Abstract
Neurodegenerative disorders of the aging population are characterized by progressive accumulation of neuronal proteins such as α-synuclein (α-syn) in Parkinson’s Disease (PD) and Amyloid ß (Aß) and Tau in Alzheimer’s disease (AD) for which no treatments are currently available. The ability to regulate the expression at the gene transcription level would be beneficial for reducing the accumulation of these proteins or regulating expression levels of other genes in the CNS. Short interfering RNA molecules can bind specifically to target RNAs and deliver them for degradation. This approach has shown promise therapeutically in vitro and in vivo in mouse models of PD and AD and other neurological disorders; however, delivery of the siRNA to the CNS in vivo has been achieved primarily through intra-cerebral or intra-thecal injections that maybe less amenable for clinical translation; therefore, alternative approaches for delivery of siRNAs to the brain is needed. Recently, we described a small peptide from the envelope protein of the rabies virus (C2-9r) that was utilized to deliver an siRNA targeting α-syn across the blood brain barrier (BBB) following intravenous injection. This approach showed reduced expression of α-syn and neuroprotection in a toxic mouse model of PD. However, since receptor-mediated delivery is potentially saturable, each allowing the delivery of a limited number of molecules, we identified an alternative peptide for the transport of nucleotides across the BBB based on the apolipoprotein B (apoB) protein targeted to the family of low-density lipoprotein receptors (LDL-R). We used an 11-amino acid sequence from the apoB protein (ApoB11) that, when coupled with a 9-amino acid arginine linker, can transport siRNAs across the BBB to neuronal and glial cells. To examine the value of this peptide mediated oligonucleotide delivery system for PD, we delivered an siRNA targeting the α-syn (siα-syn) in a transgenic mouse model of PD. We found that ApoB11 was effective (comparable to C2-9r) at mediating the delivery of siα-syn into the CNS, co-localized to neurons and glial cells and reduced levels of α-syn protein translation and accumulation. Delivery of ApoB11/siα-syn was accompanied by protection from degeneration of selected neuronal populations in the neocortex, limbic system and striato-nigral system and reduced neuro-inflammation. Taken together, these results suggest that systemic delivery of oligonucleotides targeting α-syn using ApoB11 might be an interesting alternative strategy worth considering for the experimental treatment of synucleinopathies.
Keywords: Parkinson’s disease, siRNA, blood-brain barrier, α-synuclein, low density lipoprotein receptor, apolipoprotein B, receptor mediated transytosis
Introduction
Neurodegenerative disorders of the aging population are characterized by progressive accumulation of neuronal proteins such as α-synuclein (α-syn) in Lewy body disease (LBD) (including Parkinson’s Disease (PD) and Dementia with Lewy bodies (DLB)) (McKeith et al., 2017), Amyloid ß (Aß) and Tau in Alzheimer’s disease (AD) (De Strooper and Karran, 2016; Gibbons et al., 2018; Selkoe and Hardy, 2016), huntingtin (Htt) in Huntington’s Disease (HD) (Margulis and Finkbeiner, 2014) and TDP43 in Fronto-temporal dementia (FTD) and Amyotrophic lateral sclerosis (ALS) (Cairns et al., 2007; Lee et al., 2011a). This is accompanied by death of selected neuronal populations and neuro-inflammation leading to cognitive impairment, parkinsonism and motoric disorders for which no treatments are currently available (Fu et al., 2018). The mechanisms of neurodegeneration are not fully understood but involve toxicity mediated by oligomeric forms and other multimers of the aggregating (Gibbons et al., 2018; Lashuel et al., 2013; Mucke and Selkoe, 2012) as well as cell to cell propagation (Gibbons et al., 2018; Jucker and Walker, 2018; Lee et al., 2011b; Liu et al., 2012) of the accumulating protein. It is believed that accumulation of proteins such as α-syn, Aß, Tau, Htt and TDP43 might be the result of a disequilibrium between the levels of synthesis, clearance and folding of these proteins (Brettschneider et al., 2015; Lashuel et al., 2013). Therefore, therapeutic strategies might involve decreasing synthesis, increasing clearance or promoting proper folding of these proteins (Valera et al., 2016a; Valera et al., 2016b). There has been progress in developing strategies to decrease the synthesis of proteins involved in neurological disorders by utilizing anti-sense oligonucleotides (Ghosh and Tabrizi, 2017; Schoch and Miller, 2017). Clinical trials are now underway with anti-sense oligonucleotides in HD (Dickey and La Spada, 2018; Ghosh and Tabrizi, 2017; Keiser et al., 2016) and similar strategies are being explored for PD and AD targeting α-syn (Alarcon-Aris et al., 2018) and Tau (Crunkhorn, 2017; DeVos and Hyman, 2017; Vossel et al., 2010). In addition to anti-sense, another approach for reducing gene expression includes the use of short interfering RNA (siRNA) oligonucleotides that can bind specifically to target RNAs and target them for degradation via the RISC complex have been proposed for the treatment of neurodegenerative disorders (Alves et al., 2016; Koutsilieri et al., 2007; Magen and Hornstein, 2014; White and Mallucci, 2009). This approach has shown promise as a therapeutic in vitro (Kao et al., 2004; Liu et al., 2008; Luo et al., 2004) and in mouse models of PD (Zharikov et al., 2015) and AD and other neurological disorders (Chang et al., 2018; Singer et al., 2005; Xilouri et al., 2016); however, as with anti-sense, delivery of the siRNA to the CNS in vivo is challenging and so far has been achieved primarily through intra-thecal and intra-cranial injections. Thus, the need to develop less invasive systemic approaches to deliver oligonucleotides into the CNS and across the blood-brain barrier (BBB).
The BBB controls the passage of substances from the blood into the central nervous system. Small molecules (400-500 Daltons) can pass freely (Lipinski et al., 2001); however, larger molecules are actively transported across the BBB via three main mechanisms: (a) carrier mediated transporters (CMT) allow the transport of nutrients such as sugars and amino acids from blood to the brain, (b) receptor mediated transport (RMT) allows the transport of larger proteins and carrier proteins such as transferrin (iron), apolipoprotein (lipids) and insulin from the blood to the brain, and (c) efflux transporters export drugs from the CNS to the blood e.g. p-glycoprotein and breast cancer resistance protein (Abbott et al., 2006; Begley, 2004; Begley and Brightman, 2003). Thus, a major challenge for the systemic delivery of oligonucleotides for neurodegenerative diseases is the transport to the CNS.
In the past few years, a number of receptor mediated methods to deliver oligonucleotides and peptides have been tested (Pardridge, 2017) including the use of the transferrin (Pardridge, 2016), insulin (Lajoie and Shusta, 2015) and low-density lipoprotein (LDL-R) receptors (Masliah and Spencer, 2015a; Spencer and Verma, 2007). Recently, we described a small peptide from the envelope protein of the rabies virus (C2-9r) that was utilized to deliver an siRNA targeting α-syn (siα-syn) across the BBB following systemic injection (Javed et al., 2016). With this approach, we showed reduced expression of α-syn in various brain regions and protection of the nigro-striatal system from the neurotoxic effects of MPTP in a mouse model of PD (Javed et al., 2016).
In this study we have identified an alternative peptide for the transport of nucleotides across the BBB based on the apolipoprotein B (ApoB) protein targeted to the family of LDL-R (Masliah and Spencer, 2015b; Spencer and Verma, 2007). For previous studies we utilized a 38 aa peptide (Spencer et al., 2018; Spencer et al., 2014a; Spencer et al., 2016c; Spencer et al., 2015; Spencer et al., 2014b) expressed from a lentiviral vector while for the present study we used a reduced 11-amino acid sequence from the apoB protein (ApoB11) protein, coupled with a 9-amino acid arginine linker for transporting siRNAs across the BBB to neuronal and glial cells following intra-peritoneal injection. We tested the effectiveness of this approach by delivering an siRNA targeting α-syn (siα-syn) into an α-syn tg mouse model of PD/DLB and showed reduced accumulation of α-syn associated with amelioration of the neurodegenerative and inflammatory pathology. Taken together, these results support the notion that systemic delivery of oligonucleotides targeting α-syn using ApoB11 might be an alternative strategy worth considering when developing experimental treatments for synucleinopathies of the aging population such as PD and DLB.
Results
An 11-amino acid peptide from Apolipoprotein B can deliver an siRNA to neurons and reduces α-synuclein levels in vitro
α-synuclein is a 140 amino acid synaptic protein (Iwai et al., 1995; Valera and Masliah, 2013) that plays a role as a scaffolding protein in vesicle trafficking, dopamine release and synaptic remodeling (Chandra et al., 2004). Misfolded, aggregated α-syn has been implicated in neurological disorders with parkinsonism including PD, DLB and MSA (Reviewed in (Eller and Williams, 2011)). Given the neuronal toxicity of α-syn accumulation, therapeutic strategies for synucleinopathies might include reducing expression of the mRNA through siRNA. We have previously described a small peptide from the envelope protein of the rabies virus (C2-9r) that can be used systemically to deliver siα-syn into the CNS (Javed et al., 2016). However, the rhabdovirus receptor saturates rapidly (Wunner et al., 1984), thus for this study we investigated the usefulness of ApoB as an alternative carrier protein. This is based on our previous studies showing delivery of protein and peptides to the CNS by receptor mediated transcytosis across the BBB with the LDL-R using a 38-amino acid receptor binding domain from apolipoprotein B (ApoB38) (Spencer et al., 2014a; Spencer et al., 2011; Spencer et al., 2016c; Spencer et al., 2015; Spencer et al., 2014b; Spencer and Verma, 2007). Based on literature review (Knott et al., 1986; Yang et al., 1986), we identified an 11-amino acid core domain that represented the putative minimal receptor binding domain (ApoB11) (Figure 1A). For delivery of oligonucleotides, we added 5 Gly (G) and 9 Arg (R) amino acids to the C-terminus (Javed et al., 2016). The glycine residues act as flexible anchor and the 9 arginine residues are a positively charged tail for binding negatively charged nucleotides (Figure 1A).
Figure 1. Characteristics of a non-viral peptide derived from ApoB that delivers a short RNA targeting α-syn to neuronal cells.
