Abstract
Objective:
The SVS Reporting Standards for endovascular aortic aneurysm repair (EVAR) consider the presence of a type I or III endoleak a technical failure. However, the nature and implications of these endoleaks in fenestrated EVAR (FEVAR) are not well understood.
Methods:
We performed a single-center retrospective review of 53 patients who underwent FEVAR with the Zenith Fenestrated AAA Endovascular Graft (Cook Medical) from 2013–2018. We excluded one patient without contrast-enhanced postoperative imaging who was lost to follow-up after discharge. Small, slow, type I and III endoleaks on completion angiogram were routinely observed. We identified patients with completion type I/III endoleaks by angiography review and characterized endoleak type, location, and rate of resolution on initial postoperative imaging.
Results:
Fifty-two patients were included; mean age was 75 ± 8 years, 75% were male, and 91% were white. One-hundred forty-five of 146 (99%) visceral vessels were preserved (100 renal arteries and 46 superior mesenteric arteries) with 103 fenestrations and 43 scallops; 102 (70%) target vessels were stented. After implantation of all device components, 31 patients (60%) had evidence of type I/III endoleak. Twelve patients (39%) underwent further intervention at the index procedure and three endoleaks resolved completely. Twenty-eight patients (54%) had a type I or III endoleak on completion angiogram. There were no differences between patients with and without completion endoleaks in baseline demographics, graft design, neck anatomy, or proportion of cases performed within the instructions for use (IFU) of the device. Perioperative mortality was 1.9%. On initial postoperative imaging, 27 of 28 (96%) endoleaks resolved spontaneously. One small, persistent type Ia/III endoleak was identified on postoperative day 27 and was observed. This endoleak had resolved completed on the following CTA 6-months postoperatively. In patients without a completion endoleak, one type Ia endoleak secondary to graft infolding was discovered on postoperative imaging and was successfully treated with placement of endoachors and Palmaz stent. Median follow-up was 269 days. No additional type I/III endoleaks were identified in any patient for the duration of follow-up.
Conclusions:
While completion type I and III endoleaks are common following FEVAR with the ZFEN device, nearly all of these endoleaks resolve spontaneously by the initial postoperative imaging. These results suggest that select completion endoleaks following FEVAR with the ZFEN device do not require intervention at the index procedure.
Keywords: Juxtarenal AAA, Fenestrated EVAR, Endoleak
In this retrospective study of 52 fenestrated endovascular aortic repairs type I or III endoleaks were identified on completion angiograms in 28 patients (54%); in 27 (96%) they resolved spontaneously by the initial postoperative imaging.
Introduction
Since first described in 1991, the use of endovascular abdominal aortic aneurysm repair (EVAR) has expanded rapidly and is now the predominant technique used in the repair of abdominal aortic aneurysms (AAA).1–3 As utilization of this technology has increased, so has awareness of its inherent complications. One such complication unique to EVAR is the presence of endoleaks. Studies of the long-term results of EVAR demonstrate that aneurysm-related reinterventions are more common following EVAR than open repair, and endoleaks specifically are a frequent indication for reintervention.4–6 Type I endoleaks, flow into the aneurysm sac from the proximal or distal seal zones, and type III endoleaks, flow into the sac from the overlap of two device components or a hole in the graft fabric, represent direct aortic flow into the sac and are considered more dangerous than their type II counterparts, back-bleeding into the sac via an aortic branch vessel. Persistent and late type I and III endoleaks have been associated with higher rates of conversion to open repair, aneurysm rupture, and overall mortality.7,8
In that light, the Society for Vascular Surgery (SVS) reporting standards for EVAR and thoracic endovascular aneurysm repair (TEVAR) classify presence of a type I or III endoleak at the completion of the index procedure as a technical failure.9,10 However, despite this classification, reports of completion endoleaks indicate that between 3 and 16 percent of patients undergoing EVAR will leave the operating room with evidence of a type I endoleak. Furthermore, there is a growing literature suggesting that the majority of these completion endoleaks resolve spontaneously.11–15 The recent SVS practice guidelines for management of AAA acknowledge that “a type IA endoleak may occasionally resolve after reversal of heparin and no longer be evident on postoperative CT imaging.”16 However, endovascular intervention for these endoleaks with additional ballooning, aortic cuff placement, placement of a Palmaz stent (Cordis, Milpitas, CA), and/or the placement of endoanchors is suggested. No specific treatment strategy is recommended given the lack of evidence.16
The FDA’s approval of the Zenith Fenestrated AAA Endovascular Graft (Cook Medical, Bloomington, IN) in 2012 has allowed for treatment of juxtarenal AAA with fenestrated EVAR (FEVAR).17 This graft, designed for a patient’s specific anatomy, allows for the incorporation of up to three visceral target vessels via fenestrations and/or scallops. While this innovation has broadened the extent of aneurysm that can be treated via an endovascular approach, it has also introduced multiple new sites of potential endoleak. In addition to the type Ia, Ib, and III endoleaks possible after standard EVAR, the additional endoleaks possible following FEVAR include type Ic endoleak from unstented fenestrations or the distal end of a target vessel stent, and type IIIa endoleak from the overlap of the main fenestrated graft with target vessel covered stents or the bifurcated graft.10 The natural history and clinical significance of these completion endoleaks after FEVAR is poorly understood. Furthermore, repair of these endoleaks can be more technically complex than after standard EVAR. Therefore, we evaluated the rate, location, and natural history of completion type I and III endoleaks after FEVAR at our institution.
Methods
Patient Selection:
We performed a retrospective analysis of all patients undergoing FEVAR with the Zenith Fenestrated AAA Endovascular Graft (ZFEN; Cook Medical, Bloomington, IN) at Beth Israel Deaconess Medical Center. The ZFEN device was FDA-approved for commercial use in the United States in 2012 and the first case was performed at our institution in January 2013. The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for informed consent given the retrospective nature of the design.
