Abstract
Objectives
To report procedural outcome and short-term follow-up data for the Gore septal occluder (GSO), a new device for closure of patent foramen ovale (PFO).
Background
Transcatheter closure of PFO is an established treatment modality but no current device provides a perfect solution. The GSO has a number of design features, which make it potentially attractive for closure of defects in the atrial septum.
Methods
Data from 9 centers in the United Kingdom implanting the GSO device, submitted to an electronic registry for evaluation.
Results
Two hundred twenty-nine patients undergoing PFO closure from June 2011 to October 2012 were included. Indications for closure were secondary prevention of paradoxical cerebral emboli (83.4%), migraine (2.1%), platypnoea orthodeoxia (3.9%), and other (10.5%). Median PFO size was 8 mm and 34 and 39%, respectively, had long tunnel anatomy or atrial septal aneurysms. A GSO was successfully implanted in all cases. A single device was used in 98% but in 4 patients the initial device was removed and a second device required. Procedural complications occurred in 3% and later complications (e.g., atrial fibrillation, atrial ectopics, and device thrombus) in 5.7% of cases. All patients have undergone clinical and echocardiographic follow-up and all devices remain in position. Early bubble studies (median 0 months) with Valsalva maneuver in 67.2% were negative in 89%.
Conclusions
The GSO is an effective occlusion device for closure of PFO of all types. Longer-term follow-up particularly to document later closure rates are required.
Keywords: patent foramen ovale/atrial septal defect, congenital heart, disease in adults, cerebrovascular accident
Introduction
Percutaneous closure of patent foramen ovale (PFO) is an established technique that is used widely in patients with suspected paradoxical embolism. The ideal device for occlusion of PFO should provide rapid and complete occlusion of defects of every type of anatomy, with a low risk of cardiac perforation, device related thrombus, and secondary arrhythmia. Biological