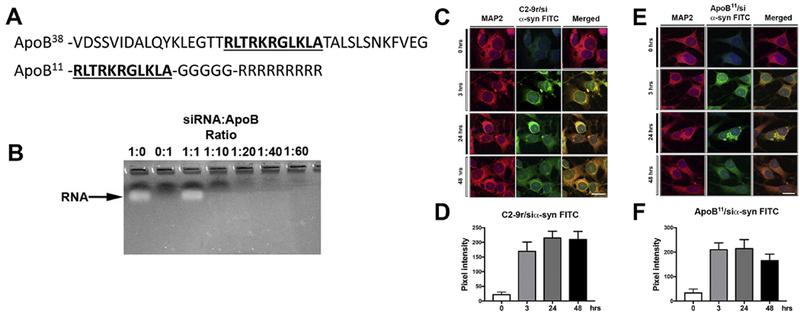
(A) Diagrammatic illustration of the original 38-amino acid peptide derived from the Apolipoprotein B protein for delivery of proteins and peptides for receptor mediated BBB transcytosis to the CNS and the novel shorter, 11 amino acid (underlined) fragment coupled to a 5 Glycine linker and a 9 Arginine positively charged tail utilized for delivering siRNA into neuronal cells. (B) The siRNA targeting α-syn was incubated with different molar ratios of the ApoB11 peptide for 30 minutes. Complexed RNA and protein were run through an agarose gel and unconjugated RNA was observed by ethidium bromide staining. (C, E) Immunocytochemical and laser scanning microscopy analysis of N2A mouse neuronal cells incubated with FITC labeled siRNA targeting α-syn added at a ratio of 1:40 siRNA:C2-9r (derived from rhabdovirus positive control) or 1:10 siRNA:ApoB11 (respectively) and analyzed at different time points for uptake of the FITC labeled siRNA. Neuronal cells were labeled with an antibody against MAP2 and visualized with Tyramide Red. (D, F) Fluorescence levels representative of FITC tagged siRNA:C2-9r or siRNA:ApoB11 (respectively) trafficking into neuronal cells were estimated after 3, 24 and 48 hrs post incubation and expressed as pixel intensity. Levels rapidly increased and plateau after 3 hrs. Scale bar represents 15μm.
To determine the ratio between the ApoB11 peptide and RNA that leads to complete complex formation, 100nM of siRNA was incubated with increasing molar ratios of ApoB11 and run on an agarose gel. Excess RNA that was not completely bound to and charge neutralized by the 9 arginine residues of the ApoB11 peptide would enter the gel and be visible by ethidium bromide staining. At a ratio of 1:0 and 1:1, unbound RNA is observable in the gel; however, at a ratio of 1:10 (0.1 μM siRNA: 1 μm ApoB11), there is no free RNA to enter the gel suggesting that all the charged RNA is neutralized by the arginine residues of the ApoB11 vector (Figure 1B). For future applications, we used a ratio of 1:10 when incubating the siRNA and ApoB11 vector prior to applications.
To ascertain if the ApoB11 peptide could deliver siRNA in a comparable manner to the recently published BBB transport peptide derived from the Rabies G envelope protein (C2-9r), the N2A mouse cholinergic neuronal cells were incubated for various time points with the C2-9r peptide with a FITC labeled siRNA targeting a conserved sequence on mouse and human α-syn (siα-syn) (Spencer et al., 2016a) at a ratio of 1:40 as previously published (Javed et al., 2016), while we incubated the FITC-siRNA with the ApoB11 vector at a molar ratio of 1:10 in vitro and then added the vector to differentiated N2A neuronal cells. Compared to controls, the C2-9r/siα-syn FITC was detected at 3 hrs and plateaued at 48 to 72 hrs (Figure 1C, D). Similarly, for the ApoB11 vector, delivery to neuronal cells was detected shortly after incubation with the ApoB11/siα-syn FITC complex, FITC label was observed throughout the cytoplasm in the neuronal cells beginning at 3 hours post-incubation (Figure 1E, F). The FITC signal appeared to become more punctate by 24 hours and at 48 hours was beginning to diminish indicating a degradation of either the FITC signal or the siRNA (Figure 1E, F).
Next, we investigated the time course and differential effects of the C2-9r and ApoB11 vectors delivering the siα-syn into neurons and modulating α-syn levels. Consistent with a previous study (Javed et al., 2016), treatment of N2A cells with the C2-9r/siα-syn resulted in a time dependent 25% and 80% reduction in α-syn at 24 hrs and 48-72 hrs respectively (Figure 2A, B). Likewise, treatment with the ApoB11/siα-syn reduced the accumulation of α-syn in a time dependent manner with 30% reduction at 24-48 hrs after treatment and nearly complete elimination of α-syn signal by immunocytochemistry at 72 hrs (Figure 2C, D). This was corroborated in a similar experiment where total protein was extracted and α-syn examined by immunoblot (Figure 2E, F). At 24 hrs post-treatment, α-syn levels was approximately 30% (C2-9r) – 35% (ApoB11) of control levels, while at 48 hrs the levels of α-syn were reduced by 70% (Figure 2F). Together, these results support the notion that ApoB11/siα-syn is as effective as the C2-9r at delivering siα-syn and reducing expression, providing support for evaluating this ApoB11/siα-syn in vivo.
Figure 2. Analysis of levels of α-syn following delivery of ApoB11siRNA targeting α-syn in neuronal cells in vitro.

(A) N2A neuronal cells were treated with 100pmol siα-syn conjugated to the (A) C2-9r peptide (derived from rhabdovirus, positive control) or to the (C) ApoB11 peptide and double immunostained with antibodies against α-syn (red) and the neuronal marker MAP2 (FITC) and analyzed with the laser confocal microscope. (B, D) Fluorescence levels representative of α-syn immunoreactivity expressed as pixel intensity following 24, 48 and 72 hrs. Levels of α-syn immunostaining started to decrease after 24 hrs reaching maximum effects after 48hrs with the C2-9r/siα-syn and 72 hrs with the ApoB11/siα-syn. (E) Representative immunoblot from replicate from N2A neuronal cells treated with siα-syn conjugated to the C2-9r peptide or the ApoB11 peptides after 24 and 48 hrs. (F) Image analysis of the levels of α-syn immunoreactive band at 14 kDa as a ratio to ß-actin after 24 and 48 hrs. Scale bar represents 10 μm.
Systemically administered ApoB11 and C2-9r vectors deliver siRNA to neurons and glial cells in non-tg and α-syn tg mice
We have previously delivered peptides and proteins across the BBB following intra-peritoneal injections when they are fused to a 38-amino acid LDL-R binding domain of ApoB (Spencer et al., 2018; Spencer et al., 2014a; Spencer et al., 2016c; Spencer et al., 2015; Spencer et al., 2014b). In contrast, experiments delivering C2-9r/siα-syn were performed following intra-venous injection (Javed et al., 2016). Therefore, we performed preliminary experiments with FITC labeled siRNA conjugated to either ApoB11 or C2-9r delivered to non-tg mouse by either intra-venous or intra-peritoneal injection. Four hours post intra-venous injection we found that compared to the siRNA-FITC alone, the siRNA conjugated to C2-9r or ApoB11 co-localized with 65% of NeuN positive neurons (Figure 3A, B). Along these lines, following intra-peritoneal injection the siRNA-FITC conjugated to C2-9r or ApoB11 co-localized with 55% of NeuN positive neurons (Figure 3C, D). Due to the ease of intra-peritoneal injection, for subsequent experiments, intra-peritoneal delivery was chosen for the route of administration.
Figure 3. Comparison of the levels of the peptide-siRNA α-syn in the mouse brain following intra-venous or intra-peritoneal delivery.
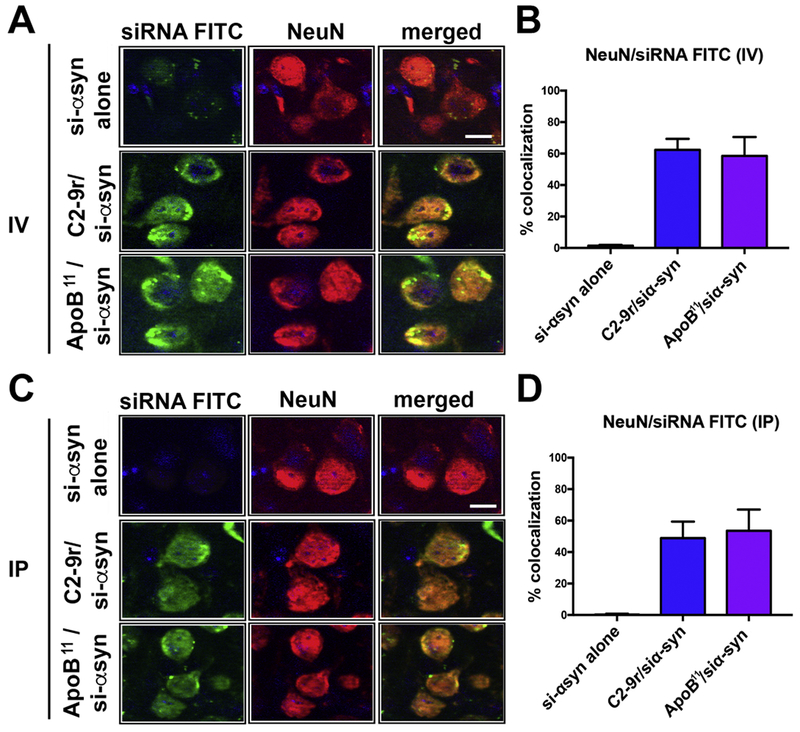
Non-tg mice received a single intravenous (IV) or intra-peritoneal (IP) injection of FITC labeled α-syn siRNA (si-αsyn) conjugated to either C2-9r, ApoB11 or naked siRNA (si-αsyn alone), 4 hours after injection, mice were sacrificed, whole brain removed, fixed and sections immunolabeled with an antibody against the neuronal marker NeuN (red). (A) Representative images from the frontal cortex following IV injection of FITC tagged si-αsyn alone or conjugated to C2-9r or ApoB11 in sections immunolabeled with NeuN and analyzed with the laser scanning confocal microscope. (B) Image analysis of the sections to estimate the co-localization with between the FITC labeled siRNA (green) and NeuN (red) expressed as % showing very little or no trafficking with the naked siRNA while comparable levels of penetration into neurons in vivo with the C2-9r, ApoB11 peptides. (C) Representative images from the frontal cortex following IP injection of FITC tagged si-αsyn. (D) Image analysis of the % co-localization between the FITC labeled siRNA (green) and NeuN (red) showing no trafficking of the naked siRNA while high levels of penetration into neurons in vivo with the C2-9r, ApoB11 peptides. n=3 non-tg (4m/o) mice per group. Scale bar represents 10 μm.