Baseline patient demographics, comorbidities, and anatomical and procedural characteristics were obtained via chart review. Glomerular filtration rate (GFR) (mL/min/1.73m2) was calculated using the CKD-EPI formula.18 Mean, peak, and final ACT before reversal with protamine were obtained from the anesthesia record. Aortic neck calcification was determined by review of the preoperative computed tomography angiography (CTA) and classified as mild (scattered calcification), moderate (less than half of circumference of aortic wall), or extensive (greater than half of circumference of aortic wall). Neck length, neck diameter, alpha angle (neck to suprarenal aorta), and beta angle (neck to aneurysm sac), were calculated using center-line reformats of the preoperative CTA (iNtuition, TeraRecon, Foster City, CA). Scans were reviewed by JM (vascular surgery fellow) and NS (general surgery resident). Neck length was defined as the distance between the lowest renal artery and the location at which the aortic diameter increased >10% from the diameter at the lowest renal artery. Neck diameter was the diameter used to size the main body device. These measurements were used to determine if the procedure was performed according to the instructions for use (IFU) of the ZFEN device (neck length ≥ 4 mm, diameter 19 – 31 mm, alpha and beta angle ≤ 45°).
Completion endoleaks were determined via review of digital subtraction angiography (DSA) aortograms performed during the index procedure. All angiograms were reviewed by NS and MS, the attending surgeon on all cases. Intraoperative interventions for endoleaks were determined via review of the operative note. Patients were grouped by presence or absence of a type I or III endoleak on completion angiogram. Subsequently, all completion endoleaks were categorized by type and location. Endoleaks were classified based on SVS endoleak definitions (Table I).10 Exact location of the endoleak was difficult to distinguish for certain endoleaks and were classified accordingly. For instance, it is difficult to distinguish between type Ia endoleaks and type III endoleaks from the overlap of the fenestrated device and a renal stent. Therefore, these endoleaks were classified as type Ia or III.
Table I.
Endoleak classification*
| Endoleak type | Description |
|---|---|
| Type I | |
| a | Leak from the proximal seal zone |
| b | Leak from the distal seal zone |
| c | Leak from a fenestration, branch end point, or branch occluding plug/coil |
| Type II | Retrograde flow from a branch artery in the excluded segment (e.g. lumbar artery) |
| Type III | |
| a | Loss of apposition or disconnect between components |
| b | Fabric tear |
| Type IV | Flow through porous fabric |
| Type V | Sac expansion (indicating sac pressurization) without identifiable endoleak |
Adopted from Fillinger et al.10
Procedures:
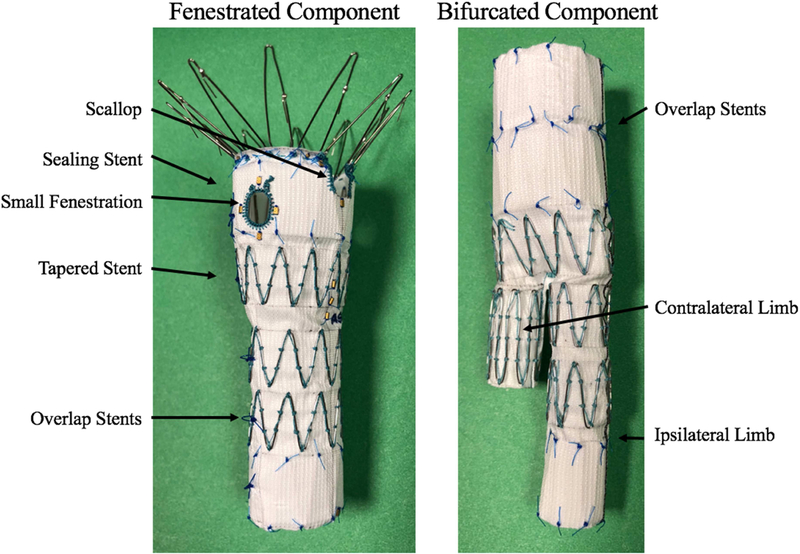
The ZFEN device is designed for each individual patient’s anatomy and is custom-made in Australia, requiring 6–8 weeks for manufacturing and shipping. It consists of a main fenestrated device, a bifurcated device, and associated iliac limbs (Figure 1). The fenestrated device allows for the incorporation of up to 3 visceral target vessels with small fenestrations, large fenestrations, or scallops (with no more of two of any type). For all patients, preoperative computed-topography angiograms (CTA) were analyzed using iNtuition software (TeraRecon). Target vessel clock-face location, relative height, and size were calculated and the ZFEN device was designed based on these measurements. We oversize the main fenestrated device 10 – 25% compared to aortic neck diameter.
Figure 1.
The Zenith Fenestrated AAA Endovascular Graft Proximal Body and Distal Bifurcated Component (Cook Medical, Bloomington, IN) with key components labeled.
All procedures were performed in a hybrid operating room using the Allura Xper fluoroscopy system (Philips Healthcare, Best, The Netherlands) with routine use of 3D image fusion.19 A percutaneous-first approach was used for bilateral groin access unless there was severe calcific atherosclerotic disease in which case groin cutdown and endarterectomy were performed. Ultrasound guidance and “pre-close” technique using two Perclose Proglide devices (Abbott Vascular, Abbott Park, IL) were used for all percutaneous femoral access. After bilateral femoral access was obtained, the main fenestrated graft was introduced. Prior to deployment, a single DSA aortogram was performed to confirm position of the 3D roadmap.
