Abstract
Division of amoebas, fungi, and animal cells into two daughter cells at the end of the cell cycle depends on a common set of ancient proteins, principally actin filaments and myosin-II motors. Anillin, formins, IQGAPs, and many other proteins regulate the assembly of the actin filaments into a contractile ring positioned between the daughter nuclei by different mechanisms in fungi and animal cells. Interactions of myosin-II with actin filaments produce force to assemble and then constrict the contractile ring to form a cleavage furrow. Contractile rings disassemble as they constrict. In some cases, knowledge about the numbers of participating proteins and their biochemical mechanisms has made it possible to formulate molecularly explicit mathematical models that reproduce the observed physical events during cytokinesis by computer simulations.
Keywords: actin, cytokinesis, contractile ring, mathematical models, myosin
INTRODUCTION
Cytokinesis is the most dramatic event in the life of a cell. After the mitotic apparatus partitions the duplicated chromosomes into separate nuclei, a cleavage furrow separates these nuclei into two daughter cells. Accurate partitioning of daughter nuclei into separate cells is required for viability and avoiding aneuploidy.
Pioneering experiments four decades ago established that animal cells assemble a contractile ring composed of actin filaments (1, 2) and myosin-II (3) around the equator of the dividing cell and that both proteins (1, 4) are required to form a cleavage furrow. Mechanical measurements established that the cleavage furrow of echinoderm eggs produces a force of ~25 pN (5), enough to divide the cell but much less than contracting muscle cells. Genetics experiments showed that cytokinesis by cellular slime mold cells (6) and fission yeast cells (7, 8) also depends on actin and myosin-II. Much has been learned about the proteins required to assemble and constrict cytokinetic contractile rings (9–11), but many fundamental questions still remain (12).
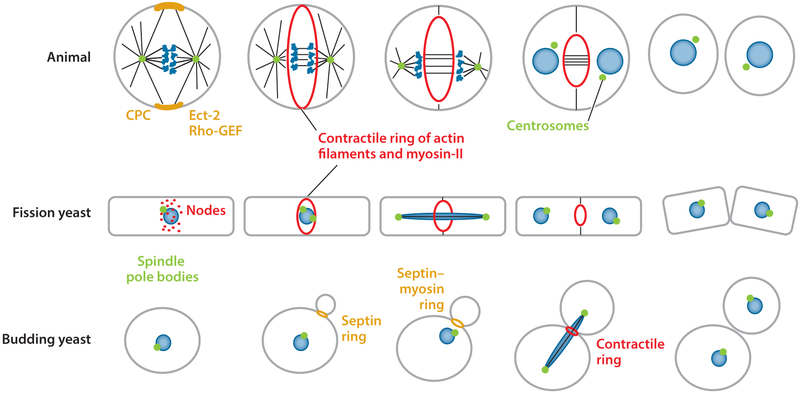
This review focuses on the assembly and mechanics of cytokinetic contractile rings (Figure 1). Contractile rings appeared in the common ancestor of animals, fungi, and amoebas approximately 1 billion years ago concurrent with the evolution of myosin-II. Plants, algae, and vast numbers of single-celled organisms lack myosin-II (13) and depend on fusion of membrane vesicles (14) or other means to divide. For example, forces produced by intracellular flagellar axonemes divide the early branching eukaryote Giardia (15). Metazoan organisms redeployed the cytokinesis proteins in contractile cells that evolved into muscle cells. Our deep knowledge of muscle contraction strongly influences thinking about cytokinesis, although some caution is required.
Figure 1.
Illustrations comparing the steps in cytokinesis by animals, budding yeast, and fission yeast. Figure modified from Reference 12. Abbreviations: CPC, chromosomal passenger complex; Rho-GEF, Rho GTPase guanine nucleotide exchange factor.
Fortunately, most genes required for cytokinesis are ancient, so insights from work on model organisms such as budding yeast, fission yeast, and nematode zygotes often apply to vertebrates. The mechanisms are not identical owing to hundreds of millions of years of divergent evolution. We evaluate what is known about the molecular mechanisms of four aspects of cytokinesis: location, assembly, constriction, and disassembly of the contractile ring. Others have reviewed abscission (16, 17), the final separation of the membranes of the daughter cells. Prokaryotes use different mechanisms for cytokinesis (18).
MECHANISMS THAT POSITION THE CLEAVAGE FURROW
Placing the plane of cell division between the daughter nuclei is essential to assure viability, so cells evolved robust, apparently redundant mechanisms to assure the fidelity of the process. These mechanisms appear to have diverged more than any other aspect of cytokinesis, so budding yeast, fission yeast, and animal cells use different strategies to separate daughter nuclei into two cells. The mitotic spindle is the prime source of positional information in animal cells (19). Fission yeast cells use cues from the poles of the cell and the nucleus to place the contractile ring. Budding yeast cells use polarity signals to position the site where the daughter cell buds from the mother cell and then later guide one set of chromosomes into the bud before cytokinesis.
Contractile Ring Placement in Animal Cells
Micromanipulation of echinoderm zygotes by Rappaport and Hiramoto showed that chemically undefined signals move from the mitotic spindle to the cortex of the cell, where after a couple of minutes, they stimulate the formation of a cleavage furrow (20). Other experiments showed that one mitotic spindle could stimulate multiple furrows. As furrows can form between spindle poles that do not straddle chromosomes, Rappaport argued that the signal must come from the poles and travel along the astral microtubules. However, equally persuasive evidence showed that the middle of the mitotic spindle also provides positioning information in small animal cells (21). Reexamination of the process in large echinoderm and frog zygotes (22) led to the conclusion that the “spindle midzone must be a source of a diffusible signal, which, under normal conditions, is spatially focused in the equator by astral microtubules” (23, p. 4049). This positioning mechanism stimulates the assembly of actin filaments and myosin-II associated with the plasma membrane in between the poles of the mitotic spindle, whether they are located centrally or positioned asymmetrically Given this conceptual framework, investigators set out to find the molecules that position the cleavage furrow.
Experiments on zygotes of the nematode Caenorhabditis elegans have been particularly informative about the signaling pathways that specify the site of cytokinesis in animal cells (19) (Figure 2). Parallel studies on flies (24), echinoderm zygotes (25), frog zygotes (26), and cultured fly cells (27) have yielded complementary information. Given the widespread distribution of these species on the phylogenetic tree, many animals must use the pathways shown in the figure but with some variation in the mechanistic details (19). No cell type has been shown to use all of the mechanisms, so our understanding of the general principles is still forming. Some aspects of the positioning mechanism have been reconstituted in frog egg extracts (28), but much work is still needed on both the biochemical and the cellular aspects of the positioning mechanisms.
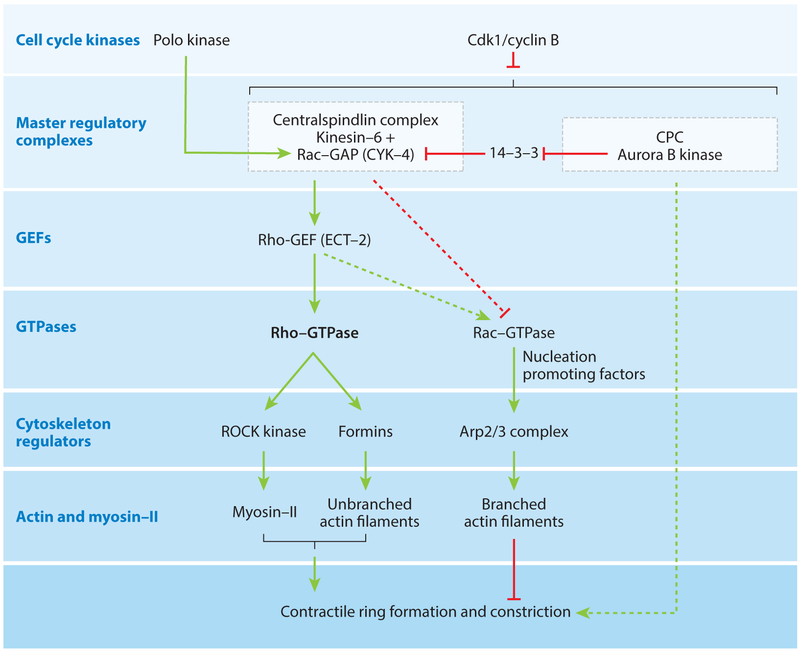
Figure 2.
Biochemical mechanisms that position the cleavage furrow in animal cells. Abbreviations: Arp2/3, actin-related protein 2 and 3; Cdk1, cyclin-dependent kinase 1; CPC, chromosomal passenger complex; GEF, guanine nucleotide exchange factor; Rac, small GTPase; Rho, small GTPase; ROCK, Rho-kinase.
Seven-level signaling pathways control cytokinesis.
Two decades of research identified multiple biochemical pathways targeting Rho GTPases to position and assemble contractile rings (Figure 2). Cell cycle kinases including both cyclin-dependent kinases (Cdks) and Polo-like kinases at the top coordinate the whole process by controlling two master regulatory complexes, the centralspindlin complex and chromosomal passenger complex (CPC). These multi-subunit complexes specify the location of the cleavage site and activate downstream signaling proteins including the key guanine nucleotide exchange factor Rho-GEF named Ect-2, Rho family GTPases, and proteins that act locally to assemble the contractile ring.
Regulation of timing by cell cycle kinases.
Strong Cdk1/cyclin B activity drives the cell into mitosis but suppresses cytokinesis until anaphase by phosphorylating sites on proteins that inhibit various steps (29–31). For example, phosphorylation of kinesin-6 in the centralspindlin complex inhibits binding to microtubules (32), and phosphorylation of Ect-2 Rho-GEF inhibits its activity and interactions with centralspindlin and membranes (33–35). Degradation of cyclin B allows the transition from metaphase to anaphase and releases the pathways controlled by the two master regulatory complexes. Simply inhibiting Cdk1 (but not other Cdks) during mitosis can drive premature cytokinesis (29, 30).
The Polo-like mitotic kinase can both inhibit and promote interactions relevant to cytokinesis. For example, phosphorylation of the microtubule cross-linking protein PRC1 inhibits formation of the central spindle until anaphase (36), whereas phosphorylation of the centralspindlin protein MgcRacGAP allows it to bind and activate Rho-GEF Ect-2 during anaphase (37).
Two master regulatory complexes.
The centralspindlin complex (38) is essential for positioning the contractile ring. It consists of a homodimer of the kinesin-6 isoform Kif23 and a homodimer of the Rac GTPase-activating protein (GAP) named Cyk-4 or MgcRacGAP (39). The kinesin-6 motor protein steps toward the plus ends of microtubules (40). MgcRacGAP has at least four functions. First, it binds kinesin-6 and promotes microtubule cross-linking in the central spindle (39). Second, the GAP domain can inactivate Rac and Cdc42 by promoting GTP hydrolysis in biochemical assays (41, 42). Third, a C1 domain interacts with acidic lipids to anchor the whole complex to the plasma membrane (43). Fourth, it binds the Rho-GEF Ect-2, the pivotal activator of the Rho-GTPase that controls the assembly of the contractile ring. This interaction depends on the Polo-like kinase phosphorylating S157 and A164 of MgcRacGAP (37), creating binding sites for the BRCT (BRCA1 C-terminal domain) domains of Rho-GEF Ect-2 (44). MgcRacGAP not only concentrates Ect-2 at the cleavage site but also activates its GEF activity (45).
The CPC consists of four proteins: borealin, survivin, INCENP, and Aurora B kinase (46). The CPC concentrates at centromeres early in mitosis, but when Cdk activity declines during anaphase, INCENP is dephosphorylated and kinesin-6/Kif20A targets CPC to the overlapping microtubules of the central spindle. Some CPC accumulates in the cortex surrounding the central spindle. During telophase, CPC concentrates in the midbody. The Aurora B kinase in the complex regulates several aspects of mitosis including attachment of microtubules to kinetochores, the mitotic spindle assembly checkpoint, and concentration of the centralspindlin complex to the spindle midzone (46). Aurora B kinase also promotes contractile ring formation by inactivating a 14—3–3 protein that inhibits the oligomerization of centralspindlin (47) (Figure 2).
