Abstract
Objective:
Evaluate the perioperative and long-term outcomes of aortic root repair (ARr) and aortic root replacement (ARR) and provide evidence for root management in acute type A aortic dissection (ATAAD).
Methods:
From 1996-2017, 491 patients underwent ARr (n=307) or ARR (n=184) (62% bioprosthesis) for ATAAD. Indications for ARR were intimal tear at the aortic root, root measuring ≥4.5 cm, connective tissue disease (CTD), or unrepairable aortic valvulopathy. Primary outcomes were in-hospital mortality, long-term survival, and reoperation rate for root pathology.
Results:
Median age was 61- and 56-years-old in ARr and ARR groups. The ARR group had more renal failure requiring dialysis, previous cardiac intervention or surgery, heart failure, coronary malperfusion syndrome, acute myocardial infarction, and severe aortic insufficiency, as well as concomitant CABG, tricuspid valve repair, and longer cardiopulmonary bypass and aortic cross-clamp times but similar arch procedures. Perioperative outcomes were similar in ARr and ARR groups including in-hospital mortality (8.5% and 8.2%), new-onset renal failure requiring permanent dialysis, stroke, myocardial infarction, and sepsis. Kaplan-Meier 10-year survival was 62% and 65%, and the 15-year cumulative incidence of reoperation was 11% and 7% in ARr and ARR groups, respectively. The primary indication for root reoperation was aortic root aneurysm in ARr group and bioprosthetic valve deterioration in ARR group.
Conclusion:
ARr and ARR are appropriate surgical options for ATAAD repair with favorable short- and long-term outcomes. ARR should be performed for ATAAD patients presenting with an intimal tear at the aortic root, root aneurysm ≥4.5 cm, CTD, or unrepairable aortic valvulopathy.
INTRODUCTION
Acute type A aortic dissection (ATAAD) is a catastrophic event associated with a high risk of operative morbidity and mortality. At present, 30-day mortality remains between 20-25%1–3. ATAAD frequently involves dissection of the aortic root, sometimes with a primary tear involving the intima of the aortic root. It is also common to see ATAAD patients with root pathology in addition to aortic dissection, such as root aneurysm or aortic valve pathology. The operative goal for ATAAD is to prevent aortic rupture with subsequent tamponade by replacing the proximal aorta; however, the appropriateness of concurrently treating any associated aortic root pathology to decrease the risk of a later reoperation remains debatable. It is a fine line between the extent of the operative procedure to salvage the patient from a catastrophic event and aggressively treating the aortic root pathology to prevent later aortic root events including aortic insufficiency, root aneurysm, and reoperation.
Criteria have yet to be established to guide surgeons on whether to perform aortic root replacement (ARR) in ATAAD 2–8. The published outcomes are inconsistent as some studies show no difference in operative mortality between ARR and aortic root repair (ARr)6–9 while another study indicates lower operative mortality in the ARr group10. Furthermore, there is inconsistency as to whether the preserved aortic root in ATAAD patients is stable 3, 11, 12 or a risk factor for later reoperation2, 5, 13, 14
In this study, we analyzed the short- and long-term outcomes of ATAAD patients that underwent ARr with preservation of the whole aortic root (including the aortic valve and all sinuses of Valsalva) or ARR with a whole root prosthesis or valve-sparing aortic root replacement (VSARR). We hypothesized that with predefined criteria for ARR or ARr, ARR would not increase perioperative mortality, and ARr would not increase the risk of late reoperation for aortic root events.
METHODS
This study was approved by the University of Michigan Institutional Review Board (Michigan Medicine, Ann Arbor, MI) and a waiver of consent was obtained.
Study Population
Between July 1996 and January 2017, 491 patients underwent ARr (n = 307) or ARR (n = 184) with inclusion/modified inclusion (n = 70, 38%), Bentall procedure (n = 69, 38%) and VSARR (n = 45, 24%) for ATAAD. The ARr group included aortic root reconstruction with preservation of the aortic valve with all native aortic root tissue (n = 307). The ARR group had a mechanical composite valve graft (n = 26, 14%), Freestyle porcine aortic root (Medtronic Inc.) (n =113, 62%), or valve-sparing root (n = 45, 24%).
Data Collection Techniques
Investigators obtained Society of Thoracic Surgery data elements from the University of Michigan Department of Cardiac Surgery Data Warehouse to determine pre-, intra-, and post-operative characteristics as previously reported15. Medical records, including operative reports, were reviewed to supplement data collection. Events of reoperation included open repair (sternotomy) or transcatheter aortic valve replacement (TAVR) for aortic root pathology only. Reoperation primarily for arch, descending thoracic, or thoraco-abdominal aortic aneurysm were not included as events of reoperation. Survival data was collected through the National Death Index database through December 31, 201516. Further survival data and reoperation data were collected from a thorough medical record review of patients’ return visits and surveys (including letters and phone calls) through January 2018. Loss of follow-up or end of the study period were treated as censors during the time to events analyses. Out of 491 patients, we had follow-up regarding reoperation and long-term events for 385 (78.4%) patients; 106 (11.6%) patients did not respond to the survey, including 33 patients who were dead and 73 patients who were still alive. The median follow-up time was 4.7 (25th and 75th percentiles: (1.8, 7.8)) years and the mean follow-up time was 5.8 years, since 62% (303/491) of all cases were performed in the second decade (2008-2017).
