Abstract
Microplastic pollution is increasingly considered to be a factor of global change: in addition to aquatic ecosystems, this persistent contaminant is also found in terrestrial systems and soils. Microplastics have been chiefly examined in soils in terms of the presence and potential effects on soil biota. Given the persistence and widespread distribution of microplastics, it is also important to consider potential evolutionary implications of the presence of microplastics in soil; we offer such a perspective for soil microbiota. We discuss the range of selection pressures likely to act upon soil microbes, highlight approaches for the study of evolutionary responses to microplastics, and present the obstacles to be overcome. Pondering the evolutionary consequences of microplastics in soils can yield new insights into the effects of this group of pollutants, including establishing ‘true’ baselines in soil ecology, and understanding future responses of soil microbial populations and communities.
Additional keywords: ecotoxicology, evolution, microbiota, selection pressures
Introduction
Microplastics are emerging as a factor of global change. These particles, generally defined as plastics <5 mm (or <1 mm) in size, have been found in a range of environments, including freshwater ecosystems (Li et al. 2018a), the oceans, arctic sea ice (Peeken et al. 2018), and also in terrestrial ecosystems and the soil (Rillig 2012; Horton et al. 2017; Machado et al. 2018a). Current studies in soils have focused on documenting the extent of pollution (e.g. Scheurer and Bigalke 2018), with data from soil lagging far behind our knowledge on oceans, where research was started a decade earlier (Thompson et al. 2004). Research has also started to document potential effects of microplastic particles on individual soil biota, such as earthworms (Huerta-Lwanga et al. 2017). Such studies are primarily aimed at understanding potential ecological consequences of this novel group of contaminants.
However, given the widespread – and likely long-term – presence of microplastics in the environment, it is also important to start considering evolutionary consequences. These have so far not been discussed, except perhaps in the context of the discovery of plastic-degrading microbes (Yoshida et al. 2016).
Here we discuss various aspects of selection pressures likely to act upon soil microbes (Fig. 1); we introduce approaches for the study of evolutionary responses, and highlight general obstacles to overcome. We argue that introducing an evolutionary perspective would present highly relevant questions to the study of these persistent contaminants in soil.
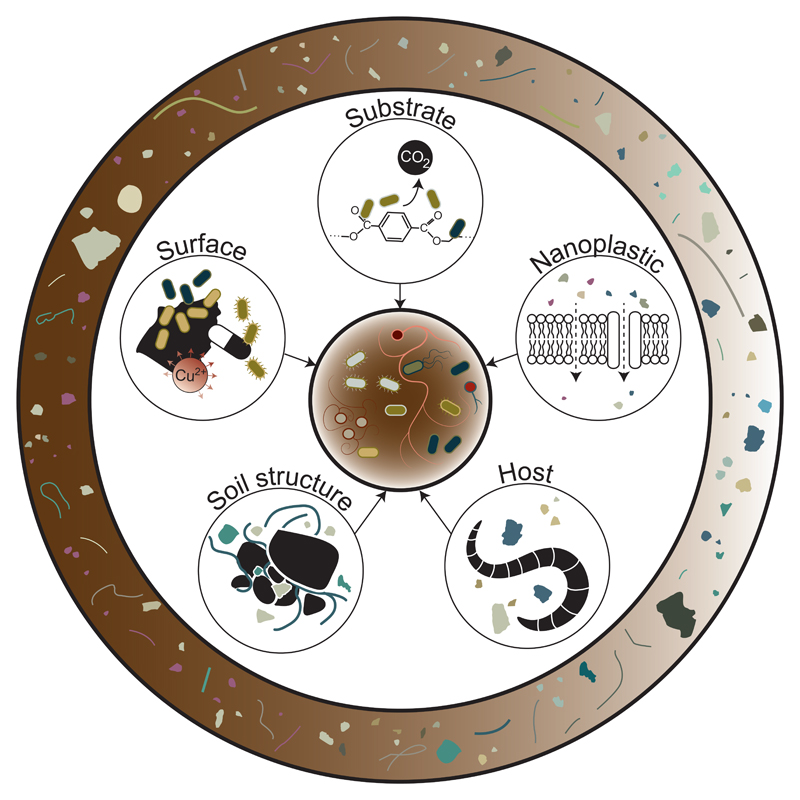
Fig. 1.
