Abstract
Invasive fungal diseases (IFD) are an important cause of morbidity and mortality in premature neonates and immunocompromised pediatric patients. Their diagnostic and therapeutic management remains a challenge. A nationwide survey was conducted among thirteen of the largest pediatric units in the UK, to obtain insight in the current management of IFD in neonates and children. All responding centers were tertiary teaching centers. The use of fungal diagnostic tools and imaging modalities varied among centers. Antifungal prophylaxis was prescribed in most centers for extreme-low birth weight infants and high risk hemato-oncologic patients, but with a huge variety in antifungals given. An empirical treatment was favored by most centers in case of febrile neutropenia. First line therapy for candidemia consists of either fluconazole or liposomal amphotericin B, with voriconazole being first line therapy for invasive aspergillosis. Disseminated invasive aspergillosis was most often mentioned as a reason to prescribe combination antifungal therapy. In conclusion, this survey reinforces the fact that there are still important aspects in the management of pediatric IFD which should ideally be addressed in pediatric clinical trials. Attention needs to be given the knowledge gaps as observed in the results of our survey to optimize the management of IFD in children and neonates.
Keywords: invasive fungal disease, invasive aspergillosis, candidemia, antifungal therapy, fungal diagnostics
Introduction
There are a number of challenges faced in the management of invasive fungal diseases (IFD) in neonates and children: a unique fungal and clinical epidemiology, the unspecific clinical presentation, the poorer performance of fungal diagnostic tests, limited pharmacokinetic data of the available antifungals with a consequent lack of dosing recommendations, and a lack of pediatric randomized control trials. The difficulties in diagnosing IFD, which are characterized by a high case-fatality rate, has resulted in an overuse of costly antifungals in high risk patient populations (1). Overuse of antifungal agents will favor antifungal resistance development and increase unwanted toxicity.
Despite international consensus guidelines about the management of IFD in neonates and children, there is still significant variability in the management of IFD and the prescribing practices vary across institutions and geographic areas (4–7). In the UK, with the exception of the guideline for the management of IFD in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation, there is a lack of national consensus guidelines addressing the management of IFD in neonates and children. We conducted a nationwide survey to be informed about the current clinical management of IFD in the UK.
Methods
A web-based survey was developed in REDCap™ to collect data from participating sites. Fifteen centers within the UK were invited to participate in April 2017. Those centers were selected due to the volume and complexity of the pediatric services, including the care and management for premature neonates and immunocompromised children. The survey was addressed to either pediatric infectious diseases clinicians or the pediatric oncologist.
The main topics addressed in the survey related to hospital characteristics including access to diagnostic facilities; use of guidelines; use of diagnostic modalities; antifungal prophylaxis; use of TDM; the management of febrile neutropenia in high risk patients; and the management of pediatric patients with invasive candidiasis (IC) and aspergillosis (IA).
The following definitions were used: high risk hemato-oncology patients were defined as those with relapsed refractory acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), severe aplastic anemia, myelodysplastic syndrome or those undergoing allogenic hematopoietic stem cell transplant (HSCT). Empiric treatment was defined when antifungals were prescribed in patients with prolonged febrile neutropenia or aspecific clinical signs only suggestive of IFD (fever driven), while pre-emptive therapy was defined as antifungal treatment started based on a positive microbiological and/or imaging abnormality suggestive of IFD (diagnostic driven).
