Abstract
In humans, perihematomal edema (PHE) is considered to be a radiological marker of secondary injury following intracerebral hemorrhage (ICH). There is also evidence that PHE might contribute to poor outcome in ICH patients. Given the rising interest in secondary injury after ICH as a therapeutic target, PHE is becoming increasingly used as a proof-of-concept surrogate measure to assess the potential efficacy of various therapeutic interventions in clinical trials.
We review the pathophysiology of PHE and its evolution, its prognostic significance and relationship to clinical outcomes, and variabilities in its detection and measurement methodologies to determine the advantages vs. shortcomings of using PHE as a translational target or radiological marker to examine the efficacy of interventions aiming to mitigate the secondary injury in ICH.
Keywords: Perihematoma, Edema, Outcome, Trials
INTRODUCTION
Brain water content, “edema”, is widely used as an endpoint to test interventions in experimental models of intracerebral hemorrhage (ICH). The development of edema in the vicinity of the hematoma, often referred to as perihematomal edema (PHE), is not uncommon in ICH patients, and is attributed to the secondary injury precipitated by the mechanical effects of the growing hematoma and toxic effects of blood and its degradation products on surrounding brain tissue [1-2]. Therefore, PHE is considered by many to represent a radiological surrogate marker of secondary injury after ICH.
Most ICH trials have aimed to limit hematoma expansion or to reduce hematoma volume because: 1) ICH size has been a consistent predictor of poor outcome in various studies [3]; 2) PHE is thought to be an epiphenomenon of ICH size; and 3) the link between PHE alone and neurological function is less clear [4]. However, PHE can lead to neurological deterioration, herniation, and death. The failure of previous interventions aiming to limit hematoma expansion to improve outcomes [5-6] coupled with rapid advances in our understanding of the underlying mechanisms of secondary injury in ICH have fueled interest in developing and testing new therapies targeting the secondary injury. As a result, PHE is becoming increasingly employed as a surrogate measure to assess the efficacy of these therapies, particularly in phase I-II trials [7-9].
However, there are several conceptual, mechanistic, analytic, study design, and radiological considerations that have to been taken into account when using PHE as an endpoint. The purpose of this review is to highlight some of these issues, their implications, and to provide recommendations for the use of PHE as a surrogate measure of efficacy in ICH trials.
THE PATHOPHYSIOLOGY OF PHE
The formation and evolution of PHE is complex and involves several processes, which are detailed elsewhere [1-2]. These include: Clot retraction & hydrostatic pressure change forcing the serum into the perihematomal space and edema formation; Thrombin formation & activation of the coagulation cascade; Inflammation; Hemolysis of red blood cells (RBCs) and subsequent hemoglobin toxicity; Complement activation; Plasma protein leakage; and blood brain barrier (BBB) disruption. Early edema within the first hours following ICH is precipitated by the outflow of electrolytes and water from the injured blood vessels, serum proteins, and clot retraction. Delayed PHE is attributed to vasogenic edema resulting from BBB disruption and cytotoxic edema attributed to neuronal death, inflammation, and thrombin production during the following 3-4 days; and lysis of RBCs and release of hemoglobin-degradation products, in particular iron, afterwards [1-2]. These pathophysiological changes related to clot retraction and water redistribution should be taken into consideration and PHE assessment correction algorithms should be used when assessing long-term evolution of PHE.
