Abstract
Background
A number of devices are available for percutaneous closure of a clinically significant patent foramen ovale (PFO). The new GORE® septal occluder (GSO) is a nonself-centering device consisting of an expanded polytetrafluoroethylene tube supported by a frame of nitinol wire conforming into a double disk. This study reports the first clinical GSO implantation experience.
Methods
GSO implantation in 20 consecutive patients is reported. Inclusion criteria were all patients referred with a significant PFO implicated in paradoxical embolism or transient right to left shunting causing desaturation. Procedures were performed under local anaesthesia and intracardiac echocardiography (ICE) in addition to fluoroscopy. Procedural data, acute and early closure rates were examined.
Results
All patients underwent successful day-case device implantation. Eleven patients had previous stroke, five had transient ischemic attacks, two had a history suspicious of PFO-related desaturation, and two had a history suspicious of PFO-related peripheral thromboembolism. Acute closure rates on IVC injection bubble testing were 100% at implant and 100% (14/14) at 1 month. Average PFO balloon size was 8.0 ± 3.6(range 2.0–16.7) mm, mean fluoroscopic implantation time 3.0 ± 1.7(range 0.7–6.3) min, radiation dose 283 ± 340 (range 6–1,431) μGym2, and total procedural time 34.8 ± 8.0 (range 22–53) min. 5 × 20 mm2, 7 × 25 m2, 8 × 30 mm2 GSO devices were implanted, aiming for device size at least twice balloon PFO size. Cases included aneurysmal septums with up to 30 mm deviation and tunnels up to 12 mm long. Removal and repositioning of two devices was performed on two occasions after uncertainty about device locking. At 1 month follow-up, two patients had brief self-terminating episodes of suspected atrial fibrillation, all had normal resting ECGs. No thromboembolic/neurological events were reported.
Conclusions
The GSO can be implanted under local anaesthesia and ICE with low procedural and fluoroscopy times with high procedural success as a day case. No residual shunts were seen. This initial experience suggests that it is a safe and effective device for PFO closure.
Keywords: patent foramen ovale, septal occluder
Introduction
Patent foramen ovales (PFOs) can be closed by a number of devices (reviewed in Ref. [1]). The most commonly used devices use a double disk type of construction which, once across the defect, endothelialize to seal the device against the interatrial septum. Concerns remain about device erosion and some devices may be technically challenging to implant or not be suited to the wide variety of PFO anatomy that exists [2–4]. Long-term reactions to implanted devices are also a concern, with the device possibly interfering with septal dynamics and causing local and systemic inflammatory reactions [5]. An optimal prosthesis should provide effective closure of the PFO, be simple to implant, easy to remove if required, have a low profile on the atrial septum and not interfere with other intracardiac structures and demonstrate good long-term biocompatibility. The GORE® septal occluder (GSO) was designed to incorporate these features. We describe the first experience in a consecutive number of patients.
Methods
Device
The GSO consists of a frame of five nickel–titanium (nitinol) wires with platinum core which is covered by a tube of expanded polytetrafluoroethylene film (ePTFE; W.L. Gore and Associates, Flagstaff, AZ) and configured in such a way as to create a double disk device. The platinum core enhances visibility on fluoroscopy. ePTFE has been a component of intracardiac grafts used over several decades with excellent biocompatibility. This device differs from the Helex septal occluder currently used in Europe and America in terms of its construction and delivery (Fig. 1) [6,7]. Similar to the Helex device, the GSO is fixed in place by an intrinsic locking mechanism which passes through the center of the device from the left atrial to right atrial disc.