Following deployment of the fenestrated graft, a DrySeal sheath (Gore Medical, Flagstaff, AZ) was introduced via the contralateral groin and fenestration and target vessel cannulation was facilitated with a TourGuide Sheath (Medtronic, Minneapolis, MN). Once target vessel cannulation was complete, the proximal seal zone of the fenestrated graft was molded with a compliant balloon. Renal fenestrations were routinely stented with iCAST Balloon Expandable Covered Stents (Atrium, Hudson, NH). Following the deployment of the renal stent, the proximal end of the iCast stent was flared with an angioplasty balloon to create overlap and full apposition between the covered stent and the fenestrated graft. SMA fenestrations were stented selectively. Following placement of the fenestrated graft and stenting of target vessels, the bifurcated component and subsequent Spiral-Z AAA Iliac Leg Grafts (Cook Medical) were deployed. The distal seal zones as well as areas of graft overlap were then molded with a compliant balloon. Protamine was routinely given at the completion of the case.
Following implantation of all devices, a completion DSA aortogram was performed to assess main body, limb, and target vessel patency and to evaluate for endoleak. Small, slow, type I and III endoleaks, as well as all type II endoleaks, were routinely observed (Video 1). Type I and III endoleaks that were deemed to large or brisk to observe were addressed with further intervention at the index procedure, consisting primarily of remolding of seal/overlap zones or placement of additional iliac limbs. Recently, we have begun to perform completion cone-beam CT scans (CBCT) in the operating room at the conclusion of the procedure to evaluate for target vessel or limb stent kinking/collapse and to better evaluate endoleaks if necessary. Presence of a type I or III endoleak on completion DSA did not alter the postoperative care of the patient, including the decision to continue/resume antiplatelet therapy or systematic anticoagulation.
Patients with concern for a perioperative complication were evaluated with a CTA during their index hospitalization. Otherwise, patients underwent routine CTA 1-month postoperatively. For patients who were evaluated with a CTA during the index hospitalization, the 1-month scan was at times deferred at the discretion of the surgeon. Patients were subsequently evaluated at 6-months intervals with CTA and occasionally duplex ultrasound in patients with renal insufficiency combined with non-contrast CT, or if target vessel stenosis severity is not certain on CTA.
Outcomes and Statistical Analysis
The primary outcome was presence of a type I or type III endoleak on initial follow-up imaging, defined as the first CTA abdomen/pelvis obtained after leaving the operating room. Secondary outcomes included perioperative mortality, perioperative complication rate, late and recurrent type I or III endoleak, and sac status at one year. Perioperative mortality and complications were defined as occurring within 30-days of the procedure or during the index hospitalization. Complications included myocardial infarction (MI; EKG changes consistent with MI or troponin increase), respiratory failure (intubation > 48 hours or reintubation), stroke (TIA or permanent neurological deficit), mesenteric ischemia requiring intervention, acute kidney injury (AKI; injury or failure as defined by the RIFLE criteria20), and access site complication (thrombosis/dissection, hematoma requiring intervention, or wound infection). Late type I or III endoleak was defined as a type I or III endoleak identified during follow-up in a patient without a type I or III endoleak on previous imaging. Recurrent type I or III endoleak was defined as a late type I or III endoleak in a patient with a type I or III endoleak on completion angiogram. Sac status was classified as stable, regressing, or expanding (regressing: diameter ≥ 5 mm smaller than initial postoperative imaging; expanding: diameter ≥ 5 mm larger than initial postoperative imaging)
We compared baseline characteristics and perioperative outcomes between patients with and without completion endoleaks using chi-squared test for categorical variables and Student’s t-test or Mann-Whitney-U test as appropriate. We used Kaplan-Meier estimates to calculate one-year survival, sac regression, and freedom-from reintervention. We used log-rank test to compare survival, freedom from reintervention, and sac regression between patients with and without completion endoleaks. All analyses were performed with Stata 14.2 (StataCorp, College Station, TX).
Results
Patients and Procedures
From 2013 to 2018, 53 patients underwent FEVAR with the ZFEN device. One patient (2%) was excluded who was lost to follow-up after discharge. For the remaining 52 patients, mean age was 75 ± 8 years, 39 (75%) were male, and 42 (91%) were white (Table II).
Table II.
Baseline patient demographics
| Demographicab | Overall (n=52) |
Completion Endoleak (n=28) |
No Completion Endoleak (n=24) |
P |
|---|---|---|---|---|
| Age (yr) | 75.2 ± 8.2 | 75.4 ± 8.9 | 75.0 ± 7.5 | .57 |
| Male | 39 (75) | 19 (68) | 20 (83) | .20 |
| White Race | 42 (91) | 22 (92) | 20 (91) | .93 |
| BMI (kg/m2) | 27.2 ± 5.2 | 26.8 ± 5.3 | 27.8 ± 5.3 | .26 |
| Hypertension | 44 (85) | 25 (89) | 19 (79) | .31 |
| CAD | 15 (29) | 8 (29) | 7 (29) | .96 |
| CHF | 5 (10) | 4 (14) | 1 (4) | .22 |
| COPD | 12 (23) | 4 (14) | 8 (33) | .10 |
| Diabetes | 8 (15) | 4 (14) | 4 (17) | .81 |
| GFR(mL/min/1.73m2) | .51 | |||
| >60 | 35 (67) | 17 (61) | 18 (75) | |
| 30 – 60 | 15 (29) | 9 (32) | 6 (25) | |
| <30 | 1 (2) | 1 (4) | 0 (0) | |
| Dialysis-dependent | 1 (2) | 1 (4) | 0 (0) | |
| Smoking Status | .09 | |||
| Current Smoker | 14 (27) | 7 (25) | 7 (29) | |
| Former Smoker | 33 (63) | 16 (57) | 17 (71) |
Data presented as mean ± standard deviation for continuous data, number (%) for categorical data
BMI = body mass index, CAD = coronary artery disease, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, GFR = glomerular filtration rate
In total, 146 visceral arteries were targeted using 103 fenestrations and 43 scallops; 100 renal arteries and 46 superior mesenteric arteries were incorporated, with a mean of 2.8 target vessels per procedure. The most common device design was a scallop for the SMA and two small fenestrations for the bilateral renal arteries, used in 37 (71%) cases. One-hundred forty-five of the 146 planned target vessels (99%) were successfully targeted. The lone technical failure was the inadvertent cannulation and stenting of an accessory renal artery leading to coverage of the main right renal artery. This patient developed AKI (injury as defined by the RIFLE criteria), however the creatinine returned to baseline by 1-month postoperatively. On routine post-operative imaging, greater than 50% of the right renal parenchyma was perfused. There was no completion endoleak and through one-year follow-up, there has been no evidence of endoleak, either type I/III or type II from the covered main renal artery.