Drivers of potential evolutionary effects of microplastics on soil microbes. The outer ring depicts microplastic particles with various properties (including size, shape, chemistry). Microbial communities (in the centre) experience various effects triggered by the microplastic particles. Typical impacts with evolutionary consequences include potential changes in soil structure, alteration of host availability or function (host microbiome), nanoplastic toxic effects, plastic particles representing a resource, and providing novel surfaces (with various chemicals attached, including heavy metals and antibiotics).
Selection pressures
Microplastic particles may affect a range of soil properties, which would present soil biota with certain selection pressures (Fig. 1). This will lead to a shift in genotypes within populations, either by selection among already existing lines, or among lines based on de novo mutations, which is evolution. The question therefore becomes: how might microplastics affect the environment in soil, and which organismal traits would become important as targets of selection?
The most obvious factor would be the microplastic as a novel resource, i.e. a source of nutrients and carbon. In fact, a microplastic may be a significant anthropogenic component of soil organic carbon already (Rillig 2018). Plastics are often made to be inert and they typically decompose very slowly; for all intents and purposes of the human time horizon, they may be regarded as persistent. However, microbiota (bacteria and fungi) genotypes with an ability to use the carbon or other elements contained in the microplastic may have a selective advantage, and such genotypes would be expected to increase in relative abundance within the population. The same is true for any other additives chemically or physically bound to the plastic polymer (e.g. plasticisers), which may be contained in microplastic particles, even though such effects may be relatively shorter-lived.
Furthermore, microplastics display an elevated ability to absorb chemical substances, such as antibiotics, heavy metals and other xenobiotics (Brennecke et al. 2016; Hirai et al. 2011; Li et al. 2018b). For example, polyamides display a particularly high adsorption capacity for antibiotics containing a carbonyl group, like tetracycline or ciprofloxacin, since strong hydrogen bonds between this carbonyl group and the amide group, as a proton donor, of the microplastics can be established (Li et al. 2018b). However, the sorption ability differs greatly between diverse plastic materials, sorbed substances and environmental conditions (Li et al. 2018b). Still, though, with increased antibiotic or heavy metal concentrations, microplastics and their surroundings can constitute microniches in the soil environment with highly selective conditions. In combination with potentially providing a novel nutrient source, microplastics can consequently serve as so called ‘hot-spots’ of horizontal gene transfer (HGT) and microbial evolution. While in water environments the additional surface introduced through microplastics is the major factor in enhancing plasmid transfer, plastic particles still favour microbial interactions to a larger extent than natural aggregates (Arias-Andres et al. 2018). Moreover, the presence of microplastics can positively alter the retention time of other introduced stressors in the soil environment and thus lead to longer lasting periods of exposure and subsequent evolution to these conditions (Sun et al. 2018).
Microplastics also have the potential to change the soil physical environment. The soil physical environment is governed by soil aggregation, a process to which many soil biota contribute (Lehmann et al. 2017). Soil aggregates are relatively stable entities whose interiors contain microhabitats with often drastically different conditions to those on aggregate surfaces. Such temporarily stable structures have recently been conceptualised as massively concurrent evolutionary incubators for microbes (Rillig et al. 2017a), which means that evolutionary processes and trajectories within aggregates are different compared with those in a non-structured soil. Following this concept, any changes in soil aggregation, that is, processes affecting rates of formation, stabilisation or disintegration of aggregates, could also be expected to have consequences for microbial evolution. Microplastics, especially linear fibres, could exert effects on these processes. A change in soil aggregation and, corresponding to these, pore distributions, could have multiple evolutionary consequences within communities that are currently difficult to predict in terms of traits and directions. In fact, changes in soil structure and pore spaces may even lead to local extinction because of microhabitat loss (Veresoglou et al. 2015). Recently, the effects of microfibers on soil aggregation were demonstrated experimentally (Machado et al. 2018b), together with the accompanying changes in bulk density and water holding capacity.
Many soil microbes interact strongly with hosts, including soil animals. Soil animals, in turn, may also interact with microplastics: earthworms have been shown to ingest polystyrene beads (Rillig et al. 2017b; Huerta Lwanga et al. 2016, 2017), and some studies have shown deleterious effects on earthworms (Huerta Lwanga et al. 2016).Microbes specialised in degrading microplastic compounds have been isolated from earthworm guts (Huerta Lwanga et al. 2018), which could be part of a newly evolved complex host-symbiont interaction in response to microplastic pollution in soils. Similarly, other soil animals may also consume these particles (e.g. Collembola; Zhu et al. 2018), with alteration in their associated microbiota. As such, we expect cascading effects of microplastics on microbiota evolution through the effects on hosts.