Operative Techniques
The indication for aortic root replacement in ATAAD patients included: (1) intimal tear at the aortic root, (2) root diameter ≥4.5 cm, (3) connective tissue disease, and (4) unrepairable aortic valve pathology. The ARR was performed as a Bentall procedure by resecting all the aortic root tissue, replacing the aortic root with a mechanical composite valve graft (St. Jude Medical, Medtronic Hall) or Freestyle stentless porcine aortic root (Medtronic Inc.), and reimplanting two separate coronary buttons with full thickness bites through the coronary buttons circumferentially. The inclusion/modified inclusion ARR was performed by including the prosthetic aortic root in the native aortic root, and two coronary ostia were reimplanted as a side-to-side fashion but with full thickness bites. VSARR was performed using the Yacoub remodeling technique (n=5) and David reimplantation technique (n=40). Valsalva graft or straight tubular grafts (two-graft technique) were used in the David procedure based on surgeon’s preference as described in our previous publication17. All three techniques of ARR, including inclusion technique, Bentall procedure, and David procedure are current practice in our group.
If patients did not meet the criteria for ARR, the aortic root was repaired by preserving the aortic valve and all sinus of Valsalva tissue to maintain the normal geometry of the aortic root, even if the aortic dissection involved all sinuses of Valsalva and extended down to the aortic annulus. Direct repair of the aortic root consisted of trimming the aortic root 3-5 mm above the sinotubular junction (STJ) or coronary ostia, whichever was more distal. All thrombus in the false lumen was removed from each sinus of Valsalva. Reconstruction of the aortic root was performed per surgeon preference and included: re-approximation of the aortic wall at the STJ with running circumferential 5-0 Prolene with no use of any surgical technical adjuncts18, inverting adventitia as a buttress for the proximal anastomosis19, or placing felt in the false lumen to create “neo-media” and using felt as a buttress for proximal anastomosis20. The direct repair of the aortic root without surgical technical adjuncts was performed by reapproximating the two layers of dissected aortic wall of the root at the STJ with 5-0 Prolene in running suture fashion circumferentially. If the dissection went down around the coronary ostia, then the aortic sinus wall was re-enforced with 5-0 Prolene in running suture fashion around the coronary ostia circumferentially as an in situ coronary button reimplantation with full thickness bites of both layers of dissected aortic sinus wall18. Biological glue was used for the aortic root reconstruction only from 1999 to 2004. We abandoned biological glue due to its toxicity and associated formation of anastomotic pseudoaneurysms. The aortic valve was resuspended at the three commissural posts with pledgeted 4-0 Prolene sutures. The repaired aortic root was then anastomosed to the Dacron graft.
Statistical Analysis
Since the ARr and ARR groups are two significantly different groups at baseline, we conducted this study as a descriptive study instead of comparing the outcomes between the two groups. Data are presented as median (interquartile range, 25th and 75th percentile) for continuous data and n (%) for categorical data. Crude survival curves for time to death since operation were estimated using the non-parametric Kaplan-Meier method. Cox proportional hazards regression was used to calculate the adjusted hazard ratios (HRs) with 95% CI for survival by adjusting age, gender, hypertension, NYHA class, CAD, acute myocardial infarction, preoperative severe aortic insufficiency, renal failure, previous cardiac intervention and surgery, and operative technique (root repair or root replacement) in the whole cohort. As patients may experience death before reoperation, cumulative incidence curves were generated to assess the reoperation rates for primarily aortic root pathology over time adjusting for death as the competing risk. Incidence rates were calculated for long-term events (such as stroke, TIA, endocarditis, reoperation for aortic root aneurysm), in which the numbers of events were divided by total patient years of follow-up. P values of less than 0.05 (2-tailed) are considered statistically significant.
RESULTS
Demographics and Preoperative Data
This is a typical cohort of patients with ATAAD. The ARR group was predominately male and younger, and had more renal failure requiring hemodialysis, previous cardiac intervention (such as percutaneous coronary intervention), previous cardiac surgery, heart failure (NYHA III-IV), acute myocardial infarction and coronary malperfusion syndrome, severe aortic insufficiency, and Marfan syndrome. Both groups had similar malperfusion syndromes in other organs including cerebral, spinal cord, celiac/hepatic, mesenteric, renal, and extremities (Table 1).
Table 1.
Demographics and Preoperative Outcomes
| Variables | Total (n=491) | Root Repair (n=307) | Root Replacement (n=184) |
|---|---|---|---|