compatibility and a low profile in the atrial septum are also desirable properties. Whilst a more robust and potentially more versatile device the GSO retains many of the desirable qualities of the low profile and soft HELEX device but is much easier to deploy. The GSO received its European CE mark and has been in clinical use in the United Kingdom since June 2011. We report the initial UK experience using the recently developed Gore septal occluder (GSO) device for occlusion of PFO.
Methods
Device and Deployment
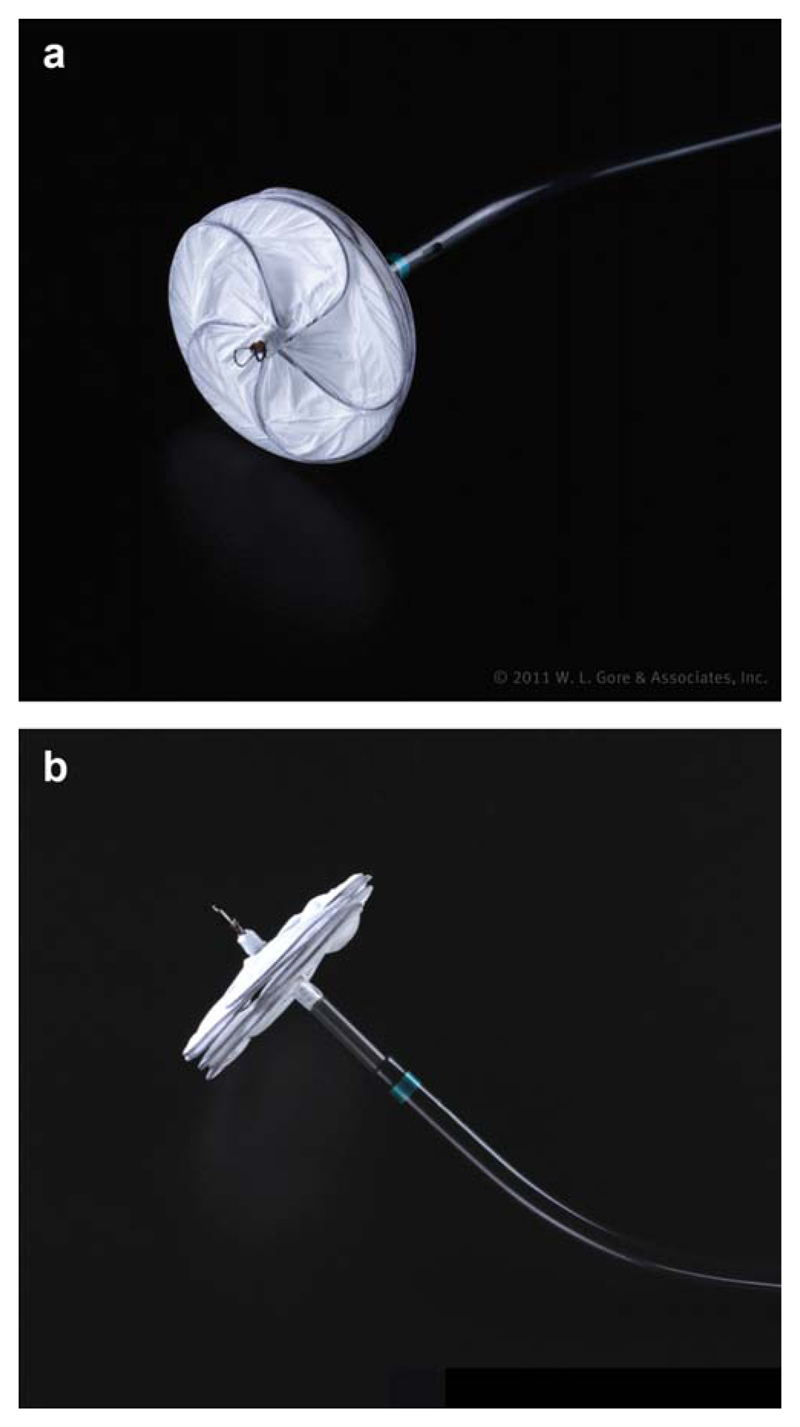
The GSO consists of a frame of 5 interlocking Nitinol wires with a platinum core wrapped within an ePTFE (Gore, Flagstaff, AZ) shell (Fig. 1a and 1b). When configured the device forms 2 concentric disks linked by a short central stalk. The GSO is available in disc diameters from 15 to 30 mm in 5 mm increments. The GSO is preassembled and loaded into a delivery catheter, which facilitates simple and intuitive delivery (Fig. 2). The delivery sheath containing the device is inserted through a short 12F femoral venous sheath and advanced into the left atrium along a super-stiff guide-wire using the preformed mono-rail port (Fig. 3). Once the tip of the delivery sheath is in the left atrium the guide-wire is removed. The deployment sequence starts with forward movement of the device using the button on the delivery catheter handle until the left atrial disk is configured and then flattened. Initially during this maneuver the straight central mandril extrudes from the sheath and projects into the atrium prior to the disc being formed (Fig. 4a and 4b). The straight section measures between 13 and 28 mm (Table I) depending on the size of the device and it is important to establish that there is adequate room within the left atrium to accommodate this structure and avoid perforation of the atrial wall.
Fig. 1.
(a) Left atrial aspect of a Gore septal occluder (GSO). (b) GSO device (lateral aspect). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
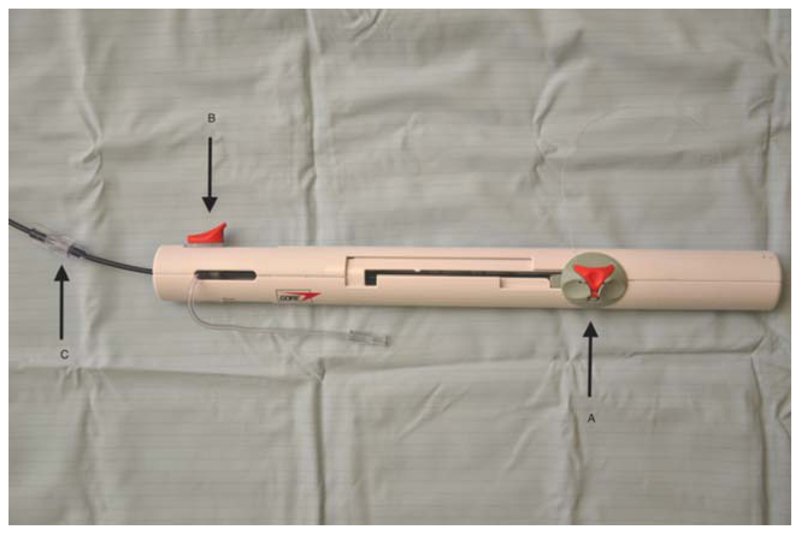
Fig. 2.