When microplastics break down further to even smaller particles, such particles may enter the nanosize range (<0.1 μm). Such nanoplastic particles may have very different properties, for example, they may be able to traverse biological membranes and thus acquire toxic properties (Machado et al. 2018a). Genotypes with better resistance to such effects would be expected to increase in abundance. These changes in community structure can further alter the complex interplay of microbial processes in the soil environment. For example, in an anaerobic digestion system, the exposure to polystyrene nanoparticles caused an inhibition in the community-wide productivity linked with significant changes in the microbial community structure (Fu et al. 2018), which is also likely to be observable in soil microbial communities.
Approaches for the study of evolutionary responses to microplastic
Several approaches are available for the study of evolutionary responses of soil biota to microplastics: experimental evolution in the laboratory, resurrection ecology, and observational studies using gradients.
Experimental evolution studies have a long tradition in microbial biology (e.g. Lenski et al. 1991; Buckling et al. 2000). Such studies use serial transfers in the laboratory to study the effects of a certain evolutionary driver. One could test using such systems if traits predicted to be favoured by the presence of a microplastic increase in abundance over time. In addition, monitoring the abundance of certain genes may be beneficial. Through its horizontal mobility across bacterial species and linkage to genes conferring diverse resistance phenotypes, the relative abundance of the class 1 integron-integrase gene intI1 is widely considered as a proxy to measure the level of and the selective pressure associated with anthropogenic pollution (Gillings et al. 2015). In environmental studies, it might be extremely difficult to disentangle the influence of microplastics on intI1 abundance from that of other potentially stronger selective agents, such as antibiotic or heavy metal residues or human associated microbial pollution (Amos et al. 2015). However, in controlled experiments, microplastics have already been shown to increase the persistence of intI1 from treated wastewater when entering a freshwater microbial community (Eckert et al. 2018). Consequently, intI1 could provide a promising target to quantitatively measure the selective pressures imposed on soil microbial communities through the addition of microplastic particles in experimental evolution experiments.
Another promising approach may be resurrection ecology (Franks et al. 2008). This is an approach where extant populations are compared with historical populations, which can be reanimated (‘resurrected’) from historical samples. In our case, this would entail the use of soil archives, for example, from agricultural experiment stations, which include samples collected before the widespread use of plastics. Populations extracted from such historical samples could be compared with extant populations from the same soil, with the caveat that other factors influencing the evolution of the target organisms may have changed concurrently.
Observational studies along established gradients of contamination, which share this basic limitation with resurrection studies, can also be used to learn about evolutionary responses of populations to the presence of microplastics. Here, correlations can be used to test for the link between predicted favoured traits and their relative abundance in populations along a microplastic contamination gradient.
Obstacles to overcome
The single most challenging aspect of studying microplastic is its diversity: microplastics come in a bewildering range and combination of chemical forms, sizes, surface properties, shapes and modifications (e.g. additives). Therefore, this is very much unlike studying specific contaminants, as this work encompasses a whole group of substances, additives and sizes with potentially very different effects. For example, the effects of beads, films and fibres on soil and soil microbes might be quite different. This imposes significant challenges on the external validity of any study, since, by necessity, these studies will be limited to few plastic types for logistical reasons.
For an understanding of the evolutionary dynamics of microplastic pollution in soil, it is important to realise that this is a gradually changing factor: microplastics arrive through various processes at the soil surface, and then accumulate gradually in the soil, because of the limited rates of decomposition. This means that, in any given soil, soil biota are not abruptly exposed to high concentrations of microplastic particles, which tends to be the current practice in experimental approaches aimed at elucidating ecological or physiological effects. Thus, it may also be useful to gradually expose soils and their biota to microplastics in experiments; evolutionary dynamics in response to gradual v. abrupt changes in the environment are expected to differ significantly.
Here, we focus on soil microbes, because they are eminently tractable experimentally. However, soil biota are enigmatically diverse and contain entire food webs. It is thus risky to focus on only particular groups of biota, since microplastics may modify trophic interactions, thus exerting differential top-down effects. Such effects would potentially be extremely important to gauge evolutionary responses; however, it is a real challenge to capture the entirety of soil biodiversity.
