Abstract
Objective
The role of tumor necrosis factor (TNF) in systemic sclerosis (SSc) remains controversial. The present study was undertaken to investigate the influence of TNF receptor (TNFR)–costimulated lymphocytes on collagen expression in fibroblasts.
Methods
TNFR expression on mononuclear cells from the dermis and blood of SSc patients was assessed by flow cytometry. Peripheral blood CD3+ lymphocytes were activated with CD3/CD28 beads and costimulated with TNFR-selective variants. Expression of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R), IL-10, and IL-13 was detected by enzyme-linked immunosorbent assay or quantitative reverse transcription–polymerase chain reaction. Healthy fibroblasts were incubated with conditioned media from TNFR-costimulated T lymphocytes, and type I collagen expression was quantified.
Results
TNFRI and TNFRII were up-regulated on dermal T lymphocytes from patients with diffuse cutaneous SSc. TNFRII expression correlated with skin thickening. After CD3/CD28 activation, peripheral blood lymphocytes from SSc patients produced more IL-6, sIL-6R, and IL-13 compared to healthy lymphocytes. Costimulation with TNFRI-selective ligands and soluble TNF further increased IL-6 expression, whereas costimulation with TNFRII led to greater release of sIL-6R. IL-10 expression, which normally occurs after TNFRII costimulation, was impaired in SSc T cells. Supernatants of TNF-costimulated SSc lymphocytes induced higher type I collagen expression in fibroblasts, which was partially reversible by dual inhibition of IL-6 and IL-13. Expression of TNFR and IL-6 in the dermis was reversible in a patient who received lymphoablative therapy prior to autologous hematopoietic stem cell transplantation.
Conclusion
TNF-costimulated T lymphocytes from SSc patients have a propensity to secrete profibrotic cytokines, while the ability to produce IL-10 is weakened. These results suggest that T lymphocytes in SSc support fibrosis, but might lack the capacity to resolve inflammation.
Systemic sclerosis (SSc) is an autoimmune connective tissue disease that leads to excessive fibrosis of the skin and internal organs. It is characterized by vasculopathy and persistent low-grade inflammation, which ultimately result in increased extracellular matrix deposition by fibroblasts and myofibroblasts (1). The inflammatory cell infiltrate in the affected dermis of SSc patients consists mainly of T lymphocytes, likely attracted by antigen-presenting cells or mast cells (2–5). Lymphocytes in SSc tissue undergo oligoclonal expansion, which indicates antigen-specific activation and costimulation (6). Teff cells in the peripheral blood of patients with SSc are also activated, as demonstrated by their expression of HLA–DR or tumor necrosis factor receptor (TNFR) (7,8). It is commonly accepted that Th2 cytokines, such as interleukin-4 (IL-4), IL-6, and IL-13, are overexpressed in SSc (7,9). In addition, it was recently suggested that functionally defective Treg cells are quantitatively increased in SSc (10). Accordingly, Treg cells producing IL-10 are reduced in both the blood (11) and the dermis (12) of SSc patients. As shown in lung fibrosis, IL-10 not only acts as an antiinflammatory mediator but also is antifibrotic, notably in association with the IL-13α decoy receptor (13).
There is growing evidence that the interaction between T cells and fibroblasts via IL-6 and IL-13 influences the deposition of extracellular matrix in SSc (14) and that T cells can trigger the differentiation of hematopoietic cells into fibrocytes (15). In SSc dermis, inflammatory cell infiltration colocalizes with collagen production and clinically correlates with skin thickening (5). T cell transfer from bleomycin-treated mice to healthy animals induces skin thickening (16), highlighting the critical role of T lymphocytes in SSc.
TNF is a pleiotropic cytokine that is mainly known for its proinflammatory properties. However, TNF also has immunosuppressive feedback effects (17,18), as well as profibrotic properties (19). Both in SSc patients and in animal models, soluble and in situ levels of TNFR are up-regulated in blood and skin, and increased levels of TNF in tissue and blood, correlating with disease activity, have been described (20–23). Soluble TNF (sTNF) has been shown to mediate the transition from pulmonary inflammation to fibrosis, e.g., by triggering transforming growth factor β (TGFβ) expression (24), and experimental lung fibrosis responds to TNF antagonist treatment (25). In clinical studies, blockade of TNF with etanercept or infliximab has been investigated in different SSc cohorts. Etanercept was effective in SSc-associated arthritis, and reduced skin thickening was reported (26). In another recent study, no significant change in the skin score was observed after treatment with infliximab (27). Given the modest clinical effect of TNF inhibitors observed in those trials, the role of TNF in SSc pathogenesis remains controversial (28). The notion that TNF does have some role is supported by the findings that TNF-treated fibroblasts exhibit reduced collagen expression and that membrane-bound TNF on nonactivated lymphocytes reduces collagen expression in fibroblasts, suggesting protection against the development of fibrosis (29).
The heterogeneous effects of TNF might be attributed to its 2 different forms: the membrane-bound form and (after cleavage by TACE) the soluble form (30). Furthermore, TNF responses are mediated via 2 different receptors, TNFRI (CD120a; p55) and TNFRII (CD120b; p75). Whereas TNFRI is efficiently activated by both forms of TNF, TNFRII is efficiently activated only by membrane-bound TNF, thus being dependent on cell contact (31). TNFRI is ubiquitously expressed at low levels on the plasma membrane, whereas TNFRII is highly regulated in its expression and primarily found on immune cells, endothelial cells, and neurons including activated Teff and Treg cells (32). Proliferation of peripheral T cells also appears to be mediated by TNFRII, indicating the role of TNFRII as a costimulatory molecule (33). Moreover, TNFRII has been shown to promote the function and expansion of Treg cells (17). In several recent reports, it was postulated that TNFRII is involved in fibrogenesis and that its overexpression and subsequent shedding by proteases exerts antiinflammatory effects by binding and inactivating sTNF (19). Indeed, collagen accumulation and proliferation in intestinal myofibroblasts induced by TNF is mediated primarily through TNFRII, as genetic deletion of TNFRII leads to reduced TNF-induced collagen production (19).
To date, the different fibrogenetic effects of TNFRI and TNFRII on SSc lymphocytes have not been addressed. The aim of this study was therefore to identify cell types with high TNFR expression in the dermis of SSc patients and to perform mechanistic studies by selective TNFR costimulation of SSc lymphocytes with subsequent cytokine analysis and assessment of the effects on collagen production by dermal fibroblasts.