RADIOLOGICAL ASSESSMENT OF PHE
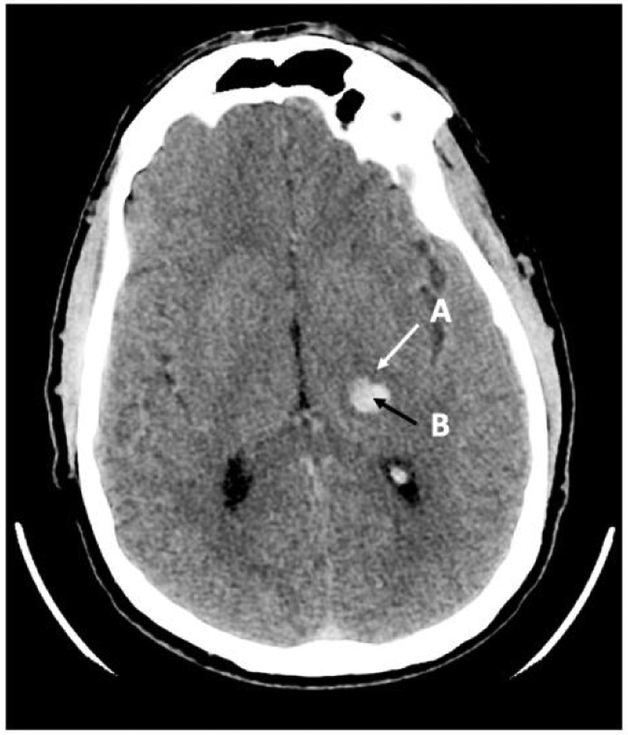
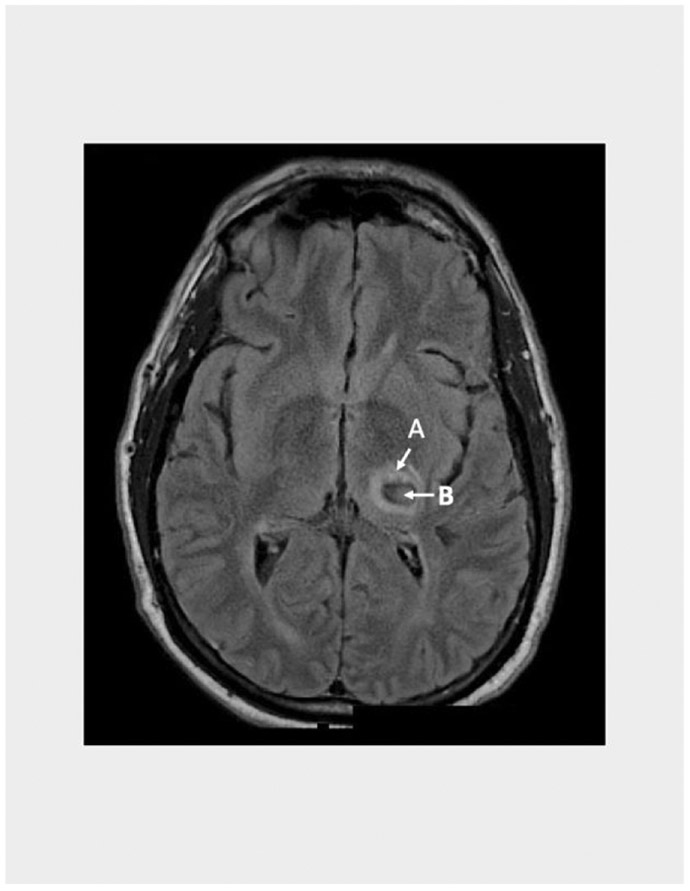
The term PHE is often used to refer to perihematomal hypodensity seen on plain Computerized Axial Tomography (CT) scan (Figure 1) or perihematomal hyperintensity on Fluid Attenuation Inversion Recovery (FLAIR) Magnetic Resonance Imaging (MRI) (Figure 2).
FIGURE 1:
CT scan of the brain showing the hyperdense hematoma (B) surrounded by an area of hyodensity (A), which represents PHE.
FIGURE 2:
MRI FLAIR sequence showing the hematoma (B) surrounded by the hyperintense PHE (A)
MRI is superior to CT for depicting PHE. As figures 1 and 2 illustrate, FLAIR MRI shows PHE with a bright signal, which facilitates delineating its borders. In contrast, delineation of PHE borders can be difficult on CT over time or in areas where it overlaps with areas of periventricular leukoaraiosis. However, CT is almost always performed as the first imaging modality in the vast majority of ICH patients in clinical practice, unlike MRI which is rarely done during the hyperacute phase. This shortcoming hinders the routine use of MRI in most clinical investigations of PHE. Furthermore, the cost of MRI, which is much more expensive than CT, can be prohibitive particularly in large scale clinical trials.