Delivery handle. A: Slider for deployment and retraction of the device. Note the fine safety suture through the red cap allowing retrieval of the device even after it has been released into its final position. B: Locking slider pulled to the left of the handle to release the device into its final orientation. C: Screw connector between braided delivery sheath and delivery handle. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
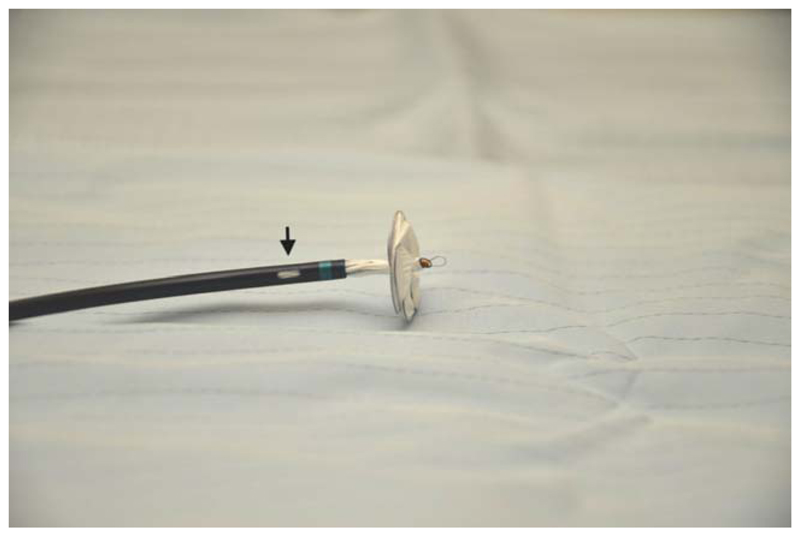
Fig. 3.
GSO and delivery sheath. Note the monorail port (arrowed) allowing the delivery sheath to be taken into the left atrium using the support of a guide wire. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
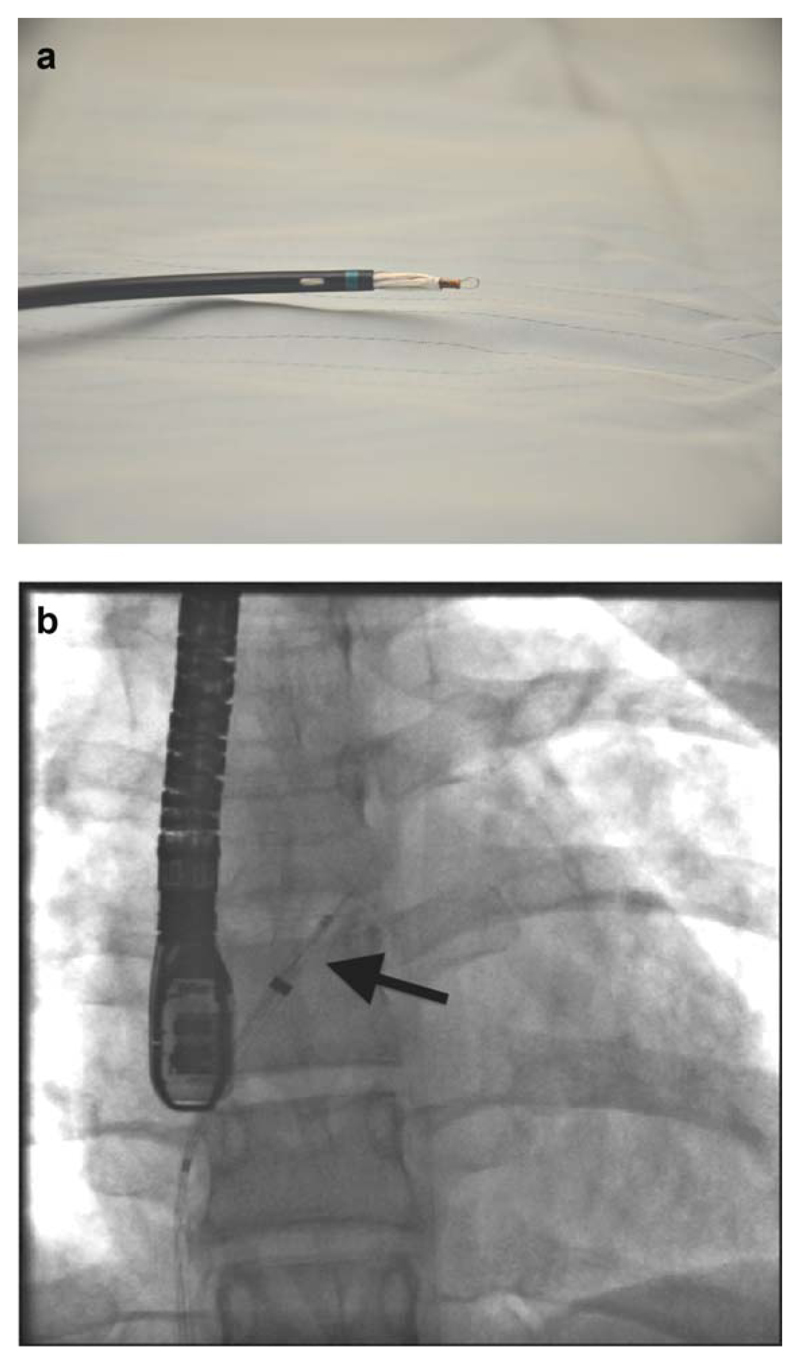
Fig. 4.
