Abstract
The omentum is a large mesenchymal fibro-fatty tissue with remarkable healing capability. It is also rich in immune cells, including macrophages, and lymphocytes, within particular structures named milky spots. Clinical observations indicate a high incidence of peritonitis after removal of the omentum suggesting that it may play a role in sepsis. To test this possibility, male CD-1 mice underwent simultaneous omentectomy and CLP, omentectomy-sham operation and CLP alone, and mortality was documented within 72h post the insults. A significant increase in mortality was observed in mice subjected to omentectomy and CLP in comparison with CLP alone. Mortality was correlated with an increase in cytokine gene expression within the lung after omentectomy and CLP as opposed to CLP alone. However, no differences in bacterial load were observed within the peritoneum or blood between groups. To test the long-term effect of omentectomy, mice were subjected to omentum removal or sham operation, allowed to recover from surgery for 14 or 28 days and then both were subjected to CLP. In these cases, no differences in mortality were observed between the groups suggesting that the lack of omentum triggers a compensatory mechanism. Finally, omentectomy and sham operation altered the composition of peritoneal immune cells with the disappearance of F4/80high macrophages and the appearance of a new population of F4/80low macrophages within 1 or 14 days post-surgery. The F4/80high positive cells reappeared after 28 days following the procedures. All of these observations suggest that the omentum plays an early role in the outcome from sepsis.
Keywords: Sepsis, shock, omentum, milky spots, inflammation, surgery
INTRODUCTION
Among all disease conditions affecting humans, sepsis is one of the top health problems affecting over 750,000 people per year in the U.S., with a mortality rate of 30–50% [1, 2]. In addition, the treatment of septic patients is a prominent economic burden to the healthcare system, exceeding a cost of more than $20 billion per year in the U.S. [3]. The morbidity and mortality associated with sepsis are often complicated by the development of secondary conditions, such as septic shock and multiple organ failure [4, 5]. Sepsis has been defined as a life-threatening organ dysfunction condition caused by a dysregulated host response to infection and injury [6, 7]. Mortality after sepsis has been associated with the inability to restore homeostasis [8], resulting in a period of immune dysfunction or anergy [9]. Currently, the only treatments for sepsis are supportive interventions such as the administration of antibiotics and fluids, which should be initiated as early as possible after a positive diagnosis as proposed by the Surviving Sepsis Campaign (http://www.survivingsepsis.org). In addition, source control is an important component in the treatment of sepsis. Overall, the conventional wisdom is that early intervention to ameliorate sepsis is a critical component in the clinical management of the disease. Therefore, the identification of initial events that are triggered during sepsis may be critical to improving therapeutic intervention and discovering new interventions. Indeed, early interventions have been shown to be important for sepsis resolution in experimental animal models [10].
The etiology of the transition from early sepsis to shock and death is not completely understood, although there is a general consensus that it is associated with an exaggerated uncontrolled inflammatory response [11]. Moreover, experimental evidence indicated that sepsis is a multifactorial condition modulated by several factors, including the initial injury, genetic background, sex and age of the subject, among other elements [12]. The function of several organ systems also contribute to the outcome from sepsis, the omentum could be one of them. The omentum is a large mesenchymal veil of fibro-fatty tissue that is suspended between the greater curvature of the stomach and the taenia coli of the transverse colon, linking these organs with the pancreas and spleen. This organ is free to migrate within the abdominal cavity due to the effects of gravity, respiration, and peristalsis of the visceral structure. This mobility allows for the omentum to contact areas of localized injury and seal visceral perforations. Historically, the omentum has been commonly referred to as “the guardian or policeman of the abdomen” because of its tremendous wound healing and regenerative properties [13, 14]. In addition, the omentum has a robust vascular system, including high endothelial venules (HEV) that are believed to be a major route for the transit of immune cells into the peritoneal cavity. Moreover, the omentum possesses complex nodules of macrophages (Mϕs) and lymphocytes, referred to as “milky spots,” which contribute to the immunological response within the peritoneum [15, 16].
Clinical observations correlated an increase in the incidence of peritonitis with patients in which the omentum was purposefully removed in an effort to decrease adhesion formation and limit subsequent bowel obstructions [17]. Experimental studies have also shown a potential involvement of the omentum in the etiology of sepsis. Thus, bacterial counts in samples obtained from mesenteric lymphoid tissue and blood in a rat model of peritonitis were elevated after omentectomy as compared to those in which the omentum was left intact [18]. Moreover, omentectomy negatively altered chemotactic indices, and the ratio of macrophages to lymphocytes [19], increasing the number of macrophages isolated from the peritoneal cavity [20]. However, the direct contribution of the omentum to the outcome from sepsis has not been clearly established. This investigation was directed at testing the hypothesis that the omentum plays a role in sepsis induced by the cecal ligation and puncture (CLP) model, which may be accomplished by healing the necrotic focus and providing an additional source of immune cells within the milky spots of this organ.