Results
Hospital characteristics
The access to the survey opened in April 2017 and was closed in July 2018. The survey was completed by clinicians from 13 teaching hospitals in the UK (response rate 87%): St. George´s Hospital, the Royal Marsden Hospital, Great Ormond Street Hospital, St. Mary´s Hospital and Evelinas Children Hospital in London, the Children´s Hospital in Oxford, Southampton Children´s Hospital, Bristol Royal Hospital for Children, Alder Hey Children´s Hospital Liverpool, Great North Children´s Hospital Newcastle, Royal Manchester Children´s Hospital, Leeds Children’s Hospital and Birmingham Children´s Hospital. Nine of those are stand-alone pediatric hospitals, three function as integrated pediatric departments within the hospital, and one is a designated specialist oncology hospital with a pediatric unit. In terms of the services provided, twelve centers have a pediatric intensive care unit (PICU), eleven a neonatal intensive care unit (NICU) and ten have a pediatric surgical unit. Twelve hospitals care for pediatric hemato-oncology patients with eight having a HSCT unit and five providing a solid organ transplant service. Each of the 13 hospitals has a Microbiology service on site, with only seven harboring a dedicated Mycology section. All the centers do have access to a Referral Mycology Laboratory if required. All the 13 hospitals have access to a Radiology department with CT and MRI imaging services.
Use of guidelines
Of the 13 responding hospitals, 10 (77%) answered to have own local guidelines for the management of IFD. Particular attention to neonates and children was given in 8 local guidelines, with 2 local guidelines addressing neonates in particular. Nine out of 13 hospitals (69%) indicated that they used one of more international guidelines for the management of IFD in neonates and children. Six (66%) centers used the IDSA 2016 Practice Guidelines for the Diagnosis and Management of Aspergillosis (8) and the IDSA 2016 Practice Guideline for the Diagnosis and Management of Candidiasis (9). Five (56%) centers each reported the use of the ESCMID Guideline for the Diagnosis and Management of Candida diseases 2012; prevention and management of invasive infections in neonates and children caused by Candida spp. (10) and the ECIL-4 Guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in pediatric patients with cancer or allogeneic hemopoietic stem-cell transplantation (11). Two (22%) centers reported the use of the IDSA 2011 Clinical Practice Guideline for the use of antimicrobial agent in neutropenic patients with cancer (12).
Management of fever and neutropenia
Three clinical scenarios were presented to obtain insight in the management strategy of high risk patients with neutropenia and persistent fever (> 96 hrs) despite antibacterial therapy. The majority of the centers reported to follow an empiric management strategy in the 3 clinical scenarios presented (table 1). Although, the 2 management strategies were not mutually exclusive as a number of centers responded that both strategies were used. Irrespective of the management strategy, all the centers reported liposomal amphotericin B as being the antifungal to be prescribed in the clinical scenarios given. One center, using exclusively a pre-emptive management strategy, adds voriconazole to the liposomal amphotericin B. Another center reported that this choice of antifungal is made per individual patient with micafungin as an alternative to liposomal amphotericin B.
Table 1. Choices of management strategies for persistent febrile neutropenia in various high-risk patient groups, presented in 3 clinical scenarios to the participants of 13 children’s hospitals in the UK.
| Clinical scenarios | Empiric strategy | Pre-emptive strategy |
|---|---|---|
| Allogenous HSCT recipient | 85% (11/13) | 54% (7/13) |
| Acute leukemia (i.e. high risk ALL, AML) | 82% (9/11) | 40% (4/10) |
| SAA/MDS | 82% (9/11) | 40% (4/10) |
HSCT: Hematopoietic Stem Cell Transplantation, ALL: Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia, SSA: sever aplastic anemia, MDS: myelodysplastic syndrome.
In case of persistent fever in high-risk neutropenic hemato-oncology patients, all centers responded that a chest X-ray is part of the standard diagnostic work-up, with 10/13 (77%) also including a high resolution CT-chest. Three centers would only perform a high resolution CT-chest when abnormalities are observed on the chest X-ray. A CT-sinuses is part of the standard diagnostic work-up in only 1 center, the other centers would only perform a CT-sinuses if clinical symptoms are present. Abdominal ultrasound (US) is performed routinely in 9 out of the 13 (69%) centers, whereas in the remaining 4 only if clinical symptoms. One center responded that a fundoscopy is performed as part of the standard diagnostic work-up.
Fungal biomarkers
Serum galactomannan testing was reported to be mainly used as a diagnostic tool in both high-risk neutropenic patients as well as non-hematology patients (table 2). Only 2 centers use serum galactomannan testing as a screening tool. β-D-glucan serum testing is used as a diagnostic tool in roughly half of the centers, with only 3 and 1 centers using the test as a screening tool in high-risk neutropenic patients and non-hematologic patients.