Ect-2 activates Rho-GTPase.
The primary positive signal to assemble the contractile ring comes from Ect-2 (GEF) that activates Rho GTPase (48, 49). In fact, local activation of RhoA induces a furrow anywhere on the cell surface (50). Autoinhibition (51) and phosphorylation of threonine 341 by Cdks (33–35) keep Ect-2 activity low during G2 and early mitotic phases. When this phosphate is removed in anaphase, Ect-2 associates with phosphorylated MgcRacGAP of the centralspindlin complex and concentrates along with active Rho-GTP on the plasma membrane adjacent to the middle of the mitotic spindle (35, 52). A PH (pleckstrin homology) domain and a region of basic residues allow Ect-2 to bind to the plasma membrane, an interaction required to activate Rho (35). Rho-GAPs named RGA-3/4 and MP-GAP/ARHGAP11A turn off activated Rho by stimulating GTP hydrolysis (53–55).
C. elegans has a second, nonessential pathway dependent on the nop-1 gene that also activates Ect-2 in early embryos (45, 56). This uncharacterized pathway complicates the analysis of the cellular functions of centralspindlin unless NOP-1 is depleted.
Rac GTPases.
Rac GTPases influence cytokinesis indirectly by activating a pathway stimulating the Arp2/3 (actin-related protein 2 and 3) complex to form branched actin filaments that may interfere with furrow formation (57). Because depleting Rac mRNAs or activators of Arp2/3 complex can compensate for certain mutations in the GAP domain of MgcRacGAP in C. elegans, the GAP activity may be responsible for inactivating Rac during furrowing (58, 59). However, other experiments suggest that the GAP domain (but not always the GAP activity) is required to activate Ect-2 (45).
Mechanisms localizing the master regulators.
The mitotic spindle determines where the master regulators—centralspindlin complex and CPC—accumulate and thus determines the position of the cleavage furrow (19). Various types of cells emphasize different aspects of the known mechanisms, which are still not fully understood.
Kinesins move both complexes toward the plus ends of microtubules. The kinesin-6 subunits move the centralspindlin complex and associated Ect-2 Rho-GEF (23, 60), whereas another kinesin-6 (Kif20AE) and a kinesin-4 (Kif4A) move the CPC (28). Consequently, both master regulators accumulate along with Ect-2 at two locations (Figure 3) where the protein PRC1 and kinesin-Kif4 cross-link the plus ends of overlapping antiparallel microtubules (61). The universal concentration of Ect-2 and centralspindlin in the middle of the anaphase mitotic spindle is striking but not required to direct equatorial furrow formation in some cells (62).
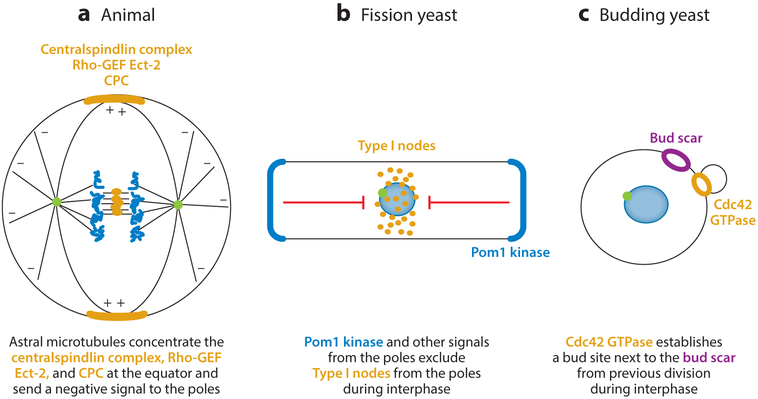
Figure 3.
Mechanisms of contractile ring formation. (a) Astral microtubules concentrate the centralspindlin complex, Rho-GEF Ect-2, and CPC at the equator and send a negative signal to the poles. (b Pom1 kinase and other signals from the poles exclude type I nodes from the poles during interphase. (c) Cdc42 GTPase establishes a bud site next to the bud scar from previous division during interphase. Figure modified from Reference 12. Abbreviations: Cdc42, small GTPase; CPC, chromosome passenger complex; GEF, guanine nucleotide exchange factor; Pom1, a fission yeast kinase; Rho, small GTPase.
More important is the localization of Ect-2 and centralspindlin on the plasma membrane, where astral microtubules overlap midway between the poles of the mitotic spindle (23). In small cells, active Ect-2 may diffuse locally from the central spindle to the membrane (23, 60), but in large egg cells, the overlapped astral microtubules are the likely source of Ect-2 on the plasma membrane (23, 60). Less is known about the accumulation of CPC on the equatorial plasma membrane, where it contributes to stabilizing the zone of active Ect-2 by promoting oligomerization of centralspindlin due to inhibitory phosphorylation of 14—3–3 by Aurora B (47) (Figure 2). Ect-2 also binds to lipids and membrane-anchored Rho-GTP. The Ect-2 activates Rho and drives the assembly of the contractile ring (63). Simultaneously, anaphase movements of chromosomes carry a PP1 protein phosphatase to the poles of the mitotic spindle, where it may contribute to relaxing the overlying cortex by dephosphorylating the ERM protein moesin and reducing actin polymerization (64).
Remarkably, some of these positioning events can be reconstituted with purified proteins (65) and in frog egg extracts with artificial asters of microtubules (28). Kinesin-4 and kinesin-6 motors concentrate the CPC, the centralspindlin complex, and active Rho-GTPase, where the plus ends of microtubules from adjacent asters overlap. Active Rho stimulates actin polymerization next to these overlap zones. In the extracts, the microtubules stop growing when they encounter oppositely polarized microtubules from an adjacent aster. Thus, cross-links formed by PRC1 between antiparallel microtubules may regulate the elongation of the microtubules and help to localize the proteins that control the assembly of the contractile ring.
In addition to positive signals guided by the mitotic apparatus, 50 years of biological observations suggested that astral microtubules participate in generating a negative signal that suppresses furrow formation around the poles of the cell but allows the activation of Rho around the equator (19). A candidate mechanism is recruitment of the protein TPXL-1 to the tips of astral microtubules, where it activates Aurora A kinase to inhibit the local accumulation of contractile ring proteins by uncharacterized pathways (66).
Contractile Ring Placement in Fission Yeast Cells
Fission yeast cells establish the cleavage plane early in the G2 phase of the cell cycle by positioning the precursors of the contractile ring in the middle of the rod-shaped cells (9) (Figure 3). These precursors are protein assemblies termed nodes bound to the inside of the plasma membrane. Two types of nodes comprised of different proteins form separately but join together during the G2 phase of the cell cycle and mature into the cytokinesis nodes that form the contractile ring (see the next section) (67).
Type I nodes form around the equator early in the G2 phase. The Cdr2 kinase is the main constituent and scaffold of type I nodes; smaller numbers of the protein kinases Cdr1p and Wee1p bind Cdr2p and contribute to regulating cell size (68–70). C-terminal lipid-binding sites anchor Cdr2p to the plasma membrane. During interphase, anillin Mid1p leaves the nucleus and joins the stationary type I nodes around the equator (71). Adjacent C2 and PH domains at the C terminus of Mid1p interact with membrane lipids (72).
Type II nodes consist of the membrane-binding scaffold protein Blt1p, Rho-GEF Gef2p, and kinesin Klp8 (variously classified as a kinesin-3 or -10). These nodes are present in the constricting contractile ring and are released on the sides of the cleavage furrow into the daughter cells as the ring disperses at the end of cytokinesis (70). They diffuse from the division site along the plasma membrane until they encounter and bind a stationary type I node near the center of the cell (67). The combined type I and type II nodes mature into cytokinesis nodes by accumulating the proteins that assemble the contractile ring.
Signals from both ends of the cell exclude type I nodes from the poles, restricting them to the middle of the cell. The Pom1 kinase generates part of this repulsive signal (68, 69, 73). Gradients of Pom1p extending from both poles are established by three mechanisms: microtubules transport Pom1p along with Tea1p and Tea4p to the poles; a PP1 phosphatase associated with Tea4p removes a phosphate from Pom1, which allows it to bind the membrane; and Pom1p phosphorylates other Pom1p molecules at the poles to promote dissociation from the membrane (74). Simulations of a mathematical model of these reactions reproduced the observed gradient of Pom1p at the tips of the cell (74).
Pom1p repels type I nodes in proportion to its local concentration by phosphorylating Cdr2p, which reduces its interactions with the membrane and Mid1p and inhibits its kinase activity (75). Therefore, nodes only form in the middle of the cell where the Pom1p concentration is low (68, 69). However, other factors must participate, because cells lacking Pom1p exclude type I nodes from only one pole of the cell (68, 69, 76, 77). Another kinase, Kin1p, concentrates at the opposite pole from that with more Pom1p and phosphorylates similar substrates but on different residues (78).
Manipulation of the position of the nucleus inside living cells shows that it can secondarily adjust the division site. Normally this is not required, as the type I nodes are located around the nucleus in the middle of the cell during interphase. Dynamic instability of microtubules attached to the nuclear envelope positions the nucleus in the middle of the cell by pushing against the ends of the cell (79). Centrifugation or optical traps can displace the nucleus if microtubules are depolymerized with a drug or absent from the cytoplasm as in the late G2 phase (80, 81). A contractile ring forms at the appropriate time wherever the displaced nucleus is located.
As the absence of anillin Mid1p results in poorly positioned contractile rings (82, 83), and as Mid1p moves from the nucleus to nodes during interphase (71, 84), many authors have proposed that the local transfer of Mid1 from the nucleus to nodes in the adjacent cortex dominates over the repulsive signal from the poles to position the contractile ring as the cell approaches mitosis. At that point, polo kinase Plo1p phosphorylates one of the nuclear export signals of Mid1p and promotes its transport from the nucleus (85). Consistent with this hypothesis, cortical nodes marked with Mid1p-green fluorescent protein (GFP) move adjacent to a displaced nucleus (80). The simplest explanation would be that interphase nodes with Cdr2p and Mid1p move intact or turn over and reappear next to the displaced nucleus. However, the type I interphase node scaffold protein Cdr2p is reported to stay behind, in the middle of the cell, when the nucleus is displaced (86). Another experiment supporting the local transfer hypothesis used a mutated Mid1p lacking both nuclear localization signals, so it never entered the nucleus. These cells place nodes with mutant Mid1p and the contractile ring correctly in the middle of the cell, even if the nucleus is displaced to one end (presumably because no Mid1p is available for local transfer from the nucleus) (86). We do not yet understand how this nuclear positioning mechanism works (87).
During mitosis the septation initiation network (SIN) of signaling proteins, the yeast version of the animal Hippo pathway (88), disperses the type I node proteins from the cytokinesis nodes into the cytoplasm by phosphorylating the Cdr2p kinase (89). Type I nodes reassemble on the plasma membrane around the daughter nuclei when the SIN activity declines at the end of mitosis (90).
Contractile Ring Placement in Budding Yeast Cells
Budding yeast cells proliferate by growing a cellular protrusion termed a bud on the side of a mother cell. During the G1 phase of the cell cycle, a bud emerges near the site of the previous cytokinesis (Figures 1 and 3). This well-studied example of cellular polarization depends on Cdc42, the most conserved of the small GTPases, and a positive feedback mechanism that concentrates active Cdc42-GTP on the inside surface of the plasma membrane (91) (Figure 3). The positive feedback works like this: Cdc42-GTP, associated with the plasma membrane by a C-terminal prenyl modification, binds a cytoplasmic kinase named PAK that is associated with a scaffold protein Bem1 and Cdc24, the GEF for Cdc42. The GEF activates nearby Cdc42 proteins, which in turn bind more of the activating GEF, to amplify the process. Simulations of mathematical models showed that rapid diffusion of the activating complex in the cytoplasm, binding to active Cdc42 on the membrane, and local activation of more slowly diffusing Cdc42 on the membrane build up a high concentration of active Cdc42 (92). In keeping with this mechanism, optogenetic targeting of Bem1 or Cdc24 to a spot on the plasma membrane can trigger the self-amplifying local accumulation of active Cdc42-GTP (93).