Patients and Methods
Patient recruitment
Patients with SSc were recruited from Freeman Hospital in Newcastle upon Tyne, UK and James Cook University Hospital in Middlesbrough, UK. Ethics approval and written informed consent were obtained. All patients were age 18 years or older and fulfilled the American College of Rheumatology (ACR) criteria for SSc (34). Skin biopsy samples were obtained from 12 patients. (Patient characteristics are described in Supplementary Table 1, on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1529-0131.) Six of these patients had diffuse cutaneous SSc (dcSSc), 5 had limited cutaneous SSc, and 1 had prescleroderma with Raynaud’s phenomenon, anticentromere antibodies, and abnormal results on nailfold capillaroscopy. One patient with dcSSc who received lymphoablative therapy followed by hematopoietic stem cell transplantation (HSCT) underwent two skin biopsies, one before and one 6 months after HSCT.
T cells from peripheral blood were obtained from a further 8 patients who fulfilled the ACR criteria for SSc. Healthy control skin samples were obtained from patients undergoing breast reduction surgery.
Flow cytometry and immunohistochemistry analysis of skin biopsy samples
Skin cells from the forearm of 12 SSc patients were analyzed by multicolor flow cytometry using a modification of a technique described by Haniffa et al (35). Briefly, a 4-mm punch biopsy was performed with local anesthesia. The skin was digested for 1 hour with 10 units/ml Dispase (Gibco BRL) in X-Vivo medium (Cambrex). The epidermis was removed with a scalpel and the dermis was further digested in 1.6 mg/ml type IV collagenase (Worthington) overnight in X-Vivo medium. Cells were resuspended and incubated for 10 minutes at 4°C in 1% mouse IgG (Sigma). They were then stained with directly fluorophore-coupled antibodies to CD45, HLA–DR, CD14, CD1a, CD3, CD4, CD19 (all from Becton Dickinson), and TNFRI and TNFRII (R&D Systems) for 1 hour on ice. Multicolor flow cytometry with prior adequate compensation procedures was performed using a BD LSRII cytometer and FACS Diva software. Macrophages were identified on the basis of autofluorescence at 488/610 nm. TNFR expression was quantified by calculating the relative mean fluorescence intensity.
For immunohistochemistry, skin biopsy samples were fixed in 4% paraformaldehyde and embedded in paraffin. After sectioning, the samples were deparaffinized and then stained with hematoxylin and eosin or with an anti-CD3 antibody (Abcam) for 1 hour after blocking with 3% bovine serum albumin. For detection, the specimens were incubated for 30 minutes with horseradish peroxidase–labeled secondary goat anti-mouse antibodies (Invitrogen).
Cell culture
For CD3+ cell isolation, whole blood (40 ml) was collected in EDTA-containing tubes (BD Bioscience) at the time of routine outpatient visits. The samples were processed within 4 hours of collection. Peripheral blood mononuclear cells (PBMCs) were separated from whole blood by Ficoll-Hypaque density-gradient centrifugation (Axis Shield). CD3+ lymphocytes were isolated from total PBMCs using a CD3+ MACS Beads Isolation Kit according to the protocol recommended by the manufacturer (Miltenyi Biotec). Briefly, PBMCs (4 × 107) were suspended in phosphate buffered saline (PBS) supplemented with 1% fetal calf serum (FCS; Sigma) and 2 mM EDTA plus 40 μl of anti-CD3+ Miltenyi magnetic beads for 20 minutes on ice. The total mixture was passed through a magnetic isolation column. Purified CD3+ monocytes were removed from the column and tested for purity by flow cytometry, which demonstrated >95% purity. Cells were then cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM) in an incubator in 5% CO2 at 37°C. CD3+ cells (1 × 106/well) were placed in 24-well culture plates in DMEM supplemented with 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM) and cultured for 24 hours with CD3/CD28 stimulatory T cell expander beads (Invitrogen) at a 1:50 bead:T cell ratio, without the addition of any extra cytokines or growth medium. Primary fibroblasts were derived from healthy skin biopsy samples obtained during breast reduction surgery. The dermis was placed in a culture flask and the fibroblasts grew from the explants within. Primary fibroblasts were cultured in Iscove’s modified Eagle’s medium (IMEM) supplemented with 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), and l-glutamine (4 mM). All fibroblast cultures were used at passages 1–4.
TNFR costimulation
For selective stimulation with TNFRI and TNFRII, recombinant CysTNF32W/86T and CysTNF143T/145R (36), respectively, were used. Recombinant human TNF (2 × 107 units/mg) was provided by Knoll. CD3+ cells were incubated with sTNF or the different TNF variants at a concentration of 100 ng/ml for 24 hours, either directly after isolation or in costimulation studies after incubation with CD3/CD28 beads as described above.
Gene expression studies
Expression of genes for IL-6 and collagen was assessed by quantitative reverse transcription–polymerase chain reaction (qRT-PCR). RNA from CD3+ T lymphocytes was isolated using an RNA Mini kit according to the instructions of the manufacturer (Qiagen). RNA (200–750 ng) was treated with DNase for 30 minutes at 37°C, then reverse transcribed to complementary DNA using random hexamers (Invitrogen) and Moloney murine leukemia virus reverse transcriptase enzyme (Invitrogen). TaqReady Master Mix (Sigma) was used to analyze expression of IL-6. Samples were analyzed in triplicate and normalized to the housekeeping gene 18S using AB7500 software (Applied Biosystems). The primers used for IL-6 were as follows: forward 5′-TACCCCCAGGAGAAGATT-3′, reverse 5′-AAGGTTCAGGTTGTTTTC-3′ (universal probe library; Roche Applied Science). Collagen gene–specific primers were 5'-CAAGAGGAAGGCCAAGTCGAGG-3′ (forward) and 5′-CGTTGTCGCAGACGCAGAT-3′ (reverse).
Enzyme-linked immunosorbent assay (ELISA)
For ELISA, recombinant IL-13 and soluble IL-6 receptor (sIL-6R) were purchased from R&D Systems. An in-house–generated ELISA was used to measure IL-6 concentrations and was performed as described previously (37). Samples were analyzed in triplicate. The IL-10 ELISA was performed according to the instructions of the manufacturer (MSD).
Conditioned media and cytokine neutralization experiments
Isolated primary fibroblasts were cultured in 24-well plates in IMEM supplemented with 10% FCS, penicillin (100 units/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM) until confluent. After being washed twice with PBS, fibroblasts were incubated overnight with 200 μl conditioned medium from T cells that were left untreated or treated for 24 hours with sTNF or TNFR-selective ligands at a concentration of 100 ng/ml. Supernatants were then removed, fibroblasts were washed with prewarmed PBS, and RNA was isolated.
