxybenzen), resveratrol, p-coumaric, honokiol, | Inhibiting PLA2 | [71, 72, 74–76] |
| Luteolin, galangin, morin, apigenin | Inhibiting COX enzyme | [78, 79] | |
| Quercetin, kaempferol, myricetin, morin | Inhibiting LOX enzyme | [80] | |
| NF-κB signaling pathway | Resveratrol, quercetin, genistein, EGCG | Modulating NF-κB signaling pathway which alters the genetic regulation of COX enzyme, pro-inflammatory cytokines and chemokines | [86, 92, 94, 138] |
| MAPKs pathway | Quercetin, EGCG, resveratrol, apigenin, luteolin | Suppressed the phosphorylation of NF-κB, ERK1/2, JNK and p38 pathway proteins which alters the production and expression of proinflammatory cytokines | [98, 99, 101, 106, 108] |
| Hesperidin, naringin, | Inhibit the p38 MAPK signaling pathway2 | [107] | |
| Kaempferol, chrysin | Attenuation of the JNK activity | [108] | |
| Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway | Resveratrol, quercetin, EGCG | Downregulating the expression of proinflammatory cytokines by alteration of PI3K/Akt signaling pathway | [112–114, 116] |
| Silibinin | Reduced the expression of pro-inflammatory cytokines and COX-2 by PI3K/Akt inhibition | [115] | |
| Isorhapontigenin | Cytokine inhibitory effects through modulation of corticosteroid insensitive PI3K/Akt pathway | [117] | |
| Ellagic acid | Protect HUVECs from apoptosis mediated by PI3K/Akt activation | [118] | |
| Resveratrol | Neuroprotection in rats undergoing ischemia induced cerebral damage by activating the PI3K/Akt survival pathway | [119] | |
| Epigenetic modulation (DNA methylation, histone modifications and posttranscriptional regulation by miRNAs) | Resveratrol | Activation of SIRT1 resulting in the inhibition of NFκB and its downstream genes such as COX-2 and iNOS | [123, 124] |
| Fisetin | Increase SIRT1 and SIRT3 expression | [58, 126] | |
| Curcumin | Inhibiting DNMTs Regulation of HATs and HDACs Regulate miRNA expression (miR-181b) Reactivate the neprilysin gene leading to inhibition of NFκB | [127, 132, 134] | |
| Quercetin, myricetin | Downregulate c-Myc, PHD2 and β-catenin expressions via SIRT1 activation in a manner that mimics hypoxic preconditioning | [128] | |
| EGCG | Hypoacetylation of p65 by inhibiting the activity of HAT which results in reduction the activity of NFκB | [129] | |
| Resveratrol | Restore LINE1 methylation in human retinal pigment epithelial cells through modulation of SIRT1 and DNMT Regulate miRNA expression (miR-21, miR-Let7A) | [131, 135, 136] |
cyanidin, delphinidin malvidin, peonidin and petunidin
No effect on ERK and JNK
Phospholipases A2 (PLA2); Cyclooxygenase (COX); Lipoxygenase (LOX); nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB signalling pathway); epigallocatechin-3-gallate (EGCG); Mitogen-activated protein kinases (MAPKs pathway); extracellular signal–regulated kinases (ERK1/2); c-Jun N-terminal kinase pathway (JNK); human umbilical vein endothelial cells (HUVECs); members of sirtuin family (SIRT1 and SIRT3); DNA methyltransferase (DNMTs); Histone acetyltransferases (HATs); Histone deacetylases (HDACs); micro RNAs (miRNAs); oncogenes (c-Myc); prolyl hydroxylase domain-containing protein 2 (PHD2); long interspersed nuclear element-1 (LINE1).
Figure 2.
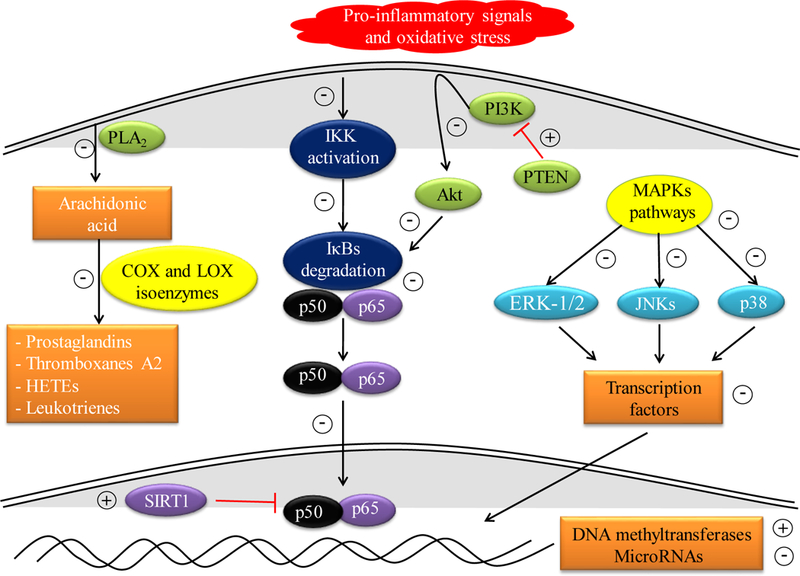
Main proposed points of action of polyphenols in autoimmune diseases (+ means activation and - inhibition. Akt, protein kinase B; COX, cyclooxygenase; ERK-1/2, extracellular-signal-regulated kinases 1/2; IκBs, inhibitory proteins of κB; IKK, IκBs kinase; JNKs, c-Jun amino-terminal kinases; LOX, lipoxygenase; MAPKs, mitogen-activated protein kinases; p38, p38-mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PLA2, phospholipase A2; PTEN, phosphatase and tensin homolog; SIRT1, sirtuin 1.
Figure 3.