Both positive and negative cues assure that the new bud emerges next to the bud scar where the mother and daughter cells separated (91). A Cdc42 GAP named Rga1 is left behind at the cleavage site as a negative cue to block Cdc42 accumulation. Positive cues are positioned next to the cleavage site. They include transmembrane proteins from the secretory pathway that accumulate around or next to the cleavage site. One of these proteins binds and activates a GTPase named Rsr1, which activates Cdc24 GEF to trigger a local accumulation of Cdc42-GTP. Active Cdc42 recruits effector proteins including formins, which contribute to the assembly of the precursor of the contractile ring (see the next section).
MECHANISMS THAT ASSEMBLE CONTRACTILE RINGS
Contractile rings from amoebas to humans are comprised of the same major proteins. In all well-studied systems, formins assemble the actin filaments, and myosin-II produces force to arrange the filaments in a continuous ring at the cleavage site. Biochemical reconstitution established that the main components of contractile rings can self-assemble; polymerizing actin with a crowding agent (methylcellulose) produces rings of actin filaments inside aqueous droplets lined with membrane lipids and surrounded by oil (94). Including myosin-II with actin promotes the formation and constriction of rings of actin filaments.
Contractile ring proteins are present at constant concentrations throughout the cell cycle, at least in fission yeast (95), so conditions change during mitosis to direct the assembly of the contractile ring. The following sections explain the different strategies used by fission yeast, budding yeast, and animal cells.
Contractile Ring Assembly in Fission Yeast Cells
Fission yeast assemble a contractile ring during a period of 10–12 min early in mitosis. Fungi lack a cortical actin filament network during interphase, so the contractile ring actin filaments are assembled de novo. During the 10 min prior to separation of the spindle pole bodies in prophase, cytokinesis nodes formed during interphase from type I and type II nodes accumulate the proteins required to form a contractile ring: IQGAP Rng2p links anillin Mid1p to the C terminus of myosin-II Myo2 (96), and F-BAR protein Cdc15p interacts with Blt1p, Mid1p, Rng2p, and formin Cdc12p (97). Super-resolution fluorescence microscopy of live cells expressing fluorescent fusion proteins showed that a cluster of approximately 10 Mid1p proteins anchors approximately 10 dimers each of IQGAP Rng2, F-BAR Cdc15p, and myosin-II Myo2 to the plasma membrane with a smaller number of formin Cdc12p dimers. A bouquet of myosin heads project into the cytoplasm (Figure 4). An actin filament grows from each formin (82, 98), which presumably anchors its barbed end to the node. Formin For3p contributes additional actin filaments (99). If an actin filament growing from a formin comes close to another node, Myo2 can bind and pull the two nodes together (100). A myosin-V Myo51, located between nodes augments the force produced by Myo2 (101, 102).
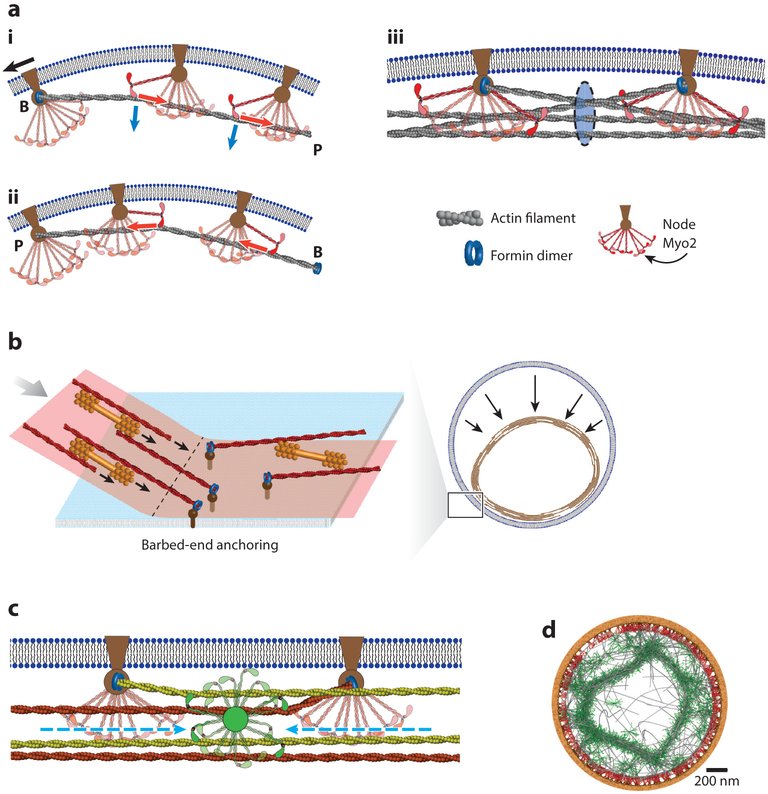
Figure 4.
Radial and transverse anchoring in constricting contractile rings in fission yeast. (a) Nodes anchor myosin-II Myo2 and the formin dimer Cdc12p to the plasma membrane. The formin anchors the actin filament barbed end (B) to the node (i). Stepping of Myo2 toward the barbed end produces tension, if lateral anchors produce a drag force (black arrow) opposing the myosin forces (red arrows). Myo2 also anchors curved actin filaments radially, producing inward Laplace forces (blue arrows). The ring tension is the sum of the tensions of all filaments passing through a cross section of the ring (blue, iii). Anchoring the pointed end of the actin filament (P) would generate compression rather than tension (ii). (b) A contractile ring segment detaches from the membrane and shortens in a permeabilized fission yeast protoplast (right). At the anchored-unanchored interface, barbed-end–anchored actin filaments point into and reel in the tensionless unanchored segment at approximately the load-free velocity of myosin-II (left). Orange bipolar filaments represent unanchored myosin-II. (c) Myosin-II Myp2 may be unanchored in the fission yeast ring. An unanchored cluster of Myp2 (green) is depicted exerting tensile force after migrating to a location with equal numbers of actin filament orientations to the right (green) and left (red). (d) model of detachment of contractile ring segments in cells with the myo2-E1 mutation where Myo2 binds actin filaments weakly. Straight bundles of actin filaments (gray) and Myp2 (green) detach from the plasma membrane, leaving Myo2 (red) behind on the membrane.
Simulations of a molecularly explicit mathematical model based on the number of proteins present and their biochemical activities showed that a search, capture, and pull mechanism assembles rings in 10 min as observed in cells, providing the connections between the nodes break approximately every 20 s to avoid clumping of nodes (100). Experiments with cofilin mutants with low severing activity showed that cofilin is required to break these connections (103). This release step is an error-correcting mechanism. A 3D simulation of ring assembly on a cylindrical representation of the cell produced contractile rings from nodes polymerizing semiflexible actin filaments bundled by cross-linking proteins (104). After assembly, the ring adds unconventional myosin-II Myp2, more F-BAR Cdc15p, and more polymerized actin during a 20-min maturation period before the onset of constriction (95, 99). Anillin Mid1p is replaced by a related protein Mid2p.
Contractile Ring Assembly in Budding Yeast Cells
The GTPase Cdc42 serves as the polarity marker to specify the assembly of a stable collar of septins on the plasma membrane at the border between the mother cell and bud (105). The septins organize the assembly of the contractile ring. First, septins bind an adapter protein named Bni5 that links to the tail of myosin-II (named Myo1). Later, polo kinase stimulates a GEF to recruit the GTPase Rho1, which activates formin Bni1p to polymerize actin filaments. As the cell cycle progresses, IQGAP takes over from Bni5 to anchor Myo1, and the collar of septins splits into two rings with the contractile ring in between.
Contractile Ring Assembly in Animal Cells
Active Rho-GTP drives contractile ring assembly in animal cells by activating Rho-kinase (ROCK) to phosphorylate the regulatory light chains of myosin-II (106) and by activating formins to assemble actin filaments (107). Myosin-II accumulates in bipolar filaments (108, 109) at the site of furrowing independent of actin filaments (110, 111) and even in the presence of the myosin inhibitor blebbistatin (112). The myosin tethering mechanism is not known. Genetics identified the crucial formins in C. elegans (Cyk-1) (24) and Drosophila (diaphanous) (113), but multiple formins appear to contribute in vertebrate cells (114). In addition, preexisting cortical actin filaments formed by the Arp2/3 complex (115) may also contribute to contractile rings.
Interactions of myosin-II with actin filaments produce forces that generate cortical flow of actin filaments and clusters of myosin toward the future cleavage site and align the filaments around the equator (116). Electron micrographs confirmed that myosin filaments interact with actin filaments during the formation of contractile rings in sea urchin zygotes (109). This arrangement is consistent with a search, capture, and pull mechanism, but the molecular details must be verified and incorporated into models and simulations of contractile ring assembly for animal cells (116).
MECHANISMS THAT CONSTRICT CONTRACTILE RINGS
Contractile rings containing actin, myosin, and other components (as described above) constrict and generate inward contractile force (5, 117–120) that mechanically drives or guides furrow ingression and cell division. Rappaport (5) measured an inward radial force of ~25 nN in the cleavage furrow of echinoderm eggs, and Schroeder identified actin filaments in the contractile ring and suggested a sliding filament mechanism similar to muscle (2, 121). Subsequently myosin-II was identified in the contractile ring (3) and shown to be required for constriction (4).
Although the evidence pointed to a muscle-like mechanism of tension generation and constriction, many questions remained. First, how does tension emerge from the organization of contractile rings, which appears remote from the spatially periodic sarcomeric organization of striated muscle? Second, given tension production in the ring, what mechanism sets the constriction rate and what is the role of other processes that accompany constriction such as change in cell shape, remodeling of the cell cortex in animal cells and amoebas, and septum growth in organisms with cell walls? Third, as contractile rings disassemble as they constrict (95, 121), how do they maintain functionality as a contractile machine while shedding their parts?
Here, we review the current status of understanding of these questions for four model systems. Investigators have proposed models of ring tension and constriction that can be tested by computer simulations and perturbing live cells. We emphasize the importance of experimental measurements of the organization of components in the ring and of ring tension, if realistic quantitative models of the fundamental mechanisms are to emerge.
Contractile Ring Tension and Constriction in Fission Yeast Cells
For fission yeast significant information on the composition and structure of constricting rings is available, so that realistic models are now feasible. At the onset of constriction, the fission yeast contractile ring has a cross section of ~ 125 nm and a circumference of 11 μm. It consists of approximately 200,000 molecules of actin (500 μm of polymer) (99, 122, 123), 5,000 myosin-II polypeptides [3,000 of Myo2 (1,500 dimers) and 2,000 of Myp2], 650 IQGAP dimers, 250 α-actinin dimers, and 200 formin Cdc12p dimers (95, 99, 124). Assuming every formin dimer initiates an actin filament, the ~350 filaments have a mean length of ~ 1.4 μm in a bundle of ~50 filaments in a cross section (96).
Punctate structures similar in size and composition to cytokinesis nodes persist in constricting rings (96) and anchor actin filaments and Myo2 to the plasma membrane (see the sidebar titled Radial Anchoring of Constricting Rings). The number of nodes is likely 120–140, as the number of Myo2 molecules changes little during maturation (95). Myp2 lies farther from the membrane than Myo2 (102, 124), and its presence in the ring depends on actin filaments (125). In cells with the myo2-E1 temperature-sensitive mutation, straight bridges containing actin filaments and Myp2 detach from the plasma membrane (102) (Figure 4d). Nevertheless, despite Myo2p-E1 having minimal ATPase activity (126), these rings appear tensile as they constrict in myo2-E1 protoplasts (120). Thus, even when manifestly unanchored, Myp2 still appears to exert tension, so that being unanchored is likely its normal state (see also Figure 4c).
RADIAL ANCHORING OF CONSTRICTING RINGS.
