Abstract
Background
Danon disease is an X-linked disturbance of autophagy manifesting with cognitive impairment and disordered heart and skeletal muscle. After a period of relative stability, patients deteriorate rapidly and may quickly become ineligible for elective heart transplantation - the only life-saving therapy.
Methods
We report a large pedigree with diverse manifestations of Danon disease in hemizygotes and female heterozygotes.
Results
Malignant cardiac arrhythmias requiring amiodarone treatment induced thyroid disease in two patients; intractable thyrotoxicosis, which enhances autophagy, caused the death of a 21 year-old man. Our patients also had striking elevation of serum troponin I during the accelerated phase of their illness (p<0.01) and rising concentrations heralded cardiac decompensation. We argue for changes to cardiac transplantation eligibility criteria.
Conclusion
Danon disease causes hypertrophic cardiomyopathy - here we propose a common pathophysiological basis for the metabolic and structural effects of this descriptive class of heart disorders. We also contend that troponin I may have prognostic value and merits exploration for clinical decision-making including health warning bracelets. Rapamycin (Sirolimus®), an approved immunosuppressant which also influences autophagy, may prove beneficial. In the interim, while new treatments are developed, a revaluation of cardiac transplantation eligibility criteria is warranted.
Keywords: Hypertrophic cardiomyopathy, Troponin, Thyrotoxicosis, LAMP2, Learning difficulties, Obesity, Danon disease, Autophagy, Rapamycin
1. Introduction
Hypertrophic cardiomyopathy (HCM) occurs in multisystem disorders, including amyloidosis and diverse inborn errors of metabolism [1]. While it may be asymptomatic, early-onset heart failure, rhythm abnormalities and sudden death are all features of this condition. HCM can result from defects in structural proteins affecting the integrity of the sarcomere [2], and from metabolic conditions, but for more than a third of patients a definitive molecular diagnosis remains elusive [3]. Identifying a hereditary condition has immediate implications for prognosis, counselling and potential treatment options [4].
Danon disease is one such inborn error of lysosomal metabolism with prominent effects on the heart. The exact prevalence is unknown but mutations in the LAMP2 gene responsible for Danon's disease, have been found in about 1% of patients with HCM [5]. Danon is inherited as an X-linked trait with clinical expression in heterozygous females (OMIM# 309060); characteristically it presents in boys and young men with the triad of mild skeletal myopathy, impaired learning ability and hypertrophic cardiomyopathy [6]. The HCM is the predominant feature in affected males [7] with massively increased cardiac mass reported [8,9]. Sudden death occurs in the third decade of life in affected hemizygous men and, apart from the use of implantable defibrillators and anti-arrhythmic drugs, cardiac transplantation appears to be the only effective treatment [7,10,11].
As a consequence of X-chromosome inactivation, female heterozygotes for Danon disease are somatic mosaics; thus while the condition is highly penetrant, clinical expression is attenuated compared with that of the hemizygous male. They typically develop late-onset dilated cardiomyopathy with atrial fibrillation and survive beyond the fourth decade of life [8].
Here we report a single large pedigree affected by Danon disease with marked clinical diversity even within hemizygous males and in this light, suggest that transplant criteria, troponin I levels as well as novel therapeutic approaches merit further consideration.
2. Case summaries
2.1. Case A
The proband and oldest of three brothers was investigated for exercise-induced myalgia aged ten years with learning and behavioural difficulties throughout childhood. Elevated alanine transaminase activity at 224–285 iu/L (<40) in the face of a normal γ-glutamyl transpeptidase activity at 22 iu/L (<50) was highly suggestive of a skeletal myopathy or mysositis. Electromyography was normal, but a persistently elevated serum creatine kinase activity 360–560 iu/L (<195) prompted muscle biopsy.
