Abstract
Objective::
We assessed the changes that have resulted from the latest breast cancer staging guidelines and the potential impact on prognosis.
Background:
Contemporary data suggest that combining anatomic staging and tumor biology yields a predictive synergy for determining breast cancer prognosis. This forms the basis for the American Joint Committee on Cancer’s (AJCC) Staging Manual, 8th edition. We assessed the changes that have resulted from the new staging guidelines and the potential impact on prognosis.
Methods:
Women with stages I to III breast cancer from 2010 to 2014 in the National Cancer Data Base were pathologically staged according to the 7th and 8th editions of the AJCC Staging Manual. Patient characteristics and restaging outcomes were summarized. Unadjusted overall survival (OS) was estimated, and differences were assessed. Cox proportional-hazards models were utilized to estimate the adjusted association of stage with OS.
Results:
After restaging the 493,854 women identified, 6.8% were upstaged and 29.7% were downstaged. The stage changes varied by tumor histology, receptor status, tumor grade, and Oncotype DX scores (all P < 0.0001). Applying the 8th edition criteria yielded an incremental reduction in survival for each increase in stage, which was not consistently seen in the 7th edition. In a subgroup analysis based on hormone receptor (HR) status, those with stages II and III, and HR− disease had a worse OS than those with HR+ disease.
Conclusions:
Applying the 8th edition staging criteria resulted in a stage change for >35% of patients diagnosed with invasive breast cancer and refined OS estimates. Overall, the transition to the 8th edition is expected to better drive clinical care, treatment recommendations, and future research.
Keywords: breast cancer, prognosis, staging
Breast cancer staging provides a universal and consistent foundation upon which providers can efficiently communicate factors that influence patient treatment recommendations and prognosis. While staging was initially developed to describe the “life history” of a cancer,1 it is now used as a communication tool to relay a concise summary of a patient’s disease and prognosis with appropriate management. As our understanding and treatment of breast cancer has evolved, contemporary data suggest that the combination of traditional anatomic staging and tumor biology yield a predictive synergy for predicting prognosis. As such, the recently released American Joint Committee on Cancer’s (AJCC) Breast Cancer Staging Manual (8th edition) now includes additional tumor characteristics and molecular biomarkers that have been validated to have critical prognostic significance.
More specifically, the AJCC Cancer Staging Manual (8th edition) includes 2 staging systems: anatomic stage and prognostic stage.2 Although TNM staging remains especially important for local-regional treatments such as surgery and radiation, endocrine therapy and other targeted therapies are increasingly recommended on the basis of tumor phenotype. Thus, the new prognostic stage now incorporates the historical anatomic TNM factors, and also tumor grade, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, and tumor multigene panel testing.2 With the inclusion of these tumor characteristics and molecular biomarkers, some disease stages will undergo important and notable shifts. Previous studies have evaluated the rates of upstaging and downstaging with the new guidelines in single-institution cohorts and population databases, although both had significant limitations related to inadequate staging.2,3 One such study was unable to assign prognostic stages to 6.8% to 13.6% of patients,3 and they did not use tumor multigene panel testing in their stage reassignment,3 both of which may have resulted in significant changes in their findings. Thus, our main objective was to determine the proportion of patients who would have a different disease stage using the new guidelines and how that restaging will impact the association of tumor stage and overall survival (OS) for breast cancer.