Finally, technical challenges remain, chiefly in respect to adequately quantifying types and amounts of microplastics in the soil matrix. These are certainly not unique to studies with an evolutionary focus, but will also limit such studies, for example, as far as observational studies are concerned, and in terms of establishing true baseline levels of contamination in experiments.
Concluding remarks
Pondering evolutionary consequences of microplastics in soils can lead to new questions (Table 1) and yield new insights into the effects of this group of pollutants. By studying the selection pressures experienced by a range of soil biota, we can learn about the ways soil biota may adapt in future soils. Importantly, this can also include interactions with other factors of global change. However, when we now measure soil biota traits or process rates, we may actually already be unknowingly capturing such responses: this therefore becomes an issue of understanding ‘true’ baselines in soil biology.
Table 1. Examples of questions on evolutionary consequences of microplastic contamination in soils.
| Question | Explanation/background |
|---|---|
| Has the presence of the microplastic in soil already affected evolutionary trajectories of soil microbiota? For example, has the microplastic created new niches for soil microbes? | Persistence of the microplastic in soil, and the finding that the microplastic appears to be ubiquitous in soil samples even from relatively non-human influenced ecosystems (Scheurer and Bigalke 2018) |
| Can evolutionary changes to the microplastic within populations buffer against or exacerbate changes in microbial community composition? How do these changes interact with phenotypic plasticity? | Eco-evolutionary dynamics |
| Does the microplastic lead to local extinctions of microbial populations? | Changes in soil physical structure (as a consequence of possible effects on soil aggregation) can lead to local exclusion of biota, for example soil animals, which may host specific microbes (Veresoglou et al. 2015; Zhu et al. 2018) |
| How does the microplastic (and microplastic type) interact with other evolutionary drivers affecting soil microbial populations? | Global change is inherently a multifactorial phenomenon; also within cities or on agricultural fields there are multiple evolutionary drivers that co-occur with microplastic contamination |
Much of what we discuss here may also be applicable to aquatic systems; however, there the provision of a surface will likely be a dominant factor (Arias-Andres et al. 2018), with the possibility of novel interactions in the particle eco-corona, including plasmid exchange.
Environmental context.
Microplastic particles are increasingly recognised as human-caused pollutants in soil with potential harmful effects on soil microorganisms. Microplastics may also have evolutionary consequences for soil microbes, because the particles may alter conditions in the soil and hence selection pressures. Including an evolutionary perspective in an environmental assessment of microplastics could lead to new questions and novel insights into responses of soil microbes to this anthropogenic stressor.
Acknowledgements
M.R. acknowledges support from the ERC Advanced Grant ‘Gradual Change’ (694368). UK received funding from the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement no. 751699.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- Amos GC, Gozzard E, Carter CE, Mead A, Bowes MJ, Hawkey PM, Zhang L, Singer AC, Gaze WH, Wellington EMH. Validated predictive modelling of the environmental resistome. The ISME Journal. 2015;9:1467–1476. doi: 10.1038/ismej.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Andres M, Klümper U, Rojas-Jimenez K, Grossart H-P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environmental Pollution. 2018;237:253–261. doi: 10.1016/j.envpol.2018.02.058. [DOI] [PubMed] [Google Scholar]
- Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuarine, Coastal and Shelf Science. 2016;178:189–195. doi: 10.1016/j.ecss.2015.12.003. [DOI] [Google Scholar]
- Buckling A, Kassen R, Bell G, Rainey PB. Disturbance and diversity in experimental microcosms. Nature. 2000;408:961–964. doi: 10.1038/35050080. [DOI] [PubMed] [Google Scholar]
- Eckert EM, Di Cesare A, Kettner MT, Arias-Andres M, Fontaneto D, Grossart HP, Corno G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environmental Pollution. 2018;234:495–502. doi: 10.1016/j.envpol.2017.11.070. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Avise JC, Bradshaw WE, Conner JK, Etterson JR, Mazer SJ, Shaw RG, Weis AE. The Resurrection Initiative: storing ancestral genotypes to capture evolution in action. Bioscience. 2008;58:870–873. doi: 10.1641/B580913. [DOI] [Google Scholar]
- Fu SF, Ding JN, Zhang Y, Li YF, Zhu R, Yuan XZ, Zou H. Exposure to polystyrene nanoplastic leads to inhibition of anaerobic digestion system. The Science of the Total Environment. 2018;625:64–70. doi: 10.1016/j.scitotenv.2017.12.158. [DOI] [PubMed] [Google Scholar]