MATERIALS AND METHODS
Animals
CD-1 (Crl:CD1 (ICR)) mice, corresponding to an outbred line, were obtained from Charles River and maintained under specific, pathogen–free conditions at the University of California, San Diego Animal Facility (La Jolla, CA). Mice were used one week after arrival to allow them to acclimate to the new environment. They have access to food and water ad libitum on a 12-hour light cycle. Experiments were carried out on 9-week-old male mice. All animal procedures were approved by the UCSD Institutional Animal Care and Use Committee.
Cecal ligation and puncture
Cecal ligation and puncture (CLP) were performed as previously described [10]. CD-1 mice were fasted for 16 h before the procedure. Animals were anesthetized with isoflurane (vaporizer at 1.5 to 2.5 MAC), and, under sterile conditions, a 2 cm incision was made in the lower abdominal region and the cecum was exposed. The distal portion of the cecum was ligated 1.5 cm from the end with a 4–0 silk suture and punctured once with a 16-gauge needle. The cecum was placed back into the peritoneal cavity and squeezed to place a small portion of its contents (bacteria and feces) into the peritoneum. Then, the peritoneal wall and skin were closed in two layers with silk sutures. Mice were resuscitated with a 1 ml subcutaneous injection of sterile saline (0.9%). After the procedure, the mice had access to water and food ad libitum. As a control, mice were sham operated as described above except that the cecum was neither ligated nor perforated. Non-operated mice were also used as a second control. For survival experiments, a small thermal probe was placed under the skin during the procedure and CD-1 mice were placed in individual cages and the body temperature was continuously recorded using an external receiver (VitalView data acquisition system). The time of mortality was recorded when the core body temperature reached room temperature as previously described [10]. Animals were monitored for any major sign of pain or distress and sacrificed immediately if any was noted. For some experiments, the lung was harvested at 3 h or 6 h after CLP or CLP+Omx procedures. It was perfused with PBS immediately after euthanasia to minimize the presence of polymorphonuclear leukocytes in circulation within the tissue, then excised, flash frozen in liquid nitrogen, and then stored at −80oC for subsequent processing.
Omentectomy:
Mice were secured in the supine position. They were prepared for aseptic surgery by shaving approximately one square centimeter of the abdomen with a rechargeable electric clipper in a designated area separate from the operative field. The skin was disinfected by swabbing with Chlorhexidine or 10% Betadine solution followed by a wiped with an alcohol rinse prior to the incision. The abdomen was entered using a 2–3 cm vertical lower midline incision. The stomach was identified in the right upper quadrant allowing for visualization of the omentum that was elevated to expose the vascular pedicle and removed by cauterization. The stomach was returned to the peritoneal cavity. This procedure was followed with either CLP or sham operation. The abdomen was closed into two layers (muscle and skin). Following closure of the abdominal wall and prior to skin closure, a transmitter was implanted subcutaneously beneath the abdominal skin. The area of the transmitter implantation was visually monitored for any signs of adverse reactions.
Determination of Bacterial load
Bacteremia in blood and the peritoneum was determined in CLP or CLP+Omx-operated animals. Samples were obtained by peritoneal lavage (5 ml per animal) or from blood via cardiac puncture (0.5 ml) were collected aseptically 3h and 6 h after the procedures, in mice under anesthesia and sacrificed immediately afterward. Samples were serially diluted in sterile PBS and 100 μl of each dilution was spread on Trypticase Soy Agar plates containing 5% Sheep Blood (BD, Franklin Lakes, NJ, USA). All plates were incubated for 24 h at 37°C. The number of bacterial colonies was counted and expressed as CFU per milliliter of blood or peritoneal lavage fluid.