For cytokine neutralization, conditioned media were preincubated with rat anti-human IL-6, rat anti-human IL-13 neutralizing IgG (each at 1 μg/ml; BD Biosciences), both antibodies combined, or matched isotype control IgG. As a further control, the isotype concentration was increased to 2 μg/ml for 30 minutes before incubation with healthy dermal fibroblasts. The next day, qRT-PCR analysis for collagen was performed as described above. For STAT-3 experiments, fibroblasts were serum starved overnight and subsequently incubated in serum-free DMEM in the presence of IL-6 (20 ng/ml), sIL-6R (25 ng/ml), or IL-13 (100 ng/ml) (all from R&D Systems), with or without preincubation with the selective STAT-3 inhibitor S31-201 (Selleck) at 50 μM.
Statistical analysis
Results are shown as the mean ± SEM. Data were evaluated by Student’s t-test or analysis of variance. For nonparametric analysis, the Mann-Whitney U test and the Kruskal-Wallis test were performed. P values less than or equal to 0.05 were considered significant.
Results
TNFR expression on dermal leukocyte subsets
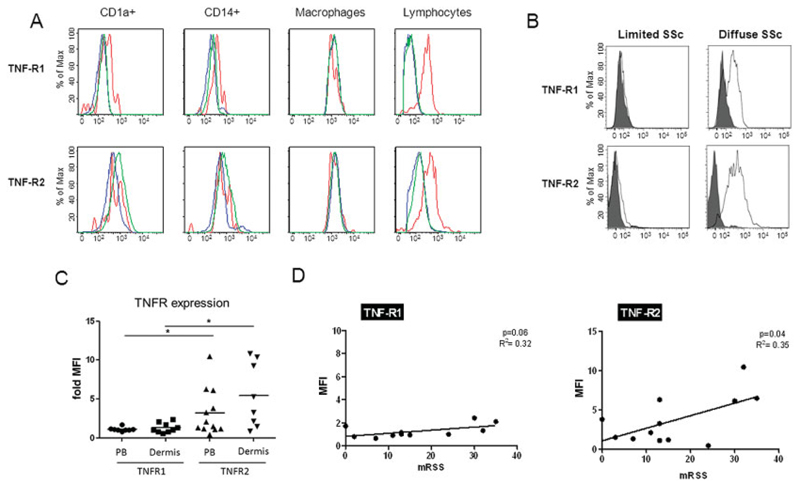
Expression of TNFRI and TNFRII in the dermis was analyzed by flow cytometry on CD45+HLA–DR–SSClowCD3+lymphocytes, CD45+HLA–DR+AF+ macrophages, CD45+HLA–DR+AF–CD14+ monocytes, and CD45+HLA–DR+AF–CD1a+ dendritic cells (SSC represents side scatter, AF represents auto-fluorescence) (Figure 1A). While dermal CD1a+ dendritic cells and CD14+ monocytes weakly expressed TNFRI, no obvious expression of TNFRII was observed in these cells. In contrast, T lymphocytes from patients with severe dcSSc exhibited high levels of TNFRI and TNFRII expression (Figure 1B). TNFRI and TNFRII expression on dermal lymphocytes was detected on both the CD4 and the CD8 subsets (data not shown), with significantly higher levels of TNFRII expressed on the cell surface in both subsets, compared to TNFRI (P < 0.05). Compared to lymphocytes from the peripheral blood, there was a trend toward greater expression of both TNFRI and TNFRII on lymphocytes from the dermis (Figure 1C), suggesting further activation of these cells in the tissue. TNFRII levels correlated significantly (P = 0.04), and TNFRI expression showed a trend toward correlation (P = 0.06), with skin thickening as determined with the modified Rodnan skin thickness score (38) (Figure 1D). Interestingly, in the patient with prescleroderma with severe Raynaud’s phenomenon of recent onset, positive anticentromere antibodies, and abnormal nailfold capillaroscopy findings but no skin thickening, we found the highest expression of both TNFRI and TNFRII.
Figure 1.
High levels of tumor necrosis factor receptor I (TNFRI) and TNFRII on lymphocytes from patients with diffuse cutaneous systemic sclerosis (dcSSc). A, Dermal skin biopsy specimens from 12 SSc patients (red lines) were digested and processed for flow cytometry to identify CDA1a+ dendritic cells, CD14+ monocytes, macrophages, and CD3 lymphocyte populations, with fluorescein isothiocyanate–labeled anti-TNFRI and phycoerythrin-labeled anti-TNFRII added to the antibody panel. Experiments were also performed using normal skin specimens (green lines) and staining with isotype control (blue lines). B, Expression of TNFRI and TNFRII on lymphocytes from patients with limited cutaneous SSc and from patients with dcSSc was compared. Lymphocytes from patients with severe dcSSc exhibited higher levels of both TNFRI and TNFRII. C, Levels of TNFRI and TNFRII were measured in dermal and peripheral blood (PB) lymphocytes from patients with SSc. Lymphocytes from both the dermis and the peripheral blood of SSc patients exhibited significantly higher expression of TNFII than of TNFI (* = P < 0.05). In addition, there was a trend toward higher expression of the TNFRs on lymphocytes from the dermis as compared to lymphocytes from the peripheral blood. Symbols represent individual patients; horizontal bars show the mean. D, Skin thickening was determined using the modified Rodnan skin thickness score (MRSS), and the relationship between TNFR expression levels and skin thickening was assessed. MFI = mean fluorescence intensity.
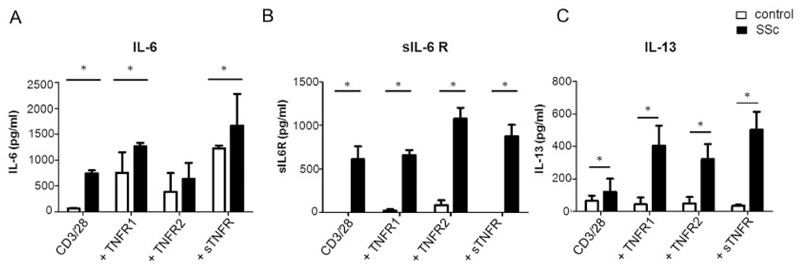
Effect of selective TNFR costimulation on IL-6, sIL-6R, and IL-13 expression in CD3/CD28-activated peripheral blood lymphocytes
CD3+ lymphocytes were isolated from the peripheral blood of SSc patients and healthy controls. Cells were left untreated or were stimulated with CD3/CD28 beads prior to coactivation with TNF variants selectively stimulating TNFRI and TNFRII, respectively, or with sTNF, which primarily stimulates TNFRI. Soluble IL-6R was measured, as fibroblasts do not express the membrane-bound form of IL-6R and need sIL-6R to signal. Levels of IL-6, sIL-6R, and IL-13 protein secretion are shown in Figure 2. CD3/CD28-activated T cells from SSc patients produced significantly higher levels of all 3 cytokines at baseline. Further secretion of IL-6 was induced by TNFR stimulation both in SSc patients and in healthy individuals (Figure 2A). Conversely, only marginal sIL-6R and IL-13 expression was induced by TNF in healthy donors (Figures 2B and C). Costimulation with soluble TNF had the strongest effect on IL-6 and IL-13 secretion, whereas sIL6R release mainly occurred after selective TNFRII ligation. There was a significant difference in sIL-6R levels (P < 0.05), but not in IL-6 and IL-13 levels, between TNFRI- and TNFRII-stimulated T cells from SSc patients. In lymphocytes from healthy donors without bead activation and subsequent TNFRI, TNFRII, and sTNF stimulation, sIL-6R could not be detected, indicating that in vitro activation with CD3/CD28 beads is necessary for the shedding of sIL-6R.