Besides the spatial difficulties encountered with manual delineation of regions of interest (ROIs) on CT, manual delineation of PHE tends to be labor intensive and to have variable reproducibility. Advances in imaging software technology have allowed for fully automated delineation of ROIs or voxel-based assessments. However, these methodologies are also limited by potential inaccuracies related to spatial normalization of the brain. In our experience, a semi-automated method seems to minimize the drawbacks of both manual and fully automated analyses. Volbers et al. [10] suggested that a semi-automated threshold-based CT, as opposed to manual tracing of PHE on CT, could address the aforementioned concerns. By using a fixed lower Hounsefield Unit (HU) of 5 with the upper value adjusted by the reader up to an absolute maximum of 33 HU to obtain the best delineation of the PHE and avoid leukoaraiosis artifact, they found good correlation with simultaneous MRI measurements (R2=0.96), and high inter- (0.96) and intra-observer (0.96) reliability. In contrast, manually traced PHE on CT was often larger with larger standard deviation, and lower inter- and intra-observer reliability [10].
However, it is not entirely clear that the changes in the perihematomal region accurately reflect brain edema or mass effect. Zazulia et al. [11] reported poor correspondence between increase in PHE and change in ipsilateral hemispheric volume, suggesting that other processes such as clot retraction, diffusion of serum from the clot, and redistribution of existing water from the hematoma might contribute to the radiological signal abnormalities in the perihematomal region on CT and MRI.
MEASUREMENT OF PHE
Studies evaluating PHE have used several varying parameters and definitions to assess PHE [12-14]. These studies also exhibited variabilities in the timing (single time point vs. peak) and method of assessing PHE progression (absolute increase vs. percent change vs. rate or speed). The various parameters used to assess PHE and its progression include:
PHE: As mentioned earlier, this often refers to the area of hypodensity on plain CT or hyperintensity on FLAIR MRI surrounding the hematoma (Figure 1 B).
Absolute PHE: This refers to the sum total of pixels of the perihematomal lesion (Figure 1 B).
Relative PHE (rPHE): This refers to the ratio of absolute PHE compared to hematoma lesion, i.e. absolute PHE divided by hematoma volume (Figure 1 B/A).
-
Edema Extension Distance (EED): This refers to the difference between the radius of a sphere equal to the absolute PHE volume and the radius of a sphere equal to the ICH volume alone [13]. This can be calculated using the following formula:
where PHE volume corresponds to absolute edema volume and π corresponds to the radius of a sphere equal to the combined volume of PHE and ICH and π the radius of a sphere equal to the volume of the ICH alone.
Rate OF PHE Growth: This refers to the difference between the initial and follow-up of any of the above definitions of PHE volumes divided by the time interval between the two scans [14]. This can be expressed as millimeter per time (hour or day) or percent change in volume per time.
Each of these parameters has its advantages and limitations. PHE and its growth are strongly related to the size of the underlying ICH [4]. Therefore, assessment of PHE alone may not account for this intermixed relationship between PHE and ICH. While rPHE accounts for the volume of the underlying ICH, rPHE can be disproportionally large in smaller ICH which may render it unsuitable to examine the relationship between PHE and outcome in some cases. Similarly, rPHE may not accurately reflect the extent of brain hemorrhage volume in patients with a small ICH and large intraventricular extension. Examining the rate of PHE growth over time has biological plausibility, since it is intuitive that the speed at which a brain mass lesion expands is important in determining neurological injury and outcome [13]. Edema Extension Distance is based on the premise that diffusion of blood products within the hematoma into the brain parenchyma results in pro-inflammatory effects and PHE around the hematoma border. It is, therefore, thought that EED is mostly influenced by the intensity of the inflammatory reaction and not ICH volume [14]. The measurement of EED is also based on the assumption that PHE is contained within an ellipsoid encapsulating a smaller ellipsoid representing the hematoma. Therefore, the use of EED may not be suitable in patients with irregular-shaped hematomas. However, the use of EED may have advantages from a clinical trial perspective. Parry-Jones et al. [14] examined the data from the control arms of the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage (INTERACT 1) trial and the Minimal Invasive Surgery Plus rt-PA for ICH Evacuation Phase 2 (MISTIE 2) trial, and found that using up to 30% reduction in EED at day 3 or 4 post-ICH instead of absolute PHE or rPHE as the primary endpoint could reduce the required sample size by approximately 75%. The authors suggested that hematoma shape is unlikely to have an important impact on EED variability, and acknowledged that this requires further confirmation.