(a, b) Maximal excursion of the left atrial elements of the device (a) with fluoroscopic correlate, (b) arrowed before the device begins to adopt a disc configuration (NB: the left atrium must be large enough to accommodate this structure or myocardial perforation is a risk). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I. Length of Mandril Excursion into Left Atrium During Initial Development of the Left Atrial Disc.
| Device size | Mandril excursion (mm) |
|---|---|
| 15 | 13 |
| 20 | 18 |
| 25 | 25 |
| 30 | 28 |
Once formed the left disc is apposed to the atrial septum following, which further forward movement of the button along the handle develops the right atrial disk. Once fully deployed the central eyelets of the device are “locked” together by pulling paired buttons on the outside of the catheter handle (Fig. 2B). At this stage the device detaches from the delivery mandril, which removes tension from the system and allows a degree of “reorientation” to a final position relative to the atrial septum (Fig. 5a and 5b). The device can be retrieved or redeployed at any stage during delivery until it is locked. Even after locking and release from the mandril, a safety cord (Fig. 2) allows the device to be unlocked and removed, although this maneuver destroys the integrity of the device and following this it cannot be reused.
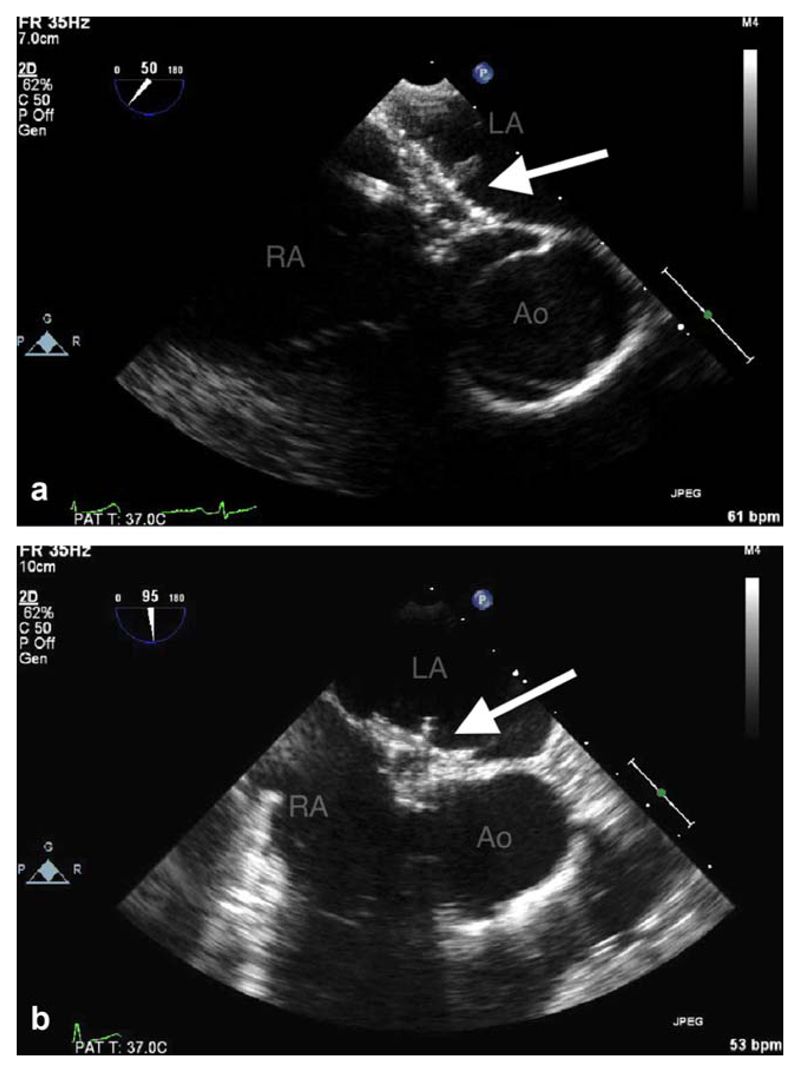
Fig. 5.
(a, b) Transesophageal echo-cardiogram (TEE) at (a) 50° and (b) 90°. GSO within the atrial septum (arrowed). LA = left atrium, RA = right atrium, Ao = aorta. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Data Collection
Electronic data were collected retrospectively within a voluntary registry from 9 participating centers in the UK. Centers were required to submit data on all cases in which PFO closure with a GSO was attempted. Implant technique and follow-up were at the discretion of the supervising clinician.
Results
Indications and Demographics
Data on 229 patients undergoing closure of a PFO (median 18 implants per center, range 4–85) with a GSO device from June 2011 to October 2012 were submitted for analysis.