METHODS
Women (age ≥18) diagnosed with stages I to III invasive breast cancer between 2010 and 2015 with histology codes identified by World Health Organization (WHO) Classification of Tumors4 were initially selected from the National Cancer Data Base (NCDB), using the 2015 Participant Use Data File (PUF). Patients with unknown or missing tumor characteristics (pathological T/N, ER, PR, HER2 status, grade), or with stage 0 or IV disease were excluded. Patients receiving any neoadjuvant therapy or with missing survival data were excluded, which included all women diagnosed in 2015 due to unavailable survival data within the NCDB. Patients were staged using the pathological staging information reported and the AJCC Cancer Staging Manuals, 7th (pathological staging) and 8th editions (prognostic pathological staging).2,5 Although the original version of the 8th edition was released in 2016, it did not include all possible permutations of variables, and an updated version was subsequently released in 2017, which was used for this study.2 The Oncotype Dx Recurrence Score (Genomic Health; Redwood, CA) was included in the 8th edition restaging when available, using the cut-offs defined by the 8th edition of the AJCC Staging Manual and TAILORx study (Trial Assigning Individualized Options for Treatment), where a low risk is equivalent to a score <11.2,6 As per guidelines, recurrence scores were used for assigning prognostic stages to those with Tl-2 N0 M0, ER+, HER2− disease.2 Given that the 8th edition staging guidelines do not mandate Oncotype DX scores to determine prognostic stage, patients with missing Oncotype DX scores were not excluded.2
Patient characteristics were summarized with N (%) for categorical variables and median [interquartile range (IQR)] for continuous variables. Patient tumor characteristics were stratified by stage change, and the chi-square test was used to compare categorical variables. OS was defined as the time from diagnosis to death or last follow-up, and patients who did not die were censored at the date of last follow-up. Kaplan–Meier (K-M) curves were used to visualize unadjusted OS, and the log-rank test was used to test for differences based on the different staging criteria or by hormone receptor (HR) status. The cohort was then divided into subgroups for 2 separate secondary analyses: age groups—<50 or ≥50; and HR status—positive (ER+ and/or PR+) or negative (ER− and PR−). Cox proportional-hazards models were utilized to estimate the association of stage with OS, after adjustment for known covariates (age, race/ethnicity, year of diagnosis, Charlson–Deyo comorbidity score, facility type, receipt of radiation/chemotherapy/endocrine therapy, and insurance status); age group (<50/≥50) was substituted for age. A robust sandwich covariance estimator was included in all Cox models to account for the correlation of patients treated at the same hospital. A P value <0.05 was considered significant; no adjustments were made for multiple analyses. All statistical analyses were conducted with SAS, version 9.4 (SAS Institute; Cary, NC), or R, version 3.3.2 (R Foundation for Statistical Computing; Vienna, Austria).
RESULTS
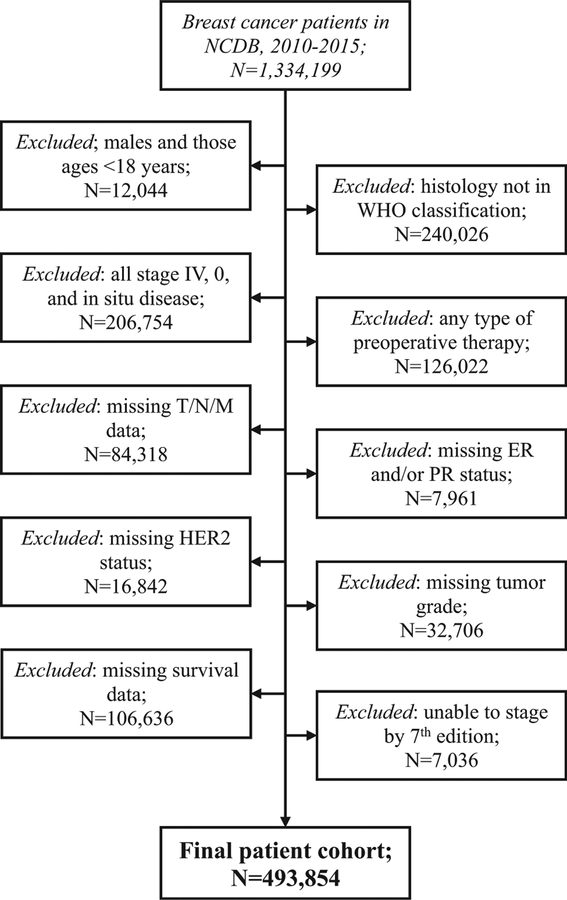
The final study population consisted of 493,854 women (Fig. 1). Patient, tumor, and treatment characteristics are summarized in Table 1. The median age at diagnosis was 62 years (IQR 52–71 years), and the majority of women were non-Hispanic whites (77.4%). Most patients had a Charlson–Deyo comorbidity score of 0 (82.6%). The median follow-up for all patients was 43.7 months [95% confidence interval (CI) 43.6–43.8, mean 44.2, range 0–87.2].
FIGURE 1.
Patient selection diagram from the National Cancer Database (NCDB) from 2010 to 2015, applying the defined inclusion and exclusion criteria.
TABLE 1.