| Patient Age (years) | 59 (49, 68) | 61 (52, 69) | 56 (44, 67) |
| ≤60-years-old | 269 (55) | 153 (50) | 116 (63) |
| Gender, female | 147 (30) | 109 (36) | 38 (21) |
| BSA | 2.1 (1.9, 2.2) | 2.0 (1.9, 2.2) | 2.1 (1.9, 2.2) |
| Pre-existing Comorbidities | |||
| Hypertension | 343 (70) | 234 (76) | 109 (59) |
| Diabetes Mellitus | 33 (6.7) | 23 (7.5) | 10 (5.4) |
| History of Smoking | 269 (55) | 176 (57) | 93 (51) |
| CAD | 87 (19) | 50 (17) | 37 (21) |
| COPD | 47 (9.6) | 26 (8.5) | 21 (11) |
| History of Stroke | 13 (2.6) | 7 (2.3) | 6 (3.3) |
| History of Renal Failure | 23 (4.7) | 11 (3.6) | 12 (6.5) |
| On Dialysis | 12 (2.4) | 6 (2.0) | 6 (3.3) |
| MFS | 13 (2.6) | 0 (0) | 13 (7.1) |
| LDS | 1 (0.2) | 0 (0) | 1 (0.5) |
| PVOD | 61 (12) | 38 (12) | 23 (13) |
| Previous Cardiac Intervention | 68 (14) | 36 (12) | 32 (17) |
| Previous Cardiac Surgery | 41 (8.4) | 20 (6.5) | 21 (11) |
| Aortic Valve Morphology | |||
| Unicuspid/Bicuspid | 44 (9.0) | 9 (2.9) | 35 (19) |
| Tricuspid | 380 (77) | 264 (86) | 116 (63) |
| Quadricuspid | 1 (0.2) | 1 (0.3) | 0 (0) |
| Unknown | 66 (13) | 33 (11) | 33 (18) |
| Pre-operative AI | |||
| None | 121 (26) | 97 (32) | 24 (13) |
| Trace | 50 (11) | 44 (14) | 6 (3.3) |
| Mild | 90 (19) | 68 (22) | 22 (12) |
| Moderate | 81 (17) | 50 (16) | 31 (17) |
| Severe | 122 (26) | 33 (11) | 89 (48) |
| Ejection Fraction | 55 (50, 60) | 55 (50, 60) | 55 (50, 60) |
| NYHA Function Class | |||
| III/IV | 107 (23) | 57 (19) | 50 (27) |
| Acute Myocardial Infarction | 17 (3.5) | 6 (2.0) | 11 (6.0) |
| Acute Stroke | 22 (4.5) | 13 (4.2) | 9 (4.9) |
| Acute Renal Failure | 67 (14) | 44 (14) | 23 (12.5) |
| Acute Paralysis | 8 (1.6) | 7 (2.3) | 1 (0.5) |
| Cardiogenic Shock | 48 (9.8) | 26 (8.5) | 22 (12) |
| Tamponade | 47 (9.6) | 31 (10) | 16 (8.7) |
| Pre-op Creatinine | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.3) | 1.0 (0.8, 1.4) |
| Malperfusion | |||
| Coronary | 17 (3.5) | 7 (2.3) | 10 (5.4) |
| Cerebral | 21 (4.1) | 12 (3.9) | 9 (4.9) |
| Spinal Cord | 8 (1.6) | 7 (2.3) | 1 (0.5) |
| Celiac/Hepatic | 8 (1.6) | 4 (1.3) | 4 (2.2) |
| Mesenteric | 42 (8.6) | 28 (9.1) | 14 (7.6) |
| Renal | 36 (7.3) | 22 (7.2) | 14 (7.6) |
| Extremity | 42 (8.6) | 30 (10) | 12 (6.5) |
| Delayed Operation | 72 (15) | 47 (15) | 25 (14) |
Data presented as median (25 %, 75 %) for continuous data and n (%) for categorical data.
Abbreviations: AI, aortic insufficiency; BSA, body surface area; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; LDS, Loeys-Dietz syndrome; MFS, Marfan syndrome; NYHA, New York heart association; PVOD, peripheral vascular occlusive disease.
Intraoperative Data
The ARr group had more arch replacement, but the utilization of ACP or RCP was similar in both groups. The ARR group had more concomitant CABG and tricuspid valve repairs as well as longer cardiopulmonary bypass and aortic cross-clamp times (Table 2).
Table 2.
Intra-Operative Outcomes
| Variables | Total (n=491) | Root Repair (n=307) | Root Replacement (n=184) |
|---|---|---|---|
| No Arch Replacement | 30 (6.1) | 7 (2.3) | 23 (12.5) |
| Arch Replacement | 461 (93.9) | 300 (97.7) | 161 (87.5) |
| Hemi-arch | 295 (60) | 178 (58) | 117 (64) |
| Zone 1 Arch | 40 (7.9) | 24 (7.8) | 16 (8.7) |
| Zone 2 Arch | 95 (20) | 75 (24) | 20 (11) |
| Total Arch (Zone 3) | 31 (6.3) | 23 (7.8) | 8 (4.3) |
| Elephant Trunk | 24 (4.9) | 17 (5.5) | 7 (3.8) |
| CPB time (minutes) | 223 (182, 274) | 203 (169, 241) | 265 (224, 300) |
| Cross Clamp time (minutes) | 159 (117, 204) | 129 (106, 159) | 214 (181, 248) |
| HCA | 463 (94) | 300 (98) | 163 (89) |
| HCA time (minutes) | 34.5 (27, 45) | 36 (28, 45) | 32 (24, 42) |
| ACP or RCP | |||
| ACP | 149 (30) | 99 (32) | 50 (27) |
| RCP | 187 (38) | 111 (36) | 76 (41) |
| Both ACP and RCP | 124 (25) | 87 (28) | 37 (20) |
| Concomitant Operations | |||
| CABG | 29 (5.9) | 13 (4.2) | 16 (8.7) |
| MV | 2 (0.4) | 0 (0) | 2 (1.1) |
| TV | 7 (1.4) | 2 (0.7) | 5 (2.7) |
| Blood Transfusions (PRBCs) | |||
| 0 units | 95 (20) | 64 (22) | 31 (18) |
| 1 unit | 35 (7.5) | 21 (7.2) | 14 (8.0) |
| 2 units | 41 (8.8) | 24 (8.2) | 17 (10) |
| ≥ 3 units | 297 (63) | 183 (63) | 114 (65) |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
Abbreviations: ACP, antegrade cerebral perfusion; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; HCA, hypothermic circulatory arrest; MV, mitral valve; PRBCs, packed red blood cells; RCP, retrograde cerebral perfusion; TV, tricuspid valve. Hemiarch: aortic arch was resected from the base of innominate artery to the base of left subclavian artery, then lesser curvature; no reimplantation of arch branches. Zone 1 arch: aortic arch was divided between innominate artery and left common carotid artery (LCC) with reimplantation of innominate artery or right common carotid artery(RCC) and right subclavian artery (RScA) separately. Zone 2 arch: aortic arch was divided between LCC and left subclavian artery (LScA) with reimplantation of innominate artery and LCC; or RScA, RCC and LCC separately Zone 3 arch: aortic arch was divided distal to the LScA with reimplantation of all arch branches.