Both radial and lateral anchoring feature in the organization of contractile rings. Lateral anchoring may underlie the ring tension mechanism (see the sidebar titled A Mechanism for Ring Tension). Radial anchoring prevents detachment from the membrane and localizes the ring at the furrow to exert centripetal force that drives furrow ingression (Figures 4a and 6a). In fission yeast, the radial anchors of the core actomyosin bundle consist of membrane proteins linked to nodes containing Myo2 that interacts with actin filaments (96). Membrane-anchored Myo2 is ideally suited to anchoring a dynamic bundle of polymerizing, tensile actin filaments (Figure 4a), because ~6 Myo2 heads interact with each μm of translocating actin filament (99). These interactions oppose inward Laplace forces from filament tensions, as illustrated by the Myo2-E1 mutant, which binds actin weakly, allowing straight bundles of actin filaments to pull away from the membrane (2, 102) (Figure 4d).
Rings shed material as they constrict while increasing the densities (number per ring length) of node components (Myo2, IQGAP Rng2p, F-BAR Cdc15p, and formin Cdc12p), of Myp2, and of α-actinin (95). The number of polymerized actin molecules falls in proportion to the circumference (99), suggesting the mean filament length decreases.
Measurements of ring tension in fission yeast.
Contractile ring tension was measured in protoplasts (see the sidebar titled Fission Yeast Protoplasts), whose cell walls were removed by enzyme digestion (120). A mean ring tension of ~400 pN was inferred from a force balance at the furrow produced by the contractile ring, using the membrane tension measured by micropipette aspiration during interphase. A more recent study found that the membrane tension is higher during mitosis, showing that the average ring tension was ~640 pN and increased ~2-fold during constriction (S. Wang, H.F. Chin, S. Thiyagarajan, Z. McDargh, E. Karatekin, et al., unpublished manuscript). Average ring tensions were 400 pN in protoplasts of Δmyp2 cells and 220 pN in protoplasts of myo2-E1 cells that lack Myo2 ATPase activity, suggesting that both myosin-II isoforms contribute to the total tension in wild-type cells.
FISSION YEAST PROTOPLASTS: A LABORATORY TO STUDY CONTRACTILE RINGS.
Fission yeast protoplasts allow contractile rings to be studied in extraordinary but revealing nonphysiological circumstances using strains with mutations and fluorescent fusion proteins. First, they provide a method to measure ring tension (120). Second, constricting rings slide laterally along the plasma membrane, as fission yeast lacks an actin cortex. Sliding–constriction dynamics were measured and mathematically modeled in protoplasts with a variety of shapes (120, 175). Third, the biochemical environment of rings can be controlled at will in permeabilized protoplasts, where the extraordinary constriction dynamics of partially unanchored rings can be studied (139; S. Wang, B. O’Shaughnessy, unpublished manuscript) (Figure 4b).
Mechanism of ring tension production in fission yeast.
Stochastic simulations of molecularly explicit 2D (120) and 3D (S. Wang, H.F Chin, S. Thiyagarajan, Z. McDargh, E. Karatekin, et al., unpublished manuscript) models produced tensions similar to the experimental measurements in protoplasts. In the initial 2D model, the amounts and the biochemical properties of proteins in the ring were taken from experiment, and formins and node-like clusters of Myo2 were assumed to be independently anchored to the plasma membrane. The model did not include Myp2. Myo2 exerted 1 pN per head on an actin filament it binds, similar to muscle myosin (127).
The simulations generated ring tension of ~400 pN, because formins associated with nodes anchored the barbed ends of the actin filaments to the plasma membrane. Thus, every actin-myosin interaction pulled the filament (productive tensile contribution) rather than pushing it (compressive contribution) (Figure 4a; see the sidebar titled A Mechanism for Ring Tension). The mechanism does not require regular muscle-like architecture, as actin filaments are made tense almost independently. Consistent with the mechanism, nodes marked by Myo2 move bidirectionally at ~22 nm/s in constricting rings (96), much slower than the ~350 nm/s maximum load-free actin gliding velocity (128). Thus, forces opposing node motion (likely drag on membrane anchors) present sufficient resistance for myosin-II to operate close to the maximum stall force. Simulations showed that turnover of actin and myosin is required to generate tension, because without turnover, ring components aggregated into unproductive clusters.
A MECHANISM FOR RING TENSION: LATERAL ANCHORING OF ACTIN BARBED ENDS.
Perhaps the oldest question about the contractile ring is, How does it generate tension? Interactions of myosin-II with actin filaments are unproductive unless anchors restrain actin filaments to develop tension when pulled by myosin-II. Striated muscles solve this problem by anchoring actin filament barbed ends at sarcomere Z-discs. For contractile rings, a conceptually simple solution requiring no regular organization is lateral anchoring of barbed ends parallel to the plasma membrane. Fission yeast appears to adopt this solution, using membrane-anchored nodes with formins to anchor the barbed end of each actin filament (129) (Figure 4a). These filaments are aligned in a bundle and oriented clockwise or counterclockwise (99, 122, 123), resulting in two node populations with opposite motions (96). Consistent with this tension mechanism, unanchored ring segments shorten ~30-fold faster in permeabilized protoplasts than rings in normal cells (2, 121, 139), as predicted by a model in which barbed-end anchored filaments reel in unanchored segments (S. Wang, B. O’Shaughnessy, unpublished manuscript) (Figure 4b). The tension mechanism may be conserved, as contractile rings in much larger cells are similar in thicknesses to the yeast ring, so the membrane remains accessible.
The simulations also explained how rapid turnover and self-organization enable rings to disassemble as they constrict without loss of functionality (Figure 5b; see the sidebar titled Disassembly and Maintenance of the Constricting Ring). Dissociating components were replaced by incoming components that continuously self-assembled into the ring organization, a tight actomyosin bundle maximizing actin-myosin interactions (120). A key self-organizing process was Myo2-mediated zippering of formin-nucleated actin filaments into the bundle. Thus, every ~30 s the ring disassembles and reassembles itself, a mechanism that would enable it to quasi-statically shorten during the ~20 min of constriction without traumatizing the organization and compromising ring tension. This mechanism requires turnover and self-assembly rates to far exceed the relative constriction rate.
Figure 5.
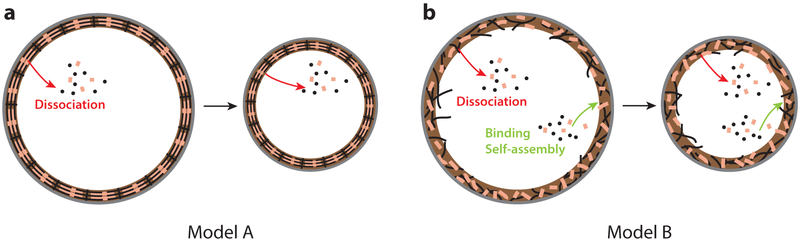
Two mechanisms for regulated disassembly of constricting contractile rings. (a) In model A, the ring consists of many similar or identical contractile units and shortens by controlled dissociation of proteins from each unit without compromising global structure. Memory of the initial structure is maintained throughout constriction. (b) In model B, the ring is continuously rebuilt as it constricts and as incoming components self-assemble and replace dissociated components more rapidly than constriction. The memory time is the turnover time. Both models preserve the organization of the ring and its ability to generate tension.
DISASSEMBLY AND MAINTENANCE OF THE CONSTRICTING RING.
Unlike muscle, contractile rings disassemble as they constrict (95, 121), raising the question, How can the bundle of actin filaments and myosin-II disassemble while maintaining its organization and function? One proposal is that its structure, comprising many sarcomere-like repeat units, is never forgotten (Figure 5a). The ring shortens through local disassembly that shortens units without compromising global structure. A modified sliding filament mechanism proposed disassembling actin filaments (121), and a similar picture was invoked to explain equal constriction times during successive divisions of C. elegans embryos (57). A second proposal is that the ring is rebuilt in tens of seconds by rapid turnover and self-assembly of incoming components (120) (Figure 5b). Provided constriction is slower than turnover-reassembly, the ring continuously rebuilds itself while preserving its organization and contractility In fission yeast, this mechanism is supported by the rapid turnover of components (<1 min) (176) and by simulations showing continuous self-organization of incoming components (120).
A new 3D molecularly explicit, stochastic simulation of constricting fission yeast rings (S. Wang, H.F. Chin, S. Thiyagarajan, Z. McDargh, E. Karatekin, et al., unpublished manuscript) included Myo2 and Cdc12p colocalized in membrane-bound nodes, as shown by super-resolution microscopy (95, 96), and with Myp2 binding only actin filaments (Figure 4c). The molecule numbers and turnover rates of components were set by experiment (95, 99, 130). During simulations, the components self-organized into an actomyosin bundle ~130 nm in diameter with a dense core of 30–35 filaments bundled by the anchored Myo2 and a corona of ~10 additional filaments cross-linked to the core by unanchored Myp2. With stall forces ~1.7 pN and 1.1 pN per Myo2 or Myp2 head, respectively, simulations reproduced the mean tension of ~640 pN, the increasing tension as rings constrict, and the loss of tension and organization in mutants of Myo2 and Myp2. The tension mechanism required actin barbed-end anchoring (Figure 4a; see the sidebar titled A Mechanism for Ring Tension), but unanchored Myp2 contributed one-third of the total tension by migrating to locations of zero net polarity in the actin bundle (131) (Figure 4c).
An analytical 1D model of constricting rings based on nodes anchoring Myo2 and formin Cdc12p reproduced the observed node motions and showed that contractile rings require lateral anchoring for organizational stability and tension (129) (see the sidebar titled A Mechanism for Ring Tension). Solutions of partial differential equations evolved bidirectional movements of nodes at ~22 nm/s, as observed (96, 132). A realistic ~2 pN of stall force per Myo2 head reproduced the experimental tension of 390 pN (120). Anchor drag resisted Myo2 pulling, enabling filaments to develop tension (Figure 4a). Half of the tension derived from a stochastic version of the sliding filament concept (2, 121), with Myo2 clusters pulling sliding actin filaments past them. The other fixed filament contribution was produced by chains of interconnected like-oriented nodes encircling the ring and also required firm lateral node anchoring, without which this contribution was rapidly destroyed by clumping instability.
Another molecularly explicit 3D simulation of the fission yeast contractile ring represented myosin-II as both two-headed membrane-anchored and four-headed unanchored molecules (133). Beads representing myosin heads pull actin filaments represented by flexible chains of beads, cross-linked by springs. The numbers of myosins and actin filaments in the cross section match experimentally measured values (95, 123). After testing different anchoring and clustering schemes, the authors favored a model with unclustered, anchored myosins, unanchored myosin, and unanchored actin. This model generated ~250 pN of tension. A node-like organization (clustered anchored myosin and actin) was rejected, because it produced severe membrane puckering. The node-induced puckering appears to result from a model rule that the surface representing the membrane and cell wall grows inward only where force is applied, but we are unaware of evidence supporting such a rule in cells.
Mechanism of ring constriction in fission yeast.
Fungi are enclosed by a cell wall, so cytokinesis must couple ring constriction to growth of a new cell wall, termed the septum, in the wake of the constricting ring (134, 135) (Figure 6b). The membrane is pinned against the cell wall by an internal turgor pressure of ~ 1.5 MPa (136), and the leading edge of the septum, the membrane lining furrow, and the contractile ring constrict together at the rate of inward radial septum growth, ~1.1 nm/s (130, 137).
Figure 6.
The relationship between ring tension and constriction rate. (a) By Laplace’s law, a contractile ring of radius R with tension T exerts an inward radial force T/R per ring length, tending to constrict the ring. The constriction rate is set by a balance of this force with opposing osmotic, elastic, and viscous cytoplasmic forces, tensile and viscous cortical forces in animal cells, and force associated with the septum in organisms with cell walls. (b) Fission yeast grows cell wall material termed the septum inward behind the contractile ring as it constricts. Confocal fluorescence micrographs of the division plane of a fission yeast cell expressing β-glucan synthase GFP-Bgs1 (labeling septum) and myosin-II light chain Rlc1-tdTomato (labeling the contractile ring). (c) In fission yeast, ring tension is too small to deform the septum mechanically, so the constriction rate is presumably set by the rate the septum is grown by Bgs1 and other glucan synthases in the membrane. The activity of Bgs1 may be mechanosensitive, with faster septum synthesis where curvature and the inward force from ring tension are high (green arrows). This mechanism would suppress rough features and ensure the septum is almost circular.