Patient, Tumor, and Treatment Characteristics‡
| All Patients (N = 411,372) | |
|---|---|
| Age | |
| Median (IQR) | 62 (52–71) |
| Age group | |
| <50 | 91,020 (18.4%) |
| ≥50 | 402,834 (81.6%) |
| Race/ethnicity | |
| Hispanic black | 594 (0.1%) |
| Hispanic other | 1289 (0.3%) |
| Hispanic white | 22,046 (4.5%) |
| Non-Hispanic black | 48,111 (9.7%) |
| Non-Hispanic other | 19,489 (3.9%) |
| Non-Hispanic white | 382,210 (77.4%) |
| Charlson-Deyo comorbidity score | |
| 0 | 408,140 (82.6%) |
| 1 | 69,943 (14.2%) |
| ≥2 | 15,771 (3.2%) |
| Tumor histology | |
| Invasive ductal carcinoma | 438,334 (88.8%) |
| Invasive lobular carcinoma | 51,334 (10.4%) |
| All other invasive carcinomas | 4186 (0.8%) |
| Tumor size (mm) | |
| Median (IQR) | 15 (9–22) |
| Pathological T stage | |
| T0 | 181 (0%) |
| Tl* | 348,821 (70.6%) |
| T1IS | 3 (0%) |
| T2 | 126,225 (25.6%) |
| T3 | 15,140 (3.1%) |
| T4 | 3484 (0.7%) |
| LNs examined | |
| Median (IQR) | 3 (2–6) |
| Had LNs examined | |
| Yes | 484,182 (98%) |
| No or unknown | 9380 (1.9%) |
| Positive LNs | |
| Median (IQR) | 2(1–4) |
| Pathological N stage | |
| N0 | 371,787 (75.3%) |
| N1 | 78,296 (15.9%) |
| N1MI | 11,981 (2.4%) |
| N2 | 21,702 (4.4%) |
| N3 | 10,088 (2%) |
| Grade | |
| 1 | 126,024 (25.5%) |
| 2 | 223,832 (45.3%) |
| 3 | 143,998 (29.2%) |
| ER status | |
| ER+ | 419,706 (85%) |
| ER− | 74,148 (15%) |
| PR status | |
| PR+ | 373,896 (75.7%) |
| PR− | 119,958 (24.3%) |
| HER2 status | |
| HER2+ | 60,155 (12.2%) |
| HER2− | 423,339 (85.7%) |
| HER2 equivocal† | 10,360 (2.1%) |
| Oncotype Dx score | |
| 0–10 (low risk) | 24,759 (5%) |
| 11–100 (all other known valid scores) | 84,223 (17.1%) |
| Unknown or invalid number | 384,872 (77.9%) |
| Surgery type | |
| Lumpectomy | 295,048 (59.7%) |
| Mastectomy | 197,746 (40%) |
| Received radiation therapy | |
| No | 193,333 (39.1%) |
| Yes | 298,237 (60.4%) |
| Surgery type (with radiation therapy) | |
| Lumpectomy + radiation | 254,217 (51.5%) |
| Mastectomy + radiation | 43,732 (8.9%) |
| Received chemotherapy | |
| No | 299,983 (60.7%) |
| Yes | 182,624 (37%) |
| Received endocrine therapy | |
| No | 130,452 (26.4%) |
| Yes | 349,664 (70.8%) |
| Received endocrine therapy, if ER+ or PR+ | |
| No (ER+ or PR+) | 65,214 (13.2%) |
| Yes (ER+ or PR+) | 347,384 (70.3%) |
The percentages listed are of the entire patient population (N = 493,854).
LN, lymph nodes.
T1 includes Tlmi.
lncluded as HER2− in later analyses.
All percentages are based on the whole population, and may not add up to 100% because of missing values.
Pathological T stages included: T0 <0.01%, T1 70.6%, T2 25.6%, T3 3.1%, and T4 0.7%; the median tumor size was 1.5 cm (IQR 0.9–2.2cm). Most patients underwent some type of lymph node evaluation (98%); the median number of lymph nodes examined was 3 nodes (IQR 2–6 nodes). Pathological N stages included: N0 75.3%, N1/N1mi 18.3%, N2 4.4%, and N3 2%; the median number of positive lymph nodes was 2 (IQR 1–4 nodes). The majority of breast cancers were invasive ductal carcinomas (88.8%), grade 2 (45.3%), ER+ (85%), PR+ (75.7%), and HER2− (85.7%). Of the 283,293 women who met criteria for sending an Oncotype Dx for staging (T1–2 N0 M0, ER+, HER2− disease), 36.7% (n = 104,029) had the test sent and reported in the NCDB. Of those meeting criteria and tested, 19.5% (n = 20,275) had a low-risk score (0–10).
Regarding adjuvant treatments, 59.7% of patients underwent lumpectomy, and 37% received chemotherapy. Of the lumpectomy patients, 86.1% received radiation, whereas 22.1% of mastectomy patients received radiation. Of those with ER+ or PR+ tumors, 70.3% received endocrine therapy; however, the completeness of systemic therapy data has not been validated for the NCDB.
Restaging
Overall, 36.6% of patients were restaged when transitioning from the 7th to 8th edition; 6.8% were upstaged and 29.7% were downstaged (Table 2). Among 7th edition stage 1B patients, 94.1% were downstaged to IA using the new 8th edition criteria. Similarly, 71.8% of 7th edition stage IIA patients and 71% of 7th edition stage IIB patients were downstaged. Significant downstaging was also noted for stage III disease, where 70.8% of 7th edition stage IIIA patients and 82.5% of stage IIIC patients were downstaged (Table 2).