To determine if the peptides could transport siRNA across the BBB following intra-peritoneal delivery in both non-tg and α-syn tg mice, animals were treated with either C2-9r /siα-syn FITC, ApoB11/siα-syn FITC or naked siα-syn FITC. Compared to mice treated with siα-syn FITC, 4 hours after systemic injection, both the C2-9r and ApoB11 delivered FITC tagged siα-syn was detected in neuronal cells as shown by co-labeling with the neuronal marker NeuN, both in the brains of non-tg (Figure 4A) and α-syn tg mice (Figure 4B). Labeling was primarily peri-nuclear in punctate patterns but was also nuclear (Figure 4A, B). Interestingly, while the C2-9r co-localized with approximately 40% of NeuN positive cells in non-tg and α-syn tg mice, the ApoB11 siRNA was present in approximately 55 and 60% of the NeuN positive neurons in non-tg and α-syn tg mice respectively, with the difference reaching significance in the α-syn tg group (Figure 4C). No labeling was observed in neuronal cells in the mice treated with FTC tagged naked siRNA (Figure 4C). Moreover, compared to mice treated with siα-syn FTC alone, after intra-peritoneal injection, both the C2-9r/ and ApoB11/siα-syn FTC localized to approximately 20-25% of astrocytes as shown by co-labeling with the astroglial cell marker GFAP, both in the brains of non-tg (Figure 4D, F) and α-syn tg mice (Figure 4E, F). No labeling was observed in astroglial cells in the mice treated with FTC tagged naked siRNA (Figure 4F).
Figure 4. Cellular distribution of the peptide-siRNA α-syn in the brains of non-transgenic and α-syn transgenic mice following intra-peritoneal injections.
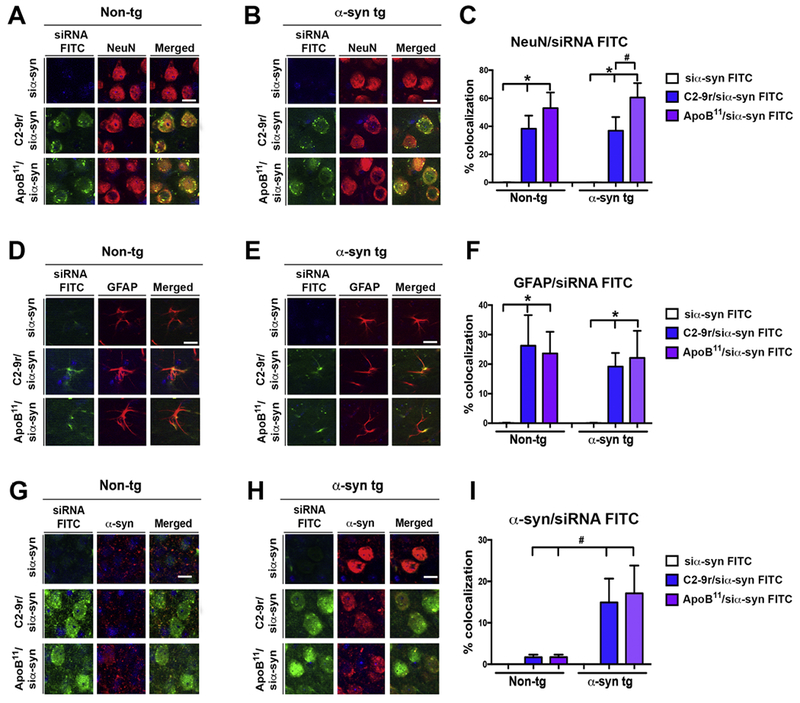
Non-tg and α-syn tg mice received a single IP injection of FITC labeled α-syn siRNA (si-αsyn) (green) conjugated to either C2-9r, ApoB11 or naked siRNA (si-αsyn alone), 4 hours after injection, mice were sacrificed, whole brain removed fixed and sections immunolabeled with antibodies against the neuronal marker NeuN, astroglial cells GFAP and (red) α-syn. (A, B) Representative images of the frontal cortex of non-tg and α-syn tg mice (respectively) following IP injection of FITC tagged si-αsyn alone or conjugated to C2-9r or ApoB11 in sections immunostained with NeuN (red) and analyzed with the laser scanning confocal microscope. (C) Image analysis of the sections to estimate the co-localization between the FITC labeled siRNA (green) and NeuN (red) expressed as % showing comparable high levels in neurons in vivo with the C2-9r, ApoB11 peptides in non-tg and α-syn tg mice. (D, E) Representative images of the frontal cortex of non-tg and α-syn tg mice (respectively) following IP injection of FITC tagged si-αsyn alone or conjugated to C2-9r or ApoB11 in sections immunostained with GFAP (red) and analyzed with the laser scanning confocal microscope. (F) Image analysis of the sections to estimate the co-localization between the FITC labeled siRNA (green) and GFAP (red) expressed as % showing comparable colocalization in astroglial cells in vivo between the C2-9r or ApoB11 peptides in non-tg and α-syn tg mice. (G, H) Representative images of the frontal cortex of non-tg and α-syn tg mice (respectively) following IP injection of FITC tagged si-αsyn alone or conjugated to C2-9r or ApoB11 in sections immunostained with α-syn (red) and analyzed with the laser scanning confocal microscope. (F) Image analysis of the sections to estimate the co-localization between the FITC labeled siRNA (green) and α-syn (red) expressed as % showing comparable colocalization in cells with α-syn aggregates in vivo between the C2-9r or ApoB11 peptides in non-tg and α-syn tg mice. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing in between groups. * indicates statistical significance p < 0.05 compared to siRNA alone. # indicates statistical significance p < 0.05 compared to C2-9r siRNA. n=3 non-tg (4m/o) mice per group. Scale bar represents 10 μm.
Next, we investigated the co-localization of the C2-9r/siα-syn FTC and ApoB11/siα-syn FTC to neurons accumulating α-syn. For this purpose, co-labeling experiments were performed with the Syn-1 antibody that cross reacts with mouse and human α-syn since the siRNA chosen for these experiments also recognizes both species. Compared to the siα-syn FTC alone, both the C2-9r and ApoB11 delivered FTC tagged siα-syn was detected in the brains of non-tg (Figure 4G) and α-syn tg mice (Figure 4H). In the non-tg mice, α-syn was detected in the neuropil with a punctate pattern and co-localized to a low extent with the C2-9r and ApoB11 (Figure 4I). In contrast, in the α-syn tg mice, α-syn immunostaining concentrated in the cell bodies reflecting the abnormal accumulation of this synaptic protein in the tg mice (Figure 4H). As expected no signal or co-localization was observed in tg mice treated with siα-syn FTC alone (Figure 4H, I); however, in α-syn tg mice treated with C2-9r/siα-syn FTC or ApoB11/siα-syn FTC, there was close co-localization to neurons exhibiting α-syn accumulation (Figure 4H, I).
Together, these studies indicate that similar to C2-9r, the ApoB11 vector is capable of delivering the siα-syn across the BBB to α-syn expressing cells, reaching the correct target.
ApoB11 and C2-9r mediated delivery of siRNA reduces α-syn accumulation in α-syn tg mice
We have previously shown that systemic delivery of siRNA targeting α-syn with the C2-9r peptide protects wild-type mice from the neurotoxic effects of MPTP (Javed et al., 2016), so for the present study, the objective was to evaluate the neuroprotective effects following systemic delivery of C2-9r and the novel peptide ApoB11 in an α-syn tg model of synucleinopathy. For this purpose, non-tg and PDGF/α-syn tg mice were treated with either si-scrambled (sc) RNA or siα-syn conjugated with either ApoB11 or C2-9r peptides. The si-sc was an RNA with the same ratio of nucleotides as the siα-syn and incubated at the same ratio of 1:10 with ApoB11 or 1:40 with C2-9r in vitro prior to in vivo delivery. Mice were treated twice weekly for 4 weeks by intraperitoneal injection of either C2-9r or ApoB11/siα-syn or si-sc.
Since the siα-syn that we designed targets both mouse and human α-syn (Figure 2), we evaluated the effects of the C2-9r and ApoB11 vectors carrying si-sc or siα-syn on levels of α-syn accumulation using the Syn-1 antibody against total α-syn. As expected, in the non-tg mice treated with either the C2-9r or ApoB11 si-sc, α-syn was detected with a punctuate pattern in the neuropil in multiple brain regions with the highest levels detected in the hippocampus (Figure 5A, upper two panels). Compared to the C2-9r or ApoB11 si-sc group (Figure 5A, upper panel), in non-tg mice treated with either the C2-9r or ApoB11 siα-syn there was a 45-50% reduction in the levels of α-syn immunoreactivity in the neocortex (Figure 5B), hippocampus (Figure 5C) and striatum (Figure 5D). In the α-syn tg mice treated with si-sc there was abundant accumulation of α-syn in neuropil and neuronal cell bodies in the neocortex, hippocampus and striatum (Figure 5A, lower 3rd and 5th panels), with the neocortex and hippocampus displaying higher levels of α-syn accumulation (Figure 5B, C) compared to the striatum (Figure 5D). Remarkably, treatment with the C2-9r or ApoB11 siα-syn resulted in a 65-70% reduction in the levels of α-syn immunoreactivity in the neocortex (Figure 5B), 40-50% in the hippocampus (Figure 6C) and 50-55% in the striatum (Figure 5D). Overall, treatment with ApoB11/siα-syn displayed a trend toward a greater effect than C2-9r/siα-syn.
Figure 5. Immunocytochemical analysis of the effects of systemic delivery of the siα-syn conjugated to C2-9r or ApoB11 peptides on accumulation of α-syn in transgenic mice.