The indication for PFO closure was generally secondary prevention of a cryptogenic stroke or transient ischaemic attack (TIA) (191 cases) but also included a smaller number of closures for other indications such as platypnea-orthodeoxia syndrome, desaturation related to previously repaired congenital heart disease, migraine, or secondary prevention of emboli to organs other than the brain. GSO’s were implanted to close residual shunts in two patients who had previously undergone PFO closure with an Amplatzer device. Demographic and implant information are shown in Table II. Median time from implant to follow-up was 9.8 months (1.2–17.3 months).
Table II. Demographic and Implant Data.
| Median age at implant (range) | 45 years (14–72) |
| Indication for closure | |
| Stroke/TIA | 191/229 |
| Migraine | 5/229 |
| Platypnea-deoxy syndrome | 9/229 |
| Othera | 24/229 |
| Median PFO balloon size (range) | 8 mm (2–17) |
| Atrial septal aneurysm (%) | 89/229 (39%) |
| Long tunnel anatomy (%) | 79/229 (34%) |
| Median procedural time (min) (range) | 32 (5–105) |
| Median X-ray dose (cGy/cm2) (range) | 366 (5–7186) |
Including coronary paradoxical embolus, and embolus to other (noncerebral) organs, residual shunt after previous closure.
PFO Anatomy
Median balloon size was 8 mm (2–17 mm). 79/229 (34%) of cases were reported by the implanting clinician as having “long tunnel” type anatomy at balloon interrogation and 89/229 (39%) had atrial septal aneurysms.
PFO Closure
X-ray fluoroscopy was used in all cases with 19 (8%) cases performed with no additional imaging. 102/229 (45%) closure procedures were guided by transesophageal echocardiography (TEE), 107/229 (47%) by intracardiac echocardiography (ICE), and a single case by transthoracic echocardiography (TTE).
Median procedure time was 32 min (5–105 min) and median procedural X-ray dose was 366 cGy/cm2 (5–7186) (Table I). The procedural X-ray dose exceeded 3000 cGy/cm2 in 8 cases, in the majority because of procedural complexity (either removal of a device and replacement with a larger device or implantation of a GSO alongside an existing occluder).
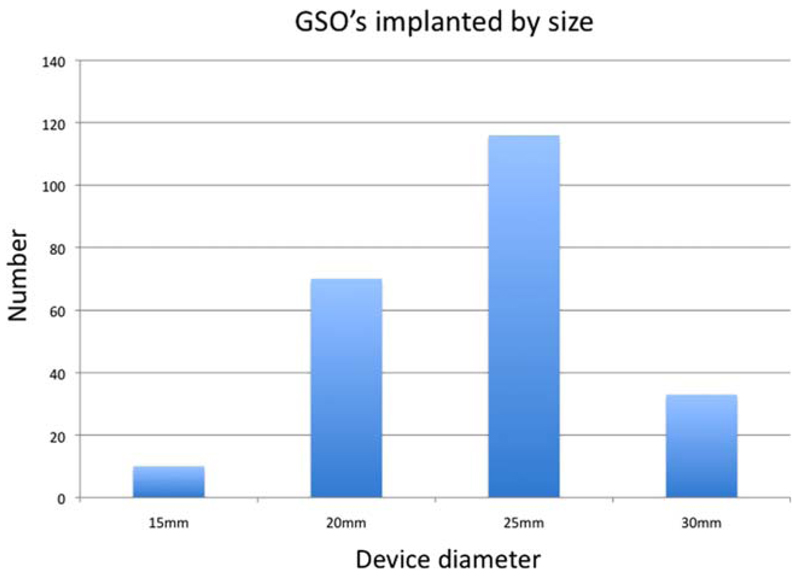
In the majority of cases a device diameter greater than or equal to twice the estimated foramen diameter was used and this was the case regardless of the anatomy (long tunnel, aneurysm, etc.). Median balloon to device size ratio for the whole group was 3.1:1 (range 1.8–10). Numbers of implanted devices by size are shown in Fig. 6.
Fig. 6.
GSO devices implanted by size. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Procedural Success
A GSO was successfully implanted in all 229 cases. 225/229 (98%) of device closures were successful with a single device but in 2 cases with a thick septum secundum the initial GSO was removed because of an unsatisfactory appearance and then successfully replaced with a larger device. In 2 other cases there was suspected failure of locking of the initially implanted devices; both devices were successfully replaced with a second device of the same size with an acceptable final result.
Complications
In-hospital complications occurred in 7 (3%) patients (Table III). The majority (4/7) were related to the venous access site and were not specific to the device. In one case an unrecognized kink developed in the delivery sheath whilst advancing the sheath from the groin to the heart. This caused perforation of the femoral vein above the inguinal ligament and required insertion of a covered stent without further consequences.