Table 2. The use and purpose of galactomannan and β-D-glucan testing in serum in different patient populations from 13 children’s hospitals in the UK.
| Biomarker | Rational | High risk neutropenic hemato-oncology patients | Non-hematologic patients |
|---|---|---|---|
| GMN | Screening | 2/13 (15%) | 1/13 (8%) |
| Diagnostic | 10/13 (77%) | 9/13 (69%) | |
| BDG | Screening | 3/12 (25%) | 1/12 (8%) |
| Diagnostic | 7/12 (58%) | 6/12 (50%) |
GM: Galactomannan, BDG: β-D-Glucan
Antifungal Prophylaxis
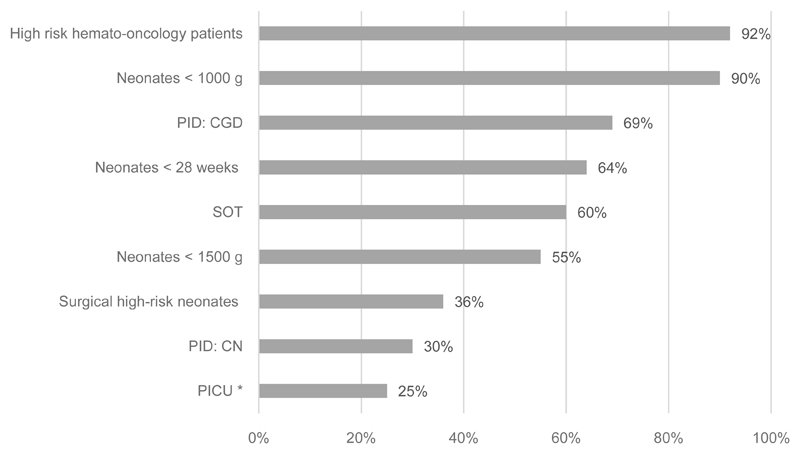
Antifungal prophylaxis is prescribed by nearly all hospitals (12/13) for high risk hemato-oncology patients with a huge variation in choice of antifungals. Four centers reported to prescribe different antifungals for prophylactic use. Liposomal amphotericin B (50%), itraconazole (42%), posaconazole (25%) and voriconazole (17%) are the most common antifungals to be prescribed for prophylaxis. For very low birth weight (VLBW) neonates, extreme LBW (ELBW) neonates and premature neonates < 28 weeks of gestation, antifungal prophylaxis is prescribed in 55%, 90% and 64% of the hospitals, respectively. Either fluconazole (70%) or nystatin (30%) was reported to be the antifungal of choice for those neonates. Itraconazole was the antifungal of choice for children with chronic granulomatous disease (response from 9 centers), with one center reporting that or itraconazole or voriconazole or posaconazole were used. Of the 5 hospitals performing solid organ transplantation in children, 60% reported to prescribe antifungal prophylaxis. Most centers (over 65%) responded that antifungal prophylaxis is not prescribed to children admitted to the PICU, or children with congenital neutropenia or neonates undergoing surgery.
Management of Candidemia
First line antifungal therapy for candidemia showed a similar pattern in the presented clinical scenarios for children being neutropenic, or admitted to the NICU or PICU (table 3). More than half of the centers responded to prescribe fluconazole in these patient groups, while the others choose to prescribe liposomal amphotericin B. Fluconazole was the preferred first line antifungal in non-neutropenic non-critically ill pediatric patients (75%). Eight out of 9 centers responded to treat for 2 weeks after the first negative blood culture obtained. In 2 centers the CVC is removed as part of the treatment; another 2 will remove the CVC when there are two positive blood cultures besides being the patient on adequate antifungal cover; with 9 centers indicating that CVC removal will dependent on the clinical condition. Additional investigations to exclude dissemination to other organs are performed in all centers responding, with an abdominal ultrasound, fundoscopy, echocardiogram and Doppler-ultrasound for signs of thrombosis performed in 100%, 92%, 77% and 46%, respectively.