Chemical structures of polyphenols used in clinical studies
5.1. Arachidonic acid dependent pathway
Arachidonic acid or eicosatetraenoic acid (AA) is a polyunsaturated fatty acid of the omega-6 series present in membrane phospholipids, from where it is released by the action of PLA2. Once in the cytoplasm, AA is targeted by various enzymes such as COX and LOX to generate prostaglandins (PGs) and thromboxanes A2 or hydroxyeicosatetraenoic acids (HETEs) and leukotrienes (LTs), respectively[69]. These molecules are lipid mediators that contribute to the inflammatory process, and by inference, their inhibition represents a therapeutic target for the reduction of inflammation. Accordingly, one of the mechanisms by which polyphenols can modulate the immune response is via direct inhibition of these pro-inflammatory enzymes [70]. Considering PLA 2 as the first enzyme in the AA cascade, it has been evidenced the inhibitory capabilities by polyphenols such as quercetin, kaempferol, and galangin, as well as some anthocyanidins (cyanidin, delphinidin malvidin, peonidin and petunidin) [71–73] Catechol (1,2-dihydroxybenzen) binds to PLA2 preventing the substrate from entering into the active site [74]. Furthermore, resveratrol and p-coumaric acid interact directly with catalytic residues of PLA2, blocking its catalytic activity [75]. An interesting study showed that quercetin and honokiol inhibited cytosolic PLA2 phosphorylation and activation in differentiated SH-SY5Y neuroblastoma cells [76]. As for COX and LOX enzymes, flavones are predominantly COX inhibitors, whereas flavanols are preferentially LOX inhibitors [77]. Thus, flavonoids, such as luteolin, galangin, morin and apigenin exert notable COX inhibitory effects [78, 79]. Flavanols, including quercetin, kaempferol, myricetin and morin, in turn, inhibit LOX enzymes [80].
5.2. NF-κB signaling pathway
NF-κB regulates the expression of a wide range of genes implicated in the inflammatory process including the inducible form of COX (COX-2), pro-inflammatory cytokines [TNFα, IL-1, IL-2, IL-6 and IL-8], chemokines [MCP-1 i.e. monocyte chemoattractant protein 1, MIP-1α, i.e. macrophage inflammatory protein, CXCL1 i.e. (C-X-C motif) ligand 1 and IL-18], adhesion molecules [VCAM-1, i.e vascular cell adhesion protein 1, ICAM-1 i.e. intercellular adhesion molecule 1] and diverse growth factors and immuno-receptors [81, 82]. Polyphenols can alter the NFκB pathway along multiple steps in the signalling cascade. Given their antioxidant action, polyphenols can reduce the levels of reactive species, mainly H2O2, thus reducing the activation of the pathway [83]. Thus, it can be assumed that these species can act as a secondary messengers facilitating the activation of the NFκB pathway by a redox-sensitive process [84]. In addition, polyphenols can block the phosphorylation and subsequent degradation of inhibitory proteins (IκBs) by altering the activation of the IκBs kinase (IKK) and preventing nuclear translocation and binding of the factor to DNA [85]. Different polyphenols including resveratrol, quercetin, genistein or epigallocatechin-3-gallate (EGCG) can inhibit the NFκB pathway. Resveratrol blocks the activation of NF-κB in macrophage RAW 264.7 cells when stimulated with LPS through avoiding IKK activation and IκB phosphorylation [86]. Furthermore, resveratrol can interfere with TLR4 oligomerization, a necessary step in the activation of the receptor [86]. In addition, resveratrol as well as oleuropein aglycone and hydroxytyrosol significantly reduce the activation of NFκB in LPS-stimulated human umbilical vein endothelial cells (HUVECs) as determined by electrophoretic mobility shift assay [87]. Studying various immune cell models (RAW264.7 macrophages and bone marrow-derived macrophages, HMC-1 human mast cells, mouse BV-2 microglia and HUVECs) the inhibitory effects of quercetin on NFκB activation has been reported, including a reduction in nuclear translocation of p50 and p65 subunits, an inhibition of the phosphorylation of IκBα and their consequent degradation, and a blockage of the IKK activation. Genistein has been shown to reduce the overproduction of TNFα and IL-6 in RAW 264.7 macrophages stimulated by LPS via inhibition of NFκB activation [92]. The mechanism of action was associated with blockage of the IKK expression, IκBα degradation and p65 translocation into the nucleus. Analogous results were noted in human synoviocyte MH7A cells stimulated with TNFα by preventing IKK phosphorylation and NFκB translocation [93]. EGCG also prevented the IKK activation and inhibited phosphorylation of the p65 subunit of NFκB in a human respiratory epithelium A549 cells stimulated by IL-1β [94]. This effect seems to be initially mediated by the inhibition of the IRAK (IL-1β-mediated IL-1β receptor-associated kinase) degradation, which prevents IKK activation. Inhibition in NFκB activation has also been documented in primary osteoarthritis chondrocytes stimulated with IL-1β [95].
5.3. Mitogen-activated protein kinases (MAPKs) pathway
MAPKs comprise a group of serine/threonine protein kinases mainly activated by stress, mitogens and growth factors with a central role in many cellular processes including the regulation of genes related to inflammation [96]. The potential effect of polyphenols on MAPKs depends on the polyphenol itself as well as the cell target. Several studies have reported an inhibitory effect of polyphenols on JNKs (c-Jun amino-terminal kinases), ERK-1/2 (extracellular-signal-regulated kinases), p38/SAPKs (stress-activated protein kinases). These inhibitory effects result in decreased expression and release of proinflammatory mediators, such as TNFα or adhesion molecules. For example, quercetin has been shown to interfere with the phosphorylation and activation of JNK on LPS-treated RAW 264.7 macrophages, thus preventing the activator protein 1 (AP-1) from binding to ADN, and inhibiting TNFα transcription [98]. Moreover, inhibition of ERK1/2 and p38 activation has been also reported in response to quercetin [98, 99]. Corroborating this study, a reduction in phosphorylated forms of ERK1/2 and p38 has been reported in an experimental rat model of autoimmune myocarditis study upon treatment with quercetin [100].