Perioperative Outcomes
In both ARr and ARR groups, 30-day mortality, in-hospital mortality, and operative mortality (including mortality within 30 days after surgery or mortality in hospital) were 8-9%. The rate of new-onset stroke, paraplegia, myocardial infarction, renal failure requiring permanent dialysis, reoperation for bleeding, sepsis, intubation time, and length of intensive care unit or hospital stay were similar between the two groups (Table 3). By 90 days, the ARr group had 30 deaths, KM survival was 90% (95% CI: 86%, 93%); the ARR group had 17 deaths, KM survival was 91% (95% CI: 86%, 94%).
Table 3.
Post-Operative Outcomes
| Variables | Total (n=491) | Root Repair (n=307) | Root Replace (n=184) |
|---|---|---|---|
| Intraoperative Mortality | 7 (1.4) | 5 (1.6) | 2 (1.1) |
| In-hospital Mortality | 41 (8.4) | 26 (8.5) | 15 (8.2) |
| 30-day Mortality | 34 (6.9) | 19 (6.2) | 15 (8.2) |
| Myocardial Infarction | 6 (1.2) | 4 (1.3) | 2 (1.1) |
| Cerebrovascular Accident | 33 (6.7) | 25 (8.2) | 8 (4.3) |
| Atrial Fibrillation | 179 (36) | 107 (35) | 72 (39) |
| Pneumonia | 86 (18) | 55 (18) | 31 (17) |
| New-Onset Renal Failure | 49 (10) | 37 (12) | 12 (6.5) |
| Requiring Dialysis | 23 (4.7) | 16 (5.2) | 7 (3.8) |
| Permanent Dialysis | 9 (1.8) | 5 (1.6) | 4 (2.2) |
| Reoperation for Bleeding | 41 (8.4) | 24 (7.8) | 17 (9) |
| Deep Sternal Infection | 11 (2.2) | 8 (2.6) | 3 (1.6) |
| Sepsis | 14 (2.9) | 9 (2.9) | 5 (2.7) |
| Limb Ischemia | 6 (1.2) | 3 (1.1) | 3 (1.6) |
| New-onset Paraplegia | 3 (0.6) | 2 (0.7) | 1 (0.5) |
| GI Complications | 39 (7.9) | 26 (8.5) | 13 (7.1) |
| Need for Tracheostomy | 17 (3.5) | 10 (3.3) | 7 (3.8) |
| Prolonged Vent | 266 (54) | 175 (57) | 91 (49) |
| Hours Intubated | 44 (23, 103) | 47 (25, 100) | 37 (21, 108) |
| Total Length of Stay (days) | 11 (7, 19) | 12 (7, 20) | 11 (7, 18) |
| Postop Length of Stay (days) | 10 (7, 16) | 10 (7, 17) | 10 (7, 16) |
Data presented as median (25%, 75%) for continuous data and n (%) for categorical data.
Long-term outcomes
There were 98 late deaths observed within the entire cohort (ARr group, n=59 and ARR group, n=39). The Kaplan-Meier 10-year and 15-year survival were 64% (95% CI: 58%, 69%) and 49% (95% CI: 40%, 57%) for the entire cohort (Figure 1A); 62% (95% CI: 54%, 70%) and 43% (95% CI: 30%, 55%) for the ARr group; and 65% (95% CI: 56%, 73%) and 54% (95% CI: 42%, 65%) for the ARR group, respectively (Figure 1B). Age, CAD, and NYHA class 3 and 4 were significant risk factors for all-time mortality after surgery with HRage = 1.04, p <0.0001, HRCAD = 1.73, P=0.007, and HRNYHA = 1.50, p=0.035. operative technique (ARr or ARR) was not a significant risk factor.
Figure 1:
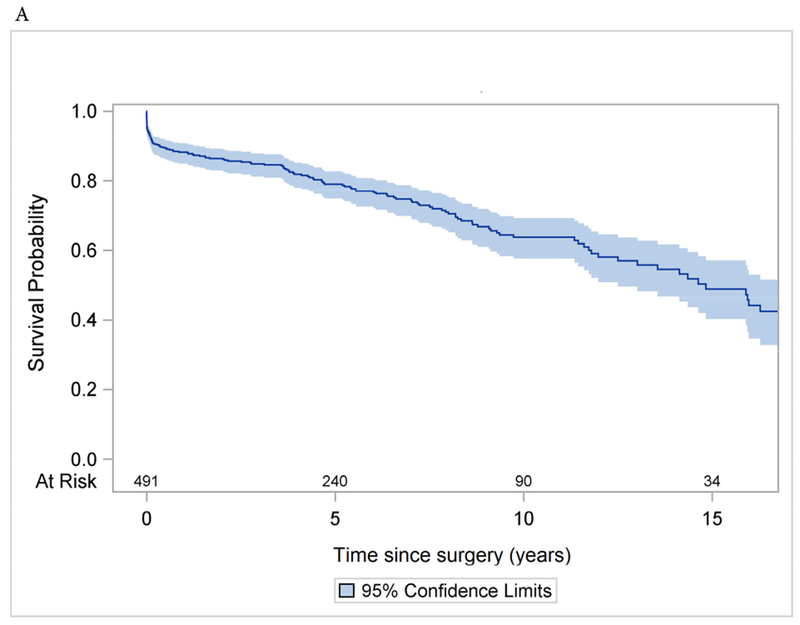
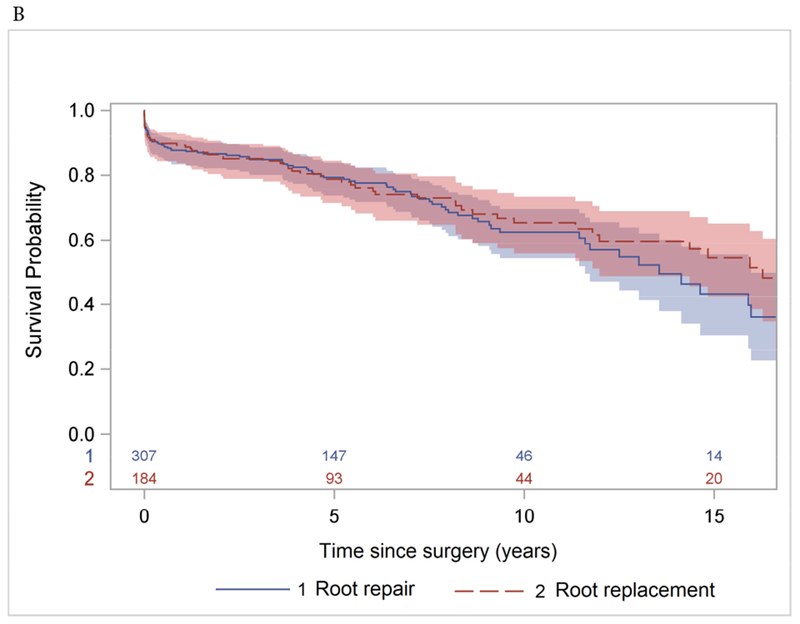
Long-term survival (Kaplan-Meier analysis) of patients with acute type A aortic dissection after aortic root repair and replacement with prosthesis. A: the whole cohort. B: separate subcohorts.