97 (98%) renal arteries and 5 (11%) superior mesenteric arteries were stented. All stented renal arteries were stented with covered-stents (96 iCAST [Atrium], 1 VIABAHN VBX [Gore Medical). For superior mesenteric arteries, one (20%) stent was covered and 4 (80%) were bare-metal (one iCAST [Atrium], two Express [Boston Scientific, Marlborough, MA], one Palmaz Blue [Cordis], one Xact [Abbott Vascular]).
Completion Endoleaks
After implantation of all devices, 31 patients (60%) had evidence of type I or III endoleak on DSA. Twelve patients (39%) underwent further intervention at the index procedure consisting of remolding of the seal/overlap zones with a compliant balloon alone (7, 58%), implantation of additional iliac limb(s) (4, 33%), or placement of an aortic cuff within the overlap zone of the fenestrated and bifurcated components (1, 8%). Following intervention, 3 endoleaks resolved completely. 28 patients (54%) had a type I or III endoleak on completion angiogram. The most common sites of completion type I/III endoleaks were type III from the overlap between the fenestrated and bifurcated components or bifurcated component and iliac limb (46%; exact location could not be determined) and type Ia or type III from the overlap of the fenestrated component and the renal artery covered stent (32%; exact location could not be determined) (Table III, Videos 1, 2).
Table III.
Location of completion type I and III endoleaks
| Location | N | % |
|---|---|---|
| la or main body and renala | 9 | 32 |
| Main body to bifurcate or bifurcate to limba | 13 | 46 |
| la or main body and renala AND main body and bifurcate | 1 | 4 |
| Bifurcate to limb | 2 | 7 |
| Ib | 2 | 7 |
| Ic from large unstented renal fenestration | 1 | 4 |
Exact location could not be determined
There were no differences in patient demographics or baseline comorbidities between patients with and without completion endoleaks (Table I). Similarly, there were no differences in graft design including fenestrated body device diameter, number of target vessels, renal stent diameter, and proportion of patients performed on IFU. During the procedure, there were no differences in mean ACT, peak ACT, or final ACT between patients with and without completion endoleaks. Finally, there were no anatomic differences between groups, specifically extensive neck calcification, neck length, neck diameter, aortic alpha and beta angle, and degree of oversizing (Table IV). A CTA was obtained during the index hospitalization for 4 patients (15%) with a completion endoleak and 7 patients (29%) without a completion endoleak (P = .24).
Table IV.
Procedural characteristics
| Characteristica | Overall (n=52) |
Completion Endoleak (n=28) |
No Completion Endoleak (n=24) |
P |
|---|---|---|---|---|
| Graft Design | ||||
| Fenestrated graft diameter (mm) | 29.9 ± 3.3 | 30.0 ± 3.4 | 29.7 ± 3.3 | .72 |
| Number of target vessels | 3 (3–3) | 3 (3–3) | 3 [3–3] | .54 |
| Right renal stent diameter (mm) | 6.2 ± 0.6 | 6.1 ± 0.5 | 6.3 ± 0.6 | .24 |
| Left renal stent diameter (mm) | 6.2 ± 0.5 | 6.2 ± 0.5 | 6.3 ± 0.5 | .39 |
| Degree of oversizing (%) | 18 ± 8 | 18 ± 9 | 18 ± 6 | .88 |
| On Instructions for Use | 32 (63) | 16 (58) | 16 (70) | .36 |
| Right distal landing zone | .60 | |||
| Common iliac artery | 43 (83) | 22 (79) | 21 (88) | |
| External iliac artery | 7 (13) | 5 (18) | 2 (8) | |
| Aorta | 2 (4) | 1 (4) | 1 (4) | |
| Left distal landing zone | .85 | |||
| Common iliac artery | 44 (85) | 24 (86) | 20 (83) | |
| External iliac artery | 6 (12) | 3 (11) | 3 (13) | |
| Aorta | 2 (4) | 1 (4) | 1 (4) | |
| Anatomy | ||||
| Neck length (mm) | 10 ± 8 | 9 ± 7 | 11 ± 8 | .34 |
| Neck diameter (mm) | 25 ± 3 | 26 ± 3 | 25 ± 3 | .57 |
| Alpha angle (degrees) | 21 ± 12 | 23 ± 12 | 19 ± 12 | .29 |
| Beta angle (degrees) | 30 ± 15 | 30 ± 14 | 31 ± 17 | .84 |
| Extensive Neck Calcification | 11 (23) | 5 (23) | 6 (23) | .98 |
| Maximum Aneurysm Diameter (mm) | 59 ± 10 | 60 ± 11 | 58 ± 9 | .58 |
| Procedural Anticoagulation | ||||
| Mean ACT (sec) | 248 ± 22 | 249 ± 25 | 248 ± 18 | .95 |
| Peak ACT (sec) | 279 [261–288] | 280 [271–297] | 273 [261–288] | .24 |
| Final ACT (sec) | 263 [233 – 277] | 258 [229 – 279] | 258 [233 – 274] | .73 |
Data presented as mean ± standard deviation for continuous data or median [interquartile range], number (%) for categorical data
Outcomes
Of the 28 completion endoleaks, 27 (96%) had resolved on initial postoperative imaging. One patient with a completion type Ia/III endoleak had a persistent endoleak, discovered on the initial postoperative CTA on postoperative day 27. The patient also had a lumbar type II endoleak on the initial postoperative scan just proximal to the aortic bifurcation. The patient underwent continued observation. On the 6-month CTA, this type Ia/III endoleak had resolved completely. The patient’s lumbar type II endoleak peristed. The maximum aortic diameter decreased to 57 mm from 61 mm on the initial postoperative CTA. Of the patients without completion endoleaks, one patient had a type Ia endoleak secondary to infolding of the proximal graft discovered on initial follow-up imaging, and subsequently underwent reintervention with placement of endoachors in the proximal seal zone and a Palmaz stent (Cordis) below the renal artery fenestrations, with subsequent resolution.
