Abstract
Natural killer (NK) cell immunosurveillance may be impaired by malignant disease, resulting in tumour escape and disease progression. Therapies that enhance NK cytotoxicity may therefore prove valuable in remission-induction and maintenance treatment regimes. Acute lymphoblastic leukaemia (ALL) has previously been considered resistant to NK cell lysis and not tractable to this approach. Our study demonstrates that Bortezomib, Valproate and Troglitzaone can upregulate NK activating ligands on a B-ALL cell line and on a proportion but not all adult primary B-ALL samples. Drug-treated ALL cells trigger higher levels of NK degranulation, as measured by CD107a expression, and this effect is dependent on signalling through the NK activating receptor NKG2D. These results suggest that Bortezomib, Valproate and Troglitzaone may have clinical utility in sensitizing ALL to NK mediated lysis in vivo.
Keywords: ALL, NK cells, NKG2D ligand
Introduction
Evidence for an anti-leukaemic effect of NK cells has been reported in a number of clinical settings including the beneficial effect of NK receptor-ligand mismatching in haplo-identical transplantation (1, 2) and efficacy of IL-2 as a maintenance treatment for acute leukaemia. Genetic studies indicate that possessing more NK activating killer-cell immunoglobulin-like receptor (KIR) genes reduces the risk of developing leukemia (3). In studies where haplo-indentical transplant donors were selected for maximal anti-leukemic potential, benefit was seen in acute myeloid leukemia (AML) more than acute lymphoblastic leukemia (ALL). In vitro studies confirm that ALL is relatively resistant to NK lysis (1, 4). However, recent evidence that activating KIRs protect against the development of childhood ALL suggests that NK cells do have some role in immune control of ALL (3). Strategies to augment NK mediated cytotoxicity and immunosurveillance of ALL may offer the potential to improve relapse free survival without adding to treatment toxicity. This is especially relevant in adult ALL where a higher frequency of adverse cytogenetics and reduced tolerance of chemotherapeutic regimens leads to high relapse rates.
NK cytotoxic granule release is determined by the integration of inhibitory and activating signals through a variety of receptors including inhibitory and activating KIRs and the activating receptors NKG2D, natural cytotoxicity receptors (NCRs) and DNAM-1. In addition to killing by cytotoxic granule release, NK cells express the death receptors TRAIL and Fas ligand, which trigger caspase-dependent apoptosis in target cells expressing their cognate ligands. Previous reports have indicated that low-level expression of activating ligands, such as the NKG2D ligands, may underpin the apparent NK-resistance of ALL (4). Modulating ligand expression on ALL cells is therefore a potential means of sensitisation to NK mediated lysis.
In the present study, we sought to increase NK degranulation in response to ALL cells using Bortezomib, Valproate and Troglitazone to alter the expression of NKG2D ligands, TRAIL ligands or HLA-class I. Previous studies have shown that Bortezomib, a proteasome inhibitor (5–10) and Valproate, a histone de-acetylase inhibitor (11–14) are capable of sensitising tumour cells to NK cytotoxicity but evidence is limited in ALL, especially for primary samples. Troglitazone, a thiazolidinedione, has not previously been explored as a sensitizing agent although it is known to have tumour cytostatic and differentiating effects [(15, 16)]. We present evidence that all three drugs enhance NK cell degranulation via an NKG2D-dependent mechanism and may have utility in potentiating NK mediated anti-leukemic effects in vivo.
Materials and Methods
Cell Lines and Clinical Samples
Human cell lines NALM-6 and K562 were provided by Newcastle Hematology Biobank. Cultures were maintained in RPMI 1640 supplemented with 10% FCS, penicillin, streptomycin and L-glutamate (RF10) at 37degrees and 5% CO2. Both lines were mycoplasma-free. Cells were subjected to drug treatment during log-phase growth. Peripheral blood or bone marrow mononuclear cells from adult B-ALL patients at diagnosis were provided by Newcastle Haematology Biobank under ethical permission from Newcastle and North Tyneside Research Ethics Committee 1. Samples were obtained with informed consent under a protocol approved by the local ethics committee. Mononuclear cells were separated from whole blood by density centrifugation and cryopreserved at -80°C.
Drug Preparation and Treatment
Bortezomib (Millennium) was provided by Newcastle hospital pharmacy at clinical concentration (3.5mg/ml in 0.9% sodium chloride with 5% mannitol). A stock solution was prepared by dilution with PBS, stored at 4°C and used within 10 weeks. Troglitazone (Sigma) was diluted in DMSO and stored at 4°C. Sodium valproate (Sigma) was diluted in PBS and stored at 4°C. Vehicle controls were PBS with 0.5% DMSO (Troglitazone) or PBS (Bortezomib and Valproate).