Table 3. Antifungal treatment for candidemia and invasive pulmonary aspergillosis in 13 children’s hospitals in the UK.
| First line drug | ||||
|---|---|---|---|---|
| Fluconazole | Liposomal amphotericin B | Echinocandins | ||
| Candidemia | NICU (n=10) | 6 (60%) | 4 (40%) | - |
| PICU (n=10) | 6 (60%) | 3 (30%) | 1 (10%) | |
| Neutropenic patients (n=11) | 5 (45%) | 6 (55%) | - | |
| Non-neutropenic patients* (n=8) | 6 (75%) | 1 (12.5%) | 1 (12.5%) | |
| Voriconazole | Liposomal amphotericin B | Both | ||
| Invasive pulmonary aspergillosis | Patients < 2 years of age (n=11) | 7 (64%) | 3 (27%) | 1 (9%) |
| Patients > 2 years of age (n=11) | 9 (82%) | 1 (9%) | 1 (9%) | |
NICU: Neonatal Intensive Care Unit, PICU: Pediatric Intensive Care Unit
not admitted to PICU
Management of Invasive Pulmonary Aspergillosis
The majority of the centers reported to prescribe voriconazole as first-line therapy for invasive pulmonary aspergillosis (table 3). In case of a probable of proven diagnosis of invasive pulmonary aspergillosis, 46% (6/13) of the centers perform imaging of the brain (CT or MRI) to screen for cerebral dissemination.
Antifungal combination therapy
Centers were asked to indicate in which clinical scenarios, antifungal combination therapy would be prescribed. Disseminated invasive aspergillosis (10/13) and coverage of a period of possible insufficient levels of azole antifungals (6/13) are the most common reasons to prescribe antifungal combination treatment. Fungal infections of the central nervous system (3/13), initially when results of diagnostic tests are awaited (2/13), Cryptococcal meningitis (2/13) and in critically-ill patients (1/13) were less common reasons to prescribe a combination of antifungal agents.
Therapeutic Drug Monitoring (TDM)
All the centers have access to perform routine TDM of antifungal agents, but seven centers do need to send their samples to another institution. The most common rational to request TDM request was routine monitoring of mold-active azoles during treatment (85%). Three centers (23%) would perform TDM as well during prophylaxis with mold-active azoles.
Discussion
The results of this national survey provides valuable information about the current management of IFD neonates and children in the UK. With a response rate of 87%, the results represent the clinical practice of 13 large third level university hospitals which captures the majority of hospital delivering care for pediatric patients at risk for developing IFD.
Our results, not surprisingly, show a substantial variation in the clinical management of IFD in neonates and children. The lack of pediatric specific evidence with respect to diagnostic measurement, management strategies, prophylactic and targeted treatment as well the lack of dosing recommendations for the newer antifungals, leaves the clinician with lots of uncertainties. Nevertheless, the results of our survey also indicates a lack of knowledge on certain aspects of the clinical management of IFD in neonates and children.
Looking into the management strategies used, the vast majority of the centers favored an empiric approach. This is most likely influenced by a lack of pediatric studies supporting a pre-emptive approach, timely access to fungal diagnostics and a prolonged turn-a-round times for specialist fungal tests, as well as the poor outcome when treatment is delayed. A diagnostic-driven approach in adult patients has shown to be feasible with more proven diagnoses made and a decrease in antifungal use without compromising outcomes (13). At the time of the survey, only supportive evidence from a single center was published for a diagnostic-driven strategy in children (14). Recently though, a randomized controlled study has shown that a pre-emptive strategy is as effective as an empirical approach in children with cancer and febrile neutropenia, and significantly reducing antifungal use (15). Obviously, employing a pre-emptive antifungal strategy is only safe and feasible if access to imaging modalities, mycological tests and the performance of a bronchoscopy and bronchoalveolar lavage can be delivered in a timely fashion.