TABLE 2.
Comparison of Breast Cancer Staging Between the 7th Edition and 8th Edition AJCC Staging Manual
| AJCC Staging Manual, 8th edition | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IA | IB | IIA | IIB | IIIA | IIIB | IIIC | |||
| AJCC Staging Manual, 7th edition | IA | 238,059 91.28% |
25,550 8.72% | 0 | 0 | 0 | 0 | 0 | 292,385 |
| IB | 11,277 94.1% |
704 5.88% | 0 | 0 | 0 | 0 | 0 | 11,981 | |
| IIA | 62,634 56.49% |
16,940 15.28% |
31,299 28.23% |
0 | 0 | 0 | 0 | 110,873 | |
| IIB | 4,198 10.45% |
17,262 42.99% |
7,049 17.55% |
7,054 17.57% |
4,591 11.43% |
0 | 0 | 40,154 | |
| IIIA | 0 | 11,955 46.80% |
1,396 5.46% |
4,746 18.58% |
4,566 17.87% |
373 1.46% |
2,511 9.83% |
25,547 | |
| IIIB | 0 | 0 | 0 | 0 | 1,067 37.76% |
1,100 38.92% |
659 23.32% |
2,826 | |
| IIIC | 0 | 0 | 0 | 0 | 4,086 40.50% |
4,235 41.98% |
1,767 17.52% |
10,088 | |
| Total | 344,994 69.86% |
72,361 14.65% |
39,744 8.05% |
11,800 2.39% |
14,310 2.90% |
5,708 1.16% |
4,937 1.00% |
493,854 | |
Percent frequency represents the percent of the row total (eg, 91.28% of 7th edition stage IA patients remained stage IA using the 8th edition criteria). Yellow boxes represent patients that were upstaged using the 8th edition, and blue boxes represent those who were downstaged using the 8th edition, whereas those in grey remained unchanged.
For patients with ductal histology, 7.3% were upstaged and 28.1% were downstaged, whereas 0.6% of those with lobular histology were upstaged and 45.7% were downstaged (P < 0.0001). For those with HR− disease (ER−/PR−), 47.9% were upstaged (1.5% downstaged), whereas 34.4% of those with HR+ (ER+ and/or PR+) disease were downstaged (<0.1% upstaged; P < 0.0001). Women with HER2+ disease had a lower frequency of upstaging compared with those with HER2− negative disease (0% of HER2+ vs 7.8% of HER2−). Including the Oncotype Dx score in the 8th edition restaging resulted in a stage change for 373 stage IBs and 36 stage IIAs, all downstaged to IA (total N = 4092, 0.1%). For patients with an Oncotype Dx score of 0 to 10 (low risk), 32.7% were downstaged, 67.2% remained the same, and 0.1% were upstaged; compared with those with a score of 11 to 100 where 30.9% were downstaged, 68.5% remained the same, and 0.6% were upstaged (P < 0.0001) (Table 3).
TABLE 3.
Tumor Characteristics by Stage Change (Upstage, Downstage, or no Change) from the 7th to the 8th Edition of the AJCC Staging Manual
| All Patients (N = 493,854) |
Downstage (n = 146,845) (29.7%) |
No Change (n = 313,375) (63.4%) |
Upstage (n = 33,634) (6.8%) |
P | |
|---|---|---|---|---|---|
| Histology | |||||
| Ductal | 438,334 | 123,014 (28.1%) | 283,353 (64.6%) | 31,967 (7.3%) | <0.0001 |
| Lobular | 51,334 | 23,464 (45.7%) | 27,564 (53.7%) | 306 (0.6%) | |
| Other | 4,186 | 367 (8.8%) | 2,458 (58.7%) | 1,361 (32.5%) | |
| Oncotype Dx score | |||||
| 0–10 | 24,759 | 8088 (32.7%) | 16,639 (67.2%) | 32 (0.1%) | <0.0001 |
| 11–100 | 84,223 | 26,028 (30.9%) | 57,723 (68.5%) | 472 (0.6%) | |
| Not available | 384,872 | 112,729 (29.3%) | 239,013 (62.1%) | 33,130 (8.6%) | |
| HR status | |||||
| HR+ | 424,016 | 145,772 (34.4%) | 278,077 (65.6%) | 167 (0%) | <0.0001 |
| HR− | 69,838 | 1073 (1.5%) | 35,298 (50.5%) | 33,467 (47.9%) | |
| Grade | |||||
| 1 | 126,024 | 28,590 (22.7%) | 97,434 (77.3%) | 0 (0%) | <0.0001 |
| 2 | 223,832 | 78,470 (35.1%) | 138,761 (62%) | 6601 (2.9%) | |
| 3 | 143,998 | 39,785 (27.6%) | 77,180 (53.6%) | 27,033 (18.8%) | |
| ER status | |||||
| ER+ | 419,706 | 145,589 (34.7%) | 273,972 (65.3%) | 145 (0%) | <0.0001 |
| ER− | 74,148 | 1256 (1.7%) | 39,403 (53.1%) | 33,489 (45.2%) | |
| PR status | |||||
| PR+ | 373,896 | 141,715 (37.9%) | 232,159 (62.1%) | 22 (0%) | <0.0001 |
| PR− | 119,958 | 5130 (4.3%) | 81,216 (67.7%) | 33,612 (28%) | |
| HER2 status | |||||
| HER2 equivocal* | 10,360 | 3532 (34.1%) | 6167 (59.5%) | 661 (6.4%) | <0.0001 |
| HER2+ | 60,155 | 17,659 (29.4%) | 42,496 (70.6%) | 0 (0%) | |
| HER2− | 423,339 | 125,654 (29.7%) | 264,712 (62.5%) | 32,973 (7.8%) | |
Percentages are based on the intrarow frequency and total for each individual variable. LN, lymph nodes.