Bortezomib was used at 100nM with NALM-6. Previous studies have demonstrated NK sensitization of various cell lines with 10nM (5), but 100nM was chosen to more closely reflect the human therapeutic plasma concentration (17)(Bros PF). Troglitazone is known to effectively target PPARg in HeLa cells at 10uM (18). 5μM (2μg/ml) was used as pharmacokinetic studies in humans suggest this is a more achievable plasma level (19). Valproate was used at a concentration of 1mM. This dose is known to upregulate MICA/B in hepatoma cell lines (20) and plasma concentrations of up to 3mM can be achieved in humans with limited toxicity (21).
NALM-6 cells were suspended at a concentration of 5*10^5/ml RF10 in a 6 well plate with 100nM Bortezomib, 5μM Troglitazone or 1mM Sodium Valproate for 48 hours. Primary blood or bone marrow samples were thawed and non-viable cells removed by negative selection (Miltenyi dead cell removal kit). Cells were suspended at 5*10^5/ml in RF10 with 50nM Bortezomib, 5μM Troglitazone or 1mM Sodium Valproate for 16 hours. The Bortezomib dose and drug incubation times were reduced for primary cells to prevent unacceptable loss of viability.
Antibodies and Flow Cytometry
The following antibodies were used for analysis of NKG2D ligand, TRAIL ligand and HLA class I expression: MICA-PE (FAB1300P), MICB-APC (FAB1599A), ULBP1-PE (IC1380P), ULBP2-APC (FAB1298A) from R&D Systems; HLA-ABC-FITC (555552), from BD Biosciences; DR4-APC (307208) and DR5-PE (307406) from BioLegend. The analysis of apoptotic cells used BD Annexin V-PE apoptosis detection kit (559763). CD107a degranulation assay used CD107a-PE (555801), CD56-FITC (340723) and CD3-APC (555335) from BD Biosciences. NKG2D blocking assays used anti-NKG2D (MAB139) from R&D Systems and src-kinase inhibition assays used PP2 (Ascent Scientific).
Cells were prepared and washed in PBS with 2% FCS and 2mM EDTA. Mouse IgG was added for 10 minutes prior to antibodies in order to block non-specific binding. Samples were analysed using a FACSCanto3 (BD Biosciences).
CD107a Degranulation Assay
The assay used IL-2 activated peripheral blood mononuclear cells as effectors. These were isolated from healthy volunteers by Lymphoprep density centrifugation and cultured for 18-24 hours in RF10 with 100units/ml IL-2.
The drug or vehicle-treated ALL cells served as targets. Cells were washed twice to remove drug and mixed with effectors at 1:1 ratio in wells of a 96-well U-bottom plate. CD107a antibody was added at 1μl/well. After 2-hour incubation at 37°C degrees, duplicate wells were pooled and cells were prepared for FACS analysis as above. In blocking experiments, effectors were pre-incubated with 10μl/ml NKG2D antibody or targets were pre-incubated with 20μl/ml PP2 for 30 minutes before degranulation.
Results
1. Upregulation of NKG2D and TRAIL Ligands on NALM-6 Cells
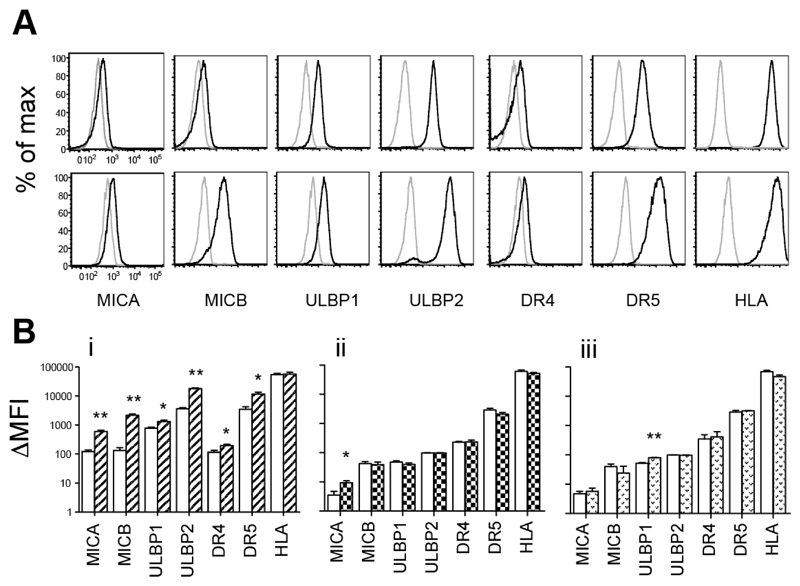
Expression of NKG2D ligands MICA, MICB, ULBP1 and ULBP2, the two activating TRAIL receptors DR4 and DR5 and MHC class I was measured on drug-treated and vehicle-treated NALM-6 cells by flow cytometry (Figure 1). Care was taken to compare ΔMFI measurements (mean fluorescence intensity with specific antibody minus no antibody) as drug treatment was found to cause significant increases in the size and auto-fluorescence of cells. Bortezomib significantly increased expression of all ligands but not MHC class I. Levels of NKG2D ligands MICA, MICB and ULBP2 were particularly enhanced, with 5.09 ± 1.32 (mean ± SD) fold higher ΔMFI for MICA, 19.5 ± 8.90 for MICB and 5.13 ± 1.33 for ULBP1 (all p<0.01) (Figure 1A and Bi). Valproate and Troglitazone were more selective and only induced significant upregulation of MICA and ULBP1 respectively.