The cumulative incidence of reoperations primarily for aortic root pathology only after the initial ATAAD procedure at 10 years and 15 years was 6.5% and 11% in the ARr group; and 4.7% and 7% in ARR group (Figure 2). The primary indication of reoperation in ARr group (n=9) was aortic root aneurysm (0.5%/year) (Table 4), the median interval time between ATAAD repair and reoperation was 6 years with 8 of 9 patients being male (Table 5), and the reoperations were all aortic root replacements (2 VSARRs, 9 bioprosthetic Bentall, and 2 mechanical Bentall). The primary indication in the ARR group (n=6) was all structural valve deterioration (0.5%/year) (Table 4), the median interval time was 8.5 years and the reoperations included 4 redo-root replacements (3 bioprosthetic and 1 mechanical Bentalls) and 2 TAVRs (CoreValve, Medtronic).
Figure 2:
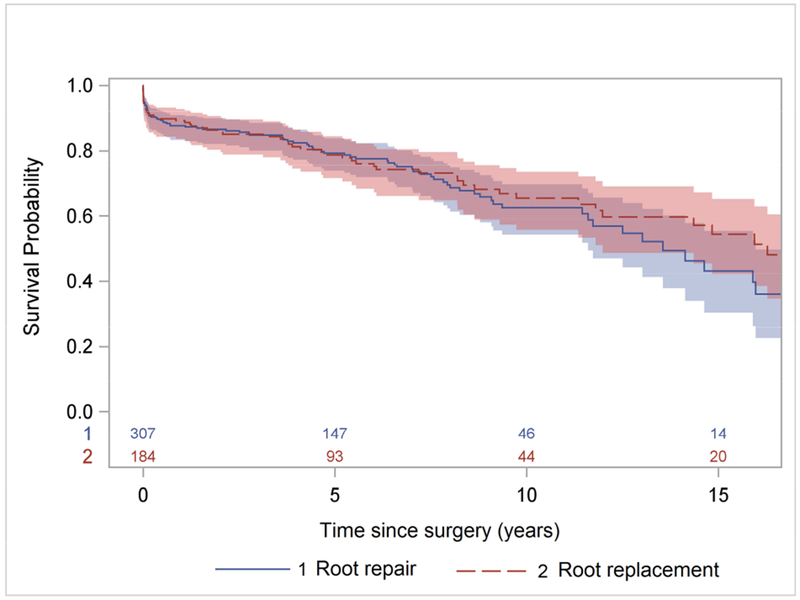
Cumulative incidence of reoperation for aortic root pathology only after acute type A dissection repair with aortic root repair or root replacement with prosthesis.
Table 4.
Long-term Events
| Variables | Root Repair | Root Replacement | ||
|---|---|---|---|---|
| (n=307) | Incidence Rate (%/patient yr) | (n=184) | Incidence Rate (%/patient yr) | |
| TIA | 3 | 0.2 | 1 | 0.1 |
| Stroke | 4 | 0.2 | 5 | 0.4 |
| Spontaneous Bleeding | 3 | 0.2 | 2 | 0.16 |
| Endocarditis | 3 | 0.2 | 1 | 0.1 |
| Reoperation for | 13 | 0.8 | 6 | 0.5 |
| Aortic Valve Dysfunction | 3 | 0.2 | 6 | 0.5 |
| Aortic Insufficiency | 3 | 5 | ||
| Aortic Stenosis | 0 | 1 | ||
| Aortic Valve Endocarditis | 0 | 0 | 0 | 0 |
| Aortic Root Aneurysm | 9 | 0.5 | 0 | 0 |
| Aortic Root Pseudoaneurysm | 1 | 0.1 | 0 | 0 |
Abbreviations: TIA, transient ischemic attack.