Septum growth sets the constriction rate, because the tension produced by a constricting contractile ring [T ~400 pN in protoplasts (120)] is no match for either the turgor pressure or the cell wall stiffness E ~50 MPa (136) (see the sidebar titled The Relation Between Ring Tension and Construction Rate). A ring 125 nm wide (96) with radius R ~1 μm produces an inward pressure ~T/wR ~2 kPa, almost three orders of magnitude below the reported turgor pressure, and negligible strains in the septum material ~T/wRE ~0.01% (130).
THE RELATION BETWEEN RING TENSION AND CONSTRICTION RATE.
The rate of constriction depends on the ring tension and forces that resist constriction (Figure 6a). In animal cells, the contractile ring is contiguous with a contractile cortex (115), so the ring constricts against cortical tension, osmotic pressure, and cortical and cytoplasmic viscous forces (163). When polar cortical tension is compromised, unbalanced ring and polar cortical tensions generate large oscillations (177). Membrane tension is much less than cortical tension (178), but membrane delivery to the division site (179) avoids lysis as the membrane area increases during division. In fungi and other organisms with cell walls, constriction requires centripetal growth of a septum (Figure 6b), which appears far too stiff (136) to be affected mechanically by ring tension (130). Thus, the constriction rate is set by the rate the septum grows into the furrow. However, ring tension could influence this rate if the enzymes synthesizing the septum are mechanosensitive (130, 138) (Figure 6c). The relation between tension and constriction rate is complex, so inferences about tension from constriction rate measurements should be made with care.
However, ring tension might influence the β-glucan and α-glucan synthases Bgs1–4 and Ags1 that synthesize the septum (134). Assuming that septal growth is mechanosensitive and mechanically coupled to the ring, a mathematical model reproduced characteristic edge roughness of septum edges in live cells and showed that ring tension would then modify septal growth rates in a curvature-dependent fashion, suppressing roughness and maintaining a nearly circular septum hole (Figure 6c) (130). The model predicted a Bgs1 mechanosensitivity of ~0.15 pN−1, the relative increase in synthesis rate per applied force (130). Consistent with curvature-dependent growth, septa grew faster in the more curved portions of elliptically shaped septum edges in mechanically deformed fission yeast cells (138).
In permeabilized protoplasts that lack cytoplasm, entire sections of rings detached from the membrane and shortened ~30-fold faster than rings in intact cells until becoming straight (139) (see the sidebar titled Fission Yeast Protoplasts). Both Myo2 and Myp2 contributed to this shortening, but neither actin assembly nor actin disassembly was required. A molecularly explicit simulation accurately reproduced and explained the experimental constriction rate and showed that segments of the bundle with actin filaments anchored by their barbed ends reel in unanchored actin filaments (S. Wang, B. O’Shaughnessy, unpublished manuscript) (Figure 4b). Simulations with other hypothetical anchoring schemes failed to constrict partially detached rings. Thus, the experimental findings and modeling explicitly support the barbed-end anchoring mechanism of tension generation (Figure 4a).
Contractile Ring Tension and Constriction in Budding Yeast Cells
Budding yeast cells assemble contractile rings from the same proteins as fission yeast but by a different pathway (explained above). Isolation of partially purified contractile rings confirmed the presence of actin, myosin-II (named Myo1), IQGAP, and an F-BAR protein (140). Electron micrographs show filaments thought to be comprised of myosin-II around the bud neck flanked by networks of septins (141).
Fluorescence recovery after photobleaching experiments showed that contractile ring proteins exchange with cytoplasmic pools at widely different rates that vary across the cell cycle (142). Tropomyosin exchanges in seconds, but myosin-II (Myo1) does not exchange until the ring constricts. Normal disassembly of the ring during constriction depends on the anaphase-promoting complex APC/C, a ubiquitin ligase that also controls the transition from metaphase to anaphase (143).
Mechanisms of ring tension production and constriction in budding yeast.
Like fission yeast, ring constriction in budding yeast is coupled to inward growth of the septum (105), but surprisingly some strains manage to divide (with difficulty) after deletion of the Myo1 gene (144). Other strains require myosin-II, but the myosin heads, which interact with actin filaments, are dispensable (145), so septum growth and closure can occur even without forces from Myo1. The essential tails of Myo1 may serve as scaffolds.
A mathematical model of disassembly driven constriction assumed that the actin filaments depolymerize from their pointed ends and that cross-links of myosin and other proteins grab retreating pointed ends of overlapping filaments, so they slide as they depolymerize, tending to restore overlap and constrict the ring (146). The model accounted for experimental data showing correlations between rates of ring disassembly and constriction, including the independence of relative constriction rate on initial ring length. Molecular aspects of this model require testing.
Contractile Ring Constriction and Disassembly in Dictyostelium
Although Dictyostelium is more distantly related to animals than fungi, it lacks a cell wall and handles cytokinesis more like an animal cell (147). The protein toolbox for cytokinesis is the same as fungi and animals and includes actin filaments, formins, IQGAP, and myosin-II (148, 149). When the cells are grown in liquid suspension, myosin-II is essential for cytokinesis (6), but these highly motile cells can divide by pulling themselves in two on a solid surface without myosin-II.
Structure of the contractile ring in Dictyostelium.
Dictyostelium has a very broad cleavage furrow associated with a network of short (100-nm average length) cross-linked actin filaments (150). Approximately 10% of total myosin-II concentrates in cortex across this broad cleavage furrow (151) as puncta similar to thick filaments (149) and exchanges with a half time of 7 s (152). The minimum force required to cleave the cell was estimated to reach a peak value of ~6 nN (151).
Mechanisms of ring tension production and constriction in Dictyostelium.
A mathematical model treated the cortex as a viscoelastic solid and the cytoplasm as a viscoelastic liquid and incorporated passive and myosin-mediated cortical stresses and forces from adhesion, protrusion, and pressure (153). In simulations, both nonadherent and adherent cells elongated and developed ingressing furrows. Without myosin-II, furrows ingressed only in adherent cells. The authors concluded that myosin or traction-mediated protrusive forces can initiate furrow ingression, whereas passive cortical tension and surface curvature forces complete ingression. An analytical model representing the cell as two lobes connected by a cylindrical furrow assumed axial compressive stresses and inward radial myosin stresses (154). The model predicted that myosin exerts a furrow stress of 0.1 nN μm−2 and that constriction rates are set by myosin forces, Laplace pressure, and resistive forces controlled by RacE and dynacortin. Mechanosensitivity of myosin-II and the cross-linker cortexillin-I helps to recruit these proteins and stabilize the furrow (147).
Contractile Ring Tension and Constriction in Animal Cells
In animal cells, a cortical zone of active RhoA drives the assembly of actin filaments and myosin-II (25). Actin filaments of both polarities are largely aligned with the ring (2, 155) and interdigitated with rods the size of myosin-II minifilaments (156). Super-resolution imaging confirmed that these rods and fluorescent spots of myosin-II in cleavage furrows (157, 158) are bipolar myosin-II filaments in contractile rings of vertebrate cultured cells (108) and sea urchin zygotes (109). The molecules anchoring the contractile ring to the plasma membrane are still under investigation (see the sidebar titled Anchoring Mechanisms).
ANCHORING MECHANISMS.
Both lipid-binding peripheral membrane proteins and transmembrane proteins are candidates to anchor contractile rings. For example, ring proteins interact with proteins that bind membrane lipids, including anillin with lipidbinding C2 and PH domains (72) and proteins with BAR or F-BAR domains (169). Membrane phosphoinositides participate in a mechanism different from the F-BAR protein Cdc15 (170). Other evidence supports anchoring roles for transmembrane enzymes that synthesize the cell wall in budding yeast (171) and fission yeast (135, 172). Cytoplasmic proteins including Sbg1p are thought to link these transmembrane proteins to the contractile ring proteins (105, 171, 173). Actin filaments in HeLa cell contractile rings converge on plasma membrane densities (156), but the anchoring proteins have not been identified. Epithelial cells use the transmembrane adhesion protein cadherin to anchor the contractile ring (174), but more work is required to characterize the anchors in most animal cells.
The concentration of myosin-II–GFP in constricting rings of four-cell C. elegans embryos was approximately constant throughout constriction (57) rather than increasing as in fission yeast (95). Photobleached zones of myosin-II–GFP in such rings regained fluorescence over 60–90 s from the sides of the bleached segment, suggesting that myosin in the ring is laterally mobile but does not exchange rapidly. The actin-sequestering drug Latrunculin A blocked ring assembly and slowed the constriction when administered after rings formed. The authors suggested that actin filaments in constricting rings shorten without replenishment from the cortex or cytoplasm (Figure 5a; see the sidebar titled Disassembly and Maintenance of the Constricting Ring). However, actin and myosin-II flow from the cortex into the cleavage furrows of single-cell C. elegans embryos (116), and the concentration of myosin-II-GFP increases exponentially over time in their rings (159), a behavior attributed to compression of the cortex as it flows into the furrow. The constriction rate per unit length of ring (minus the strain rate) increases in parallel, suggesting that myosin-II is responsible for the increasing magnitude of the strain rate.
Measurements of ring tension in echinoderm embryos.
Rappaport (5) used microneedles to measure a net inward radial force at the furrow of ~ 10–5 0 nN. Hiramoto (118) measured similar forces from the deformation of a ferrofluid droplet. The net force sums the ring tension and opposing forces from the cortex, viscous drag, and possibly other sources (Figure 6a). Yoneda & Dan (119) used axial force to compress dividing echinoderm eggs to measure cortical tension and applied an approximate force balance at the furrow to estimate the minimum ring tension for ingression. The lower bound of tension peaked at 45–60 nN and decayed as rings constricted. This approach assumed equal spherical cap lobes and uniform cortical tension, but the cortical tension is likely to vary spatially (160).
Mechanisms of ring tension production and constriction.
Antibody injection (4) and treatment with the myosin inhibitor blebbistatin (112) established that myosin-II is normally required for contractile ring constriction in animal cells. However, Cos7 cells can complete cytokinesis with one myosin-II isoform lacking ATPase activity (161), so other myosin isoforms may participate. Insufficient molecular details are known to establish the molecular mechanism of ring tension, but it likely entails myosin-II pulling actin filaments anchored to the membrane at their barbed ends (Figure 4).
Active gel models do not explicitly describe molecules but address large-scale behavior of the actomyosin cortex in a continuum framework, with force produced by myosin represented by active stress terms (162). In a model of constriction of dividing sand dollar zygotes, the cortex was treated as an incompressible viscoelastic layer with contractility from an isotropic active stress term proportional to cortex thickness, which tends to be restored locally by turnover (163). An equatorial zone with elevated active stress, assumed to represent myosin-II activated by RhoA-GTP, drove constriction, which was resisted by cortical viscosity and osmotic pressure enforcing almost constant cell volume. The model reproduced the observed evolution of cell shape and showed that isotropic equatorial activation is theoretically sufficient to drive furrow ingression.
A model of asymmetric furrowing of C. elegans embryos represented actin properties with continuous fields and described membrane- and ring-bending forces, membrane tension, osmotic pressure, and viscous forces. The model assumed a positive feedback loop where contractile forces bend the membrane, which tends to align the actin filaments and increase force locally (164). Solutions reproduced asymmetric furrowing characteristic of these cells owing to the mutually reinforcing effects of membrane curvature and filament alignment.
Constriction rates follow a scaling law in some organisms, with faster constriction in larger cells. In C. elegans embryos, shortening rates in successive divisions are proportional to initial ring length so that constriction times are invariant, suggestive of possible organization of the ring in repeat contractile units reminiscent of striated muscle sarcomeres, such that longer rings have proportionally more units (57) (Figure 5a). However, the authors concluded (159) that their new observation of parallel increases in the concentration of myosin in the ring and the constriction rate in single cell C. elegans embryos does not support the contractile unit model. Constriction rates in the filamentous fungus Neurospora crassa also increase with cell size but less than proportionally, such that constriction takes longer in larger cells (165).
Other Proposed Mechanisms of Ring Tension Production
Fission yeast and possibly animals appear to generate contractile ring tension by myosin-II exerting force on actin filaments whose barbed ends are membrane anchored (Figure 4a). Several alternative possible mechanisms have been explored theoretically (discussed below).