Additional studies reported on the inhibitory effects of EGCG against MAPKs by reducing their phosphorylation and activation, including LPS-activated macrophages or LPS-induced murine dendritic cells maturation [101–103]. EGCG have also been shown to attenuate symptoms associated with a murine model for human autoimmune Sjogren’s syndrome by reducing the phosphorylation of p38 in salivary acinar cells [104]. Resveratrol treatment inhibited p38 and JNK signalling pathways in IL-1β-stimulated rat RSC-364 synovial cells and in HUVECs treated with hydrogen peroxide [105, 106]. Similarly, other polyphenols, such as luteolin, chrysin, hesperidin, naringin and kaempferol have been shown to reduce inflammation by interfering with MAPKs pathways [107–109]. However, opposite effects, reporting on the activation of ERK and JNK signaling pathways in cultured human coronary artery endothelial cells by quercetin and catechin resulting in a downregulation of plasminogen activator inhibitor 1 (PAI-1) [110], are noteworthy and emphasize the need for future characterization on the efficacy of polyphenols in affecting MAPK signaling.
5.4. Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway
PI3K/Akt signaling pathway play an important role in the expression of pro-inflammatory mediators by inducing the degradation of IκB and the following activation of NFκB [111]. Diverse polyphenols mediate their anti-inflammatory effects, in part, through PI3K/Akt inhibition. The expression of the pro-inflammatory cytokine IL-17 was reduced by resveratrol treatment in cardiac fibroblasts in a process mediated by PI3K/Akt inhibition [112]. Resveratrol also supressed PI3K/Akt activity in IL-1β-activated human tenocytes [113]. Quercetin suppressed the phosphorylation of Akt by direct binding and inhibition of PI3K in JB6 mouse epidermal cells [114]. Silibinin, applied topically to inflamed murine ears suppressed the expression of pro-inflammatory cytokines and COX-2 by PI3K/Akt inhibition [115]. EGCG inhibited epithelial-mesenchymal transition and inflammation via the PI3K/AKT pathway by upregulating the expression of phosphatase and tensin homolog (PTEN). Since this enzyme blocks the activation of PI3K through dephosphorylation of the signaling lipid phosphatidylinositol-3,4,5-triphosphate [116]. Another study reported on the protective efficacy of isorhapontigenin against airway epithelial cell inflammation by suppressing the PI3K/Akt pathway and reducing the activation of NFκB and the release of IL-6 and chemokine CXCL8 i.e. (C-X-C motif) ligand 8 [117].
It is noteworthy that the favourable effects of polyphenols may also be associated with increased signaling in the PI3K/Akt pathway. In these studies, the activation of the pathway as a signaling mechanism for survival prevails. For instance, ellagic acid was shown to protect HUVECs from apoptosis induced by oxidized low-density lipoprotein treatment in a process mediated by PI3K/Akt activation [118]. Also, resveratrol induced neuroprotection in rats undergoing ischemia/reperfusion induced cerebral damage by activating the PI3K/Akt survival pathway [119].
5.5. Epigenetic modulation
A novel point for cellular control for polyphenols is secondary to their ability to modulate modular epigenetic mechanisms such as DNA methylation, histone modifications and posttranscriptional regulation by microRNAs, modulating the activation and differentiation of immune cells. Changes in epigenetic patterns have been evidenced in diseases including inflammatory disorders [120]. Indeed, a series of studies have reported the existence of polyphenol-induced epigenetic modifications, leading to gene activation or silencing in the absence of changes in DNA sequences [121, 122]. Among the various polyphenols, resveratrol has been shown to be a strong activator of SIRT1, resulting in the inhibition of NFκB and its downstream genes, such as COX-2 and iNOS [123, 124]. In addition, several other polyphenols, such as quercetin, curcumin, myricetin and fisetin also activate SIRT1 [125–128]. EGCG has been shown to reduce the activity of NFκB via hypoacetylation of p65 by inhibiting the activity of histone acetyl transferase [129, 130]. In addition to SIRT1, resveratrol also acts on DNA methyltransferases, increasing their expression and activities [131]. Curcumin has also been reported to reactivate the neprilysin gene (a strong inhibitor of Akt) through CpG demethylation, leading to Akt inhibition and the subsequent inhibition of NFκB in mouse neuroblastoma N2a cells [132].
MicroRNAs are small and non-coding regulatory RNAs with the capability to regulate the translocation and/or degradation of messenger RNAs [133]. Diverse studies reported the modulatory effects of polyphenols on microRNAs. However, although some studies have shown anti-inflammatory effects of the modulation of microRNAs, most research has focussed in this context on cancer outcomes. Curcumin has been found to upregulate the expression of miR-181b, which, in turn, reduces the expression of the pro-inflammatory chemokines CXCL1 and CXCL2 [134]. Another interesting microRNA is the miR-21, as it has been associated with the activity of NFκB [133]. Resveratrol has been shown to decrease miR-21 expression and NFκB activity in U251 brain tumour cells [135]. Resveratrol has also been reported to increase miR-Let7A in LPS-stimulated THP-1 cells, resulting in the reduced expression of IL-6, IL-10 and TNFα [136]. In another study performed in A/J mice lung adenoma model, EGCG has been shown to increase 12 microRNAs and to decrease 9 target genes associated with key elements of the Akt and MAPK signaling pathways [137].
6. Conclusion
Polyphenols, one of the main secondary metabolites in plants, are found to be beneficial in prevention and treatment of various diseases. In autoimmune diseases, many plant extracts or isolated individual phenolic compounds have been evaluated for their efficacy in clinical studies. Among them, extracts of G. biloba and green tea were studied for their effectiveness in vitiligo, ulcerative colitis, Multiple Sclerosis, etc. Similarly, curcumin, resveratrol, epigallocatechin gallate were also studied for their efficacy in various autoimmune diseases. Taken together, polyphenols have been shown as promising candidates for the development of novel therapeutics in autoimmune diseases. While few mechanism-based studies have been performed on their mechanism of action in autoimmune diseases, they have yet to be fully delineated.