Table III. Complications-Early.
| Access site hematoma | 2 |
| Arterio–venous fistula | 1 |
| Perforated femoral vein requiring covered stent | 1 |
| Failed locking of device (removal) | 2 |
| Vaso–vagal event following closure | 1 |
| Total | 7 |
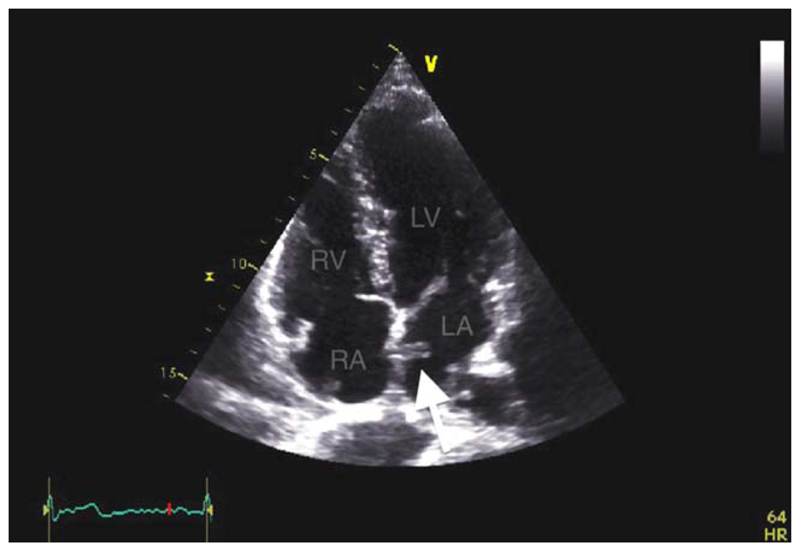
Later complications were reported in 13 (5.7%) patients (Table IV). A thrombus on the left atrial aspect of a device was detected in a single patient (Fig. 7). This patient was prescribed aspirin and clopidogrel for 6 months following PFO closure and had a normal TTE with no apparent residual shunt whilst on dual antiplatelet therapy. After 6 months he was advised to stop aspirin and continue clopidogrel long term but against advice he stopped all antiplatelet therapy. Left atrial thrombus was detected incidentally when the patient returned for a contrast TTE 11 months postimplant. The patient was anticoagulated with warfarin and the clot rapidly resolved within a week with no clinical consequences.
Table IV. Complications-Late.
| Atrial fibrillation/flutter | 5 |
| Atrial ectopics | 3 |
| Palpitations (sinus rhythm) | 3 |
| Nodal escape | 1 |
| Thrombus on left atrial disc | 1 |
| Total | 13 |
Fig. 7.
Transthoracic echocardiography (TTE). Clot on the left atrial aspect of a GSO occluder (arrowed). LA: left atrium; RA: right atrium; RV: Right ventricule; LV: left ventricle. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Atrial dysrhythmia was documented in 5/229 patients (2.2%). In 3 cases the arrhythmia was short lived, resolved spontaneously and required no specific intervention. Two out of five cases required treatment with b-blockers with both reverting to sinus rhythm. In one case medical treatment was stopped 1 month after device closure without further problems. The other case had recurrent episodes of atrial fibrillation and remains on b-blockade 6 months after closure but in sinus rhythm.
In 1 case (1/229, 0.4%) a nodal escape rhythm occurred immediately after device implantation. The patient was asymptomatic and this resolved by 3 months follow-up.
Isolated atrial ectopics were documented in 3 cases. In 3 other cases palpitations were reported but no atrial arrhythmia was demonstrated.
Patients were all treated with oral antiplatelet therapy (aspirin, clopidogrel, or dipyridamole either as monotherapy or in combination) with the length and type of treatment at the discretion of the implanting physician.
There have been no recurrent strokes or TIA’s and all devices remain in position within the atrial septum.
All patients have undergone a clinical assessment and at least one TTE examination postimplantation. 165/229 (72%) of patients have undergone 3 month follow-up assessments including echocardiography. Other than the single LA disc clot (described above) there were no reported problems on TTE and no significant intraatrial shunt on color Doppler in any patient.
Contrast TTE at rest and with Valsalva maneuver was done to assess patency of the foramen in 154/229 (67%) patients at a median 0 months (range, 0–14 months table). The short median time to contrast study reflects the practice of the largest implanting unit, which performs closure procedures under local anesthetic using ICE guidance with contrast studies with Valsalva maneuver immediately after device implantation. 137/154 (89%) of bubble studies were negative, 9/154 (5.8%) demonstrated a minor shunt (user defined, but generally less than 10 bubbles within 3 cardiac cycles) and 8/154 (5.2%) demonstrated a significant residual shunt. No anatomical features predisposed to a residual shunt.