TIME COURSE AND NATURAL HISTORY OF PHE
Observational and retrospective studies indicate that although PHE progression is fastest during the first few days after ICH onset, PHE continues to grow at a slower rate afterwards reaching its peak between 2 to 3 weeks after ictus [12; 15-17]. In a prospective study of 22 ICH patients, where serial MRIs were performed at approximately 2, 7, 14, and 21 days after ICH onset, PHE growth was fastest in the first 2 days but continued until approximately 12 days [15]. A retrospective study of 219 ICH patients who had at least 3 CT scans at variable times ranging from day 1 to >21 days after ICH onset, absolute PHE and rPHE volumes increased overtime until days 7 to 11 [12]. Another study evaluating the EED growth rate revealed an inverse relationship between edema growth and time from ICH onset, where edema growth was most rapid within the first 24h and reached approximately 60% of its peak [17].
Inflammatory processes seem to be involved in PHE evolution. In one study, the blood neutrophil-to-lymphocyte ratio correlated with 24-hour PHE growth [18]. In another study, the neutrophil-to-lymphocyte ratio on day 6 was independently associated with peak PHE volume [19].
RELATIONSHIP BETWEEN PHE AND FUNCTIONAL OUTCOME AFTER ICH
Several studies examined the relationship between PHE and functional outcomes after ICH with conflicting results, particularly regarding long-term outcomes [17; 20-24]. The disparate results are largely attributed to differences in timepoint(s) of PHE and outcome assessments, imaging, and parameters of PHE assessment in these studies. In addition, the majority of these studies were retrospective, relatively small, underpowered, and important prognostic variables and data on use of medications such as anti-edema agents were not always available.
Relationship between PHE Growth AND Outcome
A prospective population-based study involving 48 ICH patients who were imaged within 3h of onset and then 20h later found that baseline rPHE was associated with lesser odds of poor 3-month functional outcome, defined as Barthel Index score <85, but did not predict mortality [20]. On the other hand, absolute PHE did not predict functional outcome or mortality. In a pooled analysis of data from 1138 participants in INTERACT 1 and 2 trials, the growth in absolute PHE volume between baseline and 24h CT scans was associated with death or dependency at 3 months after adjustment for confounding prognostic variables (adjusted OR 1.17; 95% CI 1.02 – 1.33 per 5 milliliter increase from baseline; p =0.025) [21]. The investigators acknowledged, however, that PHE growth occurs most rapidly during the first few days after ICH and that later assessment of PHE (beyond 24h) might better assess the relationship with outcome.
Most studies assessed PHE growth after 72 hours. A meta-analysis of 21 studies (15 retrospective and 6 prospective) found that the prognostic value of absolute PHE and rPHE were conflicting in different studies [22]. A retrospective analysis of data from 596 patients contained in the Virtual International Stroke Trials Archive (VISTA) found that the absolute increase in PHE volume from baseline to 72h was associated greater odds for poor outcome, defined as modified Rankin Scale (mRS) score 3-6 at 90 days, (OR 1.78; 95% CI 1.12 - 2.64 per mL increase) [23]. PHE was only related to poor functional outcomes in basal ganglia and small (<30 mL) ICH [23]. However, this analysis included patients from various trials, and lacked a standardized tool for measurement of PHE. A hematoma volume-dependent effect of PHE on outcome, where only patients with smaller ICHs exhibited poorer outcomes with worse PHE, was demonstrated in other studies [24]. A prospective single-center study of 133 patients found a significant association between absolute PHE (but not rPHE) and functional outcome upon discharge, but only in patients with ICH volumes ≤30 ml (OR 1.123; 95% CI 1.02-1.27; p=0.03) [24].