There were no differences in perioperative mortality or complications between the two groups (Table V). Overall, there was one perioperative death (1.9%) and 10 (19%) patients experienced at least one complication.
Table V.
Perioperative outcomes
| Characteristica | Overall (n=52) |
Completion Endoleak (n=28) |
No Completion Endoleak (n=24) |
P |
|---|---|---|---|---|
| Follow-up Type I/III Endoleak | 2 (4) | 1 (4) | 1 (4) | .89 |
| Perioperative Mortalityb | 1 (2) | 0 (0) | 1 (4) | .27 |
| Any Complication | 10 (19) | 5 (18) | 5 (21) | .79 |
| Any AKIc | 9 (18) | 5 (19) | 4 (17) | .86 |
| Risk | 7 (14) | 5 (19) | 2 (9) | .32 |
| Injury | 1 (2) | 0 (0) | 1 (4) | .27 |
| Failure | 1 (2) | 0 (0) | 1 (4) | .28 |
| Bowel Ischemia | 2 (4) | 1 (4) | 1 (4) | .91 |
| Myocardial Infarction | 1 (2) | 0 (0) | 1 (4) | .28 |
| Respiratory Failure | 1 (2) | 0 (0) | 1 (4) | .28 |
| Stroke | 0 (0) | 0 (0) | 0 (0) | |
| Access Site Complication | 4 (8) | 2 (7) | 2 (8) | .87 |
Data presented as number (%)
Death occurring within 30-days of the index procedure or during the index hospitalization
AKI = Acute kidney injury; defined by the RIFLE Criteria
Over the course of median follow-up of 269 days (range: 5–1922 days), no late or recurrent type I or type III endoleaks were identified in either group. At one year, 47% of patients with completion endoleaks and 35% of patients without completion endoleaks had sac regression (P = .27; n at risk: 10 patients with completion endoleaks, 9 patients without completion endoleaks). Over the course of the study, 8 patients (15%) underwent reinterventions. Freedom-from reintervention at one-year was 91% in patients with completion endoleaks and 85% in patients without completion endoleaks (P = .65; n at risk: 13 patients with completion endoleaks, 12 patients without completion endoleaks. There was no sac expansion at one year. One patient with a completion type Ic endoleak (from an unstented large right renal fenestration) had sac expansion noted 3 years postoperatively. There was no evidence of recurrent type I or III endoleak on CTA or aortogram. This patient ultimately underwent transcaval sac embolization for a type II endoleak. There have been no other procedures for sac expansion or endoleak in either group.
Discussion
In this retrospective analysis of all patients undergoing FEVAR with the ZFEN device at our institution, we found that 54% of patients left the operating room with a type I or III endoleak on completion angiogram. Most of these endoleaks arose from two sites. The most common completion endoleak was a type III endoleak arising from either the junction of the fenestrated and bifurcated grafts or bifurcated graft and iliac limb – the exact location could not to be determined. The next most common location was proximal, either a type Ia endoleak or a type III endoleak from the junction of the fenestrated graft and renal covered stent. Importantly, nearly all the completion type I or III endoleaks (27/28, 96%) had resolved by the first postoperative CTA. One patient had a persistent type Ia/III endoleak on routine postoperative imaging, discovered on postoperative day 27, which resolved by the 6-month CTA. We did not identify any demographic, anatomic, or procedural characteristics associated with presence of completion type I or III endoleak.