Three out of 13 centers are relying on conventional X-ray of the chest to detect signs of IFD. As several studies have shown that X-chest is not sensitive enough to detect fungal nodules in the earlier phases of pulmonary infection (16–18), this is not a good practice and could potentially result in delayed diagnosis. International management guidelines strongly recommend to use (high resolution) CT imaging to detect pulmonary IFD (19,20,11).
The value of measuring fungal biomarkers in serum, e.g. galactomannan and β-d-glucan, is to exclude invasive fungal disease caused by Aspergillus sp. and Candida sp. Both tests are characterized by a high negative predictive value while the positive predictive value is low (21,22). Therefore, those tests can have an important place in screening of populations at high risk for developing IFD. In our survey, while the majority of the centers (77%) use galactomannan testing as a diagnostic test, only 2 centers (15%) use the test as a screening tool in high-risk neutropenic patients. Responses were not different with respect to use of galactomannan testing in non-hematologic patients. Is important though that galactomannan is only validated for use in neutropenic patients and its test characteristics in non-neutropenic patients is not clear. As samples for galactomannan testing are often sent to reference laboratories, prolonging the turn-around-time, this may very well affect its use as a screening tool. Half of the centers use the β–d-glucan test as a diagnostic tool with a few using it as a screening tool. Guidelines for clinical practice management in the pediatric population are discouraging the use of this test (11). Studies have shown that levels in children without IFD are higher compared to adults and a pediatric specific cut-off needs to be validated (22,23).
Antifungal prophylaxis is used by nearly all centers for high risk hemato-oncology patients and ELBW infants. Recommendations in favor of this practice can be found in international management guidelines as studies have shown that antifungal prophylaxis is effective in lowering the incidence of IFD, although no significant effect has been shown on overall outcome (10,11,20). Of note, a huge variation in choice of antifungal for prophylaxis is observed in high-risk hemato-oncology patients. This is most likely resulting from comparable quality of evidence of studies performed and strength of recommendation given in the various international management guidelines (8,11,20). Half of the centers reported to use liposomal amphotericin B for prophylaxis, although dosages are not well studied and it has no label for prophylactic use. The main reason to use it for antifungal prophylaxis in the pediatric population is the use of vincristine in various chemotherapy protocols excluding the use of azole antifungals due to well-known interactions (24,25).
The choice for liposomal amphotericin B as first line empiric therapy is in line with data from clinical trials as well as recommendations in the various international guidelines (11,26,27). First line treatment in the context of a pre-emptive strategy was reported not to be different. As most signs and symptoms suspect for IFD are non-specific with respect to the causative fungus, this seems to be a reasonable choice, with adjustments to be made when specific microbiological test results are known.
Voriconazole, being the first line treatment for IA, was reported to be the first line treatment in far most of the centers. Remarkably, this choice was more or less independent of the age of the child, although 2 out of 11 centers would prescribe liposomal amphotericin B instead of voriconazole in infants < 2 years of age. It is important to note that dosages have not been determined for infants < 2 years of age and it is not licensed for use in this age group (11,20)(10,28). If a decision is made to use voriconazole in infants < 2 years of age, this should be prescribed under strict TDM.
Fluconazole and liposomal amphotericin B were equally reported to be used as first line treatment for candidemia in both neonates and children, with the exception of non-neutropenic non-critically ill children. This is contrast with the results from an international multicenter prospective study in which fluconazole or an echinocandin were mainly used in the treatment of pediatric candidemia, with only 15% being treated with liposomal amphotericin B (28). Most effective treatment modality for pediatric candidemia is not known and is subject of a current international study carried out by the International Pediatric Fungal Network (www.ipfn.org). For neonatal candidemia a huge variation in clinical practice has been described and studies are lacking to make firm recommendations (29,30).