Relationship BETWEEN Peak PHE AND Outcome
Recent evidence suggests that peak PHE may be linked to long-term functional outcome after ICH. A retrospective cohort study of 292 ICH patients found that peak PHE volume was independently associated with day 90 outcome (OR 0.98; 95% CI 0.97-0.99) [19]. This suggests that longer assessment periods might be preferable since limiting assessment of PHE growth to 72 hours post-ictus are likely to miss the PHE peak.
Relationship BETWEEN Rate OF PHE Growth AND Outcome
The impact of PHE on neurological outcomes may depend on the rate of PHE expansion; more rapid PHE expansion may produce more damage than gradual progression. The relationship between the rate of PHE progression and outcome has been the subject of recent investigations. A retrospective single-center study of 139 patients found that PHE expansion rate between admission and 24h was a significant predictor of 90-day mortality (adjusted OR 2.21; 95% CI 1.05 – 4.64; p= 0.04), and higher 24h PHE expansion rate was associated with poorer mRS in an ordinal shift analysis (Adjusted OR 2.07; 95% CI 1.12 – 3.83; p= 0.02) [13 ]. On the other hand, there was no association between rPHE and outcomes [13]. A similar study in 596 patients in VISTA found that the odds of mortality were greater with increasing PHE expansion rate in the first 72h following ICH (OR 2.63; 95% CI 1.10 - 6.25) [25]. The rate of PHE expansion at 72 hours was associated with poor 90-day outcomes (odds ratio (OR) 1.54; 95% confidence interval (CI) 1.04 – 2.22; p =0.001) in a meta-analysis of 21 studies [22]. Another study in 861 patients from the Helsinki ICH study used the change in EED from baseline to follow-up scans to interpolate EED growth rate at 72h (centimeter per day), and employed this modeled EED growth-rate-to-time data to divide patients into higher and lower than expected EED at 72h [17]. Patients with faster edema growth at 72h had more midline shift, herniation, and higher 6-month mortality [17]. However, it is noteworthy that the EED growth rate at 72h was estimated because repeat imaging at 72h was not routinely performed in all patients. This may not have accurately represented the natural evolution of PHE in every patient. Furthermore, only 50% of the study subjects had regularly shaped and ellipsoid ICH lesions, questioning the suitability of EED for edema measurements in the remaining half of the subjects.
Few clinical studies have targeted edema formation and progression in ICH. In a retrospective, single-center, case-control study of 70 patients, treatment with mild hypothermia (35° C) within 2 days of ICH onset decreased rPHE progression between admission and day 3, but had no effect on day-90 mRS scores [26]. A randomized, placebo-controlled trial of mannitol 20% (100 mL every 4h for 5 days started within 6 days of ictus) in 128 ICH patients found no difference in 30-day mortality or 3-month Barthel Index scores between placebo-vs. mannitol-treated patients [27]. Another double-blind, randomized, placebo-controlled of 10% glycerol (administered within 24h of onset by intravenous infusion for 4 hours daily for 6 consecutive days) in 216 patients with ICH found no difference in a variety of clinical outcomes between the two treatment groups [28].