Figure 1. Induction of NK Ligands on NALM-6.
(A) MICA, MICB, ULBP1, ULBP2, DR4, DR5 and HLA expression was assessed on NALM-6 treated with vehicle control (upper) and with Bortezomib (lower) by flow cytometry. Live cells were gated for analysis based on forward and side scatter parameters. Histograms show fluorescence of unstained cells (grey line) compared with fluorescence of cells stained with specific antibody (black line). Representative data from one of 3 experiments is shown. (B) ΔMFI (mean fluorescence intensity in stained minus mean fluorescence intensity in unstained) showing mean +/- SD from 3 independent experiments with i) Bortezomib ii) Valporate and iii) Troglitazone treated NALM-6. (*p<0.05, **p<0.01 when comparing expression of each ligand in vehicle and drug-treated cells).
2. NKG2D-dependent Enhancement of NK Degranulation against NALM-6 Cells
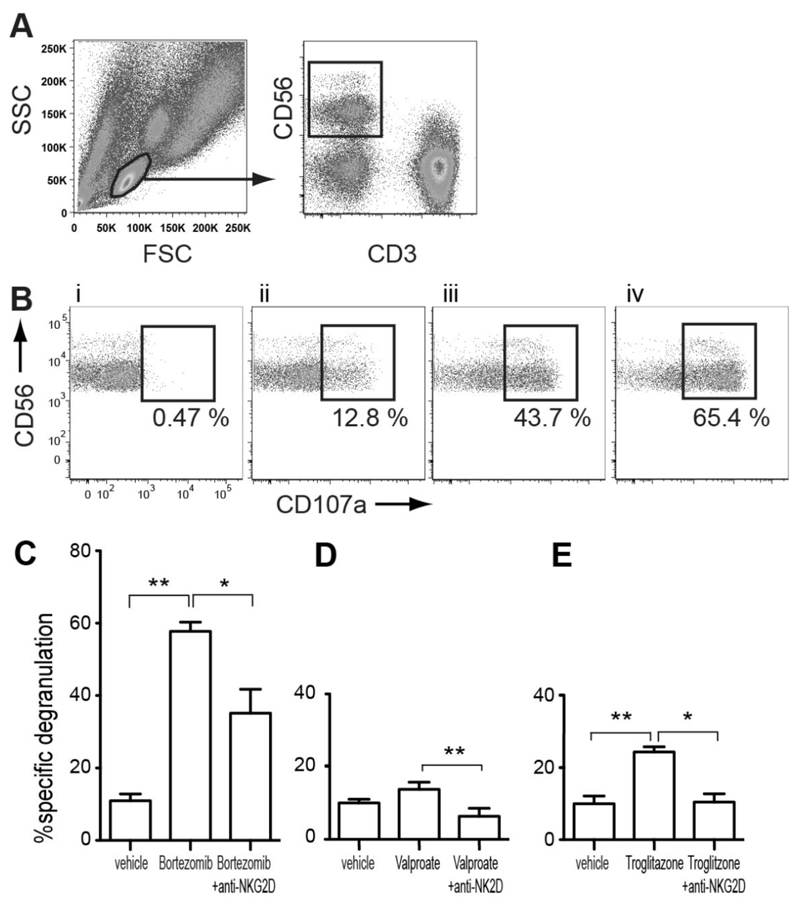
A CD107a degranulation assay was used to examine how drug treatment of targets affected signalling to NK cells. IL-2 activated NK cells were used as effectors. Without IL-2 activation, the magnitude of degranulation against any ALL target was low and virtually indistinguishable from background. NKG2D blocking antibody was added to test the NKG2D dependence of increases in degranulation induced by drug treatment. Primary NK cells in the assays were identified within the lymphocyte population demarcated by forward scatter/ side scatter gating as CD3-CD56+ cells (Figure 2A) and degranulated cells were identified as CD107a+. In all experiments, NK degranulation of targets was compared to a negative control of NK degranulation with medium alone (% specific degranulation = %CD107a+ with target - %CD107a+ with medium alone).
Figure 2. Enhancement and Blockade of NK Degranulation.