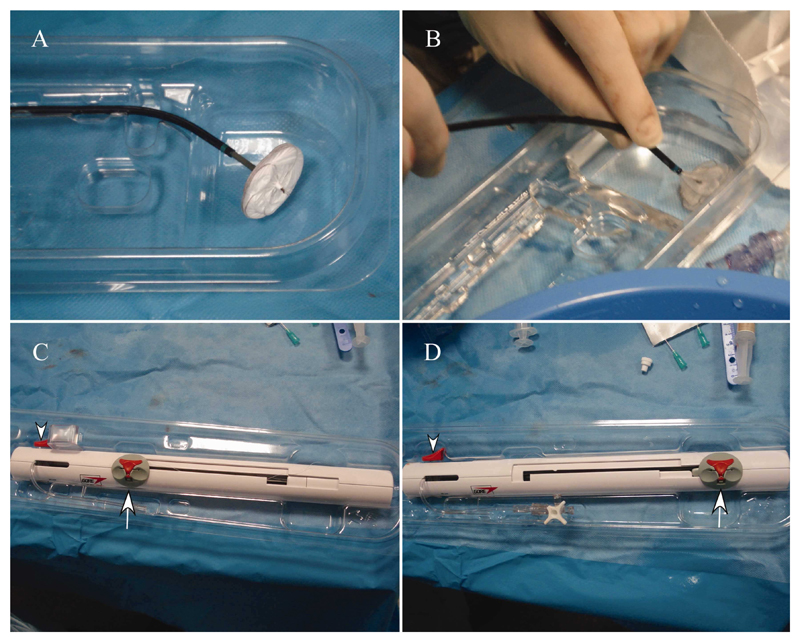
Fig. 1. The GSO delivery system.
A: Device in packaging prior to procedure showing double disc appearance prior to being loaded into the delivery catheter. B: Occluder is submerged in heparinized saline and loaded into delivery system by pushing the gray handling and deployment slider (arrow in C, D) toward the end of the handle. C: Appearance of the delivery system handle prior to device loading. The system is flushed first through the side port. D: Appearance of the delivery system after device loading with the sliding mechanism at the end of the handle. The device is thoroughly flushed again through the side port. The occluder locking mechanism (arrowhead) is not used in these stages. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Deployment Procedure
Unlike the original Helex device, the GSO is premounted on a handheld delivery system which uses a contained slider mechanism to deploy both left and right atrial discs (Fig. 1a–d). The device is first irrigated with heparinized saline and introduced through a 12Fr femoral venous sheath. It is then manipulated into the left atrium on a monorail port guidewire such as an Amplazter superstiff guidewire positioned in the left upper pulmonary vein. Once in the left atrium, the guidewire is removed and the left atrial disc deployed by a sliding action of the handle. Once the left atrial disc is formed and pulled back to the septum (Fig. 2), the right atrial disc is similarly configured by continuing the sliding action of the handle (Figs. 2 and 3). Should it be deemed necessary repositioning of the device is possible by reversing the steps of deployment. When both disks have been delivered, the device position is checked and subsequently locked using the release catch on the delivery handle, separating the device from the delivery catheter (Figs. 1 and 3). The device and septum are then free of any tension from the delivery system and further assessment of positioning can be performed. The device can still be recovered if necessary by unscrewing the outer delivery catheter from the control catheter, fixing the delivery catheter, and sliding the control catheter back to bring the device into the delivery system. The handle sliding deployment and locking mechanisms minimizes the number of steps for the operator, facilitating implantation considerably compared to the Helex device.
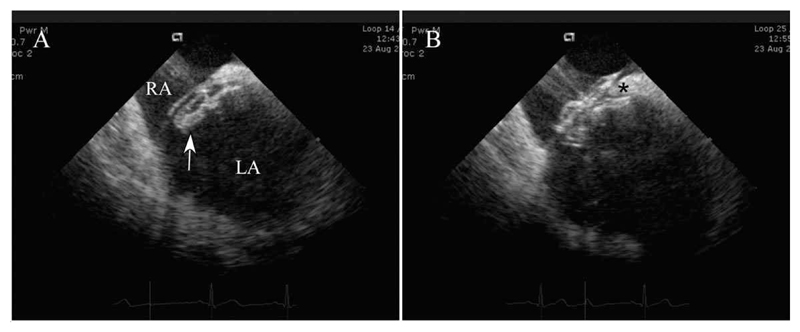
Fig. 2. ICE images of deployment.