First line combination antifungal therapy is sporadic prescribed, although disseminated IFD (2 or more organs) is for most centers (77%) a reason to use combination antifungal therapy. The pivotal randomized clinical trial comparing voriconazole versus voriconazole plus anidulafungin for primary treatment of IA did not show superiority of the combination therapy (31). Although not powered to detect meaningful differences in subsets of included patients, higher survival was observed in particular subgroups and might suggest that some patients could benefit of combination antifungal therapy. An international prospective cohort study in children with IFD showed no benefit of antifungal combination therapy but an increase in adverse events was observed (32).
In conclusion, this survey reinforces the fact that there are still important aspects in the management of pediatric IFD which should ideally be addressed in pediatric clinical trials. Attention needs to be given the knowledge gaps as observed in the results of our survey to optimize the management of IFD in children and neonates. Access to diagnostic modalities in a timely fashion with a short turnaround time of results, are urgently needed to inform clinical decision making without unnecessary delay in targeted treatment.
Figure 1. Use of antifungal prophylaxis in the different high risk patient groups in 13 children’s hospitals in the UK.
(PICU: Pediatric Intensive Care Unit. CGD: Chronic Granulomatous Disease. CN: Congenital Neutropenia. SOT: Solid Organ Transplant. *Neonates and children admitted to PICU on broad spectrum antibiotics)
Acknowledgement
We acknowledge the following members of the PASOAP (Paediatric Antifungal Stewardship: Optimising Antifungal Prescription in Paediatrics) study group for their participation in this survey: Adam Irwin (Department of Paediatric Infectious Diseases, Great Ormond Street Hospital, London, UK); Eleri Williams, Marieke Emmonts (Department of Paediatric Immunology and Infectious Diseases, Newcastle upon Tyne, UK); Stephane Paulus (Department of Paediatric Infectious Diseases, Alder Hey Children's Hospital, Liverpool, UK); Alicia Dermirjan (Department of Paediatric Infectious Diseases, Evelina Children’s Hospital, London, UK); Menie Rampola, Sally Kinsey (Department of Paediatric Oncology, General Infirmary, Leeds, UK); Simon Drysdale (Department of Paediatrics, Medical Sciences Division, University of Oxford, Oxford, UK); Paddy McMaster, Selma Mohamed (Royal Manchester Children’s Hospital, Manchester, UK); Ayad Atra (Department of Paediatric Oncology, Royal Marsden Hospital, London, UK); Stephania Vergnano (Department of Paediatric Infectious Diseases, Bristol Royal Hospital for Children, Bristol, UK); Saul Faust, Sanjay Patel, Jessica Head (Department of Paediatric Infectious Diseases, University Hospital Southampton NHS Foundation Trust, Southampton, UK); Elisabeth Whittaker, Luca Zambori (Paediatric Infectious Diseases, Imperial College Healthcare NHS Trust, London, UK); Mitu Patel (Birmingham Children’s Hospital, NHS Foundation Trust, Birmingham, UK)
Footnotes
Disclosures: The work presented in this manuscript was supported by a Gilead UK Clinical Fellowship Award. AW is supported by the Wellcome Trust Strategic Award (grant 097377) and the MRC Centre for Medical Mycology (grant MR/N006364/1) at the University of Aberdeen.