Included as HER2− in later analyses.
Associations Between Restaging and Survival
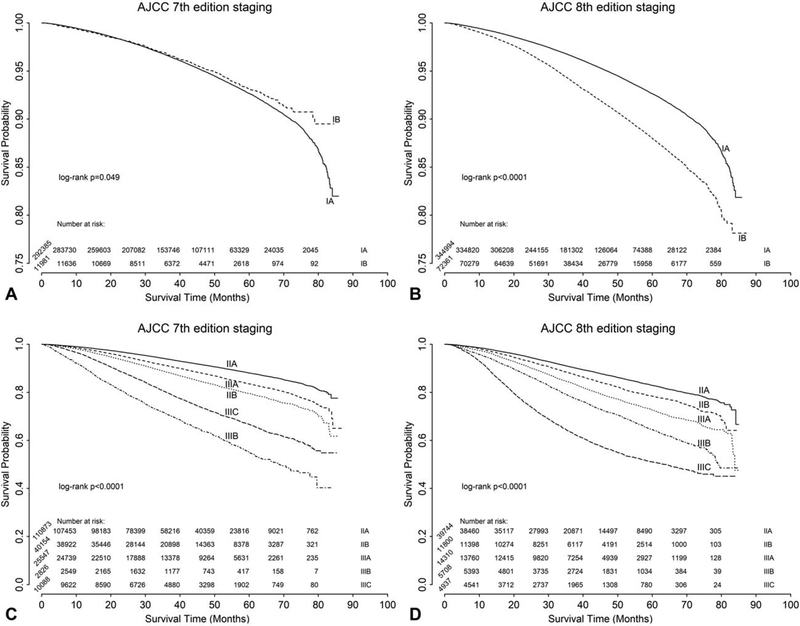
The unadjusted OS curves comparing the AJCC Staging Manual 7th and 8th editions are shown in Fig. 2. For stage I disease, the 8th edition provided better discrimination between IA and IB (log-rank P = 0.049 for the 7th edition in Fig. 2A, vs log-rank P < 0.0001 for the 8th edition in Fig. 2B). Applying the 7th edition criteria, stage IIIB disease was associated with the worst OS, whereas the 8th edition resulted in a more uniform and logical distribution of survival with stage IIIC being associated with the worst OS for the majority of the follow-up period (Fig. 2C and D). This is further demonstrated when comparing the 1 and 5-year OS rates, which consistently diminish with higher stages for the 8th edition, but fluctuate more often when using the 7th edition (Supplemental Table 1).
FIGURE 2.
Unadjusted overall survival analysis for breast cancer using the AJCC Cancer Staging Manual 7th and 8th editions for stages IA to IB (A: 7th edition; B: 8th edition) and stages IIA to IIIC (C: 7th edition; D: 8th edition).