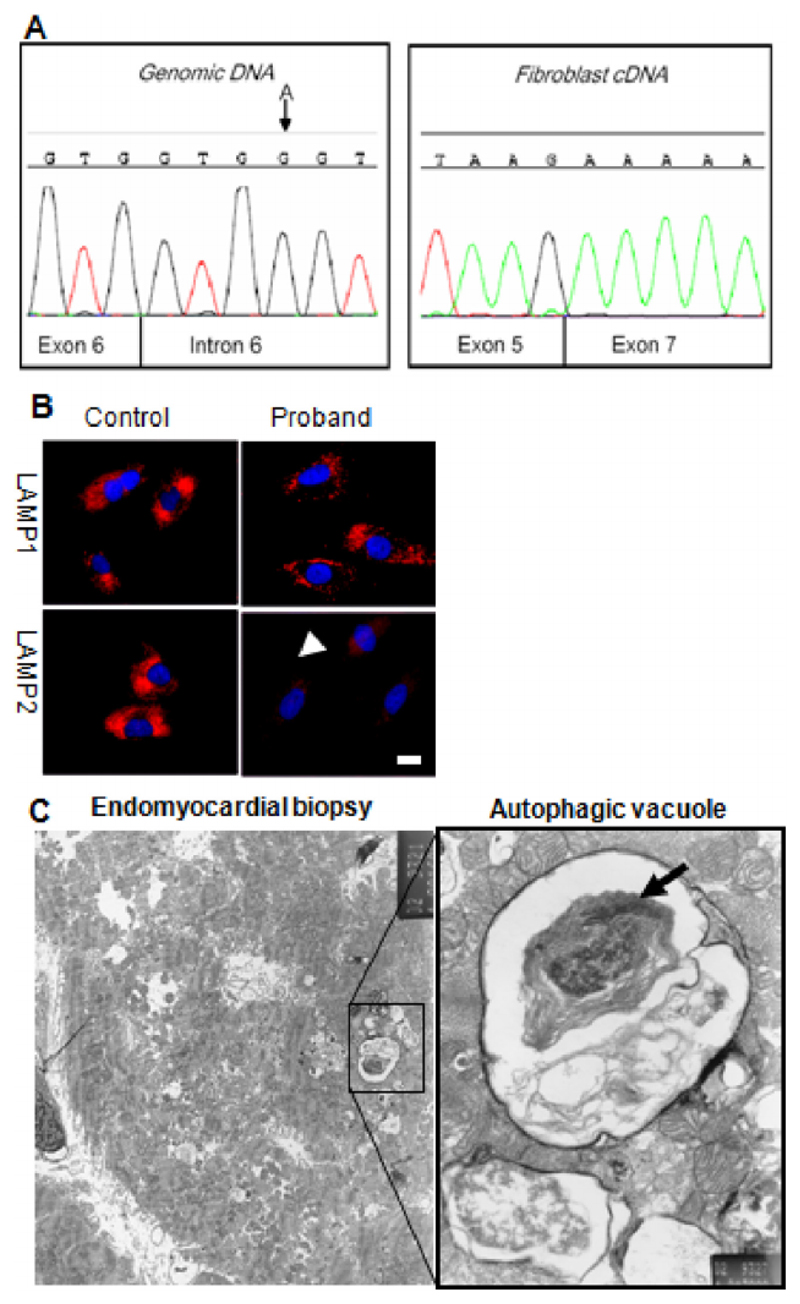
Initial histopathology of the biopsy reported non-specific changes however later review revealed prominent punctate acid phosphatase staining; electron microscopy showed a single subsarcolemmal vacuole with granular material consistent with glycogen and single limiting membrane but no definite internal layer of basal lamina-like material. Later, aged sixteen, evaluation for dyspnoea and asthma revealed a loud systolic murmur; referral to our hospital identified gross concentric left ventricular hypertrophy with outflow obstruction at rest (see Supplementary Movies 1&2). This, and the striking family history (Fig. 1), suggested Danon disease which was confirmed by reduced LAMP2 expression in cultured fibroblasts (Fig. 2b). Analysis of the gene identified a c864 + 4A>G mutation causing abnormal splicing of exon 6 and reduced abundance of the LAMP2 polypeptide (Fig. 2b). Prominent autophagic vacuoles consistent with Danon disease were seen on myocardial biopsy (Fig. 2c). EEG in the proband was normal. Cerebral MRI was not requested as the cognitive deficiencies in Danon disease are not severe [12] and MRI changes would not be expected. This has been supported by neuropathological studies revealing no macroscopic abnormalities [13].
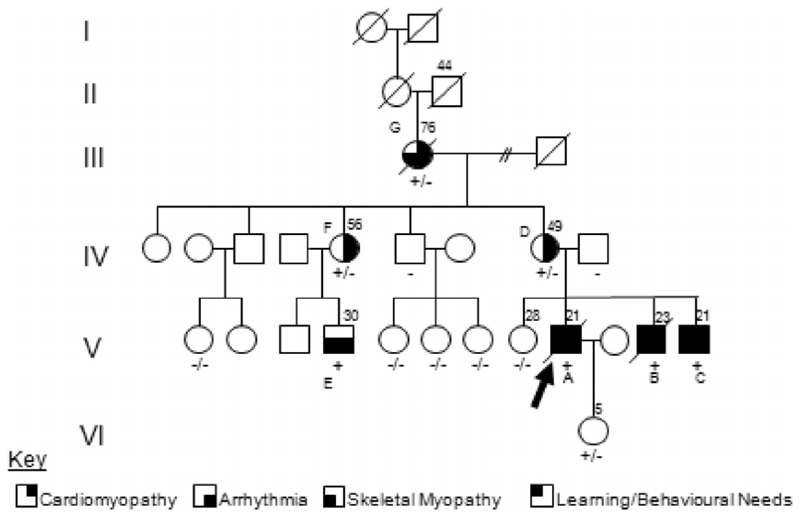
Fig. 1.