Acknowledgements
MA was supported in part by grants from the National Institute of Environmental Health Sciences, NIEHS, R01ES07331, R01ES10563 and R01ES020852.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rengasamy KRR, Khan H, Gowrishankar S, Lagoa RJL, Mahomoodally FM, Khan Z, et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol Ther 2019;194:107–31. [DOI] [PubMed] [Google Scholar]
- [2].Banchereau R, Cepika A-M, Pascual V. Systems approaches to human autoimmune diseases. Curr Opin Immunol 2013;25:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Internal Med 2015;278:369–95. [DOI] [PubMed] [Google Scholar]
- [4].Alexander T, Bondanza A, Muraro P, Greco R, Saccardi R, Daikeler T, et al. SCT for severe autoimmune diseases: consensus guidelines of the European Society for Blood and Marrow Transplantation for immune monitoring and biobanking. Bone Marrow Transplantation 2015;50:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Konforte D, Diamandis EP, van Venrooij WJ, Lories R, Ward MM. Autoimmune diseases: early diagnosis and new treatment strategies. Clin Chem 2012;58:1510–4. [DOI] [PubMed] [Google Scholar]
- [6].Javadi B, Sahebkar A. Natural products with anti-inflammatory and immunomodulatory activities against autoimmune myocarditis. Pharmacological research 2017;124:34–42. [DOI] [PubMed] [Google Scholar]
- [7].Venkatesha SH, Rajaiah R, Berman BM, Moudgil KD. Immunomodulation of autoimmune arthritis by herbal CAM. Evidence-Based Complementary and Alternative Medicine 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Busto R, Serna J, Perianes-Cachero A, Quintana-Portillo R, García-Seisdedos D, Canfrán-Duque A, et al. Ellagic acid protects from myelin-associated sphingolipid loss in experimental autoimmune encephalomyelitis. Biochimica et Biophysica Acta 2018;1863:958–67. [DOI] [PubMed] [Google Scholar]
- [9].Reglodi D, Renaud J, Tamas A, Tizabi Y, Socías SB, Del-Bel E, et al. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Progress in Neurobiology 2017;155:120–48. [DOI] [PubMed] [Google Scholar]
- [10].Wai Kan Yeung A, Aggarwal BB, Barreca D, Battino M, Belwal T, Horbańczuk OK, et al. Dietary natural products and their potential to influence health and disease including animal model studies. Animal Science Papers & Reports 2018;36. [Google Scholar]
- [11].Ganesan K, Xu B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017;9:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Belwal T, Devkota HP, Hassan HA, Ahluwalia S, Ramadan MF, Mocan A, et al. Phytopharmacology of Acerola (Malpighia spp.) and its potential as functional food. Trends in food science & technology 2018. [Google Scholar]
- [13].Petti S, Scully C. Polyphenols, oral health and disease: A review. Journal of dentistry 2009;37:413–23. [DOI] [PubMed] [Google Scholar]
- [14].Mateen S, Moin S, Zafar A, Khan AQ. Redox signaling in rheumatoid arthritis and the preventive role of polyphenols. Clinica Chimica Acta 2016;463:4–10. [DOI] [PubMed] [Google Scholar]
- [15].Pietta P-G. Flavonoids as antioxidants. Journal of natural products 2000;63:1035–42. [DOI] [PubMed] [Google Scholar]
- [16].Catarino M, Talhi O, Rabahi A, Silva A, Cardoso S. The Antiinflammatory Potential of Flavonoids: Mechanistic Aspects. Studies in Natural Products Chemistry: Elsevier; 2016: 65–99. [Google Scholar]
- [17].Jantan I, Ahmad W, Bukhari SNA. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Frontiers in plant science 2015;6:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Middleton E Effect of plant flavonoids on immune and inflammatory cell function. Flavonoids in the living system: Springer; 1998: 175–82. [DOI] [PubMed] [Google Scholar]
- [19].Wei B-L, Weng J-R, Chiu P-H, Hung C-F, Wang J-P, Lin C-N. Antiinflammatory flavonoids from Artocarpus heterophyllus and Artocarpus communis. Journal of agricultural and food chemistry 2005;53:3867–71. [DOI] [PubMed] [Google Scholar]
- [20].González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. British Journal of Nutrition 2010;104:S15–S27. [DOI] [PubMed] [Google Scholar]
- [21].Rengasamy KR, Khan H, Gowrishankar S, Lagoa RJ, Mahoomodally FM, Khan Z, et al. The role of flavonoids in autoimmune diseases: therapeutic updates. Pharmacology & therapeutics 2018. [DOI] [PubMed] [Google Scholar]
- [22].Romier B, Schneider Y-J, Larondelle Y, During A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutrition reviews 2009;67:363–78. [DOI] [PubMed] [Google Scholar]
- [23].Wu D, Wang J, Pae M, Meydani SN. Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Molecular aspects of medicine 2012;33:107–18. [DOI] [PubMed] [Google Scholar]
- [24].Oliveira A, Monteiro V, Navegantes-Lima K, Reis J, Gomes R, Rodrigues D, et al. Resveratrol role in autoimmune disease—a mini-review. Nutrients 2017;9:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dahan S, Segal Y, Shoenfeld Y. Dietary factors in rheumatic autoimmune diseases: a recipe for therapy? Nature Reviews Rheumatology 2017;13:348. [DOI] [PubMed] [Google Scholar]
- [26].Sudres M, Verdier J, Truffault F, Panse R, Berrih‐Aknin S. Pathophysiological mechanisms of autoimmunity. Annals of the New York Academy of Sciences 2018;1413:59–68. [DOI] [PubMed] [Google Scholar]
- [27].Bolon B Cellular and molecular mechanisms of autoimmune disease. Toxicologic pathology 2012;40:216–29. [DOI] [PubMed] [Google Scholar]
- [28].Hansen A, Lipsky PE, Dörner T. B-cell lymphoproliferation in chronic inflammatory rheumatic diseases. Nature Reviews Rheumatology 2007;3:561. [DOI] [PubMed] [Google Scholar]
- [29].Jäger A, Kuchroo VK. Effector and regulatory T‐cell subsets in autoimmunity and tissue inflammation. Scandinavian journal of immunology 2010;72:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aslani S, Mahmoudi M, Karami J, Jamshidi AR, Malekshahi Z, Nicknam MH. Epigenetic alterations underlying autoimmune diseases. Autoimmunity 2016;49:69–83. [DOI] [PubMed] [Google Scholar]