Table 5:
Details of patients who had reoperation for aortic root aneurysm after aortic root repair during ATAAD repair.
| First operation | ATAAD repair | Reoperation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Gender | Age (years) | AI | Aortic root (cm) | BioGlue/Felt | MPS | New diagnosis | Age (years) | AI | Aortic Root (cm) |
| 1 | M | 51 | None | 4.1 | Felt sandwich | Occlusion of the right common carotid artery | None | 60 | Moderate to severe | 5.4 |
| 2 | M | 35 | None | 3.4 | Fibrin glue | None | MFS | 47 | Moderate to severe | 6.1 |
| 3 | M | 36 | Moderate | Unknown | Unknown | Unknown | None | 50 | Mild | 6.8 |
| 4 | M | 44 | Minimal | 4.2 | None | None | None | 48 | None | 6 |
| 5 | M | 52 | Severe | 4.6 | None | Acute right sided MCA stroke | None | 53 | None | 5.8 |
| 6 | M | 55 | Mild | Unknown | Unknown | Unknown | None | 61 | Severe | 5.6 |
| 7 | F | 69 | None | Unknown | Felt sandwich | Neurological MPS | None | 70 | Moderate | Unknown |
| 8 | M | 48 | Moderate to severe | Not aneurysmal | Felt sandwich | None | MFS | 49 | Moderate | 6 |
| 9 | M | 41 | Moderate | Not aneurysmal | Felt sandwich | None | BAV | 50 | Severe | 5.6 |
Abbreviations: AI, aortic insufficiency; BAV, bicuspid aortic valve; MCA, middle cerebral artery; MFS, Marfan syndrome; MPS, malperfusion syndrome.
DISCUSSION
In this study, we reported our 20-year experience of treating 491 patients with either ARr or ARR (62% with bioprosthesis) based on predefined criteria to guide the operative approach. We report that in-hospital mortality was between 8-9% for both groups with similar long-term survival. The primary reason for reoperation was aortic root aneurysm in the ARr group and structural valve deterioration in the ARR group. (Video)
Management of the aortic root in ATAAD is very controversial. With our main priority being to maximize peri-operative survival, we used four criteria to determine whether to perform an ARR as follows:
Intimal tear in the aortic root: When the intimal flap has a tear in the sinus of Valsalva, it is very hard to repair and the repair does not sustain. Because of the tear, without aortic root replacement there is a persistent risk for rupture, aortic valvulopathy/insufficiency, or coronary artery dissection and malperfusion as well as rapid aneurysmal degeneration necessitating reoperation. The tear needs to be resected and the root needs to be replaced.
Connective tissue disease: Patients with connective tissue disease have a much higher risk of developing an aortic root aneurysm after aortic dissection even if they do not have a root aneurysm at the time of dissection; and consequently, there is a much higher risk of reoperation for an aortic root aneurysm or aortic insufficiency5, 13, 21. In our ARr group, nine patients underwent reoperation for aortic root aneurysm. Two out of 9 patients had a new diagnosis of MFS while one had a bicuspid aortic valve and root aneurysm (Table 5). These patients should undergo an ARR at the time of operation for ATAAD.
Aortic root diameter is larger than 4.5 cm: Acute aortic dissection with root involvement indicates patients have existing aortopathy. If the aortic root is larger than 4.5 cm at the time of ATAAD, it is very likely the aortic root will continue to grow and the patient will need a reoperation for either root aneurysm or aortic insufficiency. Ro and colleagues found ATAAD patients with an aortic root of 4.7 cm quickly developed moderate/severe aortic insufficiency and root aneurysm within 3 years after ATAAD repair14. This criterion has been adopted by many surgeons20, 22, 23. In patients who needed reoperation for aortic root aneurysm after ARr, the median age was 48 (41, 52) years old. This finding suggests that younger patients may have been at a higher risk of having an unknown aortopathy that led to developing aortic root aneurysm requiring reoperations. We recommend a more aggressive approach for younger patients. If patients are younger than 60 years old, ARR should be more strongly considered if the aortic root is larger than 4 cm, especially if the surgeon can perform VSARR.
Unrepairable aortic valve pathology: If patients have moderate-to-severe aortic stenosis or insufficiency, and the aortic valve is not repairable, we recommend replacement of the entire aortic root instead of only the aortic valve. One could argue to perform aortic valve replacement alone and to preserve the aortic root since it is a less complex operation than ARR. However, if the aortic root is already dissected and diseased, there is less benefit to preserving the aortic sinus segment if the aortic valve cannot also be preserved. At the University of Michigan, all ATAADs are managed by aortic surgeons who are very familiar and comfortable with aortic root procedures. This is another reason that we are more aggressive in performing ARR for ATAAD with unrepairable aortic valve pathology. As reported herein, the perioperative mortality and morbidity as well as long-term survival for the ARR group were comparable to the ARr group even though patients that underwent an ARR were sicker. This finding supports our aortic root approach for ATAAD patients. Furthermore, our findings are similar to those reported by other experienced aortic centers5, 7, 8 and in a study using IRAD data6. With aggressive ARR, the incidence rate of reoperation for root aneurysm was zero (Table 4).
Some surgeons propose that extensive dissection at the aortic root is another indication for ARR2, 4 Nishida and colleagues found that in ATAAD patients who underwent aortic root repair, dissection of 2 or more aortic sinuses was an independent predictor of late aortic root events with a hazard ratio of 2.2, including aortic root dilation ≥ 3mm/year, reoperation of the aortic root, moderate to severe aortic insufficiency, and pseudoaneurysm within 50 months10. However, we did not observe late aortic root events beyond 60 months in our previous study18. If the aortic dissection extends to the left and right sinuses of Valsalva around the coronary ostia, we re-enforce the aortic wall around the coronary ostia circumferentially with 5-0 Prolene as an in situ coronary button reimplantation18. Since biological glue was used in the Nishida study10 but not in ours18, we suggest the late aortic root events might be partially due to the toxicity of biological glue.
An ARr was performed if the patient did not have an intimal tear at the aortic root, connective tissue disease, unrepairable aortic valve pathology, or an aortic root >4.5 cm. It is important to remove all of the thrombus in the false lumen of the aortic root when performing ARr. After repair, the blood pressure inside the aortic root pushes the dissection flap against the outer layer of the dissected aorta if Teflon felt is not inserted, which keeps the dissection flap attached to the outer layer of the dissected aortic root and lets the dissected aortic wall heal18. We did not resect any part of the aortic root (Uni-Yacoub or Bi-Yacoub procedure) as described by the Stanford group5. Our previous data showed the dissected aortic root can heal completely without biological glue or Teflon felt, which maintains its natural geometry and minimizes the risk of late aortic insufficiency18.