Pedigree of family with Danon Disease. The proband is indicated by the arrow. Confirmed LAMP2 genotypes for all family members who have consented to testing are shown beneath the respective patient symbol. The principal features of the condition are represented by shaded segments within the affected patient symbols. Note that the affected males had developed the features of Danon disease before the age of twenty, in contrast to affected females who presented after the age of forty years and are yet to develop all features of the condition.
Fig. 2.
Investigations for confirming diagnosis of Danon disease. A: Genomic DNA sequencing of LAMP2 showed an A G mutation in the intron 6 splice site (left panel) a described mutation known to cause skipping of exon 6. This is confirmed in cDNA sequencing from fibroblasts (right panel) which reveals an in frame fusion of exons 5 and 7. B: Skin fibroblasts cultured from the proband were stained for the glycosylated lysosomal membrane proteins LAMP1, and LAMP2 (red) and nuclei with DAPI (blue). Cells from the proband show markedly reduced staining of immunoreactive LAMP2 polypeptide localizing to brightly staining LAMP1 positive intracellular particles (lysosomes). This is compatible with a mutation resulting in reduced abundance of the full-length protein product; this was confirmed by Western blot analysis (data not shown). Scale bar: 10 μm. C: Septal endomyocardial biopsies were taken from the right ventricle of the proband and prepared for electron microscopy. Several fibres feature autophagic vacuoles and are shown in the expanded pane to contain mitochondria (arrow), and electron-dense debris representing partially degraded cytoplasmic contents. Scale bar: 1 μm.
Risk stratification for HCM led to implantation of a cardioverter-defibrillator (ICD) but by twenty years of age he deteriorated rapidly with recurrent ventricular arrhythmias. He was assessed for cardiac transplantation. However persistent obesity and an inability to comply with dietary and lifestyle measures precluded listing for the procedure. The patient died at twenty-one years of age with cardiac failure and ventricular tachyarrhythmia.
After appropriate counselling, molecular screening for the causal mutation was carried out for this extended pedigree and those individuals harbouring the mutation were assessed for risk. The results in Fig. 1 show that all three brothers were affected, as well as a maternal male cousin; the LAMP2 defect was also demonstrated in the mother, aunt and maternal grandmother. All three brothers were issued with statements of educational need due to mild learning difficulties.
2.2. Case B
The middle brother was first assessed at the age of 14 years. He neither smoked tobacco nor ingested alcohol but also had below average scholastic achievement and mild learning difficulties; at school he had a generally poor sporting ability. Investigations showed persistent elevation of transaminases but no evidence of liver disease; his plasma creatine kinase was also elevated, indicating skeletal myopathy. Echocardiography showed moderate left ventricular hypertrophy. At 16 he suffered ventricular tachycardia and oral amiodarone was started. Thereafter he left school and was unable to continue with employment in a kitchen due to anxiety with tremor which developed with biochemical confirmation of thyrotoxicosis (which resulted from the amiodarone treatment) and carbimazole was started. An intracardiac defibrillator was also implanted but by age 20 he was assessed for cardiac transplantation. An apical thrombus complicated by embolic infarction of one kidney mandated life-long anticoagulation with warfarin. The thrombus was attributed in part to the presence of an ICD lead. He became more anxious, gained weight and was advised to lose weight as prelude to urgent cardiac transplantation. Three months later, amiodarone-induced fulminating thyrotoxicosis and an associated severe proximal myopathy supervened: urgent thyroidectomy was arranged but the patient had an unexpected asystolic arrest in hospital. Resuscitation failed and he died at 23 years of age. Interrogation of the defibrillator revealed that the heart had been refractory to several DC cardioversion shocks delivered from the implanted device.
2.3. Case C
The youngest brother was diagnosed aged 11 years. He too has had poor educational attainment. After his oldest brother's death he abjured alcohol; aged 17 an intracardiac defibrillator was implanted but he continues to suffer recurrent severe tachycardias. For three years he has received amiodarone developed thyrotoxicosis and has undergone elective thyroidectomy. Now aged twenty four years, he remains unemployed and is awaiting transplantation. However as he too remains obese and has learning difficulties this will adversely affect his eligibility and prioritisation under current transplantation guidelines. The sole surviving brother, he suffers anxiety and has become depressed.