- [31].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector T H 17 and regulatory T cells. Nature 2006;441:235. [DOI] [PubMed] [Google Scholar]
- [32].Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Annals of the rheumatic diseases 2011;70:727–32. [DOI] [PubMed] [Google Scholar]
- [33].Adami S, Cavani A, Rossi F, Girolomoni G. The role of interleukin-17A in psoriatic disease. BioDrugs 2014;28:487–97. [DOI] [PubMed] [Google Scholar]
- [34].De Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nature reviews Gastroenterology & hepatology 2016;13:13. [DOI] [PubMed] [Google Scholar]
- [35].Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain 2018;141:1900–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Teixeira B, Bittencourt VCB, Ferreira TB, Kasahara TM, Barros PO, Alvarenga R, et al. Low sensitivity to glucocorticoid inhibition of in vitro Th17-related cytokine production in multiple sclerosis patients is related to elevated plasma lipopolysaccharide levels. Clinical Immunology 2013;148:209–18. [DOI] [PubMed] [Google Scholar]
- [37].Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Science Advances 2017;3:e1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition 2002;21:495–505. [DOI] [PubMed] [Google Scholar]
- [39].Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine & growth factor reviews 2002;13:357–68. [DOI] [PubMed] [Google Scholar]
- [40].Pan H-F, Li X-P, Zheng SG, Ye D-Q. Emerging role of interleukin-22 in autoimmune diseases. Cytokine & growth factor reviews 2013;24:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL-23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends in immunology 2013;34:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chong WP, Horai R, Mattapallil MJ, Silver PB, Chen J, Zhou R, et al. IL-27p28 inhibits central nervous system autoimmunity by concurrently antagonizing Th1 and Th17 responses. Journal of autoimmunity 2014;50:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nalawade SA, Ji N, Raphael I, Pratt A III, Kraig E, Forsthuber TG. Aire is not essential for regulating neuroinflammatory disease in mice transgenic for human autoimmune-diseases associated MHC class II genes HLA-DR2b and HLA-DR4. Cellular immunology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sollid LM, Pos W, Wucherpfennig KW. Molecular mechanisms for contribution of MHC molecules to autoimmune diseases. Current opinion in immunology 2014;31:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Goris A, Liston A. The immunogenetic architecture of autoimmune disease. Cold Spring Harbor perspectives in biology 2012;4:a007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhernakova A, Withoff S, Wijmenga C. Clinical implications of shared genetics and pathogenesis in autoimmune diseases. Nature Reviews Endocrinology 2013;9:646. [DOI] [PubMed] [Google Scholar]
- [47].de la Rica L, Urquiza JM, Gómez-Cabrero D, Islam AB, López-Bigas N, Tegnér J, et al. Identification of novel markers in rheumatoid arthritis through integrated analysis of DNA methylation and microRNA expression. Journal of autoimmunity 2013;41:6–16. [DOI] [PubMed] [Google Scholar]
- [48].Morey C, Avner P. Genetics and epigenetics of the X chromosome. Annals of the New York Academy of Sciences 2010;1214:E18–E33. [DOI] [PubMed] [Google Scholar]
- [49].Zhou Y, Yuan J, Pan Y, Fei Y, Qiu X, Hu N, et al. T cell CD40LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clinical Immunology 2009;132:362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Grootaert C, Kamiloglu S, Capanoglu E, Van Camp J. Cell systems to investigate the impact of polyphenols on cardiovascular health. Nutrients 2015;7:9229–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti‐infective properties of epigallocatechin‐3‐gallate (EGCG), a component of green tea. British journal of pharmacology 2013;168:1059–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mokni M, Limam F, Elkahoui S, Amri M, Aouani E. Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Archives of Biochemistry and Biophysics 2007;457:1–6. [DOI] [PubMed] [Google Scholar]
- [53].Joskova M, Sadlonova V, Nosalova G, Novakova E, Franova S. Polyphenols and their components in experimental allergic asthma. Respiratory Regulation-The Molecular Approach: Springer; 2013: 91–8. [DOI] [PubMed] [Google Scholar]
- [54].Bouayed J Polyphenols: a potential new strategy for the prevention and treatment of anxiety and depression. Current Nutrition & Food Science 2010;6:13–8. [Google Scholar]
- [55].Umeno A, Horie M, Murotomi K, Nakajima Y, Yoshida Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016;21:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ullah H, Khan H. Anti-Parkinson potential of silymarin: Mechanistic insight and therapeutic standing. Frontiers in pharmacology 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Du G-J, Zhang Z, Wen X-D, Yu C, Calway T, Yuan C-S, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012;4:1679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhao C, Sakaguchi T, Fujita K, Ito H, Nishida N, Nagatomo A, et al. Pomegranate-derived polyphenols reduce reactive oxygen species production via SIRT3-mediated SOD2 activation. Oxidative Medicine and Cellular Longevity 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Figueira I, Menezes R, Macedo D, Costa I, Nunes dos Santos C. Polyphenols beyond barriers: a glimpse into the brain. Current neuropharmacology 2017;15:562–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ding S, Jiang H, Fang J. Regulation of Immune Function by Polyphenols. Journal of immunology research 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Betancourt BY, Biehl A, Katz JD, Subedi A. Pharmacotherapy Pearls in Rheumatology for the Care of Older Adult Patients: Focus on Oral Disease-Modifying Antirheumatic Drugs and the Newest Small Molecule Inhibitors Rheumatic Disease Clinics of North America; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Friedman MA, Winthrop KL. Vaccines and disease-modifying antirheumatic drugs: practical implications for the rheumatologist. Rheumatic Disease Clinics 2017;43:1–13. [DOI] [PubMed] [Google Scholar]