CONCLUDING REMARKS
There are several points to consider when attempting to use PHE as a therapeutic target or a surrogate measure to assess the efficacy of potential therapeutic interventions in ICH. First, the natural history of PHE is characterized by rapid progression during the first 1 to 3 days after ICH with the maximum increase occurring during the following 7 to 10 days, and PHE growth during the early time period seems to be more clinically relevant and related to neurological deterioration. This suggests that interventions targeting PHE should be initiated early (within hours of ICH onset) and maintained for 3 to 7 days. Because one should rule out rebound increase in PHE following cessation of such interventions, a longer follow-up is needed. These concerns have cost and feasibility implications from a clinical trial design perspective.
Second, the ICH-dependent impact of PHE evolution and contribution to functional outcome requires further elucidation. Because PHE is strongly related to the size of the underlying ICH, studies targeting PHE as a therapeutic target and a primary endpoint may consider limiting enrollment to patients with moderate size ICH since these patients are more likely to exhibit critical growth of PHE. This has implications regarding enrollment rate, number of sites required, and duration of the trial.
Third, rPHE is disproportionately large in smaller ICH and may not be accurate in patients with small ICH and large intraventricular extensions. Therefore, it may not be an appropriate parameter or endpoint to analyze the impact of PHE on outcome. The rate of PHE growth and EED are emerging new parameters and might represent better endpoints than rPHE volume. However, these parameters require further investigations.
Lastly, most studies examining the relationship between PHE and clinical outcomes have inherent design limitations and drawbacks which impacts accurate interpretation of their findings. More work is needed to systematically evaluate the relationship between PHE and long-term outcomes. Prospective studies with pre-specified methods, analyses, and endpoints; consistent imaging modality and timing; and adequate sample size calculations and power are needed to accurately determine the impact of PHE on ICH outcomes. Table 1 summarizes current challenges in PHE assessments and recommendations for future research of PHE.
Table 1:
Summary of the challenges in PHE assessment and recommendations for future investigations of PHE
| Challenges in PHE Assessments | Recommendations for Using PHE in Clinical Research |
|---|---|
|
CT vs. MRI
- PHE is easier to delineate on MRI compared to CT. However, MRI is not routinely performed during the hyperacute phase and is more expensive |
ICH Size and Location
- Further investigations are needed to elucidate the impact of ICH volume and location on PHE progression and its relationship to outcomes - Consider limiting enrollment into studies using PHE as a primary endpoint to moderate-sized ICHs since these patients are more likely to exhibit critical growth of PHE PHE Parameters - Absolute PHE may be a better predictor of outcome than rPHE, particularly in patients with small ICHs - The rate of PHE growth and EED are promising parameters and require further investigations Timing - Interventions aimed at targeting PHE should be initiated within hours of ICH onset and maintained for 3-7 days - Follow-up of PHE progression/resolution a few weeks after treatment is advised to capture the full effect on the intervention on the known natural history of PHE progression |
|
Measurement of PHE
Delineation: - Manual delineation is labor intensive and has variable reproducibility - Fully-automated delineation is limited by potential inaccuracies related to spatial normalization of the brain - A semi-automated threshold-based methods may address the limitations above Parameters: - There are no consistent or uniform parameters in use. Various parameters and definitions for PHE have been used in various studies. These include: absolute PHE, relative PHE, rate of PHE growth (volume/time or % change/time), EED, rate of change in EED (volume/time or % change/time) Timing: - The timing of PHE assessments has been inconsistent. PHE progresses fast during the first few days and reaches its peak by 2-3 weeks - Variable times have been reported in the literature varying from 24h to 72h or longer after ICH | |
|
Relationship between PHE and Outcome
- Variability in timing of PHE and/or outcome assessments and parameters used makes it difficult to fully ascertain the relationship between PHE and long-term functional outcomes after ICH |
Study Design
- To accurately determine the impact of PHE on outcomes, future studies should: - Be prospective - Have pre-specified methods, parameters, and endpoints - Have adequate sample size calculations and statistical power |
In conclusion, the use of PHE as a primary endpoint in clinical trials of ICH requires caution. PHE is a reasonable marker of ongoing biological responses even if it has a questionable impact on outcome. Therefore, PHE is better suited to serve as a crude surrogate marker on an intervention’s biological activity on the brain, but is not yet ready for prime time as a primary endpoint of an intervention’s efficacy.