Figure 2.
Activated lymphocytes from patients with SSc overexpress interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R), and IL-13. CD3+ lymphocytes isolated from peripheral blood of SSc patients and healthy controls (n = 8 per group) were activated with CD3/CD28 beads and costimulated with receptor-selective TNF variants and sTNF. Levels of IL-6 (A), sIL-6R (B), and IL-13 (C) in supernatants were determined by enzyme-linked immunosorbent assay. Untreated activated T cells from SSc patients expressed significantly higher levels of IL-6, sIL-6R, and IL-13 compared to healthy lymphocytes. IL-6 expression was further induced by sTNF and TNF variant stimulation in lymphocytes both from SSc patients and from healthy controls, whereas only marginal sIL-6R and IL-13 expression was induced by sTNF or TNF variants in controls. Costimulation with sTNF had the strongest effect on IL-6 and IL-13 expression in SSc lymphocytes, whereas sIL-6R expression was mostly enhanced by selective TNFRII stimulation. Values are the mean ± SEM. * = P < 0.05. See Figure 1 for other definitions.
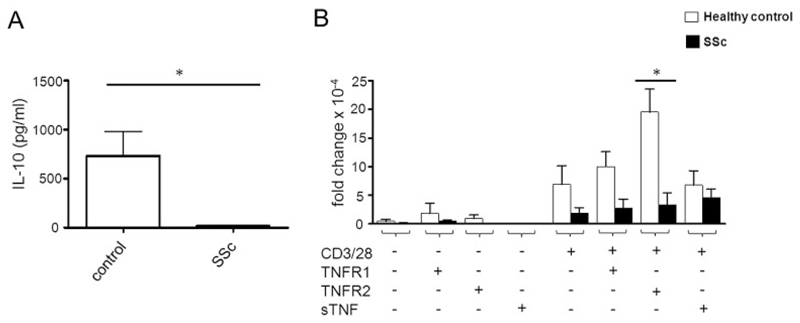
Impaired IL-10 expression in TNF-coactivated SSc lymphocytes
In CD3/CD28-activated CD3+ lymphocytes isolated from healthy individuals, the mean IL-10 level was 648 pg/ml (Figure 3A). In contrast, IL-10 production was nearly completely abrogated in lymphocytes from SSc patients (15 pg/ml). These results were consistent with data obtained from quantitative PCR analyses (Figure 3B). We found overall levels of IL-10 messenger RNA (mRNA) to be low in nonactivated T cells, independent of their stimulation with various TNF ligands. In contrast, healthy T cells activated with CD3/CD28 showed a 20-fold increase in IL-10 mRNA after stimulation with, in particular, TNFRII-selective ligands. Stimulation with sTNF or TNFRI-selective ligands resulted in no obvious up-regulation of IL-10 mRNA. Importantly, in T cells from SSc patients, IL-10 mRNA was not induced upon TNFRII-selective stimulation, although high cell surface levels of TNFRII were observed (data not shown), suggesting the presence of a TNFRII-dependent impaired antiinflammatory response in SSc.
Figure 3.
Impaired interleukin-10 (IL-10) expression in TNF-costimulated SSc lymphocytes. A, Baseline secretion of IL-10 in CD3/CD28-activated lymphocytes from SSc patients and healthy controls (n = 8 per group). IL-10 levels were significantly decreased in lymphocytes from SSc patients. B, Effects of costimulation with TNFRs or soluble TNF (sTNF) on IL-10 expression. TNFRII-mediated costimulation increased IL-10 expression in lymphocytes from controls, but not in lymphocytes from SSc patients. Values are the mean ± SEM. * = P < 0.05. See Figure 1 for other definitions.
Stimulation of type I collagen expression in fibroblasts by TNF-treated T lymphocytes
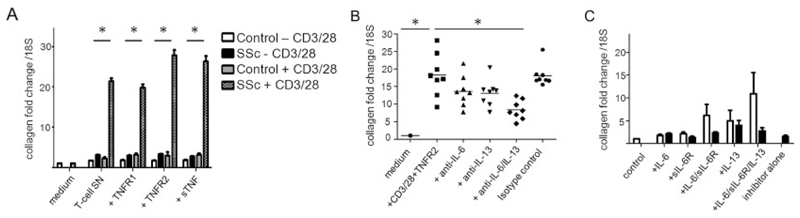
To test the profibrotic capacity of TNF-costimulated T cells, dermal fibroblasts from healthy controls were incubated with conditioned medium from the peripheral blood CD3+ T cells of SSc patients and healthy controls. CD3+ cells were incubated in the presence or absence of the 2 TNF variants and sTNF, with or without prior CD3/CD28 treatment. Type I collagen expression was analyzed by quantitative PCR (Figure 4A).
Figure 4.
Type I collagen expression in fibroblasts stimulated with T lymphocyte conditioned medium. A, Healthy fibroblasts were incubated with conditioned medium originating from peripheral blood CD3+ T cells from systemic sclerosis (SSc) patients and healthy controls (n = 8 per group). T cells were cultured with or without CD3/CD28 beads and subsequently incubated in the presence or absence of tumor necrosis factor (TNF)–selective variants and soluble TNF (sTNF), each at a concentration of 100 ng/ml. Type I collagen expression was determined by quantitative reverse transcription–polymerase chain reaction (qRT-PCR). Induction of type I collagen by supernatants (SN) from activated SSc lymphocytes was significantly greater than that observed with supernatants from activated control lymphocytes. Values are the mean ± SEM. * = P < 0.05. B, SSc T cells were activated with CD3/CD28 beads and treated with TNF receptor type II (TNFRII)–selective ligands or with medium alone, and supernatants were collected. Healthy dermal fibroblasts were then cultured in the presence of the conditioned medium. Neutralizing antibodies against interleukin-6 (IL-6), IL-13, or the combination of IL-6 and IL-13 were added to the conditioned medium prior to incubation with dermal fibroblasts. A significant reduction in collagen production after dual inhibition of IL-6 and IL-13 was observed. Symbols represent individual patients; horizontal bars show the mean. C, Healthy dermal fibroblasts were cultured overnight in serum-free medium and then stimulated with IL-6 (20 ng/ml), soluble IL-6 receptor (sIL-6R) (25 ng/ml), and IL-13 (100 ng/ml) in various combinations, with (solid bars) or without (open bars) incubation with the STAT-3 inhibitor S31-201 (50 μM). After 24 hours of stimulation, cells were harvested and type I collagen expression was analyzed by qRT-PCR, with normalization to 18S and control serum-free medium as the calibrator. Selective inhibition of STAT-3 attenuated IL-6 trans-signaling but did not significantly reduce IL-13–stimulated induction of type I collagen. Values are the mean ± SEM.