Flow cytometry analysis of peritoneal cells
Peritoneal cells were obtained by lavage of the peritoneum. Briefly, 5 ml of serum-free phenol-red free RPMI1640 were injected into the peritoneal cavity of CD-1 mice, and after gentle massage of the peritoneum to dislodge any loosely attached cells, fluid was collected. Cell suspensions were immediately centrifuged for 10 min. at 350xg and resuspended in PBS without Ca2+/Mg2+ supplemented with 0.5% BSA for counting. Peritoneal cells (5×105 cells/tube) were then incubated for 15 min with 0.5 μg of FcγR blocking antibodies (Fc block; BD Biosciences, San Jose, CA, USA) followed by antibody staining for 30 min in the dark at 4°C. Cells were then washed, centrifuged, and resuspended in FSB for analysis. Each anti-mouse antibody was added at 0.5 μg/tube and included FITC-conjugated anti-Ly6G (clone 1A8, Biolegend, San Diego, CA, USA), PE-conjugated anti-CD11b (clone M1/70, eBioscience), PE-conjugated anti-CD19 (clone 1D3, BD Bioscience) and APC-conjugated anti-F4/80 (clone BM8, eBioscience). Propidium iodide was also used to assess cell viability. Flow cytometry was performed using a FACSCanto II flow cytometer with FACSDiva software (BD Biosciences, San Jose, CA, USA). The data were analyzed using FlowJo software v.10.1 (Tree Star, Ashland, OR, USA).
Determination of inflammatory cytokine levels by quantitative real-time PCR (qPCR)
Levels of mRNA were measured by quantitative real-time PCR (qPCR). Lung samples were homogenized in TRIzol reagent (Invitrogen) using an Ultra-turrax T25 (IKA, Wilmington, NC). RNA was purified according to the manufacturer’s protocol and treated with DNase I (DNA-free kit, Ambion, Austin, TX) to remove any DNA contamination. DNA-free RNA was then reverse transcribed to cDNA using the High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Newly synthesized cDNA was further diluted and stored at −20°C. The cDNA levels of genes were measured by quantitative real-time PCR (qPCR) using the QuantiTect SYBR Green PCR kit (Qiagen) with QuantiTect validated primer sets (cat. Numbers: QT00104006 for TNF-α; QT00098875 for IL-6 and QT00106169 for IL-10, all from Qiagen). All PCR reactions were performed using the StepOnePlus Real-Time PCR System (Applied Biosystems). Melting curve analysis was performed for each primer set to ensure amplification specificity. Corresponding standard curves were added in each PCR reaction. The housekeeping gene GAPDH (QT01658692, Qiagen) was used to normalize data to cDNA inputs. The results are expressed as copy numbers of target gene per copy numbers of GAPDH.
STATISTICAL ANALYSIS
All data were analyzed using the GraphPad Prism software (GraphPad Prism Software, San Diego, CA). Significance was analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison Test or using two-way ANOVA followed by Bonferroni post-tests. A p value of < 0.05 was considered statistically significant. The statistical significance for the comparison of survival rates was analyzed by the log-rank test.
RESULTS
Removal of the omentum increased mortality after CLP.
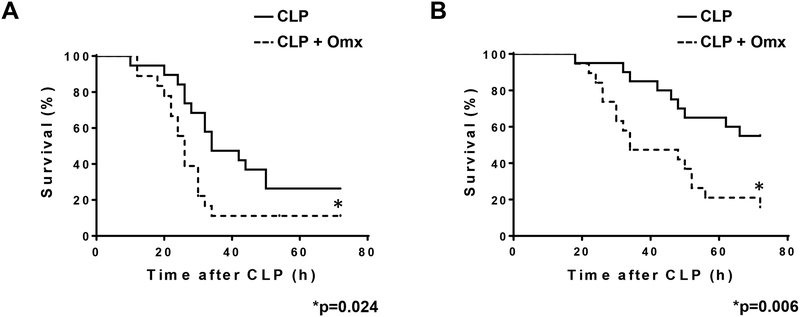
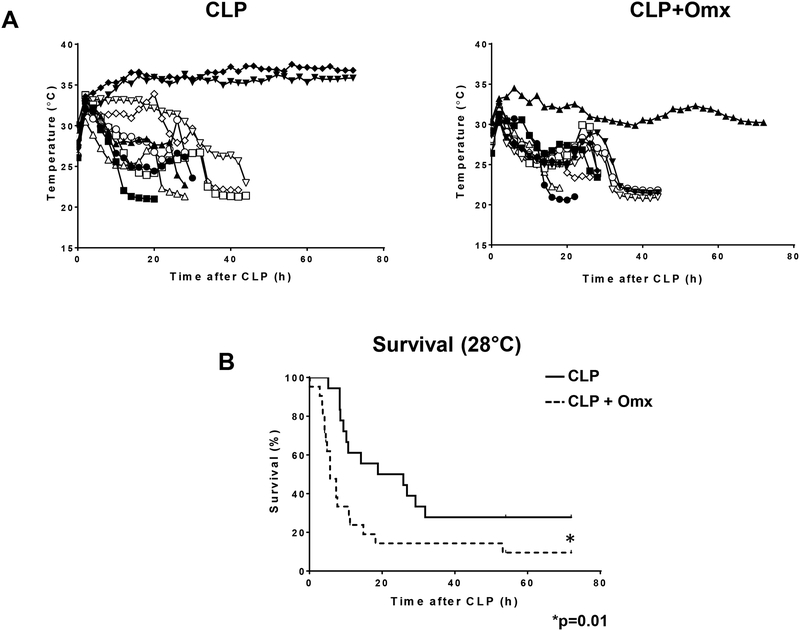
We predict that removal of the omentum during CLP is likely to compromise the outcome from sepsis. Male CD-1 mice were simultaneously subjected to omentectomy and CLP (CLP+Omx, n=20), or omentectomy-sham operation and CLP (CLP, n=20). After the insult, mice were continuously monitored by changes in their core body temperature and mortality. We have previously demonstrated that the precise time of mortality could be established when core body temperature equilibrated to the ambient temperature [10]. Certainly, removal of the omentum worsened the frequency of mortality after CLP in two different variants of this model by single puncture using a 16G (p=0.024) or an 18G (p=0.006) needle in comparison with CLP alone (Fig. 1A and B, respectively). No mortalities were observed after the omentectomy-sham operation. In addition, we have also shown that when mice after CLP reach a core body temperature of 28°C, they are predicted to die, becoming an earlier predictor of late mortality [10]. Indeed, reaching the 28°C threshold within 9 h post insult correlated with actual mortality between CLP (16G puncture) with or without omentectomy (Fig. 2A and B).