A: Left atrial disc (arrow) has been deployed in the left atrium and the whole system has been pulled against the atrial septum. B: The right atrial disc has been deployed, both the septum secundum superiorly (*) and the thinner septum primum inferiorly being held between the two atrial discs of the septal occluder. Abbreviations: LA, left atrium; RA, right atrium.
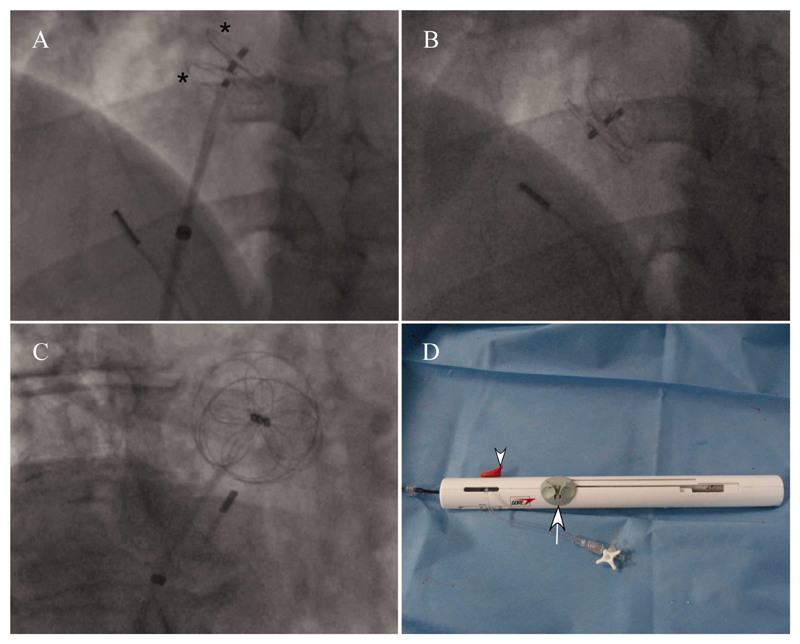
Fig. 3. Fluoroscopy images of deployment.
A and B: LAO projection, C: RAO projection. A: Both left and right atrial discs (*) have been deployed and the occluder lock mechanism has just been pulled back. Three eyelets can be seen from the right to left atrial discs. The intracardiac echo probe tip can be seen in the bottom right corner B: The retention suture has been withdrawn and the delivery catheter has fallen away from the device. C: Image of device in RAO projection showing the radio-opaque petal and circumferential appearance of the nitinol in each disk. D: Appearance of handle assembly post device deployment with the loading and deployment slider at the proximal end of the handle, the retrieval cord lock removed from the slider (arrow) and the suture removed. The occluder locking mechanism slider (arrowhead) has also been pulled back. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Implantation Procedure
Devices were deployed in 20 patients (Table I). Pretreatment with aspirin was given and, after informed consent, devices were implanted under local anaesthesia and mild sedation with 2.5–5 mg of intravenous diazepam as appropriate. Femoral venous access was achieved with 11Fr and 12Fr sheaths before giving 100 units/kg of heparin. The 11Fr sheath was used to introduce an intracardiac echo probe (Siemens, Germany) and the right atrial and interatrial septal anatomy evaluated with an Accuson Cypress ultrasound machine. A 6Fr multipurpose catheter was then positioned via the 12Fr sheath first into the superior and subsequently inferior vena cavae and the presence of right-left shunting confirmed by bubble contrast using an agitated mixture of 2 ml of the patients’ blood and 8 ml saline. The PFO was then crossed with the multipurpose catheter, guided by intracardiac echocardiography (ICE) and fluoroscopy, and positioned in the left upper pulmonary vein, where it was exchanged for an Amplatz Superstiff guidewire. Balloon sizing was performed using a 25-mm Numed balloon, measuring defect size by fluoroscopy and ICE. Device size was chosen to be more than twice the diameter of the waist seen on the sizing balloon and further oversizing was used in those patients with atrial septal aneurysm.