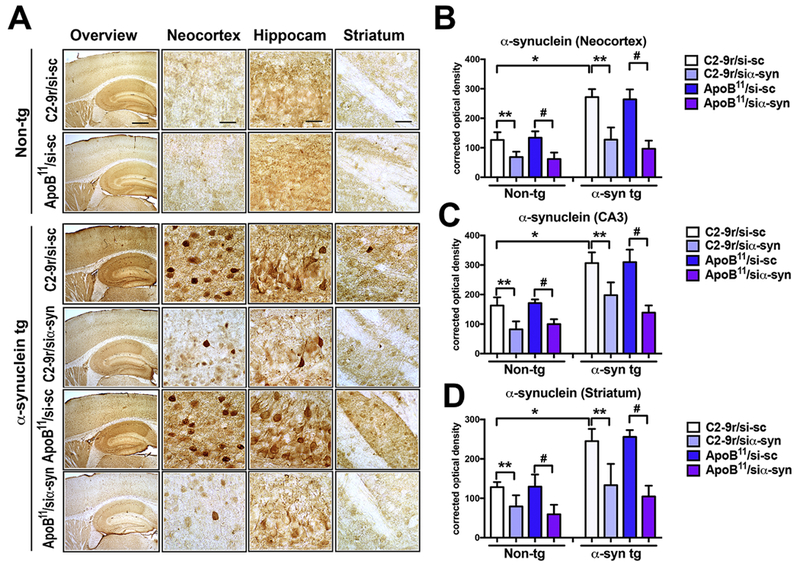
Four-month-old α-syn tg and non-tg mice received repeated intraperitoneal injections of scrambled siRNA (si-sc) or α-syn siRNA (si-αsyn) conjugated to either C2-9r, ApoB11 and were sacrificed 4 weeks later for analysis. Sections were immunolabled with an antibody against FL-α-syn and analyzed by bright field microscopy. (A) Representative images of the neocortex, hippocampus and striatum of non-tg and α-syn tg mice treated with either si-sc or si-αsyn conjugated with C2-9r or ApoB11. Image analysis of levels of expressed as corrected optical density for α-syn immunoreactivity in the (B) neocortex, (C) hippocampus (CA3), and (D) striatum. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing among groups. Statistical significance p < 0.05 compared to: * non-tg mice; ** C2-9r/si-sc; # ApoB11/si-sc. Non-tg (n=16) and α-syn tg (n=24). Scale bar represents 250 μm for low magnification panels and 25 μm for higher magnification panels.
Figure 6. Immunoblot analysis of the effects of systemic delivery of the siα-syn conjugated to C2-9r or ApoB11 peptides of α-syn and endosomal markers in α-syn tg mice.
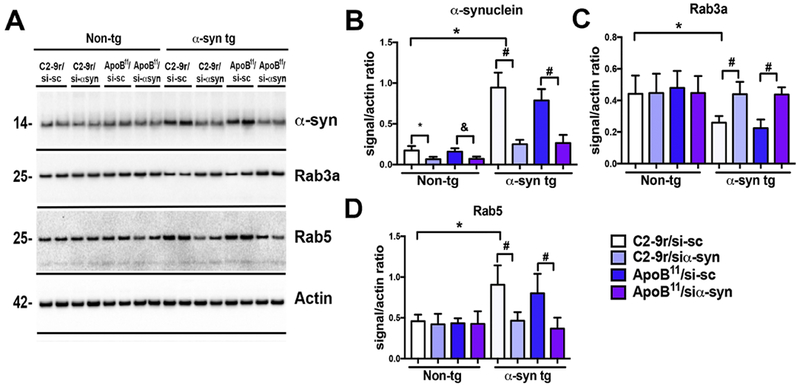
Total homogenates from the cortex were fractioned by ultracentrifugation, the membrane fraction was run for immunoblot analysis. (A) Representative, immunoblot with brain homogenates from non-tg and α-syn tg mice treated with scrambled siRNA (sc-si) or α-syn siRNA (si-αsyn) conjugated to either C2-9r or ApoB11 probed with antibodies against α-syn, endosomal markers (Rab3a, Rab5) and β-actin. Image analysis of levels of immunoreactivity for the bands corresponding to (B) α-syn (14kDa), (C) Rab3a (26kDa) and (D) Rab5 (24kDa) analyzed as ratio to β-actin signal. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing among groups. Statistical significance p < 0.05 compared to: * C2-9r/si-sc non-tg; & ApoB11/si-sc non-tg; * ApoB11/si-sc α-syn tg mice. Non-tg (n=16) and α-syn tg (n=24).
Immunoblot analysis of α-syn accumulation in mice treated with the C2-9r or ApoB11 siα-syn was done by confirming the results observed by immunohistochemistry (Figure 6). Compared to the non-tg mice, α-syn tg mice that were treated with the si-sc displayed increased levels of α-syn monomers and multimers in the cytosolic and membrane fractions (Figure 6A, B). In contrast, in the α-syn tg mice treated with C2-9r or ApoB11/siα-syn there was a 30-40% reduction in the levels of α-syn in the cytosolic and membrane fractions (Figure 6A, B). We have previously shown that the effects of α-syn reduction might be related to improvements in vesicular transport as α-syn accumulation might interfere with this process as reflected by alterations in Rab3a and Rab5 (Spencer et al., 2016a) (Fang et al., 2017). Consistent with these studies, we found that compared to the non-tg mice, in the α-syn tg mice that were treated with the si-sc there were decreased levels of Rab3a and increased levels of Rab5 in the membrane fractions (Figure 6A, C-D). In contrast, in the α-syn tg mice treated with C2-9r or ApoB11/siα-syn the levels of Rab3a and Rab5 were comparable to those found in the non-tg mice (Figure 6A, C-D). In summary, both the C2-9r and ApoB11/siα-syn reduced levels of the endogenous and transgene driven (human) α-syn in the brains of the mice that resulted in reduced levels of α-syn aggregates and improvements in vesicular transport proteins.
ApoB11 and C2-9r mediated delivery of siRNA ameliorates neurodegenerative pathology in α-syn tg mice
Next, we evaluated if reducing the accumulation of α-syn was accompanied by comparable improvements in the neurodegenerative pathology. The PDGF-α-syn tg mice displays neuronal loss and neuro-inflammation in the neocortex and hippocampal (CA3) region while the striatum and S. Nigra shows little to no change in terms of neuronal loss, but there is considerable loss of TH positive fibers in the striatum (Amschl et al., 2013; Spencer et al., 2014a). To evaluate the effects of modulating α-syn expression in the neurodegenerative pathology in the α-syn tg mice, immunocytochemical analysis was performed with antibodies against the pan-neuronal marker NeuN (Figure 7) and the marker of the dopaminergic system, TH (Figure 8). With the antibody against NeuN, in the non-tg mice (Figure 7, upper panels) no significant differences in the numbers of positive cells were observed in the neocortex (Figure 7A, B), hippocampus (Figure 7A, C) or striatum (Figure 7A, D) when comparing mice treated with C2-9r or ApoB11/si-sc vs siα-syn. In contrast and compared to non-tg, the α-syn tg mice treated with C2-9r or ApoB11/si-sc (Figure 7A, panels in 3rd and 5th rows) displayed a 20-25% decreased numbers of NeuN positive neurons in the neocortex (Figure 7A, B) and CA3 region of the hippocampus (Figure 7A, C) but not in the striatum (Figure 7A, D) as previously reported (Spencer et al., 2014a). Whereas, treatment of α-syn tg mice with the C2-9r or ApoB11/siα-syn ameliorated the loss of NeuN cell in both the neocortex (Figure 7A, B) and CA3 (Figure 7A, C) to levels comparable to non-tg mice (Figure 7A-D). With the antibody against TH, in the non-tg mice (Figure 8, upper panels) no significant differences in the density of positive fibers were observed in the striatum (Figure 8A, B), or TH positive neurons in the S. Nigra (Figure 8A, C)when comparing mice treated with C2-9r or ApoB11/si-sc vs siα-syn. In contrast and compared to non-tg, the α-syn tg mice treated with C2-9r or ApoB11/si-sc (Figure 8A, panels in 3d and 5th rows) displayed a 30-35% reduction on TH fibers in the striatum (Figure 8A, B) and no loss of TH positive fibers in the S. Nigra (Figure 8A, C) as previously reported (Amschl et al., 2013; Spencer et al., 2014a). Treatment of α-syn tg mice with the C2-9r or ApoB11/siα-syn prevented the loss of TH fibers in the striatum to levels resulting in levels comparable to non-tg mice (Figure 8A, B).
Figure 7. Immunocytochemical analysis of the effects of systemic delivery of the siα-syn conjugated to C2-9r or ApoB11 peptides on total neurons in the brain of α-syn tg mice.

Four-month-old α-syn tg and non-tg mice received repeated intraperitoneal injections of scrambled siRNA (si-sc) or α-syn siRNA (si-αsyn) conjugated with either C2-9r or ApoB11 and were sacrificed 4 weeks later for analysis. Sections were immunolabeled with an antibody against the neuronal marker NeuN and analyzed by bright field microscopy and stereology (dissector method). (A) Representative images from the neocortex, hippocampus and striatum of non-tg and α-syn tg mice treated with either si-sc or si-αsyn conjugated with C2-9r or ApoB11. Stereological estimates of total NeuN-positive neuronal counts in the hemibrain in the (B) neocortex, (C) hippocampus (CA3), and (D) striatum. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing in between groups. Statistical significance p < 0.05 compared to: * non-tg mice; ** C2-9r/si-sc α-syn-tg mice; # ApoB11/si-sc α-syn-tg mice. Scale bar represents 250 μm for low magnification panels and 25 μm for higher magnification panels. Non-tg (n=16) and α-syn tg (n=24).
Figure 8. Immunocytochemical analysis of the effects of systemic delivery of the siα-syn conjugated to C2-9r or ApoB11 peptides on tyrosine hydroxylase dopaminergic neurons and terminals in the brain of α-syn tg mice.

Four-month-old α-syn tg and non-tg mice received repeated intraperitoneal injections of scrambled siRNA (si-sc) orα-syn siRNA (si-αsyn) conjugated to either C2-9r, ApoB11 and were sacrificed 4 weeks later for analysis. Sections were immunolabeled with an antibody against the dopaminergic neuronal marker tyrosine hydroxylase (TH) and analyzed by bright field microscopy for optical density (striatum) and stereology (S. Nigra pars compacta, dissector method). (A) Representative images of TH immunostaining in the striatum and S. Nigra of non-tg and α-syn tg mice treated with either si-sc or si-αsyn conjugated with C2-9r or ApoB11. (B) Optical density analysis of TH positive fibers in the striatum. (C) Stereological analysis estimates (hemibrain) of numbers of TFI positive neurons in the S. Nigra. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing in between groups. Statistical significance p < 0.05 compared to: *non-tg mice; ** C2-9r/si-sc α-syn-tg mice; & ApoB11/si-sc α-syn-tg mice. Scale bar represents 250 μm for low magnification panels and 25 μm for higher magnification panels. Non-tg (n=16) and α-syn tg (n=24).