- [63].Szczurko O, Shear N, Taddio A, Boon H. Ginkgo biloba for the treatment of vitilgo vulgaris: an open label pilot clinical trial. BMC complementary and alternative medicine 2011;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Qa’dan F, Nahrstedt A, Schmidt M, Mansoor K. Polyphenols from Ginkgo biloba. Scientia pharmaceutica 2010;78:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clinical and Experimental Dermatology: Experimental dermatology 2003;28:285–7. [DOI] [PubMed] [Google Scholar]
- [66].Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clinical Gastroenterology and Hepatology 2015;13:1444–9. e1. [DOI] [PubMed] [Google Scholar]
- [67].Mähler A, Steiniger J, Bock M, Klug L, Parreidt N, Lorenz M, et al. Metabolic response to epigallocatechin-3-gallate in relapsing-remitting multiple sclerosis: a randomized clinical trial–. The American journal of clinical nutrition 2015;101:487–95. [DOI] [PubMed] [Google Scholar]
- [68].Malireddy S, Kotha SR, Secor JD, Gurney TO, Abbott JL, Maulik G, et al. Phytochemical antioxidants modulate mammalian cellular epigenome: implications in health and disease. Antioxidants & Redox Signaling 2012;17:327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Santangelo C, Varì R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Annali-istituto superiore di sanita 2007;43:394. [PubMed] [Google Scholar]
- [70].Yoon J-H, Baek SJ. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei medical journal 2005;46:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dreiseitel A, Korte G, Schreier P, Oehme A, Locher S, Hajak G, et al. sPhospholipase A 2 is inhibited by anthocyanidins. Journal of neural transmission 2009;116:1071–7. [DOI] [PubMed] [Google Scholar]
- [72].Lättig J, Böhl M, Fischer P, Tischer S, Tietböhl C, Menschikowski M, et al. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. Journal of computer-aided molecular design 2007;21:473–83. [DOI] [PubMed] [Google Scholar]
- [73].Lee T-P, Matteliano ML, Middleton E Jr. Effect of quercetin on human polymorphonuclear leukocyte lysosomal enzyme release and phospholipid metabolism. Life sciences 1982;31:2765–74. [DOI] [PubMed] [Google Scholar]
- [74].Dileep KV, Tintu I, Mandal PK, Karthe P, Haridas M, Sadasivan C. Binding to PLA2 May Contribute to the Anti‐Inflammatory Activity of Catechol. Chemical biology & drug design 2012;79:143–7. [DOI] [PubMed] [Google Scholar]
- [75].Shukla PK, Gautam L, Sinha M, Kaur P, Sharma S, Singh TP. Structures and binding studies of the complexes of phospholipase A2 with five inhibitors. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics 2015;1854:269–77. [DOI] [PubMed] [Google Scholar]
- [76].Chuang DY, Simonyi A, Cui J, Lubahn DB, Gu Z, Sun GY. Botanical polyphenols mitigate microglial activation and microglia-induced neurotoxicity: role of cytosolic phospholipase A2. Neuromolecular medicine 2016;18:415–25. [DOI] [PubMed] [Google Scholar]
- [77].Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. Journal of pharmacological sciences 2004;96:229–45. [DOI] [PubMed] [Google Scholar]
- [78].Makanjuola SB, Ogundaini AO, Ajonuma LC, Dosunmu A. Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo‐oxygenase‐2 and prostaglandin‐E2 blockade. International journal of rheumatic diseases 2018;21:1487–95. [DOI] [PubMed] [Google Scholar]
- [79].Baumann J, Bruchhausen Fv, Wurm G Flavonoids and related compounds as inhibitors of arachidonic acid peroxidation. Prostaglandins 1980;20:627–39. [DOI] [PubMed] [Google Scholar]
- [80].Laughton MJ, Evans PJ, Moroney MA, Hoult J, Halliwell B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives: relationship to antioxidant activity and to iron ion-reducing ability. Biochemical pharmacology 1991;42:1673–81. [DOI] [PubMed] [Google Scholar]
- [81].Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal transduction and targeted therapy 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nam N-H. Naturally occurring NF-κB inhibitors. Mini reviews in medicinal chemistry 2006;6:945–51. [DOI] [PubMed] [Google Scholar]
- [83].Simon E, Aswini P, Sameer Kumar V, Mankadath G. Curcumin and its synthetic analogue dimethoxycurcumin differentially modulates antioxidant status of normal human peripheral blood mononuclear cells. Free radical research 2018;52:583–91. [DOI] [PubMed] [Google Scholar]
- [84].Bowie A, O’Neill LA. Oxidative stress and nuclear factor-κB activation∗: A reassessment of the evidence in the light of recent discoveries. Biochemical pharmacology 2000;59:13–23. [DOI] [PubMed] [Google Scholar]
- [85].Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018;10:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al‐Abed Y, Davies P, et al. Resveratrol mitigates lipopolysaccharide‐and Aβ‐mediated microglial inflammation by inhibiting the TLR4/NF‐κB/STAT signaling cascade. Journal of neurochemistry 2012;120:461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arteriosclerosis, thrombosis, and vascular biology 2003;23:622–9. [DOI] [PubMed] [Google Scholar]
- [88].De Stefano D, Maiuri MC, Simeon V, Grassia G, Soscia A, Cinelli MP, et al. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-γ. European journal of pharmacology 2007;566:192–9. [DOI] [PubMed] [Google Scholar]
- [89].Min Y-D, Choi C-H, Bark H, Son H-Y, Park H-H, Lee S, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflammation Research 2007;56:210–5. [DOI] [PubMed] [Google Scholar]
- [90].Chen J-C, Ho F-M, Chao P-DL, Chen C-P, Jeng K-CG, Hsu H-B, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. European journal of pharmacology 2005;521:9–20. [DOI] [PubMed] [Google Scholar]