Preservation of the dissected aortic root always has a potential risk of recurrence of aortic root pathology such as recurrent aortic root dissection, aneurysm/pseudoaneurysm formation, severe aortic insufficiency, and reoperation. In our study, the primary indication of reoperation in the ARr group was aortic root aneurysm (Table 4). Eight out of 9 patients who had reoperation for the root aneurysm were male (Table 5), and Cox regression showed male patients may potentially have higher risk of reoperation for root pathology than female patients. A more aggressive root approach (such as VSARR if possible) should be considered for young male patients with ATAAD. In ARR group, a bioprosthesis was used for 62% of cases (Freestyle stentless porcine aortic root, Medtronic). Patients with bioprostheses benefited from low neurological and bleeding complications, but suffered reoperations due to the structural valve deterioration of bioprostheses (Table 4). All the patients undergoing reoperation for aortic root pathology in ARR group had a bioprosthesis (Freestyle aortic root) during the ATAAD repair. As a result, the overall cumulative incidence of reoperation was similar between the ARr and ARR groups (Figure 2).
Our study is limited by being a single-center and retrospective experience, yet with high volume and relatively long-term follow-up. Because the follow-up of reoperation was not 100% complete, we might underestimate the rate of reoperation in both the ARr and ARR groups. Bioprostheses were used in the majority (62%) of our ARR procedures, therefore our study represents the outcomes of ARr and bioprosthetic ARR in ATAAD. At the University of Michigan, the patients with ATAAD were managed mainly by aortic surgeons. Each surgeon performs 15-25 ATAAD cases per year and elective aortic root cases routinely. Our experience may not apply in general.
CONCLUSION
In ATAAD, both ARr and ARR are appropriate operations for select patients with good short- and long-term outcomes. An ARR should be considered for ATAAD patients with an intimal tear at the aortic root, root aneurysm ≥4.5 cm, connective tissue disease, or unrepairable aortic valvulopathy. ARr with preservation of the aortic root increased the potential risk of late reoperation for aortic root aneurysm.
Supplementary Material
Discussion of aortic root management in acute type A aortic dissection with power point presentation.
CENTRAL PICTURE.
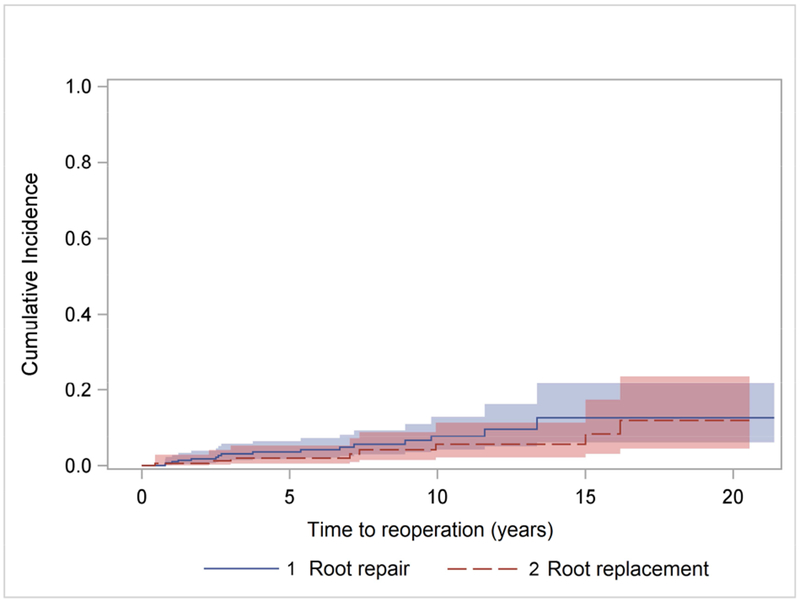
Kaplan-Meier survival of patients undergoing aortic root repair and replacement.
CENTRAL MESSAGE:
Properly selected ATAAD patients can undergo aortic root repair or replacement with favorable short- and long-term survival based on the predefined criteria of aortic root replacement.
PERSPECTIVE STATEMENT:
ATAAD patients with intimal tear at the aortic root, root aneurysm ≥4.5 cm, connective tissue disease, or unrepairable aortic valvulopathy should undergo aortic root replacement. In the absence of those criteria, aortic root repair with preservation of the aortic root is an appropriate option, but with a potential risk of reoperation for an aortic root aneurysm, especially in young male patients.
AKNOWLEDGEMENT
Dr. Yang is supported by NHLBI of NIH K08HL130614, R01HL141891, the Phil Jenkins Breakthrough Fund, and Steven Szatmari Funds. Dr. Patel is supported by the Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery.
Funding Source: NHLBI of NIH K08HL130614, R01HL141891, the Phil Jenkins Breakthrough Fund, and Darlene & Stephen J. Szatmari Funds. Joe D. Morris Collegiate Professorship, the David Hamilton Fund.