In fibroblasts treated with conditioned medium from non–bead-activated control T lymphocytes, the mean ± SEM fold change in collagen expression was 1.7 ± 0.1, and this did not change significantly upon stimulation with TNFRI (1.8 ± 0.2–fold), TNFRII (1.7 ± 0.1–fold), or sTNF (1.9 ± 0.1–fold). In contrast, conditioned medium from non–bead-activated SSc T lymphocytes induced higher levels of type I collagen mRNA in human fibroblasts independent of exogenously added TNF ligands. After CD3/CD28 activation of healthy T lymphocytes, levels of collagen expression induced in fibroblasts were similar to those in non–bead-activated T cell supernatants (2.3 ± 0.9–fold), again without a significant effect of TNF costimulation. In contrast, after CD3/CD28 activation, supernatants from SSc lymphocytes induced a 21-fold increase in type I collagen expression. While TNFRI costimulation had no further effect on collagen expression (19.8 ± 2.2–fold increase; P ≥ 0.05), TNFRII and sTNF induced significantly greater type I collagen expression in fibroblasts (27.8 ± 3.8–fold and 26.3 ± 3.91–fold, respectively; P ≤ 0.001 for both).
Dual inhibition of IL-6 and IL-13 reduces collagen expression induced by SSc lymphocyte conditioned medium
For neutralization experiments, we cultured healthy dermal fibroblasts with conditioned medium derived from SSc lymphocytes. SSc T lymphocytes were first activated with CD3/CD28 beads in the presence or absence of TNFRI and TNFRII agonists or sTNF. Neutralizing antibodies against IL-6 or IL-13, either alone or in combination, were added to the conditioned medium, and qRT-PCR analysis for collagen expression in fibroblasts was performed (Figure 4B).
Culture with SSc T lymphocyte medium alone led to a mean ± SEM 18.3 ± 3.2–fold increase in type I collagen mRNA expression. Neutralization of IL-6 resulted in a reduction in the increased collagen expression to 13.3 ± 2.3–fold (P not significant). Inhibition of IL-13 reduced collagen expression to a similar level (13.1 ± 4.5–fold change; P not significant). Combined inhibition of both IL-6 and IL-13, however, reduced the change in type I collagen expression to 8.3 ± 4.1–fold, which was a significant reduction compared to the expression level without cytokine inhibition (P ≤ 0.05) (n = 8). In contrast, treatment with matched isotype control antibody resulted in a 17.8 ± 2.4–fold change in type I collagen expression, which was not significant compared to that observed in T cells stimulated without neutralizing antibodies, thereby confirming the specificity of the neutralizing antibodies. We also performed similar experiments on resting SSc lymphocytes. Although the overall magnitude of induction was much smaller in the non–bead-activated SSc T lymphocytes, again there was clearly detectable induction of collagen expression, which was reduced slightly by the addition of neutralizing antibodies. While differences between groups were not significant, the patterns were similar to those observed in the experiments with activated lymphocytes.
To investigate the effects of IL-6 and IL-13 on collagen expression in fibroblasts, we cultured dermal fibroblasts with or without IL-6, sIL-6R, and IL-13 in various combinations (Figure 4C). Treatment with IL-6 and sIL-6R in combination, as well as IL-13 alone, led to increased collagen expression compared to control (6-fold increase and 5-fold increase, respectively; both P < 0.05), with a clear further effect of IL-6, sIL-6R, and IL-13 in combination (11-fold increase; P < 0.05), confirming the data from the IL-6/IL-13 neutralization experiments. IL-6/sIL-6R trans-signaling was inhibited by coculture with the specific STAT-3 inhibitor S31-201 (2.8-fold increase), whereas IL-13 signaling was not. Addition of TGFβ to the cocktail of IL-6, sIL-6R, and IL-13 further increased collagen production in fibroblasts (data not shown), which might explain the incomplete inhibition through IL-6 and IL-13. IL-6 alone did not elevate collagen expression in these dermal fibroblasts (1.8-fold change), potentially due to their lack of IL-6R expression (data not shown).
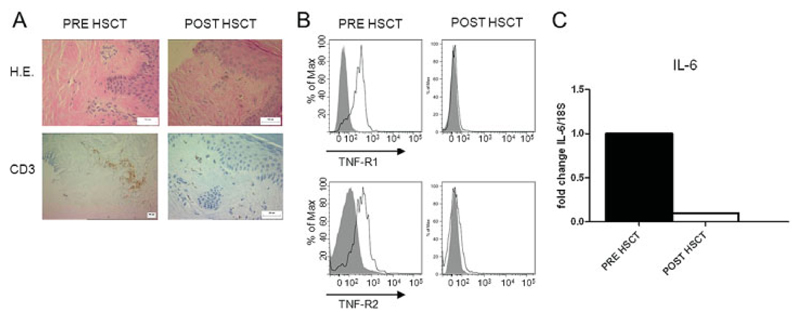
TNFR and IL-6 overexpression in the dermis is reversible after autologous HSCT
Using immunohistochemistry, we analyzed T cell infiltration in a patient with severe SSc, before and after autologous HSCT. The patient received antithymocyte globulin and high-dose cyclophosphamide. Six months after HSCT, a substantial reduction in the CD3+ cell population in the dermis was observed (Figure 5A). In addition, using flow cytometry, we determined the expression of TNFRI and TNFRII on dermal lymphocytes from the patient before and after HSCT. We observed nearly complete down-regulation of TNFRI and TNFRII after treatment (Figure 5B). IL-6 mRNA levels in the dermis were significantly down-regulated after treatment as well (Figure 5C), potentially as a result of reduced TNFR signaling.
Figure 5.