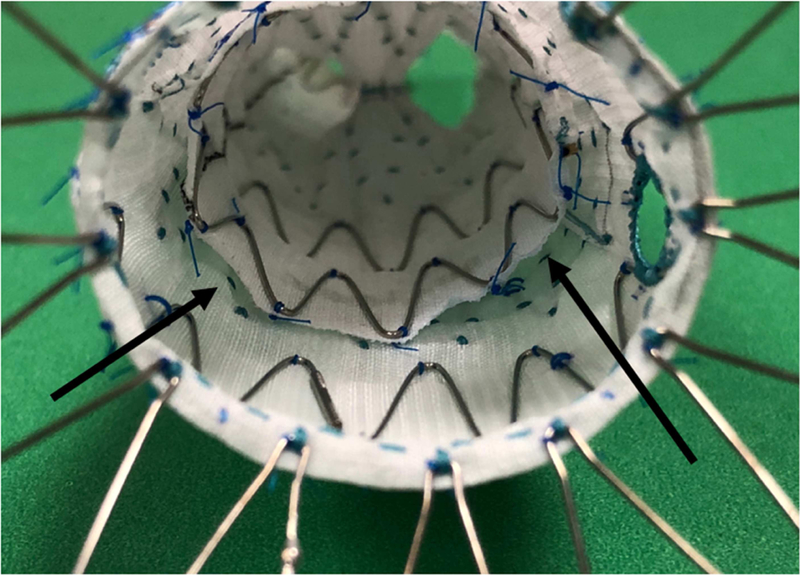
The 54% rate of completion endoleak we observed is substantially higher than reports of completion endoleaks from standard EVAR, which indicate that between 3 and 16 % of patients will leave the operating room with evidence of a type I or III endoleak11–13,15 There are two primary factors which likely contribute to this difference. First, most completion endoleaks occurring in these patients arose from graft features unique to FEVAR with the ZFEN device. The junctions of the renal artery covered stents and fenestrated graft and of the fenestrated graft to bifurcated graft are potential sources of endoleak not present during standard EVAR. While we could not determine definitively between endoleaks arising from the overlap between the fenestrated and bifurcated components and the bifurcated component and iliac limb, because we rarely see type III leaks at the junction of the Zenith main body and iliac limb during standard EVAR, we assume that most of these endoleaks arise between the proximal main body and the bifurcate. We suspect that some of these type III leaks are facilitated by landing the proximal edge of the 24mm bifurcated piece into the larger diameter of the tapered stent connecting the proximal sealing stents to the 24mm overlap stents of the fenestrated component (Figure 2). Based on this we have altered our planning process to avoid graft configurations that lead to extension of the bifurcated component into the tapered stent of the proximal component. We have not been focusing on this technical aspect of the procedure for long enough to evaluate its efficacy. Additionally, the use of a scallop or unstented fenestration may make the proximal seal zone following FEVAR more prone to completion type I endoleaks than standard EVAR.
Figure 2.
The Zenith Fenestrated AAA Endovascular Graft (Cook Medical, Bloomington, IN) with the distal bifurcated component deployed within the proximal fenestrated component, shown looking down the graft from the proximal edge. The arrows demonstrate the gaps that forms when the bifurcated component is deployed within the tapered stent of the proximal component, a potential source of type III endoleak.
Secondly, as our experience with EVAR, and more specifically FEVAR, has grown, we have observed the high rate with which these completion endoleaks resolve and have become less aggressive in treating them during the index procedure.
Prior reports of completion endoleaks following FEVAR with the ZFEN device are more limited. In their analysis of results of the ZFEN U.S. pivotal trial, Greenberg et al. report that 12 of 30 patients had evidence of type I or III endoleak on procedural angiography. Eleven of these patients underwent treatment of these endoleaks at the index procedure, with one type III completion endoleak observed.21 In their analysis of a single-center experience, Kristmudsson et al. report that 13 of 54 patients had evidence of type I or III endoleak on procedural angiography. All these endoleaks were treated with placement of a Palmaz stent and 10 resolved.22 Comparatively, we only intervened in 12 of the 31 cases with evidence of type I or III endoleak after implantation of all devices and only 3 endoleaks resolved completely during the procedure. Our method of intervention also differed from these reports, consisting primarily of repeat angioplasty alone. There are likely multiple factors contributing to this difference in practice pattern. However, one important consideration is that the ZFEN U.S. Investigational Device Exemption trial defined technical success based on the SVS reporting standards for endovascular aortic aneurysm repair, which define completion type I and III endoleaks as a technical failure, likely contributing to the high rate of intervention for these endoleaks.9,21
While 28 patients in our series left the operating room with evidence of a type I or III endoleak, all but one of these endoleaks had resolved completely prior to the first postoperative CTA, and all resolved within the first 6-months, demonstrating that properly selected type I and III endoleaks at the completion of FEVAR with the ZFEN device can be safely observed in the short-term. Continued research is necessary to evaluate if there are any long-term consequences of these postoperative endoleaks, including recurrent endoleak or changes in sac status. We consider “properly selected” endoleaks to be those that are small and slow to develop, as demonstrated in Video 1 and Video 2. Large, brisk completion endoleaks were intervened upon, and though only 3 resolved completely, all demonstrated subjective improvement after intervention. Therefore, we do continue to advocate for treatment of these endoleaks at the index operation. Unfortunately, the determination of endoleak severity is currently completely subjective, as there is no technique to quantify endoleak on DSA. Further work should be aimed at developing techniques for intraoperative endoleak quantification based on DSA. Another potential adjunct in the evaluation of completion endoleaks is on table CBCT. Our and others’ experience with completion CBCT following FEVAR with physician-modified and custom grafts has demonstrated that CBCT can be a powerful tool in assessment of technical result, especially in the evaluation of branch vessel stents.23 CBCT can also be used evaluate large completion endoleaks, especially if the origin is unclear.
The high spontaneous resolution rate of completion type I and III endoleaks observed in this study is consistent with a growing number of publications from standard EVAR. In their review of a large, single-center series, O’Donnell et al. report that 63% of type Ia endoleaks present at the completion of standard EVAR resolved by one-month follow-up. Furthermore, presence of a completion type Ia endoleak was not associated with higher long-term mortality, though it was associated with a higher odds of reintervention.12 Similarly, Millen et al. found that 31 of 33 completion type Ia endoleaks resolved spontaneously and Kim et al. found that 7 of 10 type Ia endoleaks after EVAR resolved spontaneously.11,15 Bastos Gonçalves et al. reported that 8 of 15 completion type Ia endoleaks after EVAR resolved spontaneously one-week postoperatively and all resolved by one year. However, it is important to note that one patient with a persistent type Ia endoleak died of a ruptured aneurysm during the first postoperative year.13 While the rate of spontaneous resolution is high in these cohorts, these are only endoleaks that have been selected by the surgeon as being safe to observe.