Since accumulation of α-syn in neurons and glial cells in associated with neuro-inflammatory responses that might play a role in neurodegeneration, we next investigated if reducing α-syn had effects on astrogliosis and microgliosis utilizing antibodies against GFAP (Figure 9) and Iba1 (Figure 10) respectively. As previously described with the GFAP antibody, when comparing non-tg mice treated with C2-9r or ApoB11/siα-syn or si-sc, the α-syn tg mice treated with C2-9r or ApoB11/si-sc displayed moderate astrogliosis in the neocortex and hippocampus but not in the striatum (Figure 9). Whereas, treatment of α-syn tg mice with the C2-9r or ApoB11/siα-syn reduced the astrogliosis both in the neocortex (Figure 9A, B) and hippocampus (Figure 9A, C) to levels comparable to non-tg mice (Figure 9A-D). Similarly, with the Iba1 antibody when comparing to non-tg mice, the α-syn tg mice displayed mild microgliosis in the neocortex and hippocampus but not in the striatum. In contrast, treatment of α-syn tg mice with the C2-9r or ApoB11/siα-syn reduced the microgliosis in the neocortex (Figure 10A, B) and to a lesser extent in the hippocampus (Figure 10A, C) to levels comparable to non-tg mice (Figure 10A-D).
Figure 9. Immunocytochemical analysis of the effects of systemic delivery of the siα-syn conjugated to C2-9r or ApoB11 peptides on astrogliosis in the brains of α-syn tg mice.

Four-month-old α-syn tg and non-tg mice received repeated intraperitoneal injections of scrambled siRNA (si-sc) or α-syn siRNA (si-αsyn) conjugated to either C2-9r or ApoB11 and were sacrificed 4 weeks later for analysis. Sections were immunolabeled with an antibody against the astroglial cell marker glial fibrillary acidic protein (GFAP). (A) Representative images of the patters of GFAP immunoreactivity in the neocortex, hippocampus and striatum of non-tg and α-syn tg mice treated with either si-sc or si-αsyn conjugated with C2-9r or ApoB11. Image analysis of levels of GFAP immunoreactivity expressed as corrected optical density in the (B) neocortex, (C) hippocampus (CA3), and (D) striatum. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing in between groups. * indicates statistical significance p < 0.05 compared to non-tg mice. ** p < 0.05 compared to C2-9r/si-sc. # indicates statistical significance p < 0.05 compared to α-syn-tg mice treated with ApoB11/si-sc. Scale bar represents 250 μm for low magnification panels and 25 μm for higher magnification panels. Non-tg (n=16) and α-syn tg (n=24).
Figure 10. Immunocytochemical analysis of the effects of systemic delivery of the siα-syn conjugated to C2-9r or ApoB11 peptides on microgliosis in the brains of α-syn tg mice.

Four-month-old α-syn tg and non-tg mice received repeated intraperitoneal injections of scrambled siRNA (si-sc) or α-syn siRNA (si-αsyn) conjugated to either C2-9r or ApoB11 and were sacrificed 4 weeks later for analysis. Sections were immunolabeled with an antibody against the microglia cell marker-Iba1. (A) Representative images of the neocortex, hippocampus and striatum of non-tg and α-syn tg mice treated with either si-sc or si-αsyn conjugated with C2-9r or ApoB11 of sections immunostained with the Iba1 antibody. Image analysis of the relative numbers of Iba1 immunoreactive cells per 102 in the (B) neocortex, (C) hippocampus (CA3), and (D) striatum. One-way ANOVA with poshoc Dunnet when comparing to control and Tukey-Kramer when comparing in between groups. * indicates statistical significance p < 0.05 compared to non-tg mice. ** p < 0.05 compared to C2-9r/si-sc. # indicates statistical significance p < 0.05 compared to α-syn-tg mice treated with ApoB11/si-sc. Scale bar represents 250 μm for low magnification panels and 25 μm for higher magnification panels. Non-tg (n=16) and α-syn tg (n=24).
Taken together, these results support the possibility that systemic delivery of oligonucleotides targeting α-syn using either ApoB11 or C2-9r ameliorate neurodegenerative and neuroinflammatory pathology associated with α-syn accumulation in a mouse model of PD/DLB.
Discussion
Synucleinopathies of the aging population includes DLB, PD and MSA affect over 1.5 million people in the US alone (Alafuzoff and Hartikainen, 2017). Currently no disease modifying therapies are available, but it has been proposed that since α-syn progressively accumulates and triggers toxicity in these disorders that either reducing α-syn synthesis or promoting clearance might be of therapeutic value (Kulisevsky et al., 2018; Valera and Masliah, 2016). In this study, we showed that the 11-amino acid peptide derived from the apolipoprotein B fused with a 9-amino acid Arginine tail (ApoB11) could bind to and transport an siα-syn across the BBB for delivery to neurons and astrocytes following intraperitoneal delivery. Similar to C2-9r, systemically delivered ApoB11/siα-syn effectively reduced α-syn accumulation and ameliorated neurodegeneration and reduced neuro-inflammation in a tg model of synucleinopathy.
These results agree with a previous study showing that knocking down α-syn with systemically administered C2-9r/siα-syn is neuroprotective in a toxic model of PD involving MPTP challenge (Javed et al., 2016). This is consistent with other studies showing that α-syn knock out mice are resistant to MPTP (Dauer et al., 2002; Drolet et al., 2004; Robertson et al., 2004; Tai et al., 2014), 3-nitro propionic acid (Ubhi et al., 2010) and other neurotoxins and that in the α-syn knock out background the neurotoxic effects of APP/Aß are attenuated in tg mice (Spencer et al., 2016a). This new study is different in that instead of using a toxic model of PD we utilized an α-syn tg model (line D) (Masliah et al., 2000; Rockenstein et al., 2002) that is widely used in the field and has been shown to mimic some aspects of PD/DLB. Moreover, in addition to testing C2-9r we investigated the potential value of a novel peptide vector for the delivery of the siα-syn, namely ApoB11. We chose to use this peptide because in earlier studies, we found that the parent peptide ApoB38 was highly active at delivering proteins into the CNS (Spencer et al., 2014a; Spencer et al., 2011; Spencer et al., 2016c; Spencer et al., 2015; Spencer et al., 2014b; Spencer and Verma, 2007) and to have an alternative vector to C2-9r. Overall, it was found that both peptides carrying the siα-syn behave similarly, although with ApoB11 we observed a more extensive localization to neurons (Figure 4 A-C).
Another difference we observed between the two peptides was the ratio of peptide to siRNA required for complete binding of the oligonucleotide. Previous work from Javed showed an optimal ratio of 40:1 of the C2-9r to siRNA while we showed a ratio of 10:1 for the ApoB11 to siRNA (Javed et al., 2016). It is not clear why 4 times less ApoB11 peptide is required for complete binding of the same oligonucleotide, although the ApoB11 peptide is significantly more hydrophilic than the C2 peptide. A more optimal ratio could be identified by performing sub-dilutions between the 1:1 and 1:10 that we performed in this experiment to identify a molar ratio of ApoB11 that requires less peptide and would maybe reduce the amount of unbound peptide in the injection.
Systemic delivery of peptides conjugated to siRNA may necessarily result in distribution to peripheral organs in addition to distribution to the CNS. In fact, we have previously shown that delivery of proteins and enzymes fused to the larger ApoB38 peptide are not only widely distributed across the whole CNS, but also to the liver, spleen and muscle (tissues with LDL-R expression) (Spencer et al., 2011; Spencer et al., 2014b; Spencer and Verma, 2007). Although we didn’t examine the uptake of siRNA in tissues other than the CNS in this study, we would expect a significant portion of peptide uptake to occur through the liver based on previous work (Spencer et al., 2016d). Delivery of siRNA against α-synuclein to the liver would not necessarily be detrimental as α-syn is specifically expressed in neurons; however, caution would have to be exercised when delivering siRNA against other, more ubiquitous, genes.
Recent evidence suggests that silencing α-synuclein in the adult rat brain may have some detrimental effects (Benskey et al., 2018). Bensksy et al showed delivery of an shRNA with the AAV vector directly to the S. Nigra resulted in loss of TH positive cells as well as increased microglia inflammation (Benskey et al., 2018). Previous work has shown that knockout of α-syn in the mouse has little to no detrimental effect so the knockdown in the adult animal may represent a previously unrealized pathology associated with complete knockdown of α-syn in dopaminergic cells (Abeliovich et al., 2000; Cabin et al., 2002; Dauer et al., 2002; Schluter et al., 2003). Our work in vitro and in vivo shows only approximately a 50% knockdown of the endogenous mouse α-syn. In addition, we examined the inflammation and levels of TH immunoreactivity in the striatum and the S. Nigra and saw no loss of fiber staining in either area when si-αsyn was delivered by either C2-9r or ApoB11. Thus, it is possible that the remaining α-syn is sufficient to prevent the neuronal death and subsequent inflammation observed by Benskey et al.
Earlier studies have shown that direct delivery into the nervous system of nucleotides targeting α-syn can reduce expression levels by 90% (Bourdenx et al., 2014). For example, si and shRNA targeting α-syn have been transferred into the brain of wildtype mice challenged with MPTP or in tg mice (Takahashi et al., 2015) using viral vectors (Sapru et al., 2006) or naked nucleotides (Lewis et al., 2008). Moreover, administration of naked mir-7 and mir-153 (Doxakis, 2010) or AAV delivered mir-30 has been shown to silence α-syn (Khodr et al., 2014). We have shown that delivery of a lentivirus expressing mir-101 reduces α-syn accumulation in the brains of a tg model of MSA (Valera et al., 2017). In contrast to this research where α-syn expression was abrogated by direct delivery of nucleotides into the CNS, for this study we explored the use of small peptide vectors for systemic delivery. The ApoB11 peptide could be used to deliver any form or design of nucleotides including, but not limited to: cDNA, mRNA, siRNA, shRNA, miRNA, gRNA or synthetic nucleotide sequences. Systemic delivery of oligonucleotides to the CNS could be beneficial for neurodegenerative diseases, neuro-modulatory treatments, cancers of the CNS, gene targeted editing of the CNS or anti-microbial gene delivery of the CNS.
As shown here, systemic delivery of siRNA by either the ApoB11 or C2-9r peptide (Javed et al., 2016) to the CNS occurred primarily to neurons and only to a lesser extent the astrocytes as measured by co-labeling studies with NeuN and GFAP respectively. We previously showed with the larger ApoB38 peptide fused to the protease, neurosin, that uptake occurred primarily with neurons and to a lesser extent astrocytes, microglia and oligodendrocytes (Spencer et al., 2015). This pattern closely resembled the pattern of LDL-R expression levels on these cells as the peptide relies on this receptor for endocytosis into the cell (Spencer et al., 2015). Following endocytosis, the siRNA must escape the endosome as a naked oligonucleotide to function as an inhibitor molecule. The process of this escape mechanism is unclear; however, previous studies have shown other proteins and antibodies have escaped the endosome following endocytosis with the ApoB38 peptide suggesting a mechanism must exist (Spencer et al., 2014a; Spencer et al., 2011; Spencer et al., 2016c; Spencer et al., 2015; Spencer and Verma, 2007). It is possible that binding to HDL or LDL molecules through the ApoB peptide may facilitate escape of the endosome via SR-BI receptor, thus bypassing the lysosome fusion (Lim et al., 2013; Wolfrum et al., 2007). This would allow escape of the peptide:RNA complex to the cytoplasm where it could access the mRNA for RISC mediated endonuclease digestion (Hammond, 2005).