- Gillings MR, Gaze WH, Pruden A, Smalla K, Tiedje JM, Zhu YG. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. The ISME Journal. 2015;9:1269–1279. doi: 10.1038/ismej.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Takada H, Ogata Y, Yamashita R, Mizukawa K, Saha M, Kwan C, Moore C, Gray H, Laursen D, Zettler ER, et al. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Marine Pollution Bulletin. 2011;62:1683–1692. doi: 10.1016/j.marpolbul.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. The Science of the Total Environment. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, Van Der Ploeg M, Besseling E, Koelmans AA, Geissen V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae) Environmental Science & Technology. 2016;50:2685–2691. doi: 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, van der Ploeg M, Besseling E, Koelmans AA, Geissen V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environmental Pollution. 2017;220:523–531. doi: 10.1016/j.envpol.2016.09.096. [DOI] [PubMed] [Google Scholar]
- Huerta Lwanga E, Thapa B, Yang X, Gertsen H, Salánki T, Geissen V, Garbeva P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: A potential for soil restoration. The Science of the Total Environment. 2018;624:753–757. doi: 10.1016/j.scitotenv.2017.12.144. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Zheng W, Rillig MC. Soil biota contributions to soil aggregation. Nature Ecology & Evolution. 2017;1:1828–1835. doi: 10.1038/s41559-017-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski RE, Rose MR, Simpson SC, Tadler SC. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. American Naturalist. 1991;138:1315–1341. doi: 10.1086/285289. [DOI] [Google Scholar]
- Li J, Liu H, Paul Chen J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Research. 2018a;137:362–374. doi: 10.1016/j.watres.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang K, Zhang H. Adsorption of antibiotics on microplastics. Environmental Pollution. 2018b;237:460–467. doi: 10.1016/j.envpol.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Machado AAS, Kloas W, Zarfl C, Hempel S, Rillig MC. Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biology. 2018a;24:1405–1416. doi: 10.1111/gcb.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado AAS, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC. Impacts of microplastics on the soil biophysical environment. Environmental Science & Technology. 2018b;52:9656–9665. doi: 10.1021/acs.est.8b02212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeken I, Primpke S, Beyer B, Gütermann J, Katlein C, Krumpen T, Bergmann M, Hehemann L, Gerdts G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nature Communications. 2018;9 doi: 10.1038/s41467-018-03825-5. 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig MC. Microplastic in terrestrial ecosystems and the soil? Environmental Science & Technology. 2012;46:6453–6454. doi: 10.1021/es302011r. [DOI] [PubMed] [Google Scholar]
- Rillig MC. Microplastic disguising as soil carbon storage. Environmental Science & Technology. 2018;52:6079–6080. doi: 10.1021/acs.est.8b02338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig MC, Muller LAH, Lehmann A. Soil aggregates as massively concurrent evolutionary incubators. The ISME Journal. 2017a;11:1943–1948. doi: 10.1038/ismej.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillig MC, Ziersch L, Hempel S. Microplastic transport in soil by earthworms. Scientific Reports. 2017b;7 doi: 10.1038/s41598-017-01594-7. 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurer M, Bigalke M. Microplastics in Swiss floodplain soils. Environmental Science & Technology. 2018;52:3591–3598. doi: 10.1021/acs.est.7b06003. [DOI] [PubMed] [Google Scholar]
- Sun M, Ye M, Jiao W, Feng Y, Yu P, Liu M, Jiao J, He X, Liu K, Zhao Y, Wu J, et al. Changes in tetracycline partitioning and bacteria/phage-comediated ARGs in microplastic-contaminated greenhouse soil facilitated by sophorolipid. Journal of Hazardous Materials. 2018;345:131–139. doi: 10.1016/j.jhazmat.2017.11.036. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Olson Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE. Lost at sea: Where is all the plastic? Science. 2004;304:838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- Veresoglou SD, Halley JM, Rillig MC. Extinction risk of soil biota. Nature Communications. 2015;6 doi: 10.1038/ncomms9862. 8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate) Science. 2016;351:1196–1199. doi: 10.1126/science.aad6359. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chen QL, An XL, Yang XR, Christie P, Ke X, Wu LH, Zhu YG. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biology & Biochemistry. 2018;116:302–310. doi: 10.1016/j.soilbio.2017.10.027. [DOI] [Google Scholar]