This report of the spontaneous resolution of selected completion type I and III endoleaks following FEVAR with the ZFEN device by no means intends to minimize the significance of these endoleaks when present on follow-up imaging. In our series, one patient without a completion endoleak was found to have a large type Ia endoleak at one-month follow-up secondary to posterior infolding of the proximal graft. This patient was promptly treated with placement of endoanchors anterolaterally and a Palmaz stent. The single patient in the completion endoleak group with a persistent type Ia/III endoleak one-month postoperatively underwent observation and the endoleak resolved by the subsequent CTA at 6-month follow-up. While no additional type I or III endoleaks have been discovered during the duration of follow-up in our series, late type I and III endoleaks after FEVAR with ZFEN and other devices are not uncommon and can occur at any time postoperatively.17,24–27 These late type I and III endoleaks are particularly concerning, as the aneurysm sac has not been exposed to aortic pressure since the index procedure and likely weakens over time. Persistent and late type I and III endoleaks after EVAR have been associated with higher rates of reintervention, aneurysm rupture, and overall mortality.7,8,12 Therefore, consistent, long-term follow-up with evaluation of endoleak, sac expansion, and target vessel patency is crucial in these patients and the decision to observe persistent or late type I or III endoleaks should not be taken lightly.
The results of this study must be interpreted in the context of its design. This is the experience of a single, high-volume center and therefore the results may not be generalizable. Additionally, the decision to intervene on completion endoleaks as well as their retrospective classification for this study were based on the surgeon’s subjective interpretation of their appearance and severity. There are ongoing efforts to better the classify the type and severity of endoleaks in the follow-up period, however, to our knowledge, no technique has been described to quantify the severity of an endoleak intraoperatively.28,29 As the complexity of endovascular aortic interventions continues to grow, research and development should be aimed and improving evaluation of endoleaks intraoperatively, specifically classifying severity and origin. These advances could aid in the decision to intervene, guide the type or intervention, allow for quantification of improvement after intervention, and ultimately be used to determine cutoffs for endoleaks that can be safely observed. Finally, long-term follow-up is limited in this cohort. Continued follow-up is needed to evaluate the risk of endoleak recurrence patients with completion endoleaks after FEVAR.
Conclusions:
While completion type I or III endoleaks are common following FEVAR with the ZFEN device, nearly all of these endoleaks resolve spontaneously by the initial postoperative imaging. These results suggest that select completion type I or III endoleaks following FEVAR with the ZFEN device do not require intervention at the index procedure. However, consistent, long-term follow-up with imaging to evaluate for endoleak and sac expansion remains critical in this patient population.
Supplementary Material
Video 1: Type Ia or type III endoleak. Completion digital subtraction angiography (DSA) following a fenestrated EVAR with the Zenith AAA Fenestrated Endovascular Graph (Cook Medical, Bloomington, IN) designed with a single left renal artery fenestration. This DSA demonstrates presence of a completion endoleak, either a type Ia endoleak or a type III endoleak from the junction of the fenestrated component and left renal artery covered stent, that was observed. This patient underwent CTA on postoperative day 30 by which time this endoleak had resolved.
Video 2: Type III endoleak. Completion digital subtraction angiography (DSA) following a fenestrated EVAR with the Zenith AAA Fenestrated Endovascular Graph (Cook Medical, Bloomington, IN) designed with a scallop for the superior mesenteric artery and bilateral renal artery fenestrations. This DSA demonstrates presence of a completion type III endoleak from the junction of the fenestrated component and the bifurcated component that was observed. This patient underwent CTA on postoperative day 40 by which time this endoleak had resolved.
Acknowledgments
TO and PL are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734–22
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To be presented as an oral presentation at the 2018 New England Society for Vascular Surgery Annual Meeting in Cape Neddick, ME, October 12–14, 2018
Type of Research: Retrospective cohort study.
Key Findings: Fenestrated endovascular aortic repair in 52 patients resulted in type I or III endoleaks on completion angiograms in 28 patients (54%), in 27 (96%) they resolved spontaneously.
Take Home Message: This study suggests that following FEVAR, most type I and III endoleaks resolve spontaneously.