The Apolipoprotein B protein binds to the LDL receptor at the surface of the endothelial cells on the BBB inducing endocytosis and transytosis to the CNS side where it is released for binding to receptors on neurons and astrocytes. Similarly, we believe the ApoB11 peptide binds to the LDL-R and transports siRNA to the CNS. In contrast, the RGD peptide binds to the nicotinic acetylcholine receptor (nAch) (Lentz, 1991) on neurons for uptake, although the receptor used for binding and transport across the blood-brain barrier is not known, as nAch and other known RGD binding receptors are not expressed on BBB endothelial model cells such as bEnd.3 or BCECs (Tian et al., 2015). However, other atypical nAch α-subunits have been detected by RT-PCR in rat arterial endothelial cells possibly explaining the uptake and transport mechanism at the BBB (Bruggmann et al., 2002; Bruggmann et al., 2003). These alternate receptors may also be responsible for the uptake by astrocytes. In fact, the α7 nAchR has been detected in cultured neonatal rat microglial cells (Suzuki et al., 2006). Recent studies have shown an upregulation of nAchR in the caudate nucleus of Parkinson’s patients with a concomitant downregulation in the putamen, thus potentially complicating the targeting of the C2-9r directed siRNA for Parkinson’s disease (Isaias et al., 2014). It remains to be seen what role each of these peptides may play in therapeutic interventions for different targets.
Although the t1/2 of the siRNA wasn’t examined in this study, previous studies have shown the half-life of unprotected RNA in the serum to be approximately 2 hours (Javed et al., 2016; Layzer et al., 2004) and in the rodent following intravenous delivery of 6 minutes (Soutschek et al., 2004). Once inside the cell, the siRNA has a long half-life and has shown knockdown of the target gene for as long as 3 days in dividing cells and 3-4 weeks in non-dividing cells (Bartlett and Davis, 2006; Layzer et al., 2004). In order to increase the effectiveness and therapeutic potential of siRNAs for CNS delivery, longer t1/2 in the serum would be beneficial. Introduction of 2’-fluoro or 2’-O’methyl sugar modifications function to increase endonuclease resistance and stabilizing siRNA duplexes. In fact, 2’-F modified siRNAs have an increased serum t1/2 of approximately 24 hours (Layzer et al., 2004). Currently, 2’-F modified siRNAs are approved for clinical use (Fomivirsen and Macugen) (Stein and Castanotto, 2017). Other modifications that could be considered include morpholinos, peptide nucleic acids and phosphorthioate sugars (Wang et al., 2017).
Since the transport with receptors targeted for transport across the BBB are saturable (Duffy et al., 1988; Wunner et al., 1984), identification of multiple methods of transport may be necessary for the delivery of more than one therapy simultaneously. Starzyk et al showed that the transferrin receptor could be used for transport of proteins across the BBB (Friden et al., 1991) and subsequently, the IGF-R was used to transport proteins (Coloma et al., 2000; Pardridge et al., 1995). These receptors work similarly to the LDLR family of receptors in that they (a) bind protein on the basal/ blood side, (b) transport by transytosis to the neuronal side, and (c) release the protein on the neuronal side of the BBB (Bickel et al., 2001). Targeting different receptor pathways for different therapeutics will prevent the possibility of overwhelming the transport kinetics at the BBB thus allowing simultaneous delivery of multiple therapeutic options. It remains to be seen if the 11 -amino acid ApoB peptide can function to transport larger proteins with the same efficiency as the previously published 38 amino acid ApoB38 peptide. Additional studies will be needed to determine these kinetics as well as how long lasting are the neuroprotective effects of α-syn knocked down with these two small peptide vectors. Moreover, detailed behavioral and functional analysis will be needed to further evaluate the potential therapeutic value of this approach in synucleinopathies.
In summary, we have identified a 11-amino acid LDL-R binding peptide that can transport siα-syn across the BBB as a therapeutic option for synucleinopathies such as PD and DLB. The ApoB11 peptide, along with the C2-9r peptide, represent two potential approaches for the delivery of siRNAs or other oligonucleotides to the CNS for therapies.
Methods and experimental design
Preparation of peptide vectors and siRNAs
For this study we utilized two peptide vectors to deliver the siRNA targeting α-syn. The first was a validated (as positive control) peptide namely C2-9r, (NH2CDIFTNSRGKRAGGGGrrrrrrrrr) that was developed by the addition of four Gly (G) and nine d-Arg (9r) residues to the C-terminus of the C2 peptide, derived from a 29 residue rhabdovirus glycoprotein and has been previously described (Javed et al., 2016). The second and novel vector was the ApoB11, a smaller peptide linked to a 9r (NH2RLTRKRGLKLAGGGGGRRRRRRRRR) derived from the ApoB38 peptide that we have previously shown to effectively transport antibodies, proteases and trophic factors across the BBB (Spencer et al., 2014a; Spencer et al., 2011; Spencer et al., 2016c; Spencer et al., 2015; Spencer et al., 2014b; Spencer and Verma, 2007). The peptide was synthesized to 90% purity by Karebay Biochem, Inc.
Peptides were diluted to 10mM in PBS (Life Technologies). The siα-syn (5’GAC UUU CAA AGG CCA AGG A) corresponding to nucleotides 168-186 of α-synuclein was chosen because we have previously shown targets both mouse and human α-syn (Spencer et al., 2016a). As control we used an si-scrambled (5’GGG CAU ACU GAG CUA ACA A). The siRNAs were purchased from ValueGene, Inc. and desalted. For in vitro and in vivo trafficking experiments a FITC labeled si-αsyn RNA labeled at 5’ was utilized and prepared. Oligonucleotides were re-suspended in RNAse free water (Life Technologies) at 100mM and aliquoted. The siRNA’s (si-sc and siα-syn) and peptide’s (ApoB11 and C2-9r) were mixed at specified ratios in PBS (1:0, 0:1, 1; 1, 1:10, 1:20, 1:40, 1:60) and incubated at room temperature for 30 minutes to allow RNA to bind to peptide.
Neuronal cell lines, treatments and analysis
The mouse cholinergic cell line Neuro2A (N2A) was utilized for in vitro experiments (Kojima et al., 1994). N2A cells were plated at 1 × 105 cells/well of a 12 well dish on poly L-lysine coated glass coverslips in DMEM + 1% FBS for 5 days to allow for differentiation (Spencer et al., 2016a). Cells were treated with 100pmol siRNA (FITC tagged): peptide for 0, 3, 24 and 48 hrs and then fixed in 4% paraformaldehyde. Immunolabeling was performed as previously described (Spencer et al., 2016b) with an antibody against MAP2 (Mouse monoclonal, Millipore, 1:5000) detected with Tyramide Red system (NEN Life Sciences) and analysis of uptake of the FITC labeled siRNA was performed by laser scanning confocal microscopy (BioRad, MRC1024) (Spencer et al., 2016a).
Additional experiments included treating N2A cells with untagged siRNA (100pmol): peptide for 0, 24, 48 and 72 hrs. Control experiments were performed by treating a mouse cell line with the si-sc/peptide and the siα-syn/peptide for 24 hrs. Then cells in coverslips were double immunolabeled with antibodies against MAP2 (1:200, Millipore) and α-syn (Syn-1, mouse monoclonal, 1:5000, BD Biosciences), α-syn was detected with the Tyramide Red system (NEN Life Sciences) whereas MAP2 was detected with FITC-tagged antibodies (1:75; Vector). Coverslips were imaged with a Zeiss 63× 1.4 objective on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 laser scanning confocal microscope (LSCM) system (BioRad) and analyzed with Image J to determine pixel intensity (Spencer et al., 2016a).
Animal care and tissue processing
All experiments described were carried out in strict accordance with good animal practice according to NIH recommendations, and all procedures for animal use were approved by the Institutional Animal Care and Use Committee at the University of California at San Diego (UCSD) under protocol #S07221. At the end of the 30-day treatment, the mice were sacrificed and the right hemibrains were snap-frozen and stored at −70°C for biochemical analysis, while the left hemibrains were fixed 4% paraformaldehyde for vibratome sectioning (40 μm) and neuropathological analysis.
Transgenic mouse lines and treatments
For this study, mice over-expressing α-syn from the platelet-derived growth factor β (PDGF-β) promoter (Line D) were utilized (Masliah et al., 2000; Rockenstein et al., 2002). This model was selected because mice from this line develop intraneuronal α-synuclein aggregates distributed through the neocortex and hippocampus similar to what has been described in LBD. Moreover, expression levels of the transgene are moderate (0.5-1.0 fold over baseline) allowing more clear analysis of the effects of the siRNA and these animals display loss of dopaminergic fibers in the striatum and mild motoric deficits. At first, we compared trafficking and distribution of the siRNA following intra-venous vs intra-peritoneal injection. For this purpose, non-tg mice (total, n=18) received a single intra-venous (n=9) or intra-peritoneal (n=9) injection of 50μg of 5’ FITC naked siα-syn (n=3), C9-2r/siα-syn (n=3) or ApoB11/siα-syn (n=3) and 4 hours later were terminated for analysis. Then, to study the trafficking and anatomical and cellular location of the siRNA, non-tg (n=12) and α-syn tg (total n= 12) received a single intra-peritoneal injection of 50μg of 5’ FITC labeled naked siα-syn (n=3 per genotype) or siα-syn conjugated to either C2-9r (n=3, per genotype/group) or ApoB11 (n=3), per genotype/group) and 4 hours later were sacrificed.