(A) Representative gating strategy used to identify the percentage of CD107a expressing NK cells in a degranulation assay where healthy donor PBMC are combined with a target cell. (B) CD107a expression by the NK cells where PBMC are combined with: i) medium alone (negative control), ii) MHC class I negative cell line K562 (positive control), iii) vehicle-treated NALM-6, iv) Bortezomib-treated NALM-6. NK degranulation against vehicle, drug, and drug in the presence of NKG2D blocking antibody is compared for (C) Bortezomib, (D) Valproate and (E) Troglitazone (*p<0.05, **p<0.01, n≥3 for all comparisons). % specific degranulation = %CD107a+ with target - %CD107a+ with medium alone.
After incubation with Bortezomib, NALM-6 targets elicited 5.21 ± 1.41 (mean ± SD) fold higher % specific degranulation of NK cells compared with vehicle pre-treatment, (Figure 2B). NKG2D blocking antibody reduced % specific NK degranulation against drug-treated targets by 39.8% ± 12.6, but did not reduce degranulation to the baseline of vehicle-treated targets. It follows that enhanced NKG2D signalling is an important component of Bortezomib-induced NK sensitization, but is not solely responsible for the effect. Addition of blocking antibody to untreated cells had no significant effect (data not shown). Valproate and Troglitazone showed much more modest induction of degranulation. Pre-treatment with Valproate increased % specific NK degranulation 1.37 ± 0.1 fold and blocking NKG2D completely abrogated the effect. Troglitazone increased % specific NK degranulation 2.59 ± 0.59 fold, an effect that was also reduced to baseline by NKG2D blocking-antibody. These results are in keeping with the more selective induction of NKG2D ligands by Valproate and Troglitazone.
3. Bortezomib, Valproate and Troglitazone Treatment of Primary ALL
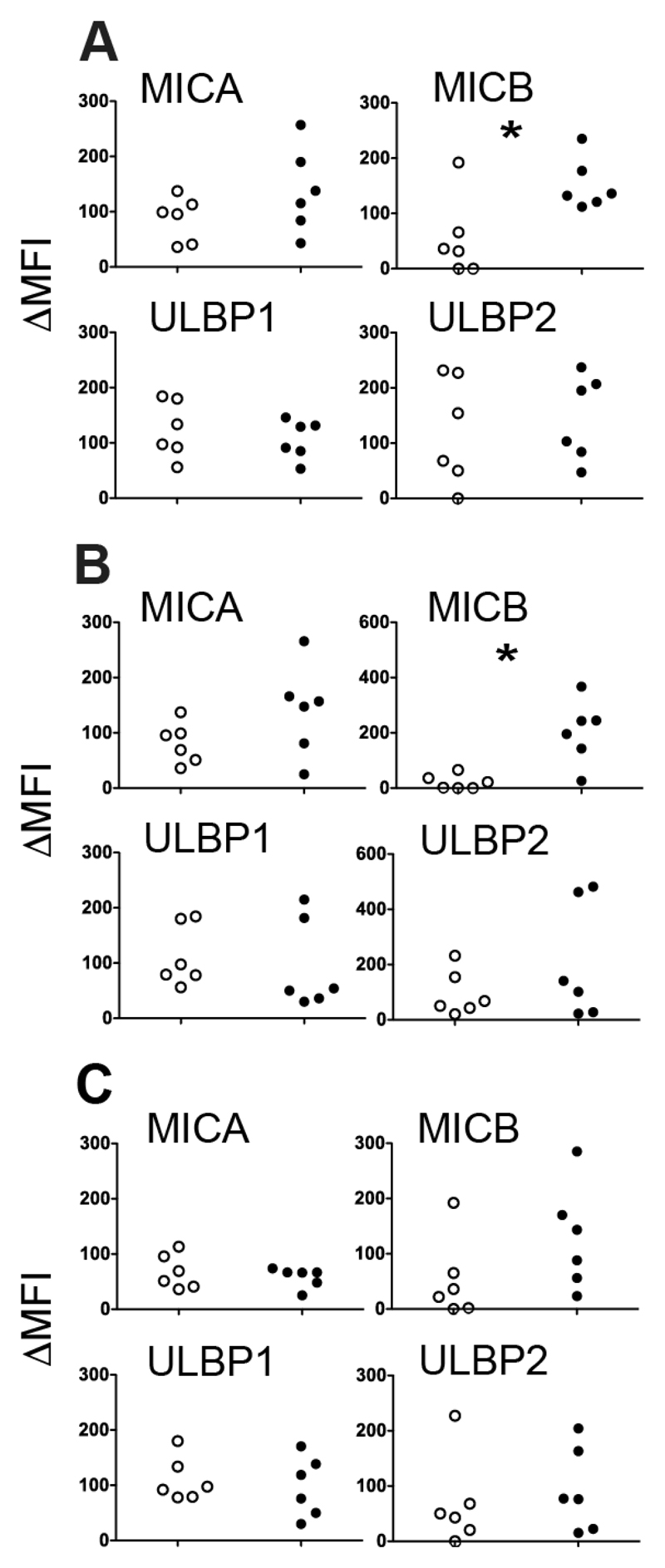
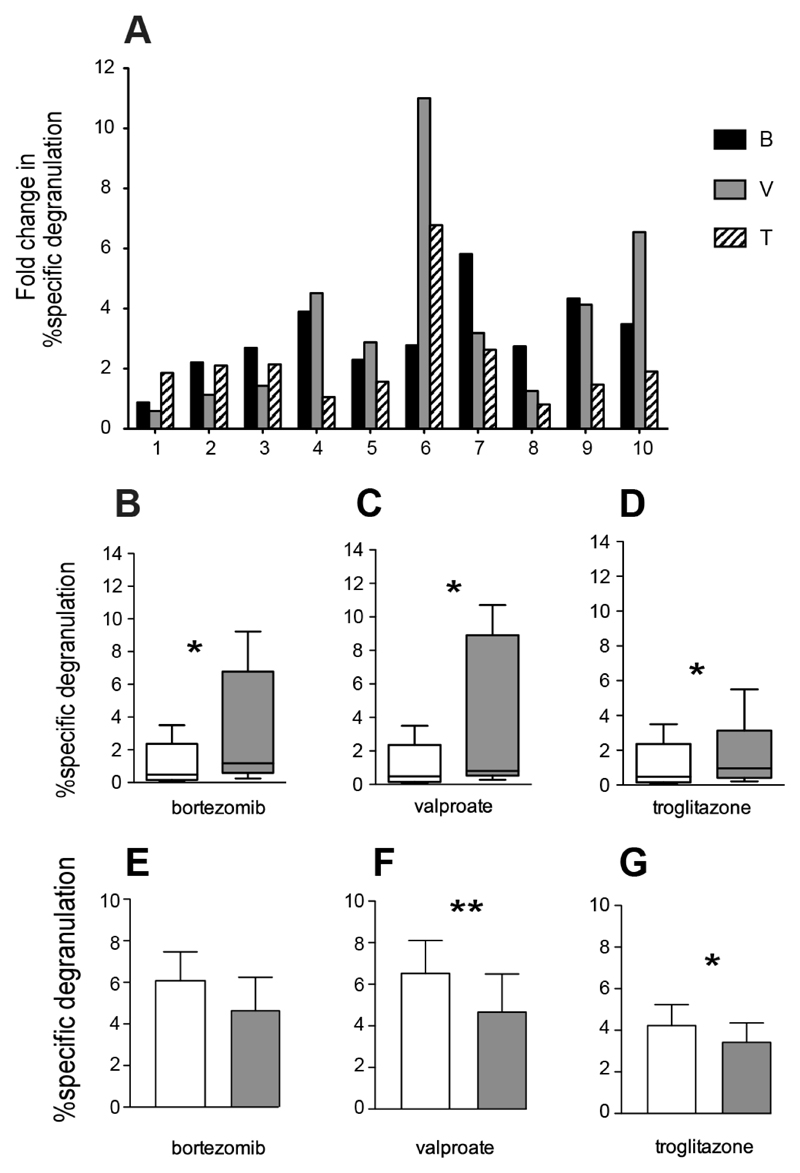