- [91].Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, et al. In vivo quercitrin anti‐inflammatory effect involves release of quercetin, which inhibits inflammation through down‐regulation of the NF‐κB pathway. European journal of immunology 2005;35:584–92. [DOI] [PubMed] [Google Scholar]
- [92].Ji G, Zhang Y, Yang Q, Cheng S, Hao J, Zhao X, et al. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS One 2012;7:e53101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Li J, Li J, Yue Y, Hu Y, Cheng W, Liu R, et al. Genistein suppresses tumor necrosis factor α-induced inflammation via modulating reactive oxygen species/Akt/nuclear factor κB and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte MH7A cells. Drug design, development and therapy 2014;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea–derived polyphenol, inhibits IL-1β-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. The Journal of nutrition 2004;134:1039–44. [DOI] [PubMed] [Google Scholar]
- [95].Akhtar N, Haqqi TM. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis research & therapy 2011;13:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kim EK, Choi E-J. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 2010;1802:396–405. [DOI] [PubMed] [Google Scholar]
- [97].Morrison DK. MAP kinase pathways. Cold Spring Harbor perspectives in biology 2012;4:a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wadsworth TL, McDonald TL, Koop DR. Effects of Ginkgo biloba extract (EGb 761) and quercetin on lipopolysaccharide-induced signaling pathways involved in the release of tumor necrosis factor-α1. Biochemical pharmacology 2001;62:963–74. [DOI] [PubMed] [Google Scholar]
- [99].Cho S-Y, Park S-J, Kwon M-J, Jeong T-S, Bok S-H, Choi W-Y, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharide-stimulated macrophage. Molecular and cellular biochemistry 2003;243:153–60. [DOI] [PubMed] [Google Scholar]
- [100].Arumugam S, Thandavarayan RA, Arozal W, Sari FR, Giridharan VV, Soetikno V, et al. Quercetin offers cardioprotection against progression of experimental autoimmune myocarditis by suppression of oxidative and endoplasmic reticulum stress via endothelin-1/MAPK signalling. Free radical research 2012;46:154–63. [DOI] [PubMed] [Google Scholar]
- [101].Li Y-F, Wang H, Fan Y, Shi H-j, Wasng Q-M, Chen B-r, et al. Epigallocatechin-3-Gallate Inhibits Matrix Metalloproteinase-9 and Monocyte Chemotactic Protein-1 Expression Through the 67-κDa Laminin Receptor and the TLR4/MAPK/NF-κB Signalling Pathway in Lipopolysaccharide-Induced Macrophages. Cellular Physiology and Biochemistry 2017;43:926–36. [DOI] [PubMed] [Google Scholar]
- [102].Ahn S-C, Kim G-Y, Kim J-H, Baik S-W, Han M-K, Lee H-J, et al. Epigallocatechin-3-gallate, constituent of green tea, suppresses the LPS-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and NF-κB. Biochemical and Biophysical Research Communications 2004;313:148–55. [DOI] [PubMed] [Google Scholar]
- [103].Ichikawa D, Matsui A, Imai M, Sonoda Y, Kasahara T. Effect of various catechins on the IL-12p40 production by murine peritoneal macrophages and a macrophage cell line, J774. 1. Biological and Pharmaceutical Bulletin 2004;27:1353–8. [DOI] [PubMed] [Google Scholar]
- [104].Hsu SD, Dickinson DP, Qin H, Borke J, Ogbureke KU, Winger JN, et al. Green tea polyphenols reduce autoimmune symptoms in a murine model for human Sjogren’s syndrome and protect human salivary acinar cells from TNF-α-induced cytotoxicity. Autoimmunity 2007;40:138–47. [DOI] [PubMed] [Google Scholar]
- [105].Lou Z, Li X, Zhao X, Du K, Li X, Wang B. Resveratrol attenuates hydrogen peroxide‐induced apoptosis, reactive oxygen species generation, and PSGL‐1 and VWF activation in human umbilical vein endothelial cells, potentially via MAPK signalling pathways. Molecular medicine reports 2018;17:2479–87. [DOI] [PubMed] [Google Scholar]
- [106].Yang G, Chang C-C, Yang Y, Yuan L, Xu L, Ho C-T, et al. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. Journal of agricultural and food chemistry 2018. [DOI] [PubMed] [Google Scholar]
- [107].Kim S-W, Kim CE, Kim MH. Flavonoids inhibit high glucose-induced up-regulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochemical and Biophysical Research Communications 2011;415:602–7. [DOI] [PubMed] [Google Scholar]
- [108].Chen C-C, Chow M-P, Huang W-C, Lin Y-C, Chang Y-J. Flavonoids inhibit tumor necrosis factor-α-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-κB: structure-activity relationships. Molecular Pharmacology 2004;66:683–93. [DOI] [PubMed] [Google Scholar]
- [109].Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS‐stimulated pathways in macrophages by the flavonoid luteolin. British journal of pharmacology 2002;136:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Pasten C, Olave NC, Zhou L, Tabengwa EM, Wolkowicz PE, Grenett HE. Polyphenols downregulate PAI-1 gene expression in cultured human coronary artery endothelial cells: molecular contributor to cardiovascular protection. Thrombosis research 2007;121:59–65. [DOI] [PubMed] [Google Scholar]
- [111].Sun H-Z, Yang T-W, Zang W-J, Wu S-F. Dehydroepiandrosterone-induced proliferation of prostatic epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway. Journal of Endocrinology 2010;204:311–8. [DOI] [PubMed] [Google Scholar]
- [112].Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. American Journal of Physiology-Heart and Circulatory Physiology 2008;294:H2078–H87. [DOI] [PubMed] [Google Scholar]
- [113].Hwang MK, Song NR, Kang NJ, Lee KW, Lee HJ. Activation of phosphatidylinositol 3-kinase is required for tumor necrosis factor-α-induced upregulation of matrix metalloproteinase-9: Its direct inhibition by quercetin. The international journal of biochemistry & cell biology 2009;41:1592–600. [DOI] [PubMed] [Google Scholar]