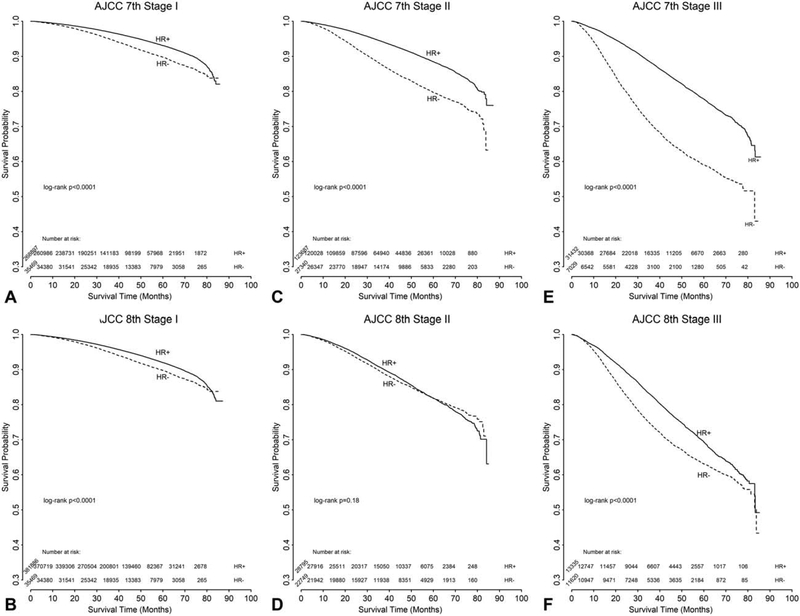
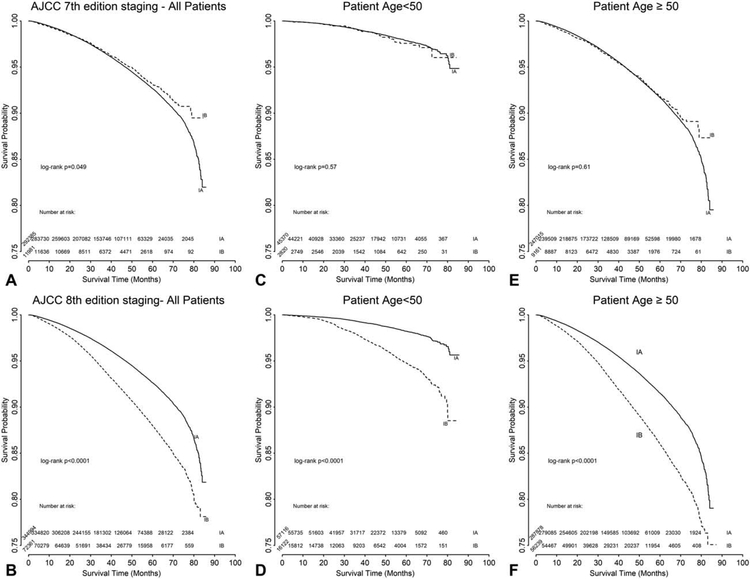
In a subgroup analysis of patients based on HR status (defined as HR+ = ER+ and/or PR+; HR− = ER− and PR−), those with stages I and III, and HR− disease had a worse OS than those with HR+ disease, while there appeared to be a less significant difference for those with stage II disease based on HR status (Fig. 3). In the subgroup analysis of stage I patients based on age, discrimination among younger patients (defined as those <50 years old) was improved with the 8th edition staging criteria (log-rank P = 0.57 for the 7th edition in Fig. 4C, vs log-rank P < 0.0001 for the 8th edition in Fig. 4D). A similar trend was noted for women ≥50 years old, but was less pronounced (log-rank P = 0.61 for the 7th edition in Fig. 4E, vs log-rank P < 0.0001 for the 8th edition in Fig. 4F).
FIGURE 3.
Unadjusted overall survival analysis for breast cancer stages I to III using the AJCC Cancer Staging Manual 7th (A, C, E) and 8th (B, D, F) editions for all patients, subdivided to stage (A and B: stage I; C and D: stage II; E and F: stage III). Hormone receptor (HR) status defined as HR+ if estrogen receptor (ER) + and/or progesterone receptor (PR) +; defined as HR− if ER− and PR−.
FIGURE 4.
Unadjusted overall survival analysis for breast cancer stage I using the AJCC Cancer Staging Manual 7th (A, C, E)and 8th (B, D, F) editions for all patients (A and B) with subgroup analysis by age <50 (C and D) or ≥50 years old (E and F).
DISCUSSION
With the new AJCC breast cancer staging system, a patient’s stage not only summarizes the anatomic extent of disease at diagnosis and/or surgery, but now also includes critical biologic tumor characteristics. Although our study population overlaps some with the cohort used to develop these new guidelines (which were based on an NCDB population from 2010 to 2012), our analysis significantly expands the original work to include a larger population with a longer follow-up. We found that inclusion of tumor grade, ER status, PR status, HER2 status, and the tumor multigene panel testing resulted in a stage change for over 35% of women (6.8% upstaged and 29.7% downstaged) with stages I to III breast cancer. For stage I patients, the 8th edition provided better discrimination between OS and disease stage, which was more apparent for women <50 years old. Similar differences may also be observed when populations are further stratified by ER/PR and HER2 status, and future studies are needed to evaluate this possibility. When comparing OS rates, application of the 8th edition staging criteria resulted in an incremental reduction in OS for each increase in stage.