Some theoretical mechanisms rely on internal cross-linking schemes between myosin and actin. A general 1D model assumed sliding actin filament pairs, represented either as rods or by a continuous density field, and using either bipolar myosin motors that track actin filament barbed ends or cross-linkers that track pointed ends of treadmilling filaments (166). Simulations generated tension, but to our knowledge, end-tracking myosins or cross-linkers have not been identified in cells. Another study assumed myosin is attached to unanchored actin filament barbed ends in interconverting monopolar and bipolar actin-myosin structures, described by continuous fields (132). Solutions for various parameters showed rings with stationary and/or moving clusters or homogeneous rings, reminiscent of experiments showing circumferentially moving clusters in fission yeast but not in HeLa cells (132). This model produces ring tension, but we are unaware of the proposed structures or kinetics in cells.
Another proposal is based on actin filament buckling. A 1D mean field model described actin filaments subject to forces from myosins assumed to follow a distribution of force-velocity relations (167). In solutions of the model, some compressive segments buckled, leaving tensile segments in the majority and rendering the bundle tensile. Some filaments buckled during contraction of reconstituted actomyosin bundles, but it remains to be seen if such buckling is relevant to cells.
Actin filament treadmilling has been proposed as a factor in ring tension production. A 1D model represented actin filaments as arcs on a circle and myosins as points with two binding sites mediating filament sliding (168). Actin filaments treadmill, and newly polymerized actin must wait to bind randomly to a free myosin site, so that myosin becomes depleted near filament barbed ends and hence tension is generated. However, actin would presumably rapidly bind myosin, as myosin-II is not known to be processive and the mechanism of actin filament turnover remains to be established.
SUMMARY POINTS.
Amoebas, fungi, and animals use a common set of proteins, including actin, myosin-II, anillin, formins, and IQGAPs, to assemble contractile rings for cytokinesis.
Cell cycle kinases coordinate mitosis with cytokinesis.
Amoebas, fungi, and animals position the cleavage site between the daughter nuclei, but the mechanisms have diverged more than any other aspect of cytokinesis.
Animal cells use the mitotic spindle, centralspindlin complex, and chromosomal passenger complex to concentrate active Rho GTPase at the cleavage site, where it stimulates formins to polymerize actin and activates myosin-II.
Signals from the poles of the cell and the nucleus position the contractile ring in fission yeast, whereas budding yeast assemble a contractile ring in the neck between the mother cell and bud.
Forces produced by myosin-II on actin filaments assemble and then constrict the contractile ring to form a cleavage furrow.
As they constrict, contractile rings disassemble by shedding polymerized actin and, in some cells, other proteins.
When sufficient information is available about the number of molecules of participating proteins and their mechanisms, computer simulations can reproduce the observed physical events and predict the outcomes of future experiments.
FUTURE ISSUES.
The inventories of cytokinesis proteins used by animal cells will be completed.
More information about the cellular organization of cytokinesis proteins will improve hypotheses about the assembly and constriction of contractile rings.
Quantitative data on the numbers of each protein molecule and mechanisms of contractile ring proteins will allow for rigorous testing of hypotheses with mathematical models and computer simulations.
Quantitative measurements on cells with mutations and depletions of contractile ring proteins will test proposed mechanisms.
Biochemical reconstitution of signaling pathways and contractile rings from purified proteins will provide orthogonal tests for mechanisms and generate new ideas.
Membrane anchors for contractile rings will be identified and characterized to understand how forces are transmitted to the membrane.
The mechanisms used by fungi to coordinate forces in the contractile ring with the biosynthesis of the septum will be characterized.
The mechanisms that coordinate constriction and disassembly of contractile rings will be identified and characterized.
ACKNOWLEDGMENTS
Research on cytokinesis in the authors’ laboratories was supported by National Institute of General Medical Sciences of the National Institutes of Health under award numbers R01GM026132 (T.D.P.) and R01GM086731 (B.O.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Michael Glotzer for advice and Sathish Thiyagarajan and Joseph Wang for helpful discussions.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Schroeder TE. 1970. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z. Zellforsch. Mikrosk. Anat 109:431–49 [PubMed] [Google Scholar]
- 2.Schroeder TE. 1973. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. PNAS 70:1688–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara K, Pollard TD. 1976. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J. Cell Biol 71:848–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mabuchi I, Okuno M. 1977. The effect of myosin antibody on the division of starfish blastomeres. J. Cell Biol. 74:251–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rappaport R 1967. Cell division: direct measurement of maximum tension exerted by furrow of echinoderm eggs. Science 156:1241–43 [DOI] [PubMed] [Google Scholar]
- 6.DeLozanne A, Spudich JA. 1987. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236:1086–91 [DOI] [PubMed] [Google Scholar]
- 7.Marks J, Hyams JS. 1985. Localization of F-actin through the cell division cycle of Schizosaccharomyces pombe. Eur. J. Cell Biol 39:27–32 [Google Scholar]
- 8.Kitayama C, Sugimoto A, Yamamoto M. 1997. Type II myosin heavy chain encoded by the myo2 gene composes the contractile ring during cytokinesis in Schizosaccharomyces pombe. J. Cell Biol. 137:1309–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheffings TH, Burroughs NJ, Balasubramanian MK. 2016. Actomyosin ring formation and tension generation in eukaryotic cytokinesis. Curr. Biol 26:R719–37 [DOI] [PubMed] [Google Scholar]
- 10.Glotzer M 2016. Cytokinesis in metazoa and fungi. Cold Spring Harb. Perspect. Biol 9:a022343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green RA, Paluch E, Oegema K. 2012. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol 28:29–58 [DOI] [PubMed] [Google Scholar]
- 12.Pollard TD. 2017. Nine unanswered questions about cytokinesis. J. Cell Biol. 216:3007–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odronitz F, Kollmar M. 2007. Drawing the tree of eukaryotic life based on the analysis of 2,269 manually annotated myosins from 328 species. Genome Biol. 8:R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller S, Jürgens G. 2016. Plant cytokinesis—no ring, no constriction but centrifugal construction of the partitioning membrane. Semin. Cell Dev. Biol 53:10–18 [DOI] [PubMed] [Google Scholar]
- 15.Hardin WR, Li R, Xu J, Shelton AM, Alas GCM, et al. 2017. Myosin-independent cytokinesis in Giardia utilizes flagella to coordinate force generation and direct membrane trafficking. PNAS 114:E5854–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mierzwa B, Gerlich DW. 2014. Cytokinetic abscission: molecular mechanisms and temporal control. Dev. Cell 31:525–38 [DOI] [PubMed] [Google Scholar]
- 17.Freémont S, Echard A. 2018. Membrane traffic in the late steps of cytokinesis. Curr. Biol 28:R458–70 [DOI] [PubMed] [Google Scholar]
- 18.Xiao J, Goley ED. 2016. Redefining the roles of the FtsZ-ring in bacterial cytokinesis. Curr. Opin. Microbiol 34:90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basant A, Glotzer M. 2018. Spatiotemporal regulation of RhoA during cytokinesis. Curr. Biol 28:R570–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rappaport R 1996. Cytokinesis in Animal Cells. Cambridge, UK: Cambridge Univ. Press [Google Scholar]
- 21.Cao LG, Wang YL. 1996. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol. Biol. Cell 7:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Dassow G, Verbrugghe KJ, Miller AL, Sider JR, Bement WM. 2009. Action at a distance during cytokinesis. J. Cell Biol 187:831–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su KC, Bement WM, Petronczki M, von Dassow G. 2014. An astral simulacrum of the central spindle accounts for normal, spindle-less, and anucleate cytokinesis in echinoderm embryos. Mol. Biol. Cell 25:4049–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swan KA, Severson AF, Carter JC, Martin PR, Schnabel H, et al. 1998. cyk-1: a C. elegans FH gene required for a late step in embryonic cytokinesis. J. Cell Sci 111:2017–27 [DOI] [PubMed] [Google Scholar]
- 25.Bement WM, Benink HA, von Dassow G. 2005. A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol 170:91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller AL, Bement WM. 2009. Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol 11:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers SL, Wiedemann U, Stuurman N, Vale RD. 2003. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol 162:1079–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen PA, Groen AC, Loose M, Ishihara K, Wühr M, et al. 2014. Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Science 346:244–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niiya F, Xie X, Lee KS, Inoué H, Miki T. 2005. Inhibition of cyclin-dependent kinase 1 induces cytokinesis without chromosome segregation in an ECT2 and MgcRacGAP-dependent manner. J. Biol. Chem 280:36502–9 [DOI] [PubMed] [Google Scholar]
- 30.Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, et al. 2006. The reversibility of mitotic exit in vertebrate cells. Nature 440:954–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canman JC, Hoffman DB, Salmon ED. 2000. The role of pre- and post-anaphase microtubules in the cytokinesis phase of the cell cycle. Curr. Biol 10:611–14 [DOI] [PubMed] [Google Scholar]
- 32.Mishima M, Pavicic V, Gruneberg U, Nigg EA, Glotzer M. 2004. Cell cycle regulation of central spindle assembly. Nature 430:908–13 [DOI] [PubMed] [Google Scholar]
- 33.Hara T, Abe M, Inoue H, Yu LR, Veenstra TD, et al. 2006. Cytokinesis regulator ECT2 changes its conformation through phosphorylation at Thr-341 in G2/M phase. Oncogene 25:566–78 [DOI] [PubMed] [Google Scholar]
- 34.Yüce Ö, Piekny A, Glotzer M. 2005. An ECT2-centralspindlin complex regulates the localization and function of RhoA. J. Cell Biol 170:571–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su KC, Takaki T, Petronczki M. 2011. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev. Cell 21:1104–15 [DOI] [PubMed] [Google Scholar]
- 36.Hu CK, Ozlü N, Coughlin M, Steen JJ, Mitchison TJ 2012. Plk1 negatively regulates PRC1 to prevent premature midzone formation before cytokinesis. Mol. Biol. Cell 23:2702–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Guo F, Brahma S, Xing Y, Burkard ME. 2014. Centralspindlin assembly and 2 phosphorylations on MgcRacGAP by Polo-like kinase 1 initiate Ect2 binding in early cytokinesis. Cell Cycle 13:2952–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishima M, Kaitna S, Glotzer M. 2002. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev. Cell 2:41–54 [DOI] [PubMed] [Google Scholar]
- 39.White EA, Raghuraman H, Perozo E, Glotzer M. 2013. Binding of the CYK-4 subunit of the centralspindlin complex induces a large scale conformational change in the kinesin subunit. J. Biol. Chem 288:19785–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nislow C, Lombillo VA, Kuriyama R, McIntosh JR. 1992. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature 359:543–47 [DOI] [PubMed] [Google Scholar]
- 41.Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, et al. 1998. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J. Biol. Chem 273:6019–23 [DOI] [PubMed] [Google Scholar]
- 42.Kimura K, Tsuji T, Takada Y, Miki T, Narumiya S. 2000. Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem 275:17233–36 [DOI] [PubMed] [Google Scholar]
- 43.Lekomtsev S, Su KC, Pye VE, Blight K, Sundaramoorthy S, et al. 2012. Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis. Nature 492:276–79 [DOI] [PubMed] [Google Scholar]
- 44.Somers WG, Saint R. 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell 4:29–39 [DOI] [PubMed] [Google Scholar]