TNFR and IL-6 overexpression in the dermis is reversible after lymphoablation followed by autologous hematopoietic stem cell transplantation (HSCT). A, Hematoxylin and eosin (H&E) and CD3 immunohistochemistry staining of the dermis of a patient with severe SSc, before and after treatment with antithymocyte globulin and high-dose cyclophosphamide followed by autologous HSCT. A substantial reduction in the CD3+ cell population was seen after HSCT. B, Dermal TNFRI and TNFRII expression, assessed by flow cytometry. In accordance with the histologic results, these analyses showed complete down-regulation of TNFRI and TNFRII after transplantation. C, Mean levels of IL-6 expression in the dermis, assessed by qRT-PCR. IL-6 was significantly down-regulated after treatment. See Figure 4 for other definitions.
Discussion
The present results highlight the importance of activated T lymphocytes in SSc pathology and confirm their profibrotic effect in association with TNF. We investigated the effect of TNF on preactivated T lymphocytes rather than the direct effect of soluble or membrane-bound TNF on fibroblasts as has been investigated in earlier studies; the latter has been described to reduce collagen expression (29). We hypothesized that in SSc-affected tissue, T lymphocytes are stimulated by antigen-presenting cells in a TNF-rich environment. Therefore, we applied CD3/CD28 stimulation with subsequent TNF costimulation in order to mimic the antigenic environment that resident T cells likely encounter in affected tissue in SSc. The use of TNFRI- and TNFRII-selective TNF variants further enabled us to study the role of the 2 distinct receptors in this setting.
We used multicolor flow cytometry to study TNFR on different leukocyte subsets in the dermis of SSc patients and demonstrated that TNFRII, and in some cases also TNFRI, was significantly up-regulated on T cells in the dermis of these patients. To our knowledge, the use of multicolor flow cytometry for such experiments has not been reported previously. Of note, TNFRII overexpression correlated with skin thickening in our patient cohort. Despite a relatively modest correlation, these results are in accordance with previous data showing up-regulation of TNFRI and TNFRII on dermal leukocytes in patients with severe SSc, as assessed by immunohistochemistry (20). Clearly, our results were influenced by the small sample size and heterogeneity of the patient cohort, with most of the patients receiving immunosuppressive treatment. Potentially, a clearer picture could have been obtained in treatment-naive patients with early SSc. Consistent with previous reports (22), we found that TNFRII expression was also upregulated in the peripheral blood of SSc patients; this up-regulation was not as great as that observed in the skin (Figure 1C). We postulate that in SSc, TNFRII up-regulation occurs during antigen encounter in the tissue. Up-regulation of TNFRII on the cell surface of CD4+ T cells after T cell receptor triggering via CD3/CD28 bead activation was also observed in our experiments (data not shown) and has been well described previously (39).
One potential explanation for the heterogeneous effects of TNF is the fact that TNF is mediated via 2 distinct receptors, TNFRI and TNFRII. By using TNF variants, we were able to stimulate TNFRI and TNFRII selectively and quantify the production of the key profibrotic cytokines IL-6, sIL-6R, and IL-13. One of our key findings was that, upon CD3/CD28 activation, lymphocytes isolated from the peripheral blood of SSc patients showed increased secretion of profibrotic cytokines when compared to lymphocytes from healthy controls. Strikingly, IL-6 and sIL-6R expression, which was barely detectable in healthy T lymphocytes, reached levels of >500 pg/ml in lymphocytes from SSc patients. These results are in accordance with those of earlier studies showing that healthy T cells do not express IL-6 upon CD3/CD28 activation alone (40). In our experiments, IL-6 secretion was mediated by TNFRI-selective and sTNF costimulation, but not by TNFRII-selective stimulation. In contrast, sIL-6R levels were higher upon TNFRII stimulation. This suggests that membrane-bound TNF, which efficiently activates both TNFRI and TNFRII, exerts a strong effect on IL-6 expression as well as sIL-6R expression, and therefore might induce IL-6 trans-signaling.
Reports of linked IL-6 and TNFRII polymorphisms in patients with lung fibrosis support the notion that the IL-6–sIL-6R interplay induced by membrane-bound TNF triggers fibrosis (41). Soluble TNF, which signals primarily through TNFRI, is a potent inducer of IL-6. The difference in production of the profibrotic cytokine IL-13 was less distinct between T lymphocytes from healthy controls and those from SSc patients after CD3/CD28 stimulation, but increased after TNF stimulation. IL-13 has been previously demonstrated to be elevated in peripheral T lymphocytes from SSc patients, in a GATA-3–dependent manner (42).
IL-13 and/or IL-6 are not exclusively stimulated by TNFR but are also triggered by other TNFR super-family members, such as OX40 and 4-1BB. OX40-mediated T cell costimulation is also involved in IL-6 production and in the pathogenesis of experimental autoimmune encephalomyelitis (43) and triggers IL-13 expression in activated umbilical cord cells (44). Polymorphisms in the OX40L region are associated with susceptibility to SSc (45). IL-13 expression is also increased by 4-1BB activation (46). We are not aware of studies on TNFR superfamily members that trigger sIL-6R expression.
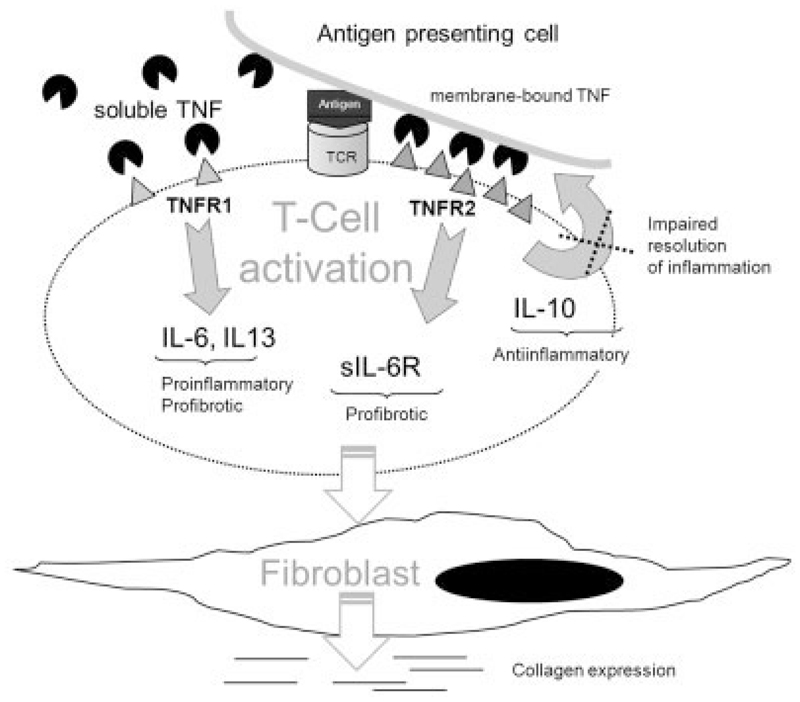
Conditioned medium from the CD3/CD28-activated CD3+ cells of SSc patients alone induced a 21-fold increase in the expression of type I collagen compared to that in untreated fibroblasts. Experiments with neutralizing antibodies revealed IL-6 and IL-13 as key cytokines mediating this effect, whereas sIL-6R is necessary for the profibrotic effect of IL-6. Our results further show that in contrast to IL-13, IL-6/sIL-6R trans-signaling is STAT-3 dependent. In animal studies, the level of IL-6–mediated STAT-3 correlates with the severity of lung fibrosis (47), and STAT-3 is currently being discussed as a potential new therapeutic target for lung fibrosis (48). The fact that dual inhibition of IL-6 and IL-13 does not completely suppress collagen expression is likely due to the presence of TGFβ carried over from the serum-containing media, as TGFβ production by SSc lymphocytes was low after CD3/CD28 stimulation (data available from the corresponding author upon request) and the profibrotic role of TGFβ in SSc is well known (49). Addition of TGFβ to IL-6, sIL-6R, and IL-13 further enhances type I collagen expression in fibroblasts. We therefore postulate that the incomplete collagen expression after IL-6 and IL-13 inhibition in our system is due to TGFβ and other growth factors, which are either produced by lymphocytes or are transferred from T cell supernatants that contained 10% FCS. A schematic diagram of the proposed effects of TNFR costimulation on cytokine production and fibroblast production in SSc is shown in Figure 6.