Fig. 1.
Removal of the omentum increased mortality after CLP. Male CD-1 mice were subjected to CLP or omentectomy+CLP using two different CLP conditions (16 or 18-gauge needle perforation). Mice were immediately resuscitated by the subcutaneous injection of 1 ml saline and survival was continuously monitored for 72 h. (A) 16-gauge needle perforation (*p=0.024; n=20 per group). (B) 18-gauge needle perforation (* p=0.006; n=20 per group). Statistical significance was analyzed by the log-rank test.
Fig.2.
A core body temperature of 28°C predicted actual mortality between CLP with or without omentectomy. Male CD-1 mice were subjected to CLP (16-gauge needle perforation) or omentectomy+CLP (16-gauge needle perforation), and a temperature probe was placed between the skin and the peritoneal membrane. Body temperature was constantly monitored every 10 minutes for up to 72 hours (A) and survival was calculated based on the time required for the animal to reach a body temperature of 28°C after CLP (B). Statistical significance was analyzed by the log-rank test with * indicating a p=0.01 (n=20 per group).
Omentectomy also altered the inflammatory response after CLP.
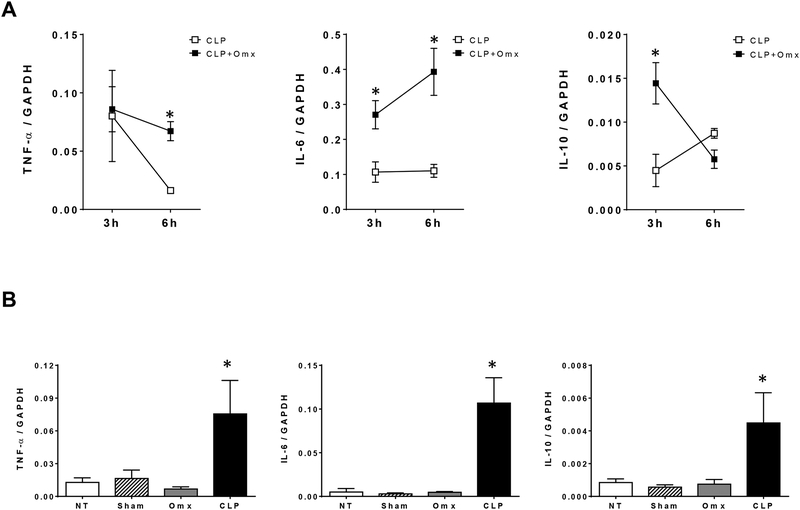
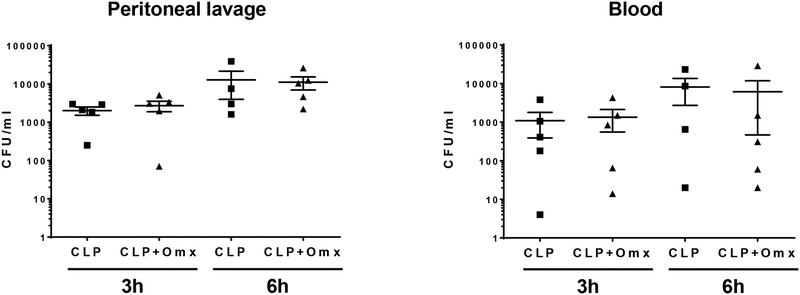
Since the outcome from sepsis has been associated with alterations of the inflammatory response, we measured cytokine mRNA levels in lung samples obtained after CLP in mice in which the omentum was removed or not. We have previously reported that measuring cytokine mRNA in target organs provided a better definition of the earlier inflammatory response after CLP [10, 21]. Higher levels of IL-6 and IL-10, but not TNFα, were observed in lung samples (n=5 per group) obtained at 3h after omentectomy and CLP (16G) in comparison with CLP (16G) alone (Fig 3A). Similar differences in IL-6 expression levels were observed in lung samples obtained at 6h after omentectomy and CLP as opposed to CLP alone. However, higher lung TNFα levels are now observed in samples obtained at 6h after omentectomy and CLP as opposed to CLP alone (Fig 3A). We did not observe differences in lung cytokine profile in mice after sham operation for CLP or omentectomy in comparison with non-operated mice. These cytokine levels were significantly low in comparison with mice undergoing CLP (Fig. 3B). In contrast to differences in cytokine levels between mice undergoing CLP (16G) and omentectomy and CLP (16G), we did not observe differences in the bacterial load within the peritoneal cavity and blood between groups (n=5 per group) obtained as described in Materials and Methods (Fig. 4).