Numerous studies have confirmed the significant correlation between tumor size, nodal burden, and survival.7 Additional studies have confirmed the importance of tumor grade and ER/PR/HER2 status, as they relate to prognosis.8–14 However, the routine incorporation of additional tumor factors and molecular biomarkers has elevated staging to the next level of precision. Yi et al15 reviewed 3728 invasive breast cancer patients diagnosed in 1997 to 2006 and reported that the combination of pathological anatomic staging, nuclear grade, and ER status was a more accurate predictor of survival than pathological anatomic staging alone. Based on this observation, a risk profile was developed,15 and subsequent evaluation of this staging system using the risk profile (Bioscore), further confirmed the importance of biologic factors in refining prognosis.16
More recently, multigene panel testing of breast cancer tumors was developed to aid with prediction of both prognosis and response to therapy. The Oncotype Dx assay is included in the 8th edition of the AJCC Staging Manual, as it had been tested in the largest prospective validation cohort at the time of the publication. Specifically, 1626 women among the 10,253 enrolled in the TAILORx trial were found to have a recurrence score <11 and received endocrine therapy alone (without other systemic therapies), and a 99.3% distant disease-free survival was noted at 5 years.6 Similarly, 17,917 women (88.4%) among the 20,275 in our study (with T1-2 N0 M0, ER+, HER2−, recurrence score <11) received endocrine therapy alone and had a 96% 5-year OS (95% CI 0.96–0.97).
Based on these studies and others, the breast cancer staging system has been updated to include traditional staging factors (TNM) and tumor-specific factors (grade, ER, PR, HER2, and tumor multigene panel testing). These updates to the 8th edition of the AJCC Cancer Staging Manual were recently validated using a single institution cohort and the California Cancer Registry.3 Weiss et al3 demonstrated that implementing the prognostic stage upstaged 29.5% of patients and downstaged 28.1%; of note, 13.6% of patients in the institutional cohort and 6.8% in the population database could not be assigned a prognostic stage. This deficiency has been updated in the current version of the 8th edition of the AJCC Staging Manual, and our study was based upon the latest edition that now assigns a stage for nearly every tumor stage (7036 patients could not be staged using the 7th edition, whereas only 734 patients could not be staged using either the 7th or the 8th edition in the present study). Furthermore, Weiss et al did not include tumor multigene testing, which may have altered their outcomes. Although reporting was limited in our NCDB population (only 36.7% had appropriate testing sent), it did result in a low risk score for 19.5% of those patients.
As the field of breast cancer advances, breast cancer staging will undoubtedly continue to evolve. For example, the MINDACT trial (Microarray in Node-Negative and 1–3 Positive Lymph Node Disease May Avoid Chemotherapy) was published shortly after the release of the 8th edition of the AJCC Staging Manual.17 Subsequently, Giuliano et al18 suggested that these findings were consistent with downstaging selected tumors with low-risk genomic profiles and may be incorporated in future updates to the AJCC Staging Manual. Furthermore, the American Society of Clinical Oncology recently provided guideline recommendations on the use of biomarkers for decision-making regarding the use of systemic therapy for early stage breast cancer.19 Taken together, future updates to the AJCC Breast Cancer Staging Manual will likely continue to include new and emerging data.
Future considerations may also include restaging after receipt of preoperative systemic therapy or after a breast cancer recurrence, as the prognostic staging guidelines are not meant to be applied to the neoadjuvant population; the 8th edition guidelines were developed using a population of patients that received surgery upfront. Although it is has been shown that women who have a complete pathological response to preoperative systemic therapy have an improved survival20 and those who experience a recurrence have a worse survival,21 there are limited and inconsistent resources available to guide one’s treatment decisions and prognosis estimates in these special circumstances. One recent study by Mittendorf et al22 introduced the Neo-Bioscore, which incorporates tumor characteristics and biomarkers, as well as treatment response, to determine one’s prognosis for those receiving preoperative systemic therapy. In a review of 4 staging systems for those receiving neoadjuvant chemotherapy, Bergquist et al23 concluded that the incorporation of chemotherapy response, tumor grade, and receptor status substantially improved the AJCC TNM staging system in discrimination of OS. In addition, the Neo-Bioscore was noted to provide the best staging discrimination.23 These areas of research will be important to consider in future revisions to the breast cancer staging system.