Figure 6.
Schematic representation of the effects of TNFR costimulation on cytokine production and subsequent fibroblast stimulation in SSc. A T cell is activated by the docking antigen-presenting cell via T cell receptor (TCR), which triggers TNFRII up-regulation. Soluble TNF stimulates primarily TNFRI, whereas membrane-bound TNF activates TNFRI and TNFRII. In SSc lymphocytes, TNFRI triggers IL-6 and IL-13. Soluble IL-6R and transforming growth factor β (TGFβ) are triggered by TNFRII (thus, by membrane-bound TNF). Secreted IL-6, sIL-6R, IL-13, and TGFβ stimulate fibroblasts to produce collagen. Antiinflammatory IL-10, which is normally expressed upon TCR activation and TNFRII costimulation, is impaired in SSc lymphocytes. See Figure 4 for other definitions.
Surprisingly, we found that, in contrast to IL-6 and IL-13, IL-10 was expressed at very low levels in SSc lymphocytes upon CD3/CD28 activation. In accordance with this, IL-10 mRNA levels were significantly upregulated by TNFRII costimulation in healthy T cells but not SSc T cells. As IL-10 is a key antiinflammatory cytokine, we interpret this finding as representing a potential inability of SSc lymphocytes to resolve inflammation.
Finally, TNFRI and TNFRII overexpression on dermal lymphocytes was analyzed in a patient with severe SSc, before and after she underwent HSCT. We found that high-dose cyclophosphamide and antithymocyte globulin substantially reduced T cell infiltration in the skin. In the remaining T cells, expression of both TNFRI and TNFRII was reversed, and IL-6 expression was subsequently reduced. In accordance with our in vitro data, this clinical observation with regard to lymphoablative treatment confirms the potential reversibility of chronic inflammation leading to TNFR over-expression and fibrosis in SSc.
Taken together, the present results show that T lymphocytes in SSc tissue overexpress TNFRII and that preactivated peripheral blood–derived lymphocytes from SSc patients are profibrotic. Costimulation of T cells via the 2 TNF receptors, in particular TNFRII, further triggers collagen production. However, production of IL-10 is down-regulated, which may impair the resolution of inflammation.
Supplementary Material
Footnotes
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Hügle had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Hügle, O’Reilly, van Laar.
Acquisition of data. Hügle, O’Reilly, Simpson, Kraaij, Bigley.
Analysis and interpretation of data. Hügle, O’Reilly, Simpson, Collin, Krippner-Heidenreich, van Laar.
References
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmajer R, Perlish JS, West WP. Ultrastructure of cutaneous cellular infiltrates in scleroderma. Arch Dermatol. 1977;113:1661–6. [PubMed] [Google Scholar]
- 3.Hugle T, Hogan V, White KE, van Laar JM. Mast cells are a source of transforming growth factor β in systemic sclerosis. Arthritis Rheum. 2011;63:795–9. doi: 10.1002/art.30190. [DOI] [PubMed] [Google Scholar]
- 4.Kalogerou A, Gelou E, Mountantonakis S, Settas L, Zafiriou E, Sakkas L. Early T cell activation in the skin from patients with systemic sclerosis. Ann Rheum Dis. 2005;64:1233–5. doi: 10.1136/ard.2004.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roumm AD, Whiteside TL, Medsger TA, Jr, Rodnan GP. Lymphocytes in the skin of patients with progressive systemic sclerosis: quantification, subtyping, and clinical correlations. Arthritis Rheum. 1984;27:645–53. doi: 10.1002/art.1780270607. [DOI] [PubMed] [Google Scholar]
- 6.Sakkas LI, Xu B, Artlett CM, Lu S, Jimenez SA, Platsoucas CD. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J Immunol. 2002;168:3649–59. doi: 10.4049/jimmunol.168.7.3649. [DOI] [PubMed] [Google Scholar]
- 7.Mavalia C, Scaletti C, Romagnani P, Carossino AM, Pignone A, Emmi L, et al. Type 2 helper T-cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol. 1997;151:1751–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteside TL, Kumagai Y, Roumm AD, Almendinger R, Rodnan GP. Suppressor cell function and T lymphocyte subpopulations in peripheral blood of patients with progressive systemic sclerosis. Arthritis Rheum. 1983;26:841–7. doi: 10.1002/art.1780260704. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly S, Hugle T, van Laar J. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford) 2012;51:1540–9. doi: 10.1093/rheumatology/kes090. [DOI] [PubMed] [Google Scholar]
- 10.Radstake TR, van Bon L, Broen J, Wenink M, Santegoets K, Deng Y, et al. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFβ expression. PLoS One. 2009;4:e5981. doi: 10.1371/journal.pone.0005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papp G, Horvath IF, Barath S, Gyimesi E, Sipka S, Szodoray P, et al. Altered T-cell and regulatory cell repertoire in patients with diffuse cutaneous systemic sclerosis. Scand J Rheumatol. 2011;40:205–10. doi: 10.3109/03009742.2010.528021. [DOI] [PubMed] [Google Scholar]
- 12.Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A, et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol. 2010;162:1056–63. doi: 10.1111/j.1365-2133.2010.09633.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MS, Elnekave E, Mentink-Kane MM, Hodges MG, Pesce JT, Ramalingam TR, et al. IL-13Rα2 and IL-10 coordinately suppress airway inflammation, airway-hyperreactivity, and fibrosis in mice. J Clin Invest. 2007;117:2941–51. doi: 10.1172/JCI31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276–81. doi: 10.1016/j.autrev.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 16.Phelps RG, Daian C, Shibata S, Fleischmajer R, Bona CA. Induction of skin fibrosis and autoantibodies by infusion of immunocompetent cells from tight skin mice into C57BL/6 Pa/Pa mice. J Autoimmun. 1993;6:701–18. doi: 10.1006/jaut.1993.1059. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–61. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 18.Kassiotis G, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427–34. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280:36099–109. doi: 10.1074/jbc.M505291200. [DOI] [PubMed] [Google Scholar]