- [114].Busch F, Mobasheri A, Shayan P, Lueders C, Stahlmann R, Shakibaei M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. Journal of Biological Chemistry 2012;287:38050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu W, Li Y, Zheng X, Zhang K, Du Z. Potent inhibitory effect of silibinin from milk thistle on skin inflammation stimuli by 12-O-tetradecanoylphorbol-13-acetate. Food & function 2015;6:3712–9. [DOI] [PubMed] [Google Scholar]
- [116].Yang N, Zhang H, Cai X, Shang Y. Epigallocatechin-3-gallate inhibits inflammation and epithelial‐mesenchymal transition through the PI3K/AKT pathway via upregulation of PTEN in asthma. International journal of molecular medicine 2018;41:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yeo SCM, Fenwick PS, Barnes PJ, Lin HS, Donnelly LE. Isorhapontigenin, a bioavailable dietary polyphenol, suppresses airway epithelial cell inflammation through a corticosteroid‐independent mechanism. British journal of pharmacology 2017;174:2043–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ou H-C, Lee W-J, Lee S-D, Huang C-Y, Chiu T-H, Tsai K-L, et al. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicology and applied pharmacology 2010;248:134–43. [DOI] [PubMed] [Google Scholar]
- [119].Abdel-Aleem GA, Khaleel EF, Mostafa DG, Elberier LK. Neuroprotective effect of resveratrol against brain ischemia reperfusion injury in rats entails reduction of DJ-1 protein expression and activation of PI3K/Akt/GSK3b survival pathway. Archives of Physiology and Biochemistry 2016;122:200–13. [DOI] [PubMed] [Google Scholar]
- [120].Rahman I, Chung S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. Personalized Nutrition: Karger Publishers; 2010: 84–94. [DOI] [PubMed] [Google Scholar]
- [121].Ayissi VBO, Ebrahimi A, Schluesenner H. Epigenetic effects of natural polyphenols: A focus on SIRT1‐mediated mechanisms. Molecular Nutrition & Food Research 2014;58:22–32. [DOI] [PubMed] [Google Scholar]
- [122].Schneider-Stock R, Ghantous A, Bajbouj K, Saikali M, Darwiche N. Epigenetic mechanisms of plant-derived anticancer drugs. Frontiers in bioscience (Landmark edition) 2012;17:129–73. [DOI] [PubMed] [Google Scholar]
- [123].He Y, Zeng H, Yu Y, Zhang J, Zeng X, Gong F, et al. Resveratrol improved the progression of chronic prostatitis via the downregulation of c-kit/SCF by activating Sirt1. Journal of agricultural and food chemistry 2017;65:5668–73. [DOI] [PubMed] [Google Scholar]
- [124].Liu Z, Jiang C, Zhang J, Liu B, Du Q. Resveratrol inhibits inflammation and ameliorates insulin resistant endothelial dysfunction via regulation of AMP‐activated protein kinase and sirtuin 1 activities. Journal of Diabetes 2016;8:324–35. [DOI] [PubMed] [Google Scholar]
- [125].Zhao L, Cen F, Tian F, Li M-J, Zhang Q, Shen HY, et al. Combination treatment with quercetin and resveratrol attenuates high fat diet‐induced obesity and associated inflammation in rats via the AMPKα1/SIRT1 signaling pathway. Experimental and therapeutic medicine 2017;14:5942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zheng W, Feng Z, You S, Zhang H, Tao Z, Wang Q, et al. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. International immunopharmacology 2017;45:135–47. [DOI] [PubMed] [Google Scholar]
- [127].Boyanapalli SS, Kong A-NT. “Curcumin, the king of spices”: epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Current pharmacology reports 2015;1:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Hong K-S, Park J-I, Kim M-J, Kim H-B, Lee J-W, Dao TT, et al. Involvement of SIRT1 in hypoxic down-regulation of c-Myc and β-catenin and hypoxic preconditioning effect of polyphenols. Toxicology and applied pharmacology 2012;259:210–8. [DOI] [PubMed] [Google Scholar]
- [129].Cordero-Herrera I, Chen X, Ramos S, Devaraj S. (−)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocytes. European journal of nutrition 2017;56:1369–73. [DOI] [PubMed] [Google Scholar]
- [130].Choi K-C, Jung MG, Lee Y-H, Yoon JC, Kwon SH, Kang H-B, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer research 2009;69:583–92. [DOI] [PubMed] [Google Scholar]
- [131].Maugeri A, Barchitta M, Mazzone M, Giuliano F, Basile G, Agodi A. Resveratrol modulates SIRT1 and DNMT functions and restores LINE-1 methylation levels in ARPE-19 cells under oxidative stress and inflammation. International journal of molecular sciences 2018;19:2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Deng Y, Lu X, Wang L, Li T, Ding Y, Cao H, et al. Curcumin inhibits the AKT/NF-κB signaling via CpG demethylation of the promoter and restoration of NEP in the N2a cell line. The AAPS journal 2014;16:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ambros V The functions of animal microRNAs. Nature 2004;431:350. [DOI] [PubMed] [Google Scholar]
- [134].Kronski E, Fiori ME, Barbieri O, Astigiano S, Mirisola V, Killian PH, et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down‐regulation of the inflammatory cytokines CXCL1 and‐2. Molecular oncology 2014;8:581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Li H, Jia Z, Li A, Jenkins G, Yang X, Hu J, et al. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Molecular and cellular biochemistry 2013;382:137–43. [DOI] [PubMed] [Google Scholar]
- [136].Song J, Jun M, Ahn M-R, Kim OY. Involvement of miR-Let7A in inflammatory response and cell survival/apoptosis regulated by resveratrol in THP-1 macrophage. Nutrition research and practice 2016;10:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhou H, Chen JX, Yang CS, Yang MQ, Deng Y, Wang H. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC genomics 2014;15:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chen L, Teng H, Jia Z, Battino M, Miron A, Yu Z, et al. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Critical reviews in food science and nutrition 2017:1–17. [DOI] [PubMed] [Google Scholar]