Our study limitations include those related to the retrospective nature of the data and those associated with using large national databases, which rely on quality of data entry. Additionally, the NCDB lacks information on breast cancer-specific survival data, which has a less defined correlation with OS in early-stage breast cancer patients and older patients with competing comorbidities. The NCDB also lacks granular data on the adjuvant therapies received, such as types of systemic therapy and/or endocrine therapy, and whether those therapies were completed as planned. Furthermore, over 84,000 patients were excluded due to missing pathological T stage, pathological N stage, and an additional 57,509 patients were excluded due to missing ER status, PR status, HER2 status, and/or tumor grade, although it is unclear whether this is likely to have changed the conclusions of the study. Furthermore, HER2 status was not routinely recorded in NCDB until 2010, and those with HER2+ disease may not have routinely received trastuzumab in the early years of this study, which may introduce some degree of bias. Of note, 7036 patients could not be staged using the 7th edition criteria, as not all permutations were associated with an overall stage, although most of these could be staged using the 8th edition (only 734 patients could not be staged using either edition). Lastly, the new staging guidelines are significantly more complicated, and implementation in the clinical setting will likely require additional resources than previously necessary for assessing disease stage, although smart phone apps have already been developed.
It should also be noted that in the 8th edition of the AJCC Staging Manual, prognostic stage groups were determined based on breast cancer populations that were offered and mostly received appropriate multidisciplinary treatment.2 Thus, further work must define how these changes will be used to guide treatment decisions for patients that are not able (or willing) to undergo standard treatments. This may become particularly apparent in resource-constrained settings24 that do not have the same treatments available as those routinely administered in the United States. However, this may also affect certain populations with limited resources in our own communities, such as the uninsured and/or lower income patients.
CONCLUSIONS
In conclusion, combining tumor biology and anatomic extent of disease in the 8th edition of the AJCC Cancer Staging Manual has resulted in several important changes to our previous understanding of breast cancer prognosis and resulted in restaging of over 35% of NCDB women with stages I to III invasive breast cancer. Expanding our knowledge of breast cancer biology will continue to advance patient care and research, which will translate to ongoing refinements in breast cancer staging. As treatments continue to evolve and include more targeted therapies, the staging system will need to be continuously re-evaluated and updated to provide the most accurate assessment of the patient’s disease and prognosis.
Supplementary Material
ACKNOWLEDGMENTS
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not been verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding: This work was supported by National Cancer Institute at the National Institutes of Health core grant (grant number 5P30-CA014236–44 to SMT and TH), “Biostatistics Shared Resource”. Dr O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 5KL2TR001115 (PI: Boulware). Dr R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors have declared no conflicts of interest related to this work.
REFERENCES
- 1.Manual for Staging of Cancer. 1st ed. Philadelphia, PA: Lippincott-Raven Publishers; 1977. [Google Scholar]
- 2.AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016. [Google Scholar]
- 3.Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation study of the American Joint Committee on Cancer Eighth Edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO/IARC Classification of Tumours. 4 ed. Vol. 4 World Health Organization; 2012. [Google Scholar]
- 5.AJCC Cancer Staging Manual. 7th ed.. New York, NY: Springer; 2009. [Google Scholar]
- 6.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. Vol. 7th. New York: Springer-Verlag; 2010. [Google Scholar]
- 8.Schwartz AM, Henson DE, Chen D, et al. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER Program. Arch Pathol Lab Med. 2014;138:1048–1052. [DOI] [PubMed] [Google Scholar]
- 9.Knight WA, Livingston RB, Gregory EJ, et al. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977;37:4669–4671. [PubMed] [Google Scholar]
- 10.Clark GM, McGuire WL, Hubay CA, et al. Progesterone receptors as a prognostic factor in stage II breast cancer. N Engl J Med. 1983;309:1343–1347. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 12.Press MF, Pike MC, Chazin VR, et al. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- 13.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. [DOI] [PubMed] [Google Scholar]
- 14.Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049–1056. [DOI] [PubMed] [Google Scholar]
- 15.Yi M, Mittendorf EA, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011;29:4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittendorf EA, Chavez-MacGregor M, Vila J, et al. Bioscore: a staging system for breast cancer patients that reflects the prognostic significance of underlying tumor biology. Ann Surg Oncol. 2017;24:3502–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375: 717–729. [DOI] [PubMed] [Google Scholar]
- 18.Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:290–303. [DOI] [PubMed] [Google Scholar]
- 19.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and longterm clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 21.Fourquet A, Campana F, Zafrani B, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys. 1989;17:719–725. [DOI] [PubMed] [Google Scholar]
- 22.Mittendorf EA, Vila J, Tucker SL, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergquist JR, Murphy BL, Storlie CB, et al. Incorporation of treatment response, tumor grade and receptor status improves staging quality in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2017;24:3510–3517. [DOI] [PubMed] [Google Scholar]
- 24.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries. Cancer. 2008; 113: 2221–2243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.