- 45.Zhang D, Glotzer M. 2015. The RhoGAP activity of CYK-4/MgcRacGAP functions non-canonically by promoting RhoA activation during cytokinesis. eLife 4:e08898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmena M, Wheelock M, Funabiki H, Earnshaw WC. 2012. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat. Rev. Mol. Cell. Biol 13:789–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basant A, Lekomtsev S, Tse YC, Zhang D, Longhini KM, et al. 2015. Aurora B kinase promotes cytokinesis by inducing centralspindlin oligomers that associate with the plasma membrane. Dev. Cell 33:204–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prokopenko SN, Brumby A, O’Keefe L, Prior L, He Y, et al. 1999. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev. 13:2301–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. 1999. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J. Cell Biol 147:921–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner E, Glotzer M. 2016. Local RhoA activation induces cytokinetic furrows independent of spindle position and cell cycle stage. J. Cell Biol 213:641–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J-E, Billadeau DD, Chen J. 2005. The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J. Biol. Chem 280:5733–39 [DOI] [PubMed] [Google Scholar]
- 52.Nishimura Y, Yonemura S. 2006. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. J. Cell Sci 119:104–14 [DOI] [PubMed] [Google Scholar]
- 53.Schmutz C, Stevens J, Spang A. 2007. Functions of the novel RhoGAP proteins RGA-3 and RGA-4 in the germ line and in the early embryo of C. elegans. Development 134:3495–505 [DOI] [PubMed] [Google Scholar]
- 54.Schonegg S, Constantinescu AT, Hoege C, Hyman AA. 2007. The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in Caenorhabditis elegans one-cell embryos. PNAS 104:14976–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zanin E, Desai A, Poser I, Toyoda Y, Andree C, et al. 2013. A conserved RhoGAP limits M phase contractility and coordinates with microtubule asters to confine RhoA during cytokinesis. Dev. Cell 26:496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tse YC, Werner M, Longhini KM, Labbé J-C, Goldstein B, Glotzer M. 2012. RhoA activation during polarization and cytokinesis of the early Caenorhabditis elegans embryo is differentially dependent on NOP-1 and CYK-4. Mol. Biol. Cell 23:4020–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carvalho A, Desai A, Oegema K. 2009. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell 137:926–37 [DOI] [PubMed] [Google Scholar]
- 58.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, et al. 2008. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science 322:1543–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuravlev Y, Hirsch SM, Jordan SN, Dumont J, Shirasu-Hiza M, Canman JC. 2017. CYK-4 regulates Rac, but not Rho, during cytokinesis. Mol. Biol. Cell 28:1258–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutterer A, Glotzer M, Mishima M. 2009. Clustering of centralspindlin is essential for its accumulation to the central spindle and the midbody. Curr. Biol. 19:2043–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subramanian R, Wilson-Kubalek EM, Arthur CP, Bick MJ, Campbell EA, et al. 2010. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell 142:433–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbrugghe KJC, White JG. 2004. SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr. Biol. 14:1755–60 [DOI] [PubMed] [Google Scholar]
- 63.Kotynkova K, Su KC, West SC, Petronczki M. 2016. Plasma membrane association but not midzone recruitment of RhoGEF ECT2 is essential for cytokinesis. Cell Rep. 17:2672–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodrigues NT, Lekomtsev S, Jananji S, Kriston-Vizi J, Hickson GR, Baum B. 2015. Kinetochore-localized PP1–Sds22 couples chromosome segregation to polar relaxation. Nature 524:489–92 [DOI] [PubMed] [Google Scholar]
- 65.Bieling P, Telley IA, Surrey T. 2010. A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell 142:420–32 [DOI] [PubMed] [Google Scholar]
- 66.Mangal S, Sacher J, Kim T, Osório DS, Motegi F, et al. 2018. TPXL-1 activates Aurora A to clear contractile ring components from the polar cortex during cytokinesis. J. Cell Biol 217:837–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akamatsu MS, Berro J, Pu K-M, Tebbs IR, Pollard TD. 2014. Cytokinetic nodes in fission yeast arise from two distinct types of nodes that merge during interphase. J. Cell Biol 204:977–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moseley JB, Mayeux A, Paoletti A, Nurse P. 2009. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature 459:857–60 [DOI] [PubMed] [Google Scholar]
- 69.Martin SG, Berthelot-Grosjean M. 2009. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature 459:852–56 [DOI] [PubMed] [Google Scholar]
- 70.Akamatsu M, Lin Y, Bewersdorf J, Pollard TD. 2017. Analysis of interphase node proteins in fission yeast by quantitative and super resolution fluorescence microscopy. Mol. Biol. Cell 28:3203–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paoletti A, Chang F. 2000. Analysis of mid1p, a protein required for placement of the cell division site, reveals a link between the nucleus and the cell surface in fission yeast. Mol. Biol. Cell 11:2757–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun L, Guan R, Lee IJ, Liu Y, Chen M, et al. 2015. Mechanistic insights into the anchorage of the contractile ring by anillin and Mid1. Dev. Cell 33:413–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang Y, Chew TG, Ge W, Balasubramanian MK. 2007. Polarity determinants Tea1p, Tea4p, and Pom1p inhibit division-septum assembly at cell ends in fission yeast. Dev. Cell 12:987–96 [DOI] [PubMed] [Google Scholar]
- 74.Hersch M, Hachet O, Dalessi S, Ullal P, Bhatia P, et al. 2015. Pom1 gradient buffering through inter-molecular auto-phosphorylation. Mol. Syst. Biol 11:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rincon SA, Bhatia P, Bicho C, Guzman-Vendrell M, Fraisier V, et al. 2014. Pom1 regulates the assembly of Cdr2-Mid1 cortical nodes for robust spatial control of cytokinesis. J. Cell Biol 206:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Celton-Morizur S, Bordes N, Fraisier V, Tran PT, Paoletti A. 2004. C-terminal anchoring of mid1p to membranes stabilizes cytokinetic ring position in early mitosis in fission yeast. Mol. Cell. Biol 24:10621–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padte NN, Martin SG, Howard M, Chang F. 2006. The cell-end factor pom1p inhibits mid1p in specification of the cell division plane in fission yeast. Curr. Biol 16:2480–87 [DOI] [PubMed] [Google Scholar]
- 78.Lee ME, Rusin SF, Jenkins N, Kettenbach AN, Moseley JB. 2018. Mechanisms connecting the conserved protein kinases Ssp1, Kin1, and Pom1 in fission yeast cell polarity and division. Curr. Biol 28:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tran PT, Doye V, Inoue S, Chang F. 2001. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 153:397–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Daga RR, Chang F. 2005. Dynamic positioning of the fission yeast cell division plane. PNAS 102:8228–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tolic-Norrelykke IM, Sacconi L, Stringari C, Raabe I, Pavone FS. 2005. Nuclear and division-plane positioning revealed by optical micromanipulation. Curr. Biol 15:1212–16 [DOI] [PubMed] [Google Scholar]
- 82.Chang F, Wollard A, Nurse P. 1996. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J. Cell Sci 109:131–42 [DOI] [PubMed] [Google Scholar]
- 83.Sohrmann M, Fankhauser C, Brodbeck C, Simanis V. 1996. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes Dev. 10:2707–19 [DOI] [PubMed] [Google Scholar]
- 84.Wu JQ, Kuhn JR, Kovar DR, Pollard TD. 2003. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5:723–34 [DOI] [PubMed] [Google Scholar]
- 85.Almonacid M, Celton-Morizur S, Jakubowski J, Dingli F, Loew D, et al. 2011. Temporal control of contractile ring assembly by Plo1 regulation of myosin II recruitment by Mid1/anillin. Curr. Biol 21:473–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Almonacid M, Moseley JB, Janvore J, Mayeux A, Fraisier V, et al. 2009. Spatial control of cytokinesis by Cdr2 kinase and Mid1/anillin nuclear export. Curr. Biol 19:961–66 [DOI] [PubMed] [Google Scholar]
- 87.Rincon SA, Paoletti A. 2012. Mid1/anillin and the spatial regulation of cytokinesis in fission yeast. Cytoskeleton 69:764–77 [DOI] [PubMed] [Google Scholar]
- 88.Simanis V. 2015. Pombe’s thirteen—control of fission yeast cell division by the septation initiation network. J. Cell Sci. 128:1465–74 [DOI] [PubMed] [Google Scholar]
- 89.Rincon SA, Estravis M, Dingli F, Loew D, Tran PT, Paoletti A. 2017. SIN-dependent dissociation of the SAD kinase Cdr2 from the cell cortex resets the division plane. Curr. Biol. 27:534–42 [DOI] [PubMed] [Google Scholar]
- 90.Pu K-M, Akamatsu M, Pollard TD. 2015. The fission yeast septation initiation network controls type 1 cytokinesis nodes. J. Cell Sci 128:441–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiou JG, Balasubramanian MK, Lew DJ. 2017. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol 33:77–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goryachev AB, Pokhilko AV. 2008. Dynamics of Cdc42 network embodies a Turing-type mechanism of yeast cell polarity. FEBS Lett. 582:1437–43 [DOI] [PubMed] [Google Scholar]
- 93.Witte K, Strickland D, Glotzer M. 2017. Cell cycle entry triggers a switch between two modes of Cdc42 activation during yeast polarization. eLife 6:e26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyazaki M, Chiba M, Eguchi H, Ohki T, Ishiwata S. 2015. Cell-sized spherical confinement induces the spontaneous formation of contractile actomyosin rings in vitro. Nat. Cell Biol 17:480–89 [DOI] [PubMed] [Google Scholar]
- 95.Wu JQ, Pollard TD. 2005. Counting cytokinesis proteins globally and locally in fission yeast. Science 310:310–14 [DOI] [PubMed] [Google Scholar]
- 96.Laplante C, Huang F, Tebbs IR, Bewersdorf J, Pollard TD. 2016. Molecular organization of cytokinesis nodes and contractile rings by super-resolution fluorescence microscopy of live fission yeast. PNAS 113:E5876–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willet AH, McDonald NA, Bohnert KA, Baird MA, Allen JR, et al. 2015. The F-BAR Cdc15 promotes contractile ring formation through the direct recruitment of the formin Cdc12. J. Cell Biol 208:391–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kovar DR, Kuhn JR, Tichy AL, Pollard TD. 2003. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol 161:875–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Courtemanche N, Pollard TD, Chen Q. 2016. Avoiding artefacts when counting polymerized actin in live cells with LifeAct fused to fluorescent proteins. Nat. Cell Biol 18:676–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vavylonis D, Wu J-Q, Hao S, O’Shaughnessy B, Pollard TD. 2008. Assembly mechanism of the contractile ring for cytokinesis by fission yeast. Science 319:97–100 [DOI] [PubMed] [Google Scholar]
- 101.Wang N, Lo Presti L, Zhu YH, Kang M, Wu Z, et al. 2014. The novel proteins Rng8 and Rng9 regulate the myosin-V Myo51 during fission yeast cytokinesis. J. Cell Biol 205:357–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laplante C, Berro J, Karatekin E, Lee R, Hernandez-Leyva A, Pollard TD. 2015. Three myosins contribute uniquely to the assembly and constriction of the cytokinetic contractile ring in fission yeast. Curr. Biol 25:1955–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Q, Pollard TD. 2011. Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J. Cell Biol 195:485–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bidone TC, Tang H, Vavylonis D. 2014. Dynamic network morphology and tension buildup in 3D model of cytokinetic ring assembly. Biophys. J 107:2618–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhavsar-Jog YP, Bi E. 2017. Mechanics and regulation of cytokinesis in budding yeast. Semin. Cell Dev. Biol 66:107–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsumura F 2005. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 15:371–77 [DOI] [PubMed] [Google Scholar]
- 107.Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, et al. 2008. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol. Biol. Cell 19:2328–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beach JR, Shao L, Remmert K, Li D, Betzig E, Hammer JA III. 2014. Nonmuscle myosin II isoforms coassemble in living cells. Curr. Biol 24:1160–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Henson JH, Ditzler CE, Germain A, Irwin PM, Vogt ET, et al. 2017. The ultrastructural organization of actin and myosin II filaments in the contractile ring: new support for an old model of cytokinesis. Mol. Biol. Cell 28:613–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schroeder TE, Otto J. 1988. Association of actin and myosin in the contractile ring. Zool. Sci 5:713–25 [DOI] [PubMed] [Google Scholar]
- 111.Dean SO, Rogers SL, Stuurman N, Vale RD, Spudich JA 2005. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. PNAS 102:13473–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, et al. 2003. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299:1743–47 [DOI] [PubMed] [Google Scholar]