Discussion
Given the evidence for indications and efficacy of PFO closure are still evolving it is essential that transcatheter delivered devices achieve effective atrial septal closure at low procedural risk [1]. Subsequent device performance is critical and new PFO closure devices require particular scrutiny to ensure that they provide equivalent or superior results as compared with existing technology [2–6].
The GSO is a new device designed for occlusion of either PFO or ostium secundum atrial septal defects (ASD’s). The device is low profile, relatively soft and constructed of biologically compatible material, features that make it particularly attractive for closure of PFO.
Previous reports of the use of the GSO are limited to a single small series (20 patients) with very short-term follow-up [7]. Our report reflects the current clinical experience in the UK and is the first large published series with this occluder.
The data were submitted on a voluntary basis to an electronic registry. Centers were required to provide information on all procedures during which deployment of a GSO was attempted. A GSO was successfully implanted in all cases, including a large proportion with both long tunnel anatomy and atrial septal aneurysm, suggesting that the GSO is a versatile device. Operators adhered almost universally to the “2:1” balloon sizing rule currently in use for the HELEX occluder, with only 3 devices implanted at a smaller ratio [2,3]. It remains to be seen whether the more robust nature of the GSO as compared with the HELEX allows safe implantation of devices at a smaller size ratio. All UK implanters already had experience with the HELEX device for atrial septal occlusion but despite this a learning curve was to be expected. Nevertheless, the implantation times and radiation doses were consistent with established devices already in use for PFO closure, reflecting the relative ease and reliability of use of the GSO and its deployment system [8].
Procedural complications were in the main related to vascular access and were not specific to the occluder itself. Only one vascular complication could be attributed to the GSO system specifically and this related to the development of a kink in the tip of the sheath whilst advancing towards the heart. The device is usually inserted along a monorail guide-wire making kinking of the sheath a possibility. This can easily be avoided by screening the delivery system up into the heart under fluoroscopic guidance. The other early complications were an unacceptable appearance of the device after “locking” the two discs leading to uncomplicated removal and replacement with another device-it is unclear whether this really related to failure of the core of the device to lock together or simply an appearance that the implanter did not feel was satisfactory. “Locking loop” failure was a recognized but generally benign occurrence with the HELEX device [2] but our data suggest that alterations in the design of the GSO primarily improved mandril stiffness have made this even rarer with this device.
A small number of later complications became evident during follow-up. The most important was the detection of thrombus attached to the left atrial disc on a single occluder, detected at 11 months postimplant, which had not been present at review 5 months previously. In this case the patient (against advice) had ceased all antiplatelet therapy. Device related thrombi are described in a number of reports with both the Amplatzer and Gore HELEX devices generally proving the least thrombogenic where specific comparisons between occluder devices have been made [9–11]. This represents a single device related thrombus in a series of 229 implants and whilst this should not be over-interpreted it perhaps emphasizes the importance of echocardiographic follow-up following device implantation.
The incidence of device related new onset atrial arrhythmia is potentially important given the link between atrial arrhythmia and the risk of thromboembolic cerebral events [12]. The incidence of atrial arrhythmia for existing devices following PFO closure varies between 0.6 and 15% depending on the series and the detection methods employed [13–18]. In this series there was confirmed evidence of new onset atrial dysrhythmia (either atrial flutter or fibrillation) in 2.2% of cases following implant of the GSO. In all but one case the arrhythmia resolved quickly and in only 2 cases was specific medical treatment required. A small number of additional patients reported palpitations but investigation failed to confirm an arrhythmia.
The five-wire frame design of the GSO provides flexibility but also allows each wire loop to conform independently to the contour of the adjacent myocardium. A potential advantage of this construction is that it may provide quicker closure of the atrial septum. Whilst there is no clear data relating residual shunts to recurrent thrombo-embolic events, clearly it is desirable that if an occlusion procedure is deemed necessary that rapid early closure of the septum is achieved. Currently, the Amplatzer family of devices achieve the highest early closure rates as assessed by postimplant bubble studies with ~93.5% of cases achieving complete or minor residual shunting 6 months following occlusion [19]. A large number of bubble studies with Valsalva were performed and reported within this registry data set demonstrating nearly 95% complete occlusion or minor shunting following PFO closure with the GSO despite the fact that on the whole the bubble studies were performed very early in keeping with current protocols within our submitting units. The HELEX by comparison is reported to have on-table closure rates of 60%, rising to 90% by six months [11].
Study Limitations
This study is limited by the non randomized and retrospective design with voluntary data submission and without specific implant protocols or standardized techniques. Follow-up times are short and in 28% of cases data is limited to the immediate postprocedural period. This reflects the fact that this device was only recently available.