Fig. 3.
Omentectomy altered the inflammatory response after CLP. Male CD-1 mice were subjected to CLP (16-gauge needle perforation) or omentectomy+CLP (16-gauge needle perforation), and lungs were harvested at 3 h or 6 h post CLP. Cytokine mRNA levels (TNF-α, IL-6, and IL-10) were measured by quantitative real-time PCR (qPCR). Results are expressed as means±SEM (n=5 in each group), and statistical analysis for the comparison between groups was performed by two-way ANOVA followed by the Bonferroni’s Multiple Comparison Test. * indicates a p<0.05 comparing CLP and omentectomy+CLP groups for each time point.
Fig. 4.
Removal of the omentum did not change bacterial loads within the peritoneal cavity or blood after CLP. Male CD-1 mice were subjected to CLP (16-gauge needle perforation) or omentectomy+CLP (16-gauge needle perforation), and peritoneal lavage and blood were collected at 3 h or 6 h post CLP. Peritoneal lavage and blood samples (n=?) were serially diluted in PBS spread on Trypticase Soy Agar plates containing 5% Sheep Blood. All plates were incubated for 24 h at 37°C. The number of bacterial colonies was counted and expressed as CFU per milliliter of blood or peritoneal lavage fluid. Statistical analysis for the comparison between groups was performed by one-way ANOVA followed by the Tukey’s Multiple Comparison Test.
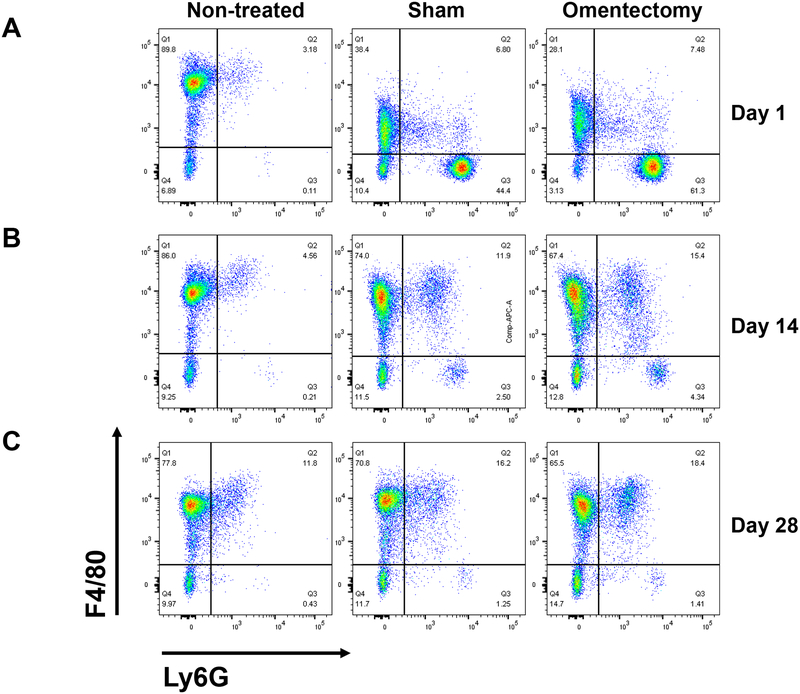
Peritoneal cellular composition is affected after omentectomy or sham operation.