GLOSSARY OF ABBREVIATIONS
- ATAAD
acute type A aortic dissection
- ARr
aortic root repair
- ARR
aortic root replacement
- CAD
coronary artery disease
- CI
confidence interval
- CABG
coronary artery bypass graft
- IRAD
international registry of acute aortic dissections
- NYHA
New York heart association
- STJ
sinotubular junction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Date and number of IRB approval: HUM00118824, approved 10/7/2016
REFERENCES
- 1.Berretta P, Patel HJ, Gleason TG, Sundt TM, Myrmel T, Desai N, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg. 2016;5:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castrovinci S, Pacini D, Di Marco L, Berretta P, Cefarelli M, Murana G, et al. Surgical management of aortic root in type A acute aortic dissection: a propensity-score analysis. Eur J Cardiothorac Surg. 2016;50:223–229. [DOI] [PubMed] [Google Scholar]
- 3.Peterss S, Dumfarth J, Rizzo JA, Bonaros N, Fang H, Tranquilli M, et al. Sparing the aortic root in acute aortic dissection type A: risk reduction and restored integrity of the untouched root. Eur J Cardiothorac Surg. 2016;50:232–239. [DOI] [PubMed] [Google Scholar]
- 4.Leshnower BG, Chen EP. When and how to replace the aortic root in type A aortic dissection. Ann Cardiothorac Surg. 2016;5:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu P, Trojan J, Tsou S, Goldstone AB, Woo YJ, Fischbein MP. Limited root repair in acute type A aortic dissection is safe but results in increased risk of reoperation. J Thorac Cardiovasc Surg. 2018;155:1–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Eusanio M, Trimarchi S, Peterson MD, Myrmel T, Hughes GC, Korach A, et al. Root replacement surgery versus more conservative management during type A acute aortic dissection repair. Ann Thorac Surg. 2014;98:2078–2084. [DOI] [PubMed] [Google Scholar]
- 7.Fleischman F, Elsayed RS, Cohen RG, Tatum JM, Kumar SR, Kazerouni K, et al. Selective Aortic Arch and Root Replacement in Repair of Acute Type A Aortic Dissection. Ann Thorac Surg. 2018;105:505–512. [DOI] [PubMed] [Google Scholar]
- 8.Halstead JC, Spielvogel D, Meier DM, Rinke S, Bodian C, Malekan R, et al. Composite aortic root replacement in acute type A dissection: time to rethink the indications? Eur J Cardiothorac Surg. 2005;27:626–632; [DOI] [PubMed] [Google Scholar]
- 9.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007;282:36112–36120. [DOI] [PubMed] [Google Scholar]
- 10.Nishida H, Tabata M, Fukui T, Takanashi S. Surgical Strategy and Outcome for Aortic Root in Patients Undergoing Repair of Acute Type A Aortic Dissection. Ann Thorac Surg. 2016;101:1464–1469. [DOI] [PubMed] [Google Scholar]
- 11.Valdis M, Adams C, Chu MWA, Kiaii B, Guo L. Comparison of outcomes of root replacement procedures and supracoronary techniques for surgical repair of acute aortic dissection. Can J Surg. 2017;60:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamohara K, Koga S, Takaki J, Yoshida N, Furukawa K, Morita S. Long-term durability of preserved aortic root after repair of acute type A aortic dissection. Gen Thorac Cardiovasc Surg. 2017;65:441–448. [DOI] [PubMed] [Google Scholar]
- 13.Saczkowski R, Malas T, Mesana T, de Kerchove L, El Khoury G, Boodhwani M. Aortic valve preservation and repair in acute Type A aortic dissection. Eur J Cardiothorac Surg. 2014;45:e220–226. [DOI] [PubMed] [Google Scholar]
- 14.Ro SK, Kim JB, Hwang SK, Jung SH, Choo SJ, Chung CH, et al. Aortic root conservative repair of acute type A aortic dissection involving the aortic root: fate of the aortic root and aortic valve function. J Thorac Cardiovasc Surg. 2013;146:1113–1118. [DOI] [PubMed] [Google Scholar]
- 15.Yang B, DeBenedictus C, Watt T, Farley S, Salita A, Hornsby W, et al. The impact of concomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J Thorac Cardiovasc Surg. 2016;152:394–400 e391. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention; National Center for Health Statistics. National Death Index. Accessed December 27, 2017.
- 17.Yang B, Patel HJ, Sorek C, Hornsby WE, Wu X, Ward S, et al. Sixteen-Year Experience of David and Bentall Procedures in Acute Type A Aortic Dissection. Ann Thorac Surg. 2018;105:779–784. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Malik A, Waidley V, Wu X, Norton EL, Williams DM, et al. Short-term outcomes of a simple and effective approach to aortic root and arch repair in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2018;155:1360–1370.e1361. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Morioka K, Li W, Yamada N, Takamori A, Handa M, et al. Adventitial inversion technique without the aid of biologic glue or Teflon buttress for acute type A aortic dissection. Eur J Cardiothorac Surg. 2005;28:864–869. [DOI] [PubMed] [Google Scholar]
- 20.Rylski B, Bavaria JE, Milewski RK, Vallabhajosyula P, Moser W, Kremens E, et al. Long-term results of neomedia sinus valsalva repair in 489 patients with type A aortic dissection. Ann Thorac Surg. 2014;98:582–588; [DOI] [PubMed] [Google Scholar]
- 21.Fann JI, Smith JA, Miller DC, Mitchell RS, Moore KA, Grunkemeier G, et al. Surgical management of aortic dissection during a 30-year period. Circulation. 1995;92:II113–121. [DOI] [PubMed] [Google Scholar]
- 22.Kunihara T, Neumann N, Kriechbaum SD, Aicher D, Schafers HJ. Aortic root remodeling leads to good valve stability in acute aortic dissection and preexistent root dilatation. J Thorac Cardiovasc Surg. 2016;152:430–436 e431. [DOI] [PubMed] [Google Scholar]
- 23.Leshnower BG, Myung RJ, McPherson L, Chen EP Midterm results of David V valve-sparing aortic root replacement in acute type A aortic dissection. Ann Thorac Surg. 2015;99:795–800; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Discussion of aortic root management in acute type A aortic dissection with power point presentation.