Having demonstrated upregulation of activating ligands and corresponding increases in NK degranulation using an ALL cell line, we explored the corresponding effects in 10 samples of primary adult B-ALL cells obtained at clinical presentation from peripheral blood or bone marrow. These experiments were much more challenging owing to the limited amount of some specimens and the low viability of primary blasts which varied from 45-93% (not shown). It would not have been possible to perform cytotoxicity assays on many of these specimens and assessment of NK degranulation provided a useful surrogate. Expression of MICA was increased by Bortezomib and Valproate and MICB by all 3 drugs in 3 ALL samples tested. NKG2D ligands ULBP1/2 were not significantly affected on average (Figure 3). Individual samples upregulated ligands in response to different drugs; no one sample was uniformly good at responding to drug treatment. There were no significant changes in expression of DR4/5 or HLA (data not shown). % specific NK degranulation was increased by all 3 drugs tested on 10 ALL samples. Almost every sample sensitized greater degranulation after one or more drug treatments (Figure 4 A). When all samples were combined, Bortezomib induced a 3.11 ± 1.35 fold increase; Troglitazone a 2.23 ± 1.68 fold increase and Valproate a 3.67 ± 3.17 fold increase. (Figure 4 B-D). Five of the primary samples were more readily sensitized by drug treatment but the pattern of sensitization varied from sample to sample. We initially attempted to block NKG2D signalling with antibody, as previously, but were unable to do so owing to an apparent increase in degranulation after pre-incubation with the antibody, attributed to Fc receptor-mediated redirected killing. This was probably not evident in NALM-6 cells used previously because of the more robust levels of degranulation. As an alternative method to explore NKG2D dependence, we pre-incubated targets with PP2. This src-kinase inhibitor has previously shown to prevent signalling downstream of NKG2D (22). Three samples with significant drug-sensitization were tested in a degranulation assay with PP2, resulting in down regulation but not complete ablation of sensitization by all drugs (Figure 4 E-G).