Our prior observations showed a worse outcome from CLP after omentectomy when both procedures were performed simultaneously. Thus, we were interested in the long-term effect of omentum removal on sepsis. To perform these experiments, we decided to establish the conditions in which mice recover after surgery and are suitable for CLP (16G) without secondary inflammatory confounding factors. Mice were subjected to omentum removal or sham operation and non-operated mice were used as controls. Mice were sacrificed at 1, 14 and 28 days after the procedure and the composition of the immune cells within the peritoneum was analyzed by flow cytometry using specific cell markers (n=4 per group). Non-operated mice show a large level of F4/80high positive peritoneal macrophages. In contrast, this subpopulation of macrophages was reduced after sham operation or omentectomy on day 1, and a new population of F4/80low positive cells was detected in both groups, which was concomitant with a massive appearance of neutrophils (LyG positive cells) within the peritoneum (Fig. 5A). At 14 days after the procedures, the number of neutrophils decreased in both omentectomized and sham-operated mice, and the population of F4/80low macrophages increased (Fig. 5B). The composition of immune peritoneal cells seemed to return to normal levels after 28 days post surgeries, with a significant reduction of neutrophils and the reappearance of F4/80high positive cells (Fig. 5C).
Fig. 5.
Omentectomy or sham operation modified the peritoneal cellular composition. Male CD-1 mice (n=4 per group) were subjected to omentectomy or sham operation, and peritoneal cells were obtained by lavage of the peritoneum at 1, 14 and 28 days post-surgery. Cells were centrifuged for 10 min at 300xg, resuspended in PBS without Ca2+/Mg2+ supplemented with 0.5% BSA and counted. A group of non-treated mice was also used as a control. Cells were stained as described in the Materials and Methods section, and flow cytometry was performed using a FACSCanto II flow cytometer with FACSDiva software. The data were analyzed using FlowJo software v.10.1. Representative Ly6G/F4/80 density plots of CD19−CD11b+ gated viable cells are shown for peritoneal cells harvested at 1 day (A), 14 days (B) or 28 days (C) post omentectomy or sham surgery.
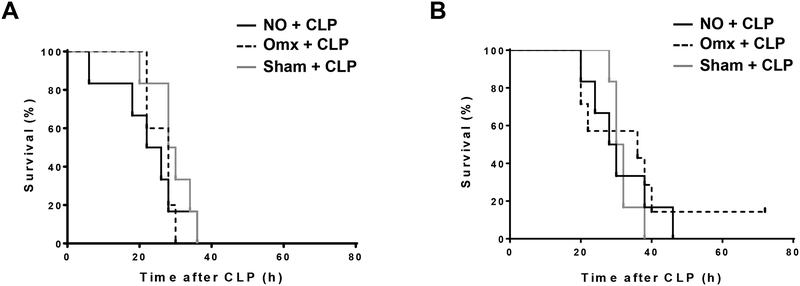
Omentectomy did not have any long-term effects on the outcome after CLP.
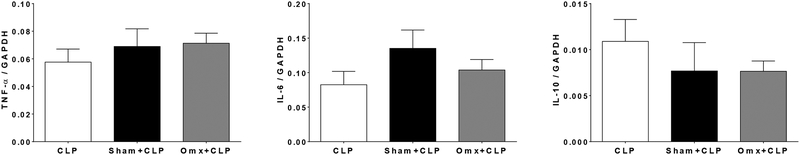
Based on the analysis of the immune peritoneal composition after omentectomy or sham operation, mice (n=6 per group) were omentectomized or sham-operated and recovered after surgery for 14 or 28 days and at these two time points they were subjected to CLP (16G). Survival was monitored as described above. No differences in survival were observed between CLP alone and CLP after omentectomy or sham operation for both recovery times, 14 or 28 days (Fig. 6A and 6B, respectively). Moreover, no differences in cytokine mRNA levels were detected among groups in lung samples harvested within 3h after CLP after omentectomy or sham operation (n=6 per group) in a different set of mice (Fig. 7).
Fig. 6.
Omentectomy did not have any long-term effects on the animal survival after CLP. CD-1 mice (n=6 per group) were subjected to omentectomy or sham operation. A group of non-treated mice was also used as a control. Mice were then subjected to CLP (16-gauge needle perforation) either 14 days (A) or 28 days (B) after the initial procedures, and survival was continuously monitored for 72 h. Statistical significance was analyzed by the log-rank test.
Fig. 7.
Omentectomy did not have any long-term effects on the inflammatory response after CLP. CD-1 mice (n=6 per group) were subjected to omentectomy or sham operation. A group of non-treated mice was also used as a control. Mice were then subjected to CLP (16-gauge needle perforation) 28 days after the initial procedures, and lungs were harvested 3 hours post CLP. Cytokine mRNA levels (TNF-α, IL-6, and IL-10) in the lungs were measured by quantitative real-time PCR (qPCR). Results are expressed as means±SEM (n=5–7 in each group), and statistical analysis for the comparison between groups was performed by one-way ANOVA followed by the Tukey’s Multiple Comparison Test.