Table I. Patient Demographics and Outcomes.
| Feature | GSO implantation data (± sd) |
|---|---|
| Median age (yrs ± SD) | 41 ± 9.8 yrs (range 21–57) |
| Male/female | 15/5 |
| Median defect size (balloon size on fluoroscopy) | 8.0 ± 3.6 mm (range 2–16.7) |
| Median radiation dose | 283 ± 340 µGym2 (range 6–1,431) |
| Median fluoroscopic time | 3.0 ± 1.7 min (range 0.7–6.3) |
| % with ASA (deviation >1 cm) | 90% (18/20) |
| Devices used | 20 mm, n = 5 25 mm, n = 7 30 mm, n = 8 |
| Median procedure time | 34.8 ± 8.0 min (range 22–53) |
| Closure achieved with bubble study post implant (IVC injection) | 20/20 |
| Shunt on transthoracic echo at 1 month | 0/14 |
| ECG changes at 1 month | 0/14 |
Patient PFO characteristics and Procedural data. Atrial septal aneurysms were defined as septal excursion greater than 10 mm measured on M-mode through the septum on intracardiac echo. Balloon sizes were all measured on fluoroscopy in LAO cranial projection. Procedural times include initial intracardiac echo assessment and superior and inferior vena caval bubble assessments. Abbreviations: GSO, Gore septal occluder; TIA, transient ischemic attack; CVA; cerebrovascular accident.
All patients were given 1 g of intravenous flucloxacillin during the procedure and advised to continue on 75 mg of aspirin for at least 6 months.
Results
Twenty consecutive patients had PFO closure with the GSO (Table I) (15 men, five women) (Table I). Mean age was 41.0 ± 9.8yrs. Eleven patients had previous stroke, five had transient ischemic attacks, two had evidence of hypoxia on exertion with a positive bubble study suggestive of a PFO, and two had a history of thromboembolism. Mean balloon size was 8.0 ± 3.6mm (range 2–16.7 mm) on fluoroscopy with mean fluoroscopy times for each case 3.0 ± 1.7 min (range 0.67–6.27 min) and radiation dose 245 ± 240 μGym2 (range 6–1431 μGym2) (Table I). The patient with highest radiation dose was the heaviest patient with weight 122 kg (mean patient weight was 83.1 ± 18.3 kg, range 61.6–122 kg). Mean procedure time was 32.1 ± 8.5 min (range 22–53 min).
Device placement was possible in all patients. In two patients, there was concern about whether the device had locked properly after deployment but before the safety suture was removed. In both cases, the device was easily retrieved. New septal occluders were then deployed without incident in both cases. No thromboembolic or neurological events were seen. In all cases, 100% closure was attained with absence of shunt on bubble contrast study from an inferior vena cava injection (Fig. 4). All patients were discharged on the same day of the procedure, following clinical and femoral access site review and echo. All were advised to take 75 mg of aspirin for at least the next 6 months and endocarditis prophylaxis measures also for 6 months.
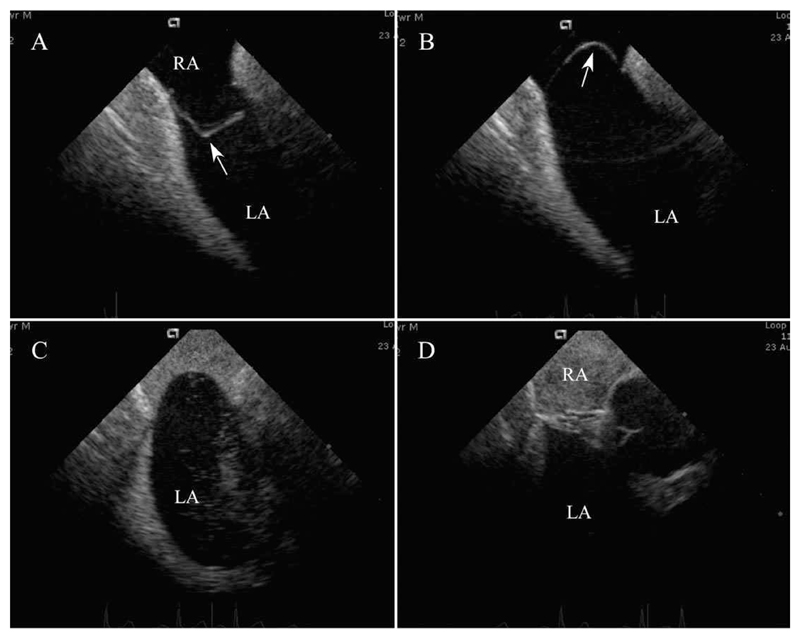
Fig. 4. Closure of PFO with large atrial septal aneurysm.