To determine the ability of the systemically administered siRNA/protein conjugate to ameliorate neurodegeneration and α-syn pathology groups of non-tg (n=16; 4 m/o) and α-syn tg (n=24; 4 m/o) mice received intra-peritoneal injections of 50μg of C2-9r/ si-sc (n=4 per group non-tg and n=6 per group α-syn tg); ApoB11/si-sc (n=4 per group non-tg and n=6 per group α-syn tg); C9-2r/siα-syn (n=4 per group non-tg and n=6 per group α-syn tg)or ApoB11/ siα-syn (n=4 per group non-tg and n=6 per group α-syn tg) twice weekly for four weeks. Following NIH guidelines for the humane treatment of animals, mice were anesthetized with chloral hydrate and flush-perfused transcardially with 0.9% saline. Brains and peripheral tissues were removed and divided sagitally. The right hemibrain was post-fixed in phosphate-buffered 4% PFA (pH 7.4) at 4°C for 48 hours for neuropathological analysis, while the left hemibrain was snap-frozen and stored at −70°C for subsequent protein analysis.
Immunoblot analysis
Cell lysates and mouse hemibrains were homogenized as previously described (Crews et al., 2010; Spencer et al., 2009). For immunoblot analysis, 20 μg of total protein per lane was loaded on 4-12% Bis-Tris SDS-PAGE gels and blotted onto polyvinylidene fluoride membranes. Membranes were probed with antibodies against total α-syn (Syn-1, BD Biosciences). Incubation with primary antibody was followed by species-appropriate incubation with secondary antibody tagged with horseradish peroxidase (Santa Cruz Biotechnology) and visualization with enhanced chemiluminescence. Analysis of all immunoblot was performed with a Versadoc XL imaging apparatus (BioRad) using β-actin (Sigma) levels as a loading control.
Immunocytochemical and neuropathological analyses
Briefly as previously described (Spencer et al., 2016a) blind-coded sagittal brain vibratome sections were treated at 4°C for overnight with primary antibodies against α-syn (Syn-1, BD Biosciences, mouse monoclonal, 1:500), neuronal marker NeuN (Millipore, mouse monoclonal, 1:1000), dopaminergic marker tyrosine hydroxylase (TH) (Millipore, mouse monoclonal, 1:1000), astroglial marker GFAP (Millipore, mouse monoclonal, 1:1000) and microglial cell marker Iba-1 (Abcam, goat polyclonal, 1:500) respectively. Following overnight incubation, the sections were incubated with biotinylated-secondary antibodies and detected with avidin D-FIRP (ABC Elite, Vector Laboratories, Burlingame, CA). To determine α-syn neuropathology (Syn-1) and astrogliosis (GFAP), the brain sections were imaged by Olympus BX41 microscope. The levels of immunoreactivity were determined by optical density analysis using Image Quant 1.43 program (NIH). From each section a total of 10 digital fields 1024×1024 pixels in the neocortex and hippocampus were analyzed. Levels of optical density were corrected to background using sections stained in the absence of the primary antibody. The numbers of α-syn inclusion positive cells were determined per field (100 μm2). To estimate neuronal cell numbers briefly as previously described (Overk et al., 2014), the numbers of NeuN-immunoreactive neurons in the hippocampus were estimated utilizing unbiased stereological methods. The CA3 region of the hippocampus was outlined using an Olympus BX51 microscope running Stereo Investigator 8.21.1 software (Micro-BrightField, Cochester, VT) (grid sizes 150 × 150 μm and the counting frames were 30 × 30 μm). The average coefficient of error for each region was below 0.1. Sections were analyzed using a 100× 1.4 PlanApo oil-immersion objective. The average mounted tissue thickness allowed for 2 μm top and bottom guard-zones and a 5 μm high dissector. Similarly, the number of TH (S. Nigra) immunoreactive neurons were estimated using unbiased stereological methods (Overk et al., 2014). In addition, the density of TH positive axonal fibers in the striatum was analyzed by optical density as described above. To analyze microglial cell morphology briefly as previously described (El-Agnaf et al., 2017), sections labeled with Iba-1 were analyzed utilizing the Image-Pro Plus program (Media Cybernetics, Silver Spring, MD) (10 digital images 1024×1024 pixels per section) and analyzed in order to estimate the average number of microglial cells per unit area (100 μm2) and ramifications of Iba-1 positive microglia (Morrison and Filosa, 2013).
To determine the distribution and cellular localization of the naked or C2-9r or ApoB11 FITC tagged siRNA, co-labeling experiments were performed as previously described (Spencer et al., 2013). For this purpose, vibratome sections were immunolabeled with antibodies against α-syn (Syn-1, BD Biosciences, mouse monoclonal, 1:5000), NeuN (Millipore, mouse monoclonal, 1:10,000) and GFAP (Millipore, mouse monoclonal, 1:10,000) followed by detection with the Tyramide Signal Amplification™-Direct (Red) system (NEN Life Sciences) while the siRNA was directly visualized by the appended FITC label. Sections were imaged with a Zeiss 63× (N.A. 1.4) objective on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 LSCM system (BioRad) (Masliah et al., 2000).
Statistical analysis
All experiments were performed blind coded and in triplicate. Values in the figures are expressed as means ± SEM. To determine the statistical significance, values were compared by using the one-way ANOVA with posthoc Dunnet when comparing the non-tg controls vs α-syn tg mice. Additional comparisons among groups treated with C2-9r or ApoB11/siα-syn or si-sc were done using Tukey-Krammer or Fisher posthoc tests. The differences were considered to be significant if p values were less than 0.05.
Highlights.
An 11aa peptide can transport an siRNA across the BBB to knockdown gene expression.
Widespread distribution of siRNA can be accomplished.
α-synuclein accumulation can be reduced and neuro-inflammation reversed with siRNA.
Nonviral delivery of siRNA is possible without intracranial or intrathecal injection.
Acknowledgements
Supported by NIH grants AG05131 and AG18440.
Abbreviations
- α-syn
α-synuclein
- LBD
Lewy body disease
- PD
Parkinson’s Disease
- DLB
Dementia with Lewy bodies
- Aß
Amyloid ß
- AD
Alzheimer’s disease
- Htt
huntingtin
- HD
Huntington’s Disease
- FTD
Fronto-temporal dementia
- ALS
Amyotrophic lateral sclerosis
- BBB
blood-brain barrier
- CMT
carrier mediated transporters
- RMT
receptor mediated transport
- LDL-R
low-density lipoprotein receptor
- siα-syn
siRNA targeting α-syn
- C2-9r
small peptide from the envelope protein of the rabies virus
- ApoB
apolipoprotein B
- si-sc
si-scrambled (sc) RNA
- ApoB11
11-amino acid sequence from the apoB protein coupled with a 9-amino acid arginine linker
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, et al. , 2006. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 7,41–53. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, et al. , 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 25,239–52. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Hartikainen P, 2017. Alpha-synucleinopathies. Handb Clin Neurol. 145, 339–353. [DOI] [PubMed] [Google Scholar]
- Alarcon-Aris D, et al. , 2018. Selective alpha-Synuclein Knockdown in Monoamine Neurons by Intranasal Oligonucleotide Delivery: Potential Therapy for Parkinson’s Disease. Mol Ther. 26, 550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves S, et al. , 2016. Gene Therapy Strategies for Alzheimer’s Disease: An Overview. Hum Gene Ther. 27, 100–7. [DOI] [PubMed] [Google Scholar]
- Amschl D, et al. , 2013. Time course and progression of wild type alpha-synuclein accumulation in a transgenic mouse model. BMC Neurosci. 14, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DW, Davis ME, 2006. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 34, 322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DJ, 2004. ABC transporters and the blood-brain barrier. Curr Pharm Des. 10, 1295–312. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW, 2003. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 61, 39–78. [DOI] [PubMed] [Google Scholar]
- Benskey MJ, et al. , 2018. Silencing Alpha Synuclein in Mature Nigral Neurons Results in Rapid Neuroinflammation and Subsequent Toxicity. Front Mol Neurosci. 11,36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel U, et al. , 2001. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 46, 247–79. [DOI] [PubMed] [Google Scholar]
- Bourdenx M, et al. , 2014. Down-regulating alpha-synuclein for treating synucleopathies. Mov Disord. 29, 1463–5. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, et al. , 2015. Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci. 16, 109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggmann D, et al. , 2002. Multiple nicotinic acetylcholine receptor alpha-subunits are expressed in the arterial system of the rat. Histochem Cell Biol. 118, 441–7. [DOI] [PubMed] [Google Scholar]
- Bruggmann D, et al. , 2003. Rat arteries contain multiple nicotinic acetylcholine receptor alpha-subunits. Life Sci. 72, 2095–9. [DOI] [PubMed] [Google Scholar]
- Cabin DE, et al. , 2002. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 22, 8797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, et al. , 2007. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 114, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, et al. , 2004. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 101, 14966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JL, et al. , 2018. Targeting Amyloid-beta Precursor Protein, APP, Splicing with Antisense Oligonucleotides Reduces Toxic Amyloid-beta Production. Mol Ther. 26, 1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coloma MJ, et al. , 2000. Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res. 17, 266–74. [DOI] [PubMed] [Google Scholar]
- Crews L, et al. , 2010. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 5, e9313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Crunkhorn S, 2017. Alzheimer disease: Antisense oligonucleotide reverses tau pathology. Nat Rev Drug Discov. 16, 166. [DOI] [PubMed] [Google Scholar]
- Dauer W, et al. , 2002. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 99, 14524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Karran E, 2016. The Cellular Phase of Alzheimer’s Disease. Cell. 164, 603–15. [DOI] [PubMed] [Google Scholar]
- DeVos SL, Hyman BT, 2017. Tau at the Crossroads between Neurotoxicity and Neuroprotection. Neuron. 94, 703–704. [DOI] [PubMed] [Google Scholar]
- Dickey AS, La Spada AR, 2018. Therapy development in Huntington disease: From current strategies to emerging opportunities. Am J Med Genet A. 176, 842–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxakis E, 2010. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem. 285, 12726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet RE, et al. , 2004. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 25, 761–9. [DOI] [PubMed] [Google Scholar]
- Duffy KR, et al. , 1988. Human blood-brain barrier insulin-like growth factor receptor. Metabolism. 37, 136–40. [DOI] [PubMed] [Google Scholar]
- El-Agnaf O, et al. , 2017. Differential effects of immunotherapy with antibodies targeting alpha-synuclein oligomers and fibrils in a transgenic model of synucleinopathy. Neurobiol Dis. 104,85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller M, Williams DR, 2011. alpha-Synuclein in Parkinson disease and other neurodegenerative disorders. Clin Chem Lab Med. 49, 403–8. [DOI] [PubMed] [Google Scholar]
- Fang F, et al. , 2017. Synuclein impairs trafficking and signaling of BDNF in a mouse model of Parkinson’s disease. Sci Rep. 7, 3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden PM, et al. , 1991. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc Natl Acad Sci U S A. 88, 4771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, et al. , 2018. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 21, 1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Tabrizi SJ, 2017. Gene suppression approaches to neurodegeneration. Alzheimers Res Ther. 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons GS, et al. , 2018. Mechanisms of Cell-to-Cell Transmission of Pathological Tau: A Review. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, 2005. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 579, 5822–9. [DOI] [PubMed] [Google Scholar]