DISCUSSION
The omentum has tremendous healing capability, which plays a role in the spontaneous sealing of intestinal perforations and focal necrotizing enterocolitis [22]. In addition, this organ is rich in blood vessels and important aggregates of immune cells, containing macrophages and lymphocytes organized around a specialized postcapillary venule allowing for the rapid influx of inflammatory mediators into the peritoneal cavity in response to injury and infection. In addition, the omentum is a source of resident stem cells that are largely responsible for wound healing and tissue regeneration [15]. Therefore, the omentum is likely to play a major role in controlling intraabdominal infection. However, its role in the etiology of sepsis has not been clearly delineated. In the present study, we tested this possibility by using an experimental animal model of sepsis induced by CLP. We observed an increase in mortality in mice that were simultaneously subjected to CLP and omentectomy as opposed to mice that were exposed to CLP alone. In addition, we observed an increase in the inflammatory response in the lung after the combination of omentectomy and CLP in contrast to CLP alone that correlates with the increase in mortality. We did not observe differences in the inflammatory response between mice undergoing omentectomy alone, sham operation or non-operated mice that were substantially reduced in comparison with CLP alone, suggesting that the inflammatory response between omentectomy and CLP acts in a synergistic fashion rather in an additive manner. In particular, we observed highly elevated levels of IL-6 in the lung within 3 and 6 h after CLP and omentectomy in contrast to CLP alone. High levels of IL-6 have been proposed as a good predictor of mortality after sepsis [23], which is supported by our study. In addition, down-regulation of TNFα is slower after omentectomy and CLP in comparison with CLP in absence of omentectomy, in spite of elevated initial levels of IL-10 in the first group that should reduce TNFα expression. This observation may be an additional proof of dysfunction of the immune system during sepsis [9, 10, 24]. It is also possible that omentum resident stem cells may play a role in the protective ability of this organ during injury.
In spite of high mortality after the combination of omentectomy and CLP, the bacterial load in the peritoneum and their translocation into blood were not affected by the absence of the omentum suggesting that this organ does not play a role in bacterial clearance. This observation is in contrast with another study that reported an increase in bacterial counts after omentectomy in a rat model of peritonitis [18]. Thus, the increased mortality after omentectomy and CLP may be related to the incapacity to heal cecum perforations or the encapsulation of the necrotic area. An unexpected observation was that removal of the omentum has no impact on the outcome from CLP initiated several days (14–28 days) post the initial surgery when mice are recovered from the operative trauma. This finding does not sustain clinical observations indicating an increased incidence of peritonitis in patients that underwent omentectomy for the control of adhesion formation and bowel obstructions [17]. It is possible that an absence of the natural omentum triggers a compensatory mechanism to replace the healing capacity of this organ, perhaps by increasing the proliferation of fat tissue, activating an injury preventive mechanism, or just activating a preconditioning mechanism.
The omentum is rich in vascularization presenting a vast number of mesenchymal fenestrations overlying the milky spots, which are believed to be central entry ports for immune cells in response to infection via HEV [25, 26]. Indeed, the transmigration of neutrophils across HEVs has been reported to be more rapid than across conventional postcapillary venules, requiring a unique combination of selectins and cellular adhesion molecules to complete this process [27]. We did not observe any differences in the level of peritoneal neutrophil infiltration between mice that were omentectomized or sham-operated, suggesting that other active and effective pathways for neutrophil infiltration are involved in the peritoneal immune response.
An interesting observation that was obtained from this study is that the natural population of peritoneal macrophages, which are characterized as F4/80high positive cells, were not detected within peritoneal lavages following surgery, both omentectomy or sham operation. A similar observation has been described before and coined “the macrophage disappearance reaction” [28]. The reduction within this macrophage population is unclear, but may be related to an increase in their adhesion to the peritoneum, emigration from the cavity or death by apoptosis. This decrease in F4/80high positive macrophages was coincident with a massive infiltration of neutrophils into the peritoneum. In contrast, a new population of cells that are F4/80low was observed 24 h of post omentectomy or sham operation. These cells may correspond to infiltrating macrophages, perhaps derived from peripheral monocytes. This population of F4/80low cells increased dramatically within 14 days after surgery and were replaced by F4/80high macrophages with 28 days of surgery. It is possible that these F4/80high macrophages are the product of F4/80low cells. However, this possibility needs to be tested experimentally.