A and B: ICE pictures of aneurysmal septum with septum primum (arrow) deviation greater than 3 cm. Markers at side of image denote 1 cm. C: Bubble injection in inferior vena cava with opacification of right atrium and passage of bubbles into the left atrium. D: Bubble injection in inferior vena cava after device closure with opacification again seen of the right atrium but no passage of bubbles into the left atrium. Abbreviations: LA, left atrium; RA, right atrium.
Follow-up Results
So far, 14 patients have undergone 1 month review. No patients reported neurological symptoms or deterioration in exercise capacity. Two of the 14 patients reported palpitation symptoms suggestive of paroxysmal atrial fibrillation (AF), both settling spontaneously without medication. On examination all were in sinus rhythm with no new electrocardiogram changes. On echo, all devices (14/14) appeared to be well seated with no interference with the atrioventricular valves. No residual shunts were seen on color flow Doppler.
Conclusions
This is the first report of implantation and clinical outcomes with the new GORE™ septal occluder. Implantation of the GSO is safe, effective and was feasible under fluoroscopy and ICE in a day case setting. It was well tolerated with no residual shunt nor major adverse events at up to 1 month review postimplantation in a cohort of 14 patients. It was suitable for a variety of atrial septal anatomy including aneurysmal septums of up to 3 cm deviation and for tunnel length up to 12 mm. The low procedural times and fluoroscopy times compare favorably with Biostar and Amplatzer devices in septal defect closure [8] and are similar to those previously reported with the Helex device [7], despite being on the “learning curve” of new device implantation.
This device may have a number of long-term advantages. It contains a minimal amount of nitinol which imparts conformability and softness and may protect against erosions. The ePTFE membrane rapidly endothelializes and has been used in endovascular grafts for several decades with proven biocompatibility. The Helex device delivery system is less straightforward with multiple steps and some operators found it cumbersome to implant [6,9]. Residual shunts with the Helex device were thought to be related to device size and the presence of atrial septal aneurysm [10,11]. In our small cohort, successful closure was seen in all, with a range of device sizes used and many had atrial septal aneurysms. Episodes of atrial arrhythmia are thought to be less with the Helex device than other devices and longer term studies will be needed to see if this is the case with the new GSO device, of similar construction [12]. Late thrombosis is associated with incomplete endothelialization and late device dislocation tends to occur in the first month while endothelialization occurs, long-term data suggesting this is a rare event [13–16] and the need for surgical removal is low [17]. The first month results with the GSO are encouraging in this regard.
These early results suggest that the GSO is a safe effective PFO closure device that is straightforward to implant. It is quick to deploy with minimal imaging, has currently left no residual shunt on bubble study, has minimal risk of device embolization and is suitable for a range of atrial septal anatomy. Long-term follow-up will be required to investigate possible late complications such as wire fractures, device erosions, residual shunts, and long-term comparison with other devices will be helpful. However, this initial data suggests the GSO is a useful addition to the septal closure device armamentarium.
Footnotes
Conflict of interest: Nothing to report.
Disclosures: Dr. O Ormerod has a consultant agreement with WL Gore and Associates.