Figure 3. Induction of NK Ligand Expression on Primary ALL Blasts.
(A-C) Scatter plots showing effect of drug treatment on NKG2D ligand expression (ΔMFI above isotype control) for each of 3 primary ALL samples treated in duplicate with: A) 50nM Bortezomib, B) 1mM Valproate and C) 5μM Troglitazone compared with vehicle controls. Ligands MICA, MICB, ULBP1 and ULBP2 as indicated (*p<0.05, **p<0.01).
Figure 4. Induction of NK Degranulation with Primary ALL Blasts.
A) Induction of NK degranulation by 10 primary ALL samples (1-10) treated with 50nM Bortezomib (B), 1mM Valproate (V), 5μM Troglitazone (T) or vehicle control for 16 hours. Fold change in % specific degranulation = the ratio of % specific degranulation with and without drug.
B-D) Cumulative analysis of data in A) shown for each drug treatment (shaded bars) compared with untreated controls (open bars).
E-G) NK degranulation against 3 primary ALL samples treated as above, with 20µM src kinase inhibitor PP2 (shaded bars) vs no kinase inhibitor (open bars): D) 50nM Bortezomib, E) 1mM Valproate, F) 5μM Troglitazone (*p<0.05, **p<0.01).
Discussion
Our studies show that is it possible to enhance NK recognition of ALL by exposure to Bortezomib, Valproate and Troglitazone. These therapeutic agents are already licensed for clinical use and their effects on NK cells are present at concentrations achievable in vivo. Upregulation of NK degranulation was associated with increased expression of NKG2D ligands and blocked by anti-NKG2D antibodies or src inhibitor PP2. These effects were demonstrated in NALM-6, a lymphoblastic cell line, and primary isolates of ALL. Of the three agents, Bortezomib was the most effective. An NK degranulation assay was used to circumvent the issues of variable viability of primary ALL specimens and potential synergistic cytotoxic effects of the agents tested. It also allowed us to study NK activation on a small number of targets within the milieu of total mononuclear cells, which arguably constitutes a more physiological in vitro system than using highly enriched NK preparations. One disadvantage of this assay is that we were unable to test the functional significance of DR4 and DR5 upregulation as the time course and downstream mechanisms of TRAIL ligation differ from those of degranulation.
Acute lymphoblastic leukemia has previously been described as resistant to NK-mediated lysis owing to low expression of NK ligands (4). Enhancement of NK ligand expression by targets is an obvious approach to increasing NK recognition and has been explored in a number of cell lines. Bortezomib has been shown to sensitize a variety of tumour cells (5), in addition to multiple myeloma (8, 9), hepatocellular carcinoma (6), murine leukemia (7) and CD34+ CML cells (10).
Sensitization by upregulating NKG2D ligands (6, 9), TRAIL ligands (5, 7, 10) or by downregulating MHC class I (8) has been shown in different contexts. Primary isolates of ALL have not been previously tested. With an ALL cell line we also found upregulation of both NKG2D and TRAIL ligands but in primary ALL blasts the effect was much more restricted to MICB. Evidence of NK sensitization by Valproate and other HDAC inhibitors exists for a range of haematological (11–14) and non-haematological malignancies (20, 23–26). One previous report describes sensitisation of primary ALL blasts by increased expression of MICA and MICB (5, 12). Our data support this finding, although only MICB reached significance in our hands. In addition, we did not see upregulation of TRAIL ligands or reduction of MHC class I by Valproate. Finally we have shown that Troglitazone, a thiazolidinedione with tumour cytostatic and differentiating effects, is also a modest NK sensitizing agent; this has not been previously studied.
Drugs capable of sensitizing tumours to NK recognition have been shown to improve tumour clearance in animal models. Paradoxically, a number of studies highlight the potential of proteasome and HDAC inhibitors to impair other aspects of anti-tumour immunity. Both drugs may alter the phenotype, cytokine production and immunostimulatory capacity of dendritic cells (27, 28), leading to a reduction in tumour specific immunity. Bortezomib can induce resistance to antigen-specific T cells as proteosome inhibition alters processing of tumour antigen (29). Both Bortezomib and HDAC inhibitors can suppress activity of NK cells (30–32) and chronic exposure to surface and soluble NKG2D ligands can suppress NK function (33–35).
Further studies are now required to develop the concept of NK sensitisation for clinical use in ALL. As xeno-transplant models of in vivo NK cytotoxicity can be difficult to perform and the drugs tested are already in clinical use, it is reasonable to suggest that they be adopted by phase I/II clinical trials. Strategies to augment NK cytotoxicity in ALL may offer the potential to improve remission induction rates or prolong the durability of remission by maintenance therapy, without adding to treatment toxicity. This is especially relevant in adults and older patients with ALL where a higher frequency of adverse cytogenetics and reduced tolerance of chemotherapeutic regimens leads to high relapse rates.