In summary, the lack of omentum appears to play an early negative effect on the outcome from sepsis induced by CLP. These observations are in line with the clinical experience in which the omentum plays an important role in the control of infection and tissue damage. Interestingly, the lack of omentum may be compensated in the long term by an unknown mechanism that restores the healing or immunological capacity of this organ. The lack of understanding about this potential mechanism is a limitation of our study. Therefore, further studies are needed to elucidate the nature of this possible compensatory mechanism induced in absence of the omentum. Moreover, the immunological role of the omentum during inflammation and infection requires additional investigations. Finally, our observation needs to be considered in the context of results obtained in an experimental animal model that does not completely mirror the human condition. Therefore, any potential extrapolation of our results to the clinical setting is premature.
Acknowledgments
Support
This work was supported by NIH grant R01 GM114473–01.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348(16):1546–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Torio CM, Andrews RM. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011: Statistical Brief #160, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD), 2006. [PubMed] [Google Scholar]
- 4.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 369(9):840–851, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41(2):580–637, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–810, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coopersmith CM, Deutschman CS. The New Sepsis Definitions: Implications for the Basic and Translational Research Communities. Shock 47(3):264–268, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 40(4):463–475, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 15(5):496–497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cauvi DM, Song D, Vazquez DE, Hawisher D, Bermudez JA, Williams MR, Bickler S, Coimbra R, De Maio A. Period of irreversible therapeutic intervention during sepsis correlates with phase of innate immune dysfunction. J Biol Chem 287(24):19804–19815, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36(1):296–327, 2008. [DOI] [PubMed] [Google Scholar]
- 12.De Maio A, Torres MB, Reeves RH. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock 23(1):11–17, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Liebermann-Meffert D The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin North Am 80(1):275–293, xii, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Collins D, Hogan AM, O’Shea D, Winter DC. The omentum: anatomical, metabolic, and surgical aspects. J Gastrointest Surg 13(6):1138–1146, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Shimotsuma M, Takahashi T, Kawata M, Dux K. Cellular subsets of the milky spots in the human greater omentum. Cell Tissue Res 1991. 264(3):599–601, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Platell C, Cooper D, Papadimitriou JM, Hall JC. The omentum. World J Gastroenterol 6(2):169–176, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambroze WL Jr, Wolff BG, Kelly KA, Beart RW Jr, Dozois RR, Ilstrup DM. Let sleeping dogs lie: role of the omentum in the ileal pouch-anal anastomosis procedure. Dis Colon Rectum 34(7):563–565, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Uzunkoy A, Ozbilge H, Horoz M. The influence of omentectomy on bacterial clearance: an experimental study. Ulus Travma Acil Cerrahi Derg 15(6):541–545, 2009. [PubMed] [Google Scholar]
- 19.Agalar F, Savek I, Cakmakci M, Hascelik G, Abbasoglu O. Effect of omentectomy on peritoneal defence mechanisms in rats. Eur J Surg 163(8):605–609, 1997. [PubMed] [Google Scholar]
- 20.Agca B, Paksoy M, Polat E, Aksin E, Dirican A, Durgun V, Eren D. Influence of omentectomy on peritoneal defense mechanisms in an experimental model of intra-abdominal infection. Eur Surg Res 35(1):35–40, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Halbach JL, Wang AW, Hawisher D, Cauvi DM, Lizardo RE, Rosas J, Reyes T, Escobedo O, Bickler SW, Coimbra R, et al. Why Antibiotic Treatment Is Not Enough for Sepsis Resolution: an Evaluation in an Experimental Animal Model. Infect Immun 85(12):e00664–17, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diesen DL, Skinner MA. Spontaneous sealing of a neonatal intestinal perforation by the omentum. J Pediatr Surg 43(12):2308–2310, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17(6):463–467, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Remick DG. Pathophysiology of sepsis. Am J Pathol 170(5)1435-1444, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen EW, Gerber SA, Sedlacek AL, Rybalko VY, Chan WM, Lord EM. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol Res 45(2–3):185–194, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Geng X, Li Y. Milky spots: omental functional units and hotbeds for peritoneal cancer metastasis. Tumour Biol 37(5):5715–5726, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buscher K, Wang H, Zhang X, Striewski P, Wirth B, Saggu G, Lutke-Enking S, Mayadas TN, Ley K, Sorokin L, et al. Protection from septic peritonitis by rapid neutrophil recruitment through omental high endothelial venules. Nat Commun 7:10828, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barth MW, Hendrzak JA, Melnicoff MJ, Morahan PS. Review of the macrophage disappearance reaction. J Leukoc Biol 57(3):361–367, 1995. [DOI] [PubMed] [Google Scholar]