SIGNIFICANCE STATEMENT.
Intracerebral hemorrhage is a devastating condition with no specific effective therapy. Most investigations of medical therapies have focused on limiting hematoma expansion, but had little effect on final outcomes. There is growing interest in developing interventions to target the cascade of events that ensues following ICH and the secondary injury precipitated by blood and its degradation products. Perihematomal edema (PHE) is believed to represent an easy radiological marker of the secondary injury and is being increasingly employed as an endpoint to assess the efficacy of these interventions. Careful appraisal of the strengths and limitations of using PHE as a therapeutic target and surrogate measure of efficacy is essential to improve study design and interpretation of the results to successfully develop innovative and effective therapies for ICH.
Footnotes
CONFLICT OF INTEREST
The authors (M Selim and C Norton) have no conflict of interest, including any financial, personal, or other relationships with people or organizations that could influence the present article.
Support Information: Dr. Selim receives grant support from the National Institute of Neurological
Disorders and Stroke (U01-NS 074425) and American Heart Association (15CSA24540001).
REFERENCES
- 1.Lim-Hing k, Rincon F (2017). Secondary Hematoma Expansion and Perihemorrhagic Edema after Intracerebral Hemorrhage: From Bench Work to Practical Aspects. Front Neurol;8:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi G, Keep RF, Hoff JT. (2002). Pathophysiology of brain edema formation. Neurosurg Clin N Am;13(3):371–383. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. (1993). Volume of intracerebral hemorrhage: a powerful and easy-to-use predictor of 30-day mortality. Stroke;24:987–993. [DOI] [PubMed] [Google Scholar]
- 4.Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, ….Anderson CS, For the INTERACT Investigators. (2009). Significance of perihematomal edema in acute intracerebral hemorrhage: The INTERACT trial. Neurology; 73(23): 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, ….Steiner T; FAST Trial Investigators. (2008). Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med;358(20):2127–2137. [DOI] [PubMed] [Google Scholar]
- 6.Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, ….Bath PM; TICH-2 Investigators. (2018). Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage(TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet;391(10135):2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, ….Shi FD. (2014). Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol;71(9):1092–1101. [DOI] [PubMed] [Google Scholar]
- 8.Kollmar R, Staykov D, Dörfler A, Schellinger PD, Schwab S, Bardutzky J. (2010). Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke;41:1684–1689. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Yuan Q, Sun YR, Wu X, Du ZY, Li ZQ, ….Hu J. (2017). Effects of Deferoxamine Mesylate on Hematoma and Perihematoma Edema after Traumatic Intracerebral Hemorrhage. J Neurotrauma;34(19):2753–2759. [DOI] [PubMed] [Google Scholar]
- 10.Volbers B, Staykov D, Wagner I, Dörfler A, Saake M, Schwab S, Bardutzky J. (2011). Semiautomatic volumetric assessment of perihemorrhagic edema with computed tomography. Eur J Neurol;18(11):1323–1328. [DOI] [PubMed] [Google Scholar]
- 11.Zazulia AR, Videen TO, Diringer MN, Powers WJ. (2011). Poor Correlation between Perihematomal MRI Hyperintensity and Brain Swelling after Intracerebral Hemorrhage. Neurocrit Care;15(3):436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staykov D, Wagner I, Volbers B, Hauer EM, Doerfler A, Schwab S, Bardutzky J. (2011). Natural course of perihemorrhagic edema after intracerebral hemorrhage. Stroke;42:2625–2629. [DOI] [PubMed] [Google Scholar]