References
- 1.Pana ZD, Roilides E, Warris A, et al. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc. 2017;6:S3–11. doi: 10.1093/jpids/pix046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehrnbecher T, Groll AH. Invasive fungal infections in the pediatric population. Expert Rev Anti Infect Ther. 2011;9:275–278. doi: 10.1586/eri.11.1. [DOI] [PubMed] [Google Scholar]
- 5.Lehrnbecher T, Zaoutis T, Gamis A, et al. International variations in infection supportive care practices for paediatric patients with acute myeloid leukaemia. Br J Haematol. 2009;147:125–128. doi: 10.1111/j.1365-2141.2009.07844.x. [DOI] [PubMed] [Google Scholar]
- 6.Burwell LA, Kaufman D, Blakely J, et al. Antifungal prophylaxis to prevent neonatal candidiasis: a survey of perinatal physician practices. Pediatrics. 2006;118:1019–1026. doi: 10.1542/peds.2006-0446. [DOI] [PubMed] [Google Scholar]
- 7.Lestner JM, Versporten A, Doerholt K, et al. Systemic antifungal prescribing in neonates and children: outcomes from the antibiotic resistance and prescribing in european children (ARPEC) study. Antimicrob Agents Chemother. 2015;59:782–789. doi: 10.1128/AAC.04109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hope WW, Castagnola E, Groll AH, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18:38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 11.Groll AH, Castagnola E, Cesaro S, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 2014;15:327–340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 12.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 13.Fung M, Kim J, Marty FM, et al. Meta-analysis and cost comparison of empirical versus pre-emptive antifungal strategies in hematologic malignancy patients with high-risk febrile neutropenia. PLoS One. 2015;10:e0140930. doi: 10.1371/journal.pone.0140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castagnola E, Bagnasco F, Amoroso L, et al. Role of manafement strategies in reducing mortality from invasive fungal disease in children with cancer or receiving hematopoietic stem cell transplant: a single centre 30-year experience. Pediatr Infect Dis J. 2014;33:233–237. doi: 10.1097/INF.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 15.Santolaya M, Alvarez AM, Acuña M, et al. Efficacy of pre-emptive versus empirical antifungal therapy in children with cancer and high-risk febrile neutropenia: a randomized clinical trial. J Antimicrob Chemother. 2018;73:2860–2866. doi: 10.1093/jac/dky244. [DOI] [PubMed] [Google Scholar]
- 16.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 17.Heussel CP, Kauczor H, Fischer B, Mildenberger P, Thelen M. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. Am J Radiol. 1997;169:1347–1353. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 18.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19(1):253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 19.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:S1–38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Schelenz S, Barnes RA, Barton RC, et al. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet. 2015;15:461–474. doi: 10.1016/S1473-3099(15)70006-X. [DOI] [PubMed] [Google Scholar]
- 22.Lehrnbecher T, Robinson PD, Fisher BT, et al. Galactomannan, β-D-glucan, and Polymerase Chain Reaction-based assays for the diagnosis of invasive fungal disease in dediatric cancer and hematopoietic stem cell transplantation: a systematic review and meta-analysis. Clin Infect Dis. 2016;63:1340–1348. doi: 10.1093/cid/ciw592. [DOI] [PubMed] [Google Scholar]
- 23.Warris A, Lehrnbecher T. Progress in the diagnosis of invasive fungal disease in children. Curr Fungal Infect Rep. 2017;11:35–44. doi: 10.1007/s12281-017-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Schie RM, Bruggemann RJ, Hoogerbrugge PM, et al. Effect of azole antifungal therapy on vincristine toxicity in childhood acute lymphoblastic leukaemia. J Antimicrob Chemother. 2011;66:1853–1856. doi: 10.1093/jac/dkr223. [DOI] [PubMed] [Google Scholar]
- 25.Moriyama B, Henning SA, Leung J, et al. Adverse interactions between antifungal azoles and vincristine: review and analysis of cases. Mycoses. 2013;55:290–297. doi: 10.1111/j.1439-0507.2011.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–71. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 27.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 28.Palazzi DL, Arrieta A, Castagnola E, et al. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multi-national study. Pediatr Infect Dis J. 2014;33:1294–1296. doi: 10.1097/INF.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 29.Oeser C, Vergnano S, Naidoo R, et al. Neonatal invasive fungal infection in England 2004–2010. Clin Microbiol Infect. 2014;20:936–941. doi: 10.1111/1469-0691.12578. [DOI] [PubMed] [Google Scholar]
- 30.Steinbach WJ, Marr KA, Anaissie EJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65:453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Marr K, Schlamm H, Herbrecht R, et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015;162:81–89. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]
- 32.Wattier RL, Dvorak CC, Hoffman JA, et al. A prospective, international cohort study of invasive mold infections in children. J Pediatric Infect Dis Soc. 2015;4:1–10. doi: 10.1093/jpids/piu074. [DOI] [PMC free article] [PubMed] [Google Scholar]