2.4. Cases D, E, F & G
In contrast, the three affected heterozygous women in the pedigree (Fig. 1) have developed mild left ventricular systolic impairment and atrial fibrillation in the fifth decade of life, with death occurring due to cardiac failure at the age of 76 years in the maternal grandmother (Case G) of the proband. All have completed schooling and been employed and financially independent; all have been married and borne children; none has complained of exercise-induced myalgia. Recently the mother (Case D), now in her 50′s, developed deteriorating cardiac function requiring evaluation for transplantation, although smoking cessation and weight loss remain a prerequisite.
Despite harbouring identical mutations, the affected maternal aunt (Case F) and her son (Case E & cousin of the proband), have manifestations of the disease which are markedly less severe than the three brothers (A–C) and their mother (D). In striking contrast to his younger affected cousins, this 36 year-old cousin (E) has only mild myalgia after exercise; he first required cardioversion at 36 years of age and amiodarone has recently started with normal body habitus, he lives independently in his own property with a mortgage and is employed long-term as a stock manager. There is no evidence of cardiac hypertrophy either by echocardiography or dynamic cardiac MRI and electrocardiography shows only pre-excitation abnormalities.
3. Discussion
3.1. Clinical heterogeneity & implications for diagnosis
The pedigree of this family (Fig. 1) illustrates the clinical diversity in Danon disease even within males of a single extended family and also, as expected, by sex. The similar phenotype in the three brothers from the nuclear family demonstrates the high penetrance of this disorder as well as the frequent presentation with extra-cardiac manifestations including mild learning difficulties, problems with anger management and emotional relationships as well as securing opportunities for gainful employment.
The mutation identified in the LAMP2 gene affects splicing but does not completely abrogate gene function. It is thus formally possible that variable expressivity of the inherited defect in the affected male hemizygotes can be explained by differences in splicing efficiency, leading to variation in the abundance of the wild type transcript and its cognate translation product. This could theoretically account for the clinical heterogeneity seen in these patients harbouring identical mutations. However, we consider that this is unlikely to be the principal explanation since the greatest variation in severity is observed the two branches of the family. Most males with Danon disease have missense, minor deletions or splice-site mutations in the LAMP2 gene and it seems probable that complete deletion of the LAMP2 function would be fatal or at least early lethal.
In the affected brothers (A–C) the Danon disease phenotype was consistently severe. The discordance is observed principally between the two branches of this family: with discordance between the affected (heterozygous) sisters (F compared with D) as well as discordance between their respective male (hemizygous) offspring (E compared with A–C) who are first degree cousins. This suggests that factors other than intrinsic variation in splicing efficiency account for the clinical diversity of disease in this pedigree.
The most striking difference between the branches of this pedigree was in body habitus. There was an association between truncal obesity and the branch with the severe clinical features (A–C & their mother D). The three sons (A–C) and their severely affected mother (D) required anti-arrhythmic agents and implantation of intraventricular cardioverter-defibrillator devices. In contrast, neither the affected maternal aunt (F) nor male cousin (E), is obese, and cardioversion or other treatment was required until the ages of 59 and 36, respectively, despite harbouring the same disease-associated mutant LAMP2 allele as the index case1.
While the random effects of Lyonization may account for the clinical heterogeneity seen in the two sisters (D & F) who are heterozygous for the LAMP2 mutation, this process cannot account for the differences in the male cousins who are hemizygous. Their heterogeneity may result from the influence of background modifier genes and consequential metabolic effects related to obesity. This immediately prompts consideration of imprinting; a phenomenon which is strongly linked to morbid nutritional states.
A high index of suspicion for Danon disease must be maintained in any young patient with ventricular hypertrophy or conduction abnormalities - particularly if learning difficulties are present. Given the clinical and divergent expressivity of a single genetic trait amongst males and females within the pedigree as reported here, even in the absence of overt extra-cardiac manifestations, Danon disease should be considered amongst the differential diagnoses. In the proband, original investigations including skeletal muscle biopsy were unavailing, however review of this material including electron microscopy and special stains including acid phosphatase activity and dystrophin-immunoreactivity, indicated a lysosomal abnormality and are compatible with Danon disease.