- 13.Parry-Jones AR, Wang X, Sato S, Mould WA, Vail A, Anderson CS, Hanley DF. (2015). Edema Extension Distance: Outcome Measure for Phase II Clinical Trials Targeting Edema After Intracerebral Hemorrhage. Stroke;46(6):e137–140. [DOI] [PubMed] [Google Scholar]
- 14.Urday S, Beslow LA, Dai F, Zhang F, Battey TW, Vashkevich A, ….Sheth KN. (2016). Rate of Perihematomal Edema Expansion Predicts Outcome After Intracerebral Hemorrhage. Crit Care Med;44(4):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, Wijman CA. (2011). Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke;42:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inaji M, Tomita H, Tone O, Tamaki M, Suzuki R, Ohno K. (2003). Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir Suppl;86:445–448. [DOI] [PubMed] [Google Scholar]
- 17.Wu TY, Sharma G, Strbian D, Putaala J, Desmond PM, Tatlisumak T, ….Meretoja A. (2017). Natural History of Perihematomal Edema and Impact on Outcome After Intracerebral Hemorrhage. Stroke;48:873–879. [DOI] [PubMed] [Google Scholar]
- 18.Gusdon AM, Gialdini G, Kone G, Baradaran H, Merkler AE, Mangat HS, ….Murthy SB. (2017). Neutrophil-lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke;48:2589–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volbers B, Giede-Jeppe A, Gerner ST, Sembill JA, Kuramatsu JB, Lang S, …. Huttner HB. (2018). Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology; 90(12):e1005–e1012. [DOI] [PubMed] [Google Scholar]
- 20.Gebel JM Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, ….Broderick JP. (2002). Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke;33:2636–2641. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Arima H, Wu G, Heeley E, Delcourt C, Zhou J, ….Anderson CS; INTERACT Investigators. (2015). Prognostic significance of perihematomal edema in acute intracerebral hemorrhage: pooled analysis from the intensive blood pressure reduction in acute cerebral hemorrhage trial studies. Stroke;46:1009–1013 [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Ma L, Zheng J, You C. 2017. Prognostic Role of Perihematomal Edema in Intracerebral Hemorrhage: A Systematic Review. Turk Neurosurg. doi: 10.5137/1019-5149.JTN.19659-16.0. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Murthy SB, Moradiya Y, Dawson J, Lees KR, Hanley DF, Ziai WC; VISTA-ICH Collaborators. (2015). Perihematomal Edema and Functional Outcomes in Intracerebral Hemorrhage: Influence of Hematoma Volume and Location. Stroke;46:3088–3092. [DOI] [PubMed] [Google Scholar]
- 24.Appelboom G, Bruce SS, Hickman ZL, Zacharia BE, Carpenter AM, Vaughan KA,…. Connolly ES Jr. (2013). Volume-dependent effect of perihematomal oedema on outcome for spontaneous intracerebral haemorrhages. J Neurol Neurosurg Psychiatry;84:488–493. [DOI] [PubMed] [Google Scholar]
- 25.Murthy SB, Urday S, Beslow LA, Dawson J, Lees K, Kimberly WT, ….Ziai WC; VISTA ICH Collaborators. (2016). Rate of perihaematomal oedema expansion is associated with poor clinical outcomes in intracerebral haemorrhage. J Neurol Neurosurg Psychiatry;87(11):1169–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volbers B, Herrmann S, Willfarth W, Lücking H, Kloska SP, Doerfler A, ….Staykov D. (2016). Impact of Hypothermia Initiation and Duration on Perihemorrhagic Edema Evolution After Intracerebral Hemorrhage. Stroke;47:2249–2255. [DOI] [PubMed] [Google Scholar]
- 27.Misra UK, Kalita J, Ranjan P, Mandal SK. (2005). Mannitol in intracerebral hemorrhage: a randomized controlled study. J Neurol Sci;234(1-2):41–45. [DOI] [PubMed] [Google Scholar]
- 28.Yu YL, Kumana CR, Lauder IJ, Cheung YK, Chan FL, Kou M, ….Fong KY. (1992). Treatment of acute cerebral hemorrhage with intravenous glycerol. A double-blind, placebo-controlled, randomized trial. Stroke;23:967–971. [DOI] [PubMed] [Google Scholar]