- 113.Castrillon D, Wasserman S. 1994. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 120:3367–77 [DOI] [PubMed] [Google Scholar]
- 114.Watanabe S, De Zan T, Ishizaki T, Yasuda S, Kamijo H, et al. 2013. Loss of a Rho-regulated actin nucleator, mDia2, impairs cytokinesis during mouse fetal erythropoiesis. Cell Rep. 5:926–32 [DOI] [PubMed] [Google Scholar]
- 115.Chalut KJ, Paluch EK. 2016. The actin cortex: a bridge between cell shape and function. Dev. Cell 38:571–73 [DOI] [PubMed] [Google Scholar]
- 116.Reymann AC, Staniscia F, Erzberger A, Salbreux G, Grill SW. 2016. Cortical flow aligns actin filaments to form a furrow. eLife 5:e17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hiramoto Y 1970. Rheological properties of sea urchin eggs. Biorheology 6:201–34 [DOI] [PubMed] [Google Scholar]
- 118.Hiramoto Y 1975. Force exerted by the cleavage furrow of sea urchin eggs. Dev. Growth Differ 17:27–38 [DOI] [PubMed] [Google Scholar]
- 119.Yoneda M, Dan K. 1972. Tension at the surface of the dividing sea-urchin egg. J. Exp. Biol 57:575–87 [DOI] [PubMed] [Google Scholar]
- 120.Stachowiak MR, Laplante C, Chin HF, Guirao B, Karatekin E, et al. 2014. Mechanism of cytokinetic contractile ring constriction in fission yeast. Dev. Cell 29:547–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schroeder TE. 1972. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J. Cell Biol 53:419–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kamasaki T, Osumi M, Mabuchi I. 2007. Three-dimensional arrangement of F-actin in the contractile ring of fission yeast. J. Cell Biol 178:765–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Swulius MT, Nguyen LT, Ladinsky MS, Ortega DR, Aich S, et al. 2018. Structure of the fission yeast actomyosin ring during constriction. PNAS 115:E1455–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McDonald NA, Lind AL, Smith SE, Li R, Gould KL. 2017. Nanoscale architecture of the Schizosaccharomyces pombe contractile ring. eLife 6:e28865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takaine M, Numata O, Nakano K. 2015. An actin–myosin-II interaction is involved in maintaining the contractile ring in fission yeast. J. Cell Sci 128:2903–18 [DOI] [PubMed] [Google Scholar]
- 126.Lord M, Pollard TD. 2004. UCS protein Rng3p activates actin filament gliding by fission yeast myosin-II. J. Cell Biol 167:315–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Molloy JE, Burns JE, Kendrick-Jones J, Tregear RT, White DC. 1995. Movement and force produced by a single myosin head. Nature 378:209–12 [DOI] [PubMed] [Google Scholar]
- 128.Stark BC, Sladewski TE, Pollard LW, Lord M 2010. Tropomyosin and myosin-II cellular levels promote actomyosin ring assembly in fission yeast. Mol. Biol. Cell 21:989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thiyagarajan S, Wang S, O’Shaughnessy B. 2017. A node organization in the actomyosin contractile ring generates tension and aids stability. Mol. Biol. Cell 28:3286–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thiyagarajan S, Munteanu EL, Arasada R, Pollard TD, O’Shaughnessy B. 2015. The fission yeast cytokinetic contractile ring regulates septum shape and closure. J. Cell Sci 28:3672–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stachowiak MR, McCall PM, Thoresen T, Balcioglu HE, Kasiewicz L, et al. 2012. Self-organization of myosin II in reconstituted actomyosin bundles. Biophys. J 103:1265–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wollrab V, Thiagarajan R, Wald A, Kruse K, Riveline D. 2016. Still and rotating myosin clusters determine cytokinetic ring constriction. Nat. Commun 7:11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen LT, Swulius MT, Aich S, Mishra M, Jensen GJ. 2018. Coarse-grained simulations of actomyosin rings point to a nodeless model involving both unipolar and bipolar myosins. Mol. Biol. Cell 29:1318–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cortés JC, Konomi M, Martins IM, Muñoz J, Moreno MB, et al. 2007. The (1,3)β-d-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol 65:201–17 [DOI] [PubMed] [Google Scholar]
- 135.Muñoz J, Cortés J, Sipiczki M, Ramos M, Clemente-Ramos JA, et al. 2013. Extracellular cell wall β(1,3)glucan is required to couple septation to actomyosin ring contraction. J. Cell Biol 203:265–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Atilgan E, Magidson V, Khodjakov A, Chang F. 2015. Morphogenesis of the fission yeast cell through cell wall expansion. Curr. Biol 25:2150–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Proctor SA, Minc N, Boudaoud A, Chang F. 2012. Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr. Biol 22:1601–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou Z, Munteanu EL, He J, Ursell T, Bathe M, et al. 2015. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Mol. Biol. Cell 26:78–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mishra M, Kashiwazaki J, Takagi T, Srinivasan R, Huang Y, et al. 2013. In vitro contraction of cytokinetic ring depends on myosin II but not on actin dynamics. Nat. Cell Biol 15:853–59 [DOI] [PubMed] [Google Scholar]
- 140.Young BA, Buser C, Drubin DG. 2010. Isolation and partial purification of thé Saccharomyces cerevisiae cytokinetic apparatus. Cytoskeleton 67:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ong K, Wloka C, Okada S, Svitkina T, Bi E. 2014. Architecture and dynamic remodelling of the septin cytoskeleton during the cell cycle. Nat. Commun 5:5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wloka C, Vallen EA, The L, Fang X, Oh Y, Bi E. 2013. Immobile myosin-II plays a scaffolding role during cytokinesis in budding yeast. J. Cell Biol 200:271–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tully GH, Nishihama R, Pringle JR, Morgan DO. 2009. The anaphase-promoting complex promotes actomyosin-ring disassembly during cytokinesis in yeast. Mol. Biol. Cell 20:1201–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bi E, Maddox P, Lew DJ, Salmon ED, McMillan JN, et al. 1998. Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol 142:1301–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lord M, Laves E, Pollard TD. 2005. Cytokinesis depends on the motor domains of myosin-II in fission yeast but not in budding yeast. Mol. Biol. Cell 16:5346–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mendes Pinto I, Rubinstein B, Kucharavy A, Unruh JR, Li R. 2012. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell 22:1247–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Srivastava V, Iglesias PA, Robinson DN. 2016. Cytokinesis: robust cell shape regulation. Semin. Cell Dev. Biol 53:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Faix J, Weber I, Mintert U, Köhler J, Lottspeich F, Marriott G. 2001. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 20:3705–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yumura S, Fukui Y. 1985. Reversible cyclic AMP-dependent change in distribution of myosin thick filaments in Dictyostelium. Nature 314:194–96 [DOI] [PubMed] [Google Scholar]
- 150.Reichl EM, Ren Y, Morphew MK, Delannoy M, Effler JC, et al. 2008. Interactions between myosin and actin crosslinkers control cytokinesis contractility dynamics and mechanics. Curr. Biol 18:471–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robinson DN, Cavet G, Warrick HM, Spudich JA. 2002. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biol. 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yumura S 2001. Myosin II dynamics and cortical flow during contractile ring formation in Dictyostelium cells. J. Cell Biol 154:137–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poirier CC, Ng WP, Robinson DN, Iglesias PA. 2012. Deconvolution of the cellular force-generating subsystems that govern cytokinesis furrow ingression. PLOS Comput. Biol 8:e1002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang WD, Robinson DN. 2005. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. PNAS 102:7186–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sanger JM, Sanger JW. 1980. Banding and polarity of actin filaments in interphase and cleaving cells. J. Cell Biol 86:568–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maupin P, Pollard TD. 1986. Arrangement of actin filaments and myosin-like filaments in the contractile ring and of actin-like filaments in the mitotic spindle of dividing HeLa cells. J. Ultrastruct. Mol. Struct. Res 94:92–103 [DOI] [PubMed] [Google Scholar]
- 157.Maupin P, Phillips CL, Adelstein RS, Pollard TD. 1994. Differential localization of myosin-II isozymes in human cultured cells and blood cells. J. Cell Sci 107:3077–90 [DOI] [PubMed] [Google Scholar]
- 158.Zhou M, Wang YL. 2008. Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol. Biol. Cell 19:318–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khaliullin RN, Green RA, Shi LZ, Gomez-Cavazos JS, Berns MW, et al. 2018. A positive-feedback-based mechanism for constriction rate acceleration during cytokinesis in Caenorhabditzs elegans. eLife 7:e36073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hiramoto Y 1967. Observations and measurements of sea urchin eggs with a centrifuge microscope. J. Am. Vet. Med. Assoc 150:219–30 [DOI] [PubMed] [Google Scholar]
- 161.Ma X, Kovacs M, Conti MA, Wang A, Zhang Y, et al. 2012. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. PNAS 109:4509–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Joanny JF, Kruse K, Ramaswamy S. 2013. The actin cortex as an active wetting layer. Eur. Phys. J. E 36:52–58 [DOI] [PubMed] [Google Scholar]
- 163.Turlier H, Audoly B, Prost J, Joanny JF. 2014. Furrow constriction in animal cell cytokinesis. Biophys. J 106:114–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dorn JF, Zhang L, Phi TT, Lacroix B, Maddox PS, et al. 2016. A theoretical model of cytokinesis implicates feedback between membrane curvature and cytoskeletal organization in asymmetric cytokinetic furrowing. Mol. Biol. Cell 27:1286–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Calvert ME, Wright GD, Leong FY, Chiam KH, Chen Y, et al. 2011. Myosin concentration underlies cell size-dependent scalability of actomyosin ring constriction. J. Cell Biol 195:799–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zumdieck A, Kruse K, Bringmann H, Hyman AA, Jülicher F. 2007. Stress generation and filament turnover during actin ring constriction. PLOS ONE 2:e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lenz M, Thoresen T, Gardel ML, Dinner AR. 2012. Contractile units in disordered actomyosin bundles arise from F-actin buckling. Phys. Rev. Lett 108:238107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oelz D, Rubinstein B, Mogilner A. 2015. A combination of actin treadmilling and cross-linking drives contraction of random actomyosin arrays. Biophys. J 109:1818–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McDonald NA, Vander Kooi CW, Ohi MD, Gould KL. 2015. Oligomerization but not membrane bending underlies the function of certain F-BAR proteins in cell motility and cytokinesis. Dev. Cell 35:725–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Snider CE, Willet AH, Chen JS, Arpağ G, Zanic M, Gould KL. 2017. Phosphoinositide-mediated ring anchoring resists perpendicular forces to promote medial cytokinesis. J. Cell Biol 216:3041–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Meitinger F, Palani S. 2016. Actomyosin ring driven cytokinesis in budding yeast. Semin. Cell Dev. Biol 53:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arasada R, Pollard TD. 2014. Contractile ring stability in S. pombe depends on F-BAR protein Cdc15p and Bgs1p transport from the Golgi complex. Cell Rep. 8:1533–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sethi K, Palani S, Cortés JC, Sato M, Sevugan M, et al. 2016. A new membrane protein Sbg1 links the contractile ring apparatus and septum synthesis machinery in fission yeast. PLOS Genet 12:e1006383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoffman BD, Yap AS. 2015. Towards a dynamic understanding of cadherin-based mechanobiology. Trends Cell Biol 25:803–14 [DOI] [PubMed] [Google Scholar]
- 175.Mishra M, Huang Y, Srivastava P, Srinivasan R, Sevugan M, et al. 2012. Cylindrical cellular geometry ensures fidelity of division site placement in fission yeast. J. Cell Sci 125:3850–57 [DOI] [PubMed] [Google Scholar]
- 176.Pelham RJ Jr., Chang F 2001. Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nat. Cell Biol 3:235–44 [DOI] [PubMed] [Google Scholar]
- 177.Sedzinski J, Biro M, Oswald A, Tinevez JY, Salbreux G, Paluch E. 2011. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 476:462–66 [DOI] [PubMed] [Google Scholar]
- 178.Tinevez JY, Schulze U, Salbreux G, Roensch J, Joanny JF, Paluch E. 2009. Role of cortical tension in bleb growth. PNAS 106:18581–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang N, Lee IJ, Rask G, Wu JQ. 2016. Roles of the TRAPP-II complex and the exocyst in membrane deposition during fission yeast cytokinesis. PLOS Biol 14:e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]