- Isaias IU, et al. , 2014. Nicotinic acetylcholine receptor density in cognitively intact subjects at an early stage of Parkinson’s disease. Front Aging Neurosci. 6, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A, et al. , 1995. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 14, 467–75. [DOI] [PubMed] [Google Scholar]
- Javed H, et al. , 2016. Development of Nonviral Vectors Targeting the Brain as a Therapeutic Approach For Parkinson’s Disease and Other Brain Disorders. Mol Ther. 24, 746–58. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jucker M, Walker LC, 2018. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat Neurosci. 21, 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SC, et al. , 2004. BACE1 suppression by RNA interference in primary cortical neurons. J Biol Chem. 279, 1942–9. [DOI] [PubMed] [Google Scholar]
- Keiser MS, et al. , 2016. Gene suppression strategies for dominantly inherited neurodegenerative diseases: lessons from Huntington’s disease and spinocerebellar ataxia. Hum Mol Genet. 25, R53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodr CE, et al. , 2014. Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects. Brain Res. 1550, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott TJ, et al. , 1986. Complete protein sequence and identification of structural domains of human apolipoprotein B. Nature. 323, 734–8. [DOI] [PubMed] [Google Scholar]
- Kojima N, et al. , 1994. Induction of cholinergic differentiation with neurite sprouting by de novo biosynthesis and expression of GD3 and b-series gangliosides in Neuro2a cells. J Biol Chem. 269, 30451–6. [PubMed] [Google Scholar]
- Koutsilieri E, et al. , 2007. The therapeutic potential of siRNA in gene therapy of neurodegenerative disorders. J Neural Transm Suppl. 43–9. [DOI] [PubMed] [Google Scholar]
- Kulisevsky J, et al. , 2018. Update in therapeutic strategies for Parkinson’s disease. Curr Opin Neurol. 31, 439–447. [DOI] [PubMed] [Google Scholar]
- Lajoie JM, Shusta EV, 2015. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu Rev Pharmacol Toxicol. 55, 613–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, et al. , 2013. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 14, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzer JM, et al. , 2004. In vivo activity of nuclease-resistant siRNAs. RNA. 10, 766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, et al. , 2011a. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat Rev Neurosci. 13, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, et al. , 2011b. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci Res. 70, 339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz TL, 1991. Structure-function relationships of curaremimetic neurotoxin loop 2 and of a structurally similar segment of rabies virus glycoprotein in their interaction with the nicotinic acetylcholine receptor. Biochemistry. 30, 10949–57. [DOI] [PubMed] [Google Scholar]
- Lewis J, et al. , 2008. In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener. 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, et al. , 2013. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 17, 671–84. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, et al. , 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 46, 3–26. [DOI] [PubMed] [Google Scholar]
- Liu DM, et al. , 2008. RNA interference mediated silencing of alpha-synuclein in MN9D cells and its effects on cell viability. Neurosci Bull. 24, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, et al. , 2012. Trans-synaptic spread of tau pathology in vivo. PLoS One. 7, e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo HM, et al. , 2004. Down-regulation amyloid beta-protein 42 production by interfering with transcript of presenilin 1 gene with siRNA. Acta Pharmacol Sin. 25, 1613–8. [PubMed] [Google Scholar]
- Magen I, Hornstein E, 2014. Oligonucleotide-based therapy for neurodegenerative diseases. Brain Res. 1584, 116–28. [DOI] [PubMed] [Google Scholar]
- Margulis J, Finkbeiner S, 2014. Proteostasis in striatal cells and selective neurodegeneration in Huntington’s disease. Front Cell Neurosci. 8, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, et al. , 2000. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 287, 1265–9. [DOI] [PubMed] [Google Scholar]
- Masliah E, Spencer B, 2015a. Applications of ApoB LDLR-Binding Domain Approach for the Development of CNS-Penetrating Peptides for Alzheimer’s Disease. Methods Mol Biol. 1324, 331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Spencer B, Applications of ApoB LDLR-Binding Domain Approach for the Development of CNS-Penetrating Peptides for Alzheimer’s Disease In: Langel Ü, (Ed.), Cell-Penetrating Peptides. Springer, 2015b, pp. 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, et al. , 2017. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HW, Filosa JA, 2013. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ, 2012. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2, a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overk CR, et al. , 2014. Hippocampal neuronal cells that accumulate alpha-synuclein fragments are more vulnerable to Abeta oligomer toxicity via mGluR5--implications for dementia with Lewy bodies. Mol Neurodegener. 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, 2016. Re-engineering therapeutic antibodies for Alzheimer’s disease as blood-brain barrier penetrating bi-specifc antibodies. Expert Opin Biol Ther. 16, 1455–1468. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, 2017. Delivery of Biologics Across the Blood-Brain Barrier with Molecular Trojan Horse Technology. BioDrugs. 31, 503–519. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, et al. , 1995. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm Res. 12, 807–16. [DOI] [PubMed] [Google Scholar]
- Robertson DC, et al. , 2004. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 89, 1126–36. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, et al. , 2002. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 68, 568–78. [DOI] [PubMed] [Google Scholar]
- Sapru MK, et al. , 2006. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 198, 382–90. [DOI] [PubMed] [Google Scholar]
- Schluter OM, et al. , 2003. Role of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuroscience. 118, 985–1002. [DOI] [PubMed] [Google Scholar]
- Schoch KM, Miller TM, 2017. Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron. 94, 1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J, 2016. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer O, et al. , 2005. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 8, 1343–9. [DOI] [PubMed] [Google Scholar]
- Soutschek J, et al. , 2004. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 432, 173–8. [DOI] [PubMed] [Google Scholar]
- Spencer B, et al. , 2018. Selective targeting of 3 repeat Tau with brain penetrating single chain antibodies for the treatment of neurodegenerative disorders. Acta Neuropathol. 136, 69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2016a. Reducing Endogenous alpha-Synuclein Mitigates the Degeneration of Selective Neuronal Populations in an Alzheimer’s Disease Transgenic Mouse Model. J Neurosci. 36, 7971–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2014a. ESCRT-mediated uptake and degradation of brain-targeted alpha-synuclein single chain antibody attenuates neuronal degeneration in vivo. Mol Ther. 22, 1753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2016b. alpha-Synuclein interferes with the ESCRT-III complex contributing to the pathogenesis of Lewy body disease. Hum Mol Genet. 25, 1100–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2011. Peripheral delivery of a CNS targeted, metalo-protease reduces abeta toxicity in a mouse model of Alzheimer’s disease. PLoS One. 6, e16575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2013. Lentivirus mediated delivery of neurosin promotes clearance of wild-type alpha-synuclein and reduces the pathology in an alpha-synuclein model of LBD. Mol Ther. 21, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2016c. Systemic Central Nervous System (CNS)-targeted Delivery of Neuropeptide Y (NPY) Reduces Neurodegeneration and Increases Neural Precursor Cell Proliferation in a Mouse Model of Alzheimer Disease. J Biol Chem. 291, 1905–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2009. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 29, 13578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2015. A brain-targeted, modified neurosin (kallikrein-6) reduces alpha-synuclein accumulation in a mouse model of multiple system atrophy. Mol Neurodegener. 10, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2014b. A neuroprotective brain-penetrating endopeptidase fusion protein ameliorates Alzheimer disease pathology and restores neurogenesis. J Biol Chem. 289, 17917–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, et al. , 2016d. α-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease. Annals of Clinical and Translational Neurology. 3, 588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer BJ, Verma IM, 2007. Targeted delivery of proteins across the blood-brain barrier. Proc Natl Acad Sci U S A. 104, 7594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CA, Castanotto D, 2017. FDA-Approved Oligonucleotide Therapies in 2017. Mol Ther. 25, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, et al. , 2006. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 83, 1461–70. [DOI] [PubMed] [Google Scholar]
- Tai Y, et al. , 2014. Protective effect of alpha-synuclein knockdown on methamphetamine-induced neurotoxicity in dopaminergic neurons. Neural Regen Res. 9, 951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, et al. , 2015. Normalization of Overexpressed alpha-Synuclein Causing Parkinson’s Disease By a Moderate Gene Silencing With RNA Interference. Mol Ther Nucleic Acids. 4, e241. [DOI] [PubMed] [Google Scholar]
- Tian X, et al. , 2015. LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci Rep. 5, 11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, et al. , 2010. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 30, 6236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Masliah E, 2013. Immunotherapy for neurodegenerative diseases: Focus on alpha-synucleinopathies. Pharmacol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Masliah E, 2016. Therapeutic approaches in Parkinson’s disease and related disorders. J Neurochem. 139 Suppl 1, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, et al. , 2016a. Review: Novel treatment strategies targeting alpha-synuclein in multiple system atrophy as a model of synucleinopathy. Neuropathol Appl Neurobiol. 42, 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, et al. , 2016b. Immunotherapeutic Approaches Targeting Amyloid-beta, alpha-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders. Neurotherapeutics. 13, 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, et al. , 2017. MicroRNA-101 Modulates Autophagy and Oligodendroglial Alpha-Synuclein Accumulation in Multiple System Atrophy. Front Mol Neurosci. 10, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, et al. , 2010. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 330, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. , 2017. Challenges and opportunities for siRNA-based cancer treatment. Cancer Lett. 387, 77–83. [DOI] [PubMed] [Google Scholar]
- White MD, Mallucci GR, 2009. RNAi for the treatment of prion disease: a window for intervention in neurodegeneration? CNS Neurol Disord Drug Targets. 8, 342–52. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, et al. , 2007. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 25, 1149–57. [DOI] [PubMed] [Google Scholar]
- Wunner WH, et al. , 1984. Characterization of saturable binding sites for rabies virus. J Virol. 50, 691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, et al. , 2016. Impairment of chaperone-mediated autophagy induces dopaminergic neurodegeneration in rats. Autophagy. 12, 2230–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, et al. , 1986. Sequence, structure, receptor-binding domains and internal repeats of human apolipoprotein B-100. Nature. 323, 738–42. [DOI] [PubMed] [Google Scholar]
- Zharikov AD, et al. , 2015. shRNA targeting alpha-synuclein prevents neurodegeneration in a Parkinson’s disease model. J Clin Invest. 125, 2721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