Acknowledgements
This work was supported by a grant from the British Society for Haematology.
References
- 1.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. doi: 10.1126/science.1068440. Epub 2002/03/16. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–9. Epub 1999/06/25. [PubMed] [Google Scholar]
- 3.Almalte Z, Samarani S, Iannello A, Debbeche O, Duval M, Infante-Rivard C, et al. Novel associations between activating killer-cell immunoglobulin-like receptor genes and childhood leukemia. Blood. 2011;118(5):1323–8. doi: 10.1182/blood-2010-10-313791. Epub 2011/05/27. [DOI] [PubMed] [Google Scholar]
- 4.Romanski A, Bug G, Becker S, Kampfmann M, Seifried E, Hoelzer D, et al. Mechanisms of resistance to natural killer cell-mediated cytotoxicity in acute lymphoblastic leukemia. Experimental hematology. 2005;33(3):344–52. doi: 10.1016/j.exphem.2004.11.006. Epub 2005/02/26. [DOI] [PubMed] [Google Scholar]
- 5.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer research. 2006;66(14):7317–25. doi: 10.1158/0008-5472.CAN-06-0680. Epub 2006/07/20. [DOI] [PubMed] [Google Scholar]
- 6.Armeanu S, Krusch M, Baltz KM, Weiss TS, Smirnow I, Steinle A, et al. Direct and natural killer cell-mediated antitumor effects of low-dose bortezomib in hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(11):3520–8. doi: 10.1158/1078-0432.CCR-07-4744. Epub 2008/06/04. [DOI] [PubMed] [Google Scholar]
- 7.Hallett WH, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180(1):163–70. doi: 10.4049/jimmunol.180.1.163. Epub 2007/12/22. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111(3):1309–17. doi: 10.1182/blood-2007-03-078535. Epub 2007/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113(15):3503–11. doi: 10.1182/blood-2008-08-173914. Epub 2008/12/23. [DOI] [PubMed] [Google Scholar]
- 10.Yong AS, Keyvanfar K, Hensel N, Eniafe R, Savani BN, Berg M, et al. Primitive quiescent CD34+ cells in chronic myeloid leukemia are targeted by in vitro expanded natural killer cells, which are functionally enhanced by bortezomib. Blood. 2009;113(4):875–82. doi: 10.1182/blood-2008-05-158253. Epub 2008/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skov S, Pedersen MT, Andresen L, Straten PT, Woetmann A, Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer research. 2005;65(23):11136–45. doi: 10.1158/0008-5472.CAN-05-0599. Epub 2005/12/03. [DOI] [PubMed] [Google Scholar]
- 12.Kato N, Tanaka J, Sugita J, Toubai T, Miura Y, Ibata M, et al. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;21(10):2103–8. doi: 10.1038/sj.leu.2404862. Epub 2007/07/13. [DOI] [PubMed] [Google Scholar]
- 13.Diermayr S, Himmelreich H, Durovic B, Mathys-Schneeberger A, Siegler U, Langenkamp U, et al. NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK-cell lines with single KIR-HLA class I specificities. Blood. 2008;111(3):1428–36. doi: 10.1182/blood-2007-07-101311. Epub 2007/11/13. [DOI] [PubMed] [Google Scholar]
- 14.Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(4):641–8. doi: 10.1038/leu.2008.354. Epub 2009/01/20. [DOI] [PubMed] [Google Scholar]
- 15.Huang H, Wu D, Fu J, Chen G. Multiple myeloma cells undergo differentiation upon exposure to rosiglitazone and all-trans retinoic acid. Leukemia & lymphoma. 2009;50(6):966–73. doi: 10.1080/10428190902866724. Epub 2009/04/18. [DOI] [PubMed] [Google Scholar]
- 16.Saiki M, Hatta Y, Yamazaki T, Itoh T, Enomoto Y, Takeuchi J, et al. Pioglitazone inhibits the growth of human leukemia cell lines and primary leukemia cells while sparing normal hematopoietic stem cells. International journal of oncology. 2006;29(2):437–43. Epub 2006/07/06. [PubMed] [Google Scholar]
- 17.Bross PF, Kane R, Farrell AT, Abraham S, Benson K, Brower ME, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(12 Pt 1):3954–64. doi: 10.1158/1078-0432.CCR-03-0781. Epub 2004/06/26. [DOI] [PubMed] [Google Scholar]
- 18.Coyle AT, Kinsella BT. Synthetic peroxisome proliferator-activated receptor gamma agonists rosiglitazone and troglitazone suppress transcription by promoter 3 of the human thromboxane A2 receptor gene in human erythroleukemia cells. Biochemical pharmacology. 2006;71(9):1308–23. doi: 10.1016/j.bcp.2006.01.011. Epub 2006/02/28. [DOI] [PubMed] [Google Scholar]