References
- 1.MacDonald ST, Carminati M, Chessa M. Managing adults with congenital heart disease in the catheterization laboratory: State of the art. Exp Rev Cardiovasc Ther. 2010;8:1741–1752. doi: 10.1586/erc.10.165. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, Quarti A, Bianchini F, Bucari S, Costantini C, Giovagnoni A, Perna GP. Late cardiac perforation after transcatheter closure of patent foramen ovale. Ann Thorac Surg. 2006;81:e29–30. doi: 10.1016/j.athoracsur.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 3.Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: Review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 4.El-Said HG, Moore JW. Erosion by the Amplatzer septal occluder: Experienced operator opinions at odds with manufacturer recommendations? Catheter Cardiovasc Interv. 2009;73:925–930. doi: 10.1002/ccd.21931. [DOI] [PubMed] [Google Scholar]
- 5.Sigler M, Jux C. Biocompatibility of septal defect closure devices. Heart. 2007;93:444–449. doi: 10.1136/hrt.2006.098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahn EM, Wilson N, Cutright W, Latson LA. Development and testing of the Helex septal occluder, a new expanded polytetrafluoroethylene atrial septal defect occlusion system. Circulation. 2001;104:711–716. doi: 10.1161/hc3301.092792. [DOI] [PubMed] [Google Scholar]
- 7.Ponnuthurai FA, van Gaal WJ, Burchell A, Mitchell A, Wilson N, Ormerod O. Single centre experience with GORE-HELEX septal occluder for closure of PFO. Heart Lung Circ. 2009;18:140–142. doi: 10.1016/j.hlc.2007.11.141. [DOI] [PubMed] [Google Scholar]
- 8.Morgan G, Lee KJ, Chaturvedi R, Benson L. A biodegradable device (BioSTAR) for atrial septal defect closure in children. Catheter Cardiovasc Interv. 2010;76:241–245. doi: 10.1002/ccd.22517. [DOI] [PubMed] [Google Scholar]
- 9.Delaney JW, Chan KC, Rhodes JF., Jr The design and deployment of the HELEX septal occluder. Congenit Heart Dis. 2006;1:202–209. doi: 10.1111/j.1747-0803.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen SG, Smout R, Spruance SL. Transcranial doppler quantification of residual shunt after percutaneous patent foramen ovale closure: Efficacy of the GORE((R)) HELEX septal occluder. J Interv Cardiol. 2011;24:366–372. doi: 10.1111/j.1540-8183.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 11.Thaman R, Faganello G, Gimeno JR, Szantho GV, Nelson M, Curtis S, Martin RP, Turner MS. Efficacy of percutaneous closure of patent foramen ovale: Comparison among three commonly used occluders. Heart. 2011;97:394–399. doi: 10.1136/hrt.2010.203950. [DOI] [PubMed] [Google Scholar]
- 12.Taaffe M, Fischer E, Baranowski A, Majunke N, Heinisch C, Leetz M, Hein R, Bayard Y, Buscheck F, Reschke M, Hoffman I, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder) Am J Cardiol. 2008;101:1353–1358. doi: 10.1016/j.amjcard.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. doi: 10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Korabathina R, Thaler DE, Kimmelstiel C. Stroke due to late device thrombosis following successful percutaneous patent foramen ovale closure. Catheter Cardiovasc Interv. 2011 doi: 10.1002/ccd.23342. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Zhao X, Zheng X, Chen S, Xu R, Qin Y. Incomplete endothelialization and late dislocation after implantation of an amplatzer septal occluder device. Circulation. 2011;124:e188–189. doi: 10.1161/CIRCULATIONAHA.110.991836. [DOI] [PubMed] [Google Scholar]
- 16.Tomar M, Khatri S, Radhakrishnan S, Shrivastava S. Intermediate and long-term followup of percutaneous device closure of fossa ovalis atrial septal defect by the Amplatzer septal occluder in a cohort of 529 patients. Ann Pediatr Cardiol. 2011;4:22–27. doi: 10.4103/0974-2069.79618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma SK, Tobis JM. Explantation of patent foramen ovale closure devices: a multicenter survey. JACC Cardiovasc Interv. 2011;4:579–585. doi: 10.1016/j.jcin.2011.01.009. [DOI] [PubMed] [Google Scholar]