- 20.Gruschwitz MS, Albrecht M, Vieth G, Haustein UF. In situ expression and serum levels of tumor necrosis factor-α receptors in patients with early stages of systemic sclerosis. J Rheumatol. 1997;24:1936–43. [PubMed] [Google Scholar]
- 21.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, et al. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS One. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz LA, Lasky J, Hamilton RF, Jr, Holian A, Hoyle GW, Banks W, et al. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp Lung Res. 1998;24:721–43. doi: 10.3109/01902149809099592. [DOI] [PubMed] [Google Scholar]
- 23.Smith RE, Strieter RM, Phan SH, Kunkel SL. C-C chemokines: novel mediators of the profibrotic inflammatory response to bleomycin challenge. Am J Respir Cell Mol Biol. 1996;15:693–702. doi: 10.1165/ajrcmb.15.6.8969262. [DOI] [PubMed] [Google Scholar]
- 24.Oikonomou N, Harokopos V, Zalevsky J, Valavanis C, Kotanidou A, Szymkowski DE, et al. Soluble TNF mediates the transition from pulmonary inflammation to fibrosis. PLoS One. 2006;1:e108. doi: 10.1371/journal.pone.0000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter N, Collard HR, King TE., Jr Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:330–8. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- 26.Lam GK, Hummers LK, Woods A, Wigley FM. Efficacy and safety of etanercept in the treatment of scleroderma-associated joint disease. J Rheumatol. 2007;34:1636–7. [PubMed] [Google Scholar]
- 27.Denton CP, Engelhart M, Tvede N, Wilson H, Khan K, Shiwen X, et al. An open-label pilot study of infliximab therapy in diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2009;68:1433–9. doi: 10.1136/ard.2008.096123. [DOI] [PubMed] [Google Scholar]
- 28.Distler JH, Schett G, Gay S, Distler O. The controversial role of tumor necrosis factor α in fibrotic diseases [review] Arthritis Rheum. 2008;58:2228–35. doi: 10.1002/art.23645. [DOI] [PubMed] [Google Scholar]
- 29.Chizzolini C, Parel Y, De Luca C, Tyndall A, Akesson A, Scheja A, et al. Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor α Arthritis Rheum. 2003;48:2593–604. doi: 10.1002/art.11129. [DOI] [PubMed] [Google Scholar]
- 30.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 31.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Oppenheim JJ. TNF-α: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr Dir Autoimmun. 2010;11:119–34. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, et al. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993;151:4637–41. [PubMed] [Google Scholar]
- 34.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 35.Haniffa M, Ginhoux F, Wang XN, Bigley V, Abel M, Dimmick I, et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009;206:371–85. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krippner-Heidenreich A, Tubing F, Bryde S, Willi S, Zimmermann G, Scheurich P. Control of receptor-induced signaling complex formation by the kinetics of ligand/receptor interaction. J Biol Chem. 2002;277:44155–63. doi: 10.1074/jbc.M207399200. [DOI] [PubMed] [Google Scholar]
- 37.Ward C, Eger K, Diboll J, Jones D, Haniffa MA, Brodlie M, et al. Bronchial epithelial cells cultured from clinically stable lung allograft patients promote the development of macrophages from monocytes rather than dendritic cells. Thorax. 2009;64:430–5. doi: 10.1136/thx.2008.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clements P, Lachenbruch P, Seibold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 39.Scheurich P, Thoma B, Ucer U, Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-α: induction of TNF receptors on human T cells and TNF-α-mediated enhancement of T cell responses. J Immunol. 1987;138:1786–90. [PubMed] [Google Scholar]
- 40.Villiger PM, Cronin MT, Amenomori T, Wachsman W, Lotz M. IL-6 production by human T lymphocytes: expression in HTLV-1-infected but not in normal T cells. J Immunol. 1991;146:550–9. [PubMed] [Google Scholar]
- 41.Pantelidis P, Fanning GC, Wells AU, Welsh KI, du Bois RM. Analysis of tumor necrosis factor-α, lymphotoxin-α, tumor necrosis factor receptor II, and interleukin-6 polymorphisms in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;163:1432–6. doi: 10.1164/ajrccm.163.6.2006064. [DOI] [PubMed] [Google Scholar]
- 42.Medsger TA, Jr, Ivanco DE, Kardava L, Morel PA, Lucas MR, Fuschiotti P. GATA-3 up-regulation in CD8+ T cells as a biomarker of immune dysfunction in systemic sclerosis, resulting in excessive interleukin-13 production. Arthritis Rheum. 2011;63:1738–47. doi: 10.1002/art.30489. [DOI] [PubMed] [Google Scholar]
- 43.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167:2991–9. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 44.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 45.Gourh P, Arnett FC, Tan FK, Assassi S, Divecha D, Paz G, et al. Association of TNFSF4 (OX40L) polymorphisms with susceptibility to systemic sclerosis. Ann Rheum Dis. 2010;69:550–5. doi: 10.1136/ard.2009.116434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nam KO, Shin SM, Lee HW. Cross-linking of 4-1BB up-regulates IL-13 expression in CD8(+) T lymphocytes. Cytokine. 2006;33:87–94. doi: 10.1016/j.cyto.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 47.O’Donoghue RJ, Knight DA, Richards CD, Prele CM, Lau HL, Jarnicki AG, et al. Genetic partitioning of interleukin-6 signalling in mice dissociates Stat3 from Smad3-mediated lung fibrosis. EMBO Mol Med. 2012;4:939–51. doi: 10.1002/emmm.201100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prele CM, Yao E, O’Donoghue RJ, Mutsaers SE, Knight DA. STAT3: a central mediator of pulmonary fibrosis? Proc Am Thorac Soc. 2012;9:177–82. doi: 10.1513/pats.201201-007AW. [DOI] [PubMed] [Google Scholar]
- 49.Sargent JL, Milano A, Bhattacharyya S, Varga J, Connolly MK, Chang HY, et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol. 2010;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.