- 19.Young MA, Lettis S, Eastmond R. Concomitant administration of cholestyramine influences the absorption of troglitazone. British journal of clinical pharmacology. 1998;45(1):37–40. doi: 10.1046/j.1365-2125.1998.00645.x. Epub 1998/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer research. 2005;65(14):6321–9. doi: 10.1158/0008-5472.CAN-04-4252. Epub 2005/07/19. [DOI] [PubMed] [Google Scholar]
- 21.Wilimowska J, Florek E, Piekoszewski W. Disposition of valproic acid in self-poisoned adults. Basic & clinical pharmacology & toxicology. 2006;99(1):22–6. doi: 10.1111/j.1742-7843.2006.pto_417.x. Epub 2006/07/27. [DOI] [PubMed] [Google Scholar]
- 22.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nature immunology. 2003;4(6):557–64. doi: 10.1038/ni929. Epub 2003/05/13. [DOI] [PubMed] [Google Scholar]
- 23.Schmudde M, Braun A, Pende D, Sonnemann J, Klier U, Beck JF, et al. Histone deacetylase inhibitors sensitize tumour cells for cytotoxic effects of natural killer cells. Cancer letters. 2008;272(1):110–21. doi: 10.1016/j.canlet.2008.06.027. Epub 2008/08/23. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Wang Y, Zhou Z, Zhang J, Tian Z. Sodium butyrate upregulates expression of NKG2D ligand MICA/B in HeLa and HepG2 cell lines and increases their susceptibility to NK lysis. Cancer immunology, immunotherapy : CII. 2009;58(8):1275–85. doi: 10.1007/s00262-008-0645-8. Epub 2009/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Soto A, Folgueras AR, Seto E, Gonzalez S. HDAC3 represses the expression of NKG2D ligands ULBPs in epithelial tumour cells: potential implications for the immunosurveillance of cancer. Oncogene. 2009;28(25):2370–82. doi: 10.1038/onc.2009.117. Epub 2009/05/12. [DOI] [PubMed] [Google Scholar]
- 26.Yamanegi K, Yamane J, Kobayashi K, Kato-Kogoe N, Ohyama H, Nakasho K, et al. Sodium valproate, a histone deacetylase inhibitor, augments the expression of cell-surface NKG2D ligands, MICA/B, without increasing their soluble forms to enhance susceptibility of human osteosarcoma cells to NK cell-mediated cytotoxicity. Oncology reports. 2010;24(6):1621–7. doi: 10.3892/or_00001026. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 27.Straube C, Wehner R, Wendisch M, Bornhauser M, Bachmann M, Rieber EP, et al. Bortezomib significantly impairs the immunostimulatory capacity of human myeloid blood dendritic cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007;21(7):1464–71. doi: 10.1038/sj.leu.2404734. Epub 2007/05/15. [DOI] [PubMed] [Google Scholar]
- 28.Song W, Tai YT, Tian Z, Hideshima T, Chauhan D, Nanjappa P, et al. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25(1):161–8. doi: 10.1038/leu.2010.244. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lundqvist A, Su S, Rao S, Childs R. Cutting edge: bortezomib-treated tumors sensitized to NK cell apoptosis paradoxically acquire resistance to antigen-specific T cells. J Immunol. 2010;184(3):1139–42. doi: 10.4049/jimmunol.0902856. Epub 2009/12/23. [DOI] [PubMed] [Google Scholar]
- 30.Markasz L, Stuber G, Vanherberghen B, Flaberg E, Olah E, Carbone E, et al. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Molecular cancer therapeutics. 2007;6(2):644–54. doi: 10.1158/1535-7163.MCT-06-0358. Epub 2007/02/20. [DOI] [PubMed] [Google Scholar]
- 31.Feng X, Yan J, Wang Y, Zierath JR, Nordenskjold M, Henter JI, et al. The proteasome inhibitor bortezomib disrupts tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression and natural killer (NK) cell killing of TRAIL receptor-positive multiple myeloma cells. Molecular immunology. 2010;47(14):2388–96. doi: 10.1016/j.molimm.2010.05.003. Epub 2010/06/15. [DOI] [PubMed] [Google Scholar]
- 32.Ogbomo H, Michaelis M, Kreuter J, Doerr HW, Cinatl J., Jr Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS letters. 2007;581(7):1317–22. doi: 10.1016/j.febslet.2007.02.045. Epub 2007/03/14. [DOI] [PubMed] [Google Scholar]
- 33.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–8. doi: 10.1038/nature01112. Epub 2002/10/18. [DOI] [PubMed] [Google Scholar]
- 34.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nature immunology. 2005;6(9):928–37. doi: 10.1038/ni1239. Epub 2005/08/24. [DOI] [PubMed] [Google Scholar]
- 35.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106(5):1711–7. doi: 10.1182/blood-2005-03-0918. Epub 2005/05/12. [DOI] [PubMed] [Google Scholar]