References
- 1.Parodi JC, Palmaz JC, Barone HD. Transfemoral Intraluminal Graft Implantation for Abdominal Aortic Aneurysms. Ann Vasc Surg. 1991. November;5(6):491–9. [DOI] [PubMed] [Google Scholar]
- 2.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009;49(3):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dua A, Kuy S, Lee CJ, Upchurch GR, Desai SS. Epidemiology of aortic aneurysm repair in the United States from 2000 to 2010. J Vasc Surg. 2014. June 1;59(6):1512–7. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366–74. [DOI] [PubMed] [Google Scholar]
- 5.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015. July 23;373(4):328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang RW, Goodney P, Tucker LY, Okuhn S, Hua H, Rhoades A, et al. Ten-year results of endovascular abdominal aortic aneurysm repair from a large multicenter registry. J Vasc Surg. 2013;58(2):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Marrewijk C, Buth J, Harris PL, Norgren L, Nevelsteen A, Wyatt MG. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: The EUROSTAR experience. J Vasc Surg. 2002. March 1;35(3):461–73. [DOI] [PubMed] [Google Scholar]
- 8.Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, Van Marrewijk C, Laheij RJF. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: The EUROSTAR experience. J Vasc Surg. 2000;32(4):739–49. [DOI] [PubMed] [Google Scholar]
- 9.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002. May 1;35(5):1048–60. [DOI] [PubMed] [Google Scholar]
- 10.Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. 2010;52(4):1033.e15–1033. [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Ra H Do, Min S-I, Jae HJ, Ha J, Min S-K. Clinical significance of type I endoleak on completion angiography. Ann Surg Treat Res. 2014;86(2):95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donnell TFX, Corey MR, Deery SE, Tsougranis G, Maruthi R, Clouse WD, et al. Select early type IA endoleaks after endovascular aneurysm repair will resolve without secondary intervention. J Vasc Surg. 2018. January 1;67(1):119–25. [DOI] [PubMed] [Google Scholar]
- 13.Bastos Gonçalves F, Verhagen HJM, Vasanthananthan K, Zandvoort HJA, Moll FL, Van Herwaarden JA. Spontaneous delayed sealing in selected patients with a primary type-ia endoleak after endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2014;48(1):53–9. [DOI] [PubMed] [Google Scholar]
- 14.Tan TW, Eslami M, Rybin D, Doros G, Zhang WW, Farber A. Outcomes of patients with type i endoleak at completion of endovascular abdominal aneurysm repair. J Vasc Surg. 2016;63(6):1420–7. [DOI] [PubMed] [Google Scholar]
- 15.Millen AM, Osman K, Antoniou GA, McWilliams RG, Brennan JA, Fisher RK. Outcomes of persistent intraoperative type Ia endoleak after standard endovascular aneurysm repair. J Vasc Surg. 2015;61(5):1185–91. [DOI] [PubMed] [Google Scholar]
- 16.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018. January 1;67(1):2–77.e2. [DOI] [PubMed] [Google Scholar]
- 17.Oderich GS, Greenberg RK, Farber M, Lyden S, Sanchez L, Fairman R, et al. Results of the United States multicenter prospective study evaluating the Zenith fenestrated endovascular graft for treatment of juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2014;60(6):1420–1428.e5. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009. May 5;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stangenberg L, Shuja F, Carelsen B, Elenbaas T, Wyers MC, Schermerhorn ML. A novel tool for three-dimensional roadmapping reduces radiation exposure and contrast agent dose in complex endovascular interventions. J Vasc Surg. 2015. August 1;62(2):448–55. [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004. August;8(4):R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg RK, Sternbergh WC, Makaroun M, Ohki T, Chuter T, Bharadwaj P, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009;50(4):730–737.e1. [DOI] [PubMed] [Google Scholar]
- 22.Kristmundsson T, Sonesson B, Malina M, Björses K, Dias N, Resch T. Fenestrated endovascular repair for juxtarenal aortic pathology. J Vasc Surg. 2009;49(3):568–75. [DOI] [PubMed] [Google Scholar]
- 23.Tenorio ER, Mirza AK, Kärkkäinen JM, Oderich GS. Lessons learned and learning curve of fenestrated and branched endografts. J Cardiovasc Surg (Torino). 2018. September 12;In press. [DOI] [PubMed] [Google Scholar]
- 24.Wang SK, Gutwein AR, Gupta AK, Lemmon GW, Sawchuk AP, Motaganahalli RL, et al. Institutional experience with the Zenith Fenestrated aortic stent graft. J Vasc Surg. 2018. February;in press. [DOI] [PubMed] [Google Scholar]
- 25.Starnes BW, Heneghan RE, Tatum B. Midterm results from a physician-sponsored investigational device exemption clinical trial evaluating physician-modified endovascular grafts for the treatment of juxtarenal aortic aneurysms. J Vasc Surg. 2017;65(2):294–302. [DOI] [PubMed] [Google Scholar]
- 26.Oderich GS, Ribeiro M, Hofer J, Wigham J, Cha S, Chini J, et al. Prospective, nonrandomized study to evaluate endovascular repair of pararenal and thoracoabdominal aortic aneurysms using fenestrated-branched endografts based on supraceliac sealing zones. J Vasc Surg. 2017;65(5):1249–1259.e10. [DOI] [PubMed] [Google Scholar]
- 27.Roy IN, Millen AM, Jones SM, Vallabhaneni SR, Scurr JRH, McWilliams RG, et al. Long-term follow-up of fenestrated endovascular repair for juxtarenal aortic aneurysm. Br J Surg. 2017. July 1;104(8):1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommer WH, Becker CR, Haack M, Rubin GD, Weidenhagen R, Schwarz F, et al. Time-resolved CT Angiography for the Detection and Classification of Endoleaks. Radiology. 2012;263(3):917–26. [DOI] [PubMed] [Google Scholar]
- 29.Demehri S, Signorelli J, Kumamaru KK, Wake N, George E, Hanley M, et al. Volumetric Quantification of Type II Endoleaks: An Indicator for Aneurysm Sac Growth Following Endovascular Abdominal Aortic Aneurysm Repair. Radiology. 2014;271(1):282–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Type Ia or type III endoleak. Completion digital subtraction angiography (DSA) following a fenestrated EVAR with the Zenith AAA Fenestrated Endovascular Graph (Cook Medical, Bloomington, IN) designed with a single left renal artery fenestration. This DSA demonstrates presence of a completion endoleak, either a type Ia endoleak or a type III endoleak from the junction of the fenestrated component and left renal artery covered stent, that was observed. This patient underwent CTA on postoperative day 30 by which time this endoleak had resolved.
Video 2: Type III endoleak. Completion digital subtraction angiography (DSA) following a fenestrated EVAR with the Zenith AAA Fenestrated Endovascular Graph (Cook Medical, Bloomington, IN) designed with a scallop for the superior mesenteric artery and bilateral renal artery fenestrations. This DSA demonstrates presence of a completion type III endoleak from the junction of the fenestrated component and the bifurcated component that was observed. This patient underwent CTA on postoperative day 40 by which time this endoleak had resolved.