Conclusions
In this registry series the GSO was a reliable and effective device for closure of PFO of all types. Complications rates were in keeping with those reported for existing technology, which are already in use for this procedure. Clearly, further prospective data relating to device performance are required.
Footnotes
Conflict of interest: Relationship with industry: Both Dr Thomson and Dr Ormerod are consultants for GORE medical (the device manufacturer) but had no direct role in the development of this device and have no stock or financial interest in this company.
References
- 1.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 2.Heinisch C, Bertog S, Wunderlich N, et al. Percutaneous closure of the patent foramen ovale using the HELEX® Septal Occluder: Acute and long-term results in 405 patients. EuroIntervention. 2012;8:717–723. doi: 10.4244/EIJV8I6A111. [DOI] [PubMed] [Google Scholar]
- 3.Billinger K, Ostermayer SH, Carminati M, et al. HELEX Septal occluder for transcatheter closure of patent foramen ovale: multicentre experience. EuroIntervention. 2006;1:465–471. [PubMed] [Google Scholar]
- 4.Chatterjee T, Petzsch M, Ince H, et al. Interventional closure with Amplatzer PFO occluder of patent foramen ovale in patients with paradoxical cerebral embolism. J Interv Cardiol. 2005;18:173–179. doi: 10.1111/j.1540-8183.2005.04050.x. [DOI] [PubMed] [Google Scholar]
- 5.Handke M, Harloff A, Olschewski M, et al. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]
- 6.Amin Z, Hijazi ZM, Bass JL, et al. PFO closure complications from the AGA registry. Catheter Cardiovasc Interv. 2008;72:74–79. doi: 10.1002/ccd.21582. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald S, Daniels M, Ormerod O. Initial use of the new GORE septal occluder in patent foramen ovale closure: Implantation and preliminary results. Catheter Cardiovasc Interv. 2013;81:660–665. doi: 10.1002/ccd.24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagdi P, Ritter M. Patient radiation dose during percutaneous interventional closure of interatrial communications. J Cardiol. 2009;53:368–373. doi: 10.1016/j.jjcc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Krumsdorf U, Ostermayer S, Billinger K, et al. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. doi: 10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 10.von Bardeleben RS, Richter C, Otto J, et al. Long term follow up after percutaneous closure of PFO in 357 patients with paradoxical embolism: Difference in occlusion systems and influence of atrial septum aneurysm. Int J Cardiol. 2009;134:33–41. doi: 10.1016/j.ijcard.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Taaffe M, Fischer E, Baranowski A, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder) Am J Cardiol. 2008;101:1353–1358. doi: 10.1016/j.amjcard.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 12.Durrant J, Lip GY, Lane DA. Stroke risk stratification scores in atrial fibrillation: Current recommendations for clinical practice and future perspectives. Expert Rev Cardiovasc Ther. 2013;11:77–90. doi: 10.1586/erc.12.161. [DOI] [PubMed] [Google Scholar]
- 13.Spies C, Reissmann U, Timmermanns I, et al. Comparison of contemporary devices used for transcatheter patent foramen ovale closure. J Invasive Cardiol. 2008;20:442–447. [PubMed] [Google Scholar]
- 14.Szkutnik M, Lenarczyk A, Kusa J, et al. Symptomatic tachyand bradyarrhythmias after transcatheter closure of interatrial communications with Amplatzer devices. Cardiol J. 2008;15:510–516. [PubMed] [Google Scholar]
- 15.Spies C, Khandelwal A, Timmermanns I, et al. Incidence of atrial fibrillation following transcatheter closure of atrial septal defects in adults. Am J Cardiol. 2008;102:902–906. doi: 10.1016/j.amjcard.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Bronzetti G, D’Angelo C, Donti A, et al. Role of atrial fibrillation after transcatheter closure of patent foramen ovale in patients with or without cryptogenic stroke. Int J Cardiol. 2011;146:17–21. doi: 10.1016/j.ijcard.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Anzola GP, Morandi E, Casilli F, Onorato E. Does transcatheter closure of patent foramen ovale really “shut the door?” A prospective study with transcranial Doppler. Stroke. 2004;21:2140–2144. doi: 10.1161/01.STR.0000137764.07815.de. [DOI] [PubMed] [Google Scholar]
- 18.Burow A, Schwerzmann M, Wallmann D, et al. Atrial fibrillation following transcatheter closure of patent foramen ovale in patients with stroke. Cardiology. 2008;111:47–50. doi: 10.1159/000113427. [DOI] [PubMed] [Google Scholar]
- 19.Carroll JD, Saver JL, Thaler DE, et al. RESPECT PFO closure trial. Paper presented at Trans-catheter Therapeutics; Miami. 25th October 2012. [Google Scholar]