The behavioural difficulties associated with Danon disease may be overlooked; a careful study of 82 patients found cognitive disability in 100% of affected men and 50% of women, while another series did not report any in 7 severely affected patients. Since learning difficulties are a strong indicator for the diagnosis, the educational history should be carefully reviewed. Furthermore, any cognitive disability requires scrupulous attention as it will inform management and affect how, for example, reproductive counselling - which is mandatory for this fatal hereditary condition - is handled.
3.2. Troponin I as a potential biomarker in Danon disease
At the time of writing, other than ultrasound, no proven or facile biomarker that reflects the severity and prognosis of Danon disease has been validated for clinical use [14]. However serum concentrations of troponins may have predictive value for adverse outcomes in other non-ischaemic cardiac diseases such as dilated [15] and hypertrophic cardiomyopathy [16]. In the latter study of 183 patients with HCM, those with raised serum troponin T concentrations as determined by highly sensitive assays, were more likely to have adverse cardiac outcomes including death (hazard ratio 5.05). The recent introduction of novel ultrasensitive troponin assays which are capable of detecting free troponin T and I concentrations in the serum in the nanomolar range is a potential avenue to explore, since these assays appear to detect the egress of this biomarker molecule from intact cardiomyocytes in the absence of overt necrosis [17]. Hitherto, however, only a few determinations of serum troponins have been conducted in Danon disease; these were reported to be only slightly elevated above the healthy reference range [18,19].
To explore the potential significance of troponin I as an informative biomarker in the cardiomyopathy of Danon disease, we determined serial troponin I concentrations in serum obtained from key members of the pedigree described here at routine and emergency hospital visits and at the maximum ages studied. As depicted in Table 1, in the severely affected family members (three brothers and their mother), strikingly elevated steady-state troponin I concentrations were found. At the same time, troponin I concentrations in serum sampled from the mildly afflicted maternal aunt and cousin with clinically mild disease were significantly lower (p < 0.002, Student t-test). This is a notable association between outcomes, clinical manifestations and troponin I determinations; we contend that serial troponin I measurements can be a helpful adjunct in the management of these patients but cannot yet be considered neither a specific or sensitive biomarker of Danon disease.
Table 1. Serial plasma troponin I concentrations in a pedigree with Danon disease.
| Patient and Age | c864 + 4A > G Mutation Status | Clinical Severity | BMI | Mean troponin I | Range | Standard Deviation |
|---|---|---|---|---|---|---|
| Case A. Eldest brother Age 21 5 years troponins |
Hemizygous | Deceased Severe |
37 | 4.6 | 2.0–6.3 | 1.5 |
| Case B, Middle Son Aged 23 7 years of troponins |
Hemizygous | Deceased Severe |
28 | 8.7 | 0.8–40 | 11.0 |
| Case C, Youngest Son Age 21 4 years of troponins |
Hemizygous | Severe | 37 | 7.0 | 3.8–18.4 | 6.3 |
| Case D, Their mother Age 57 7 years of troponins |
Heterozygous | Moderate | 41.6 | 0.30 | 0.15–0.55 | 0.12 |
| Case G, Their grandmother Age 76 1 year of troponins |
Heterozygous | Moderate | 26.8 | 0.13 | 0.1–0.15 | 0.03 |
| Case F, Their Aunt Age 62 6 years of troponins |
Heterozygous | Mild/Moderate | 33.1 | 0.14 | 0.07–0.18 | 0.05 |
| Case E, Their cousin Age 31 6 years of troponins |
Hemizygous | Minimal | 25.4 | 0.07 | N/A | 0.05 |
Note the higher body mass index values seen in the more severely affected children.
Reference range for healthy adults: 0–0.1 μg/L.
Having found serum troponin I concentrations to be elevated to an extreme value, it was important to assay them at intervals; a record of high troponins would ensure that on any acute hospital admission, colleagues would not treat the patient unnecessarily for a suspected acute cardiac ischaemic event. Catheterisation might itself provoke an arrhythmia and death. For this reason we suggest that patients consider wearing a Medical Alert Bracelet enscribed “Danon disease with elevated serum troponin.”
3.3. Limitations of current clinical management
The management of cardiac arrhythmias in Danon's remains challenging [20] and early ICD implantation is recommended empirically. However Maron et al., found that such devices failed to prevent death in 5 of their seven patients and similar poor outcomes are described here for our patients also [8]. Three of our patients additionally received amiodarone which caused thyrotoxicosis in two – a complication with potentially catastrophic effects not previously noted in Danon disease and which contributed to the death of the brother of the proband. Uncontrolled thyrotoxicosis greatly accelerates autophagy and in this Case B, rapidly progressive proximal myopathy was induced.
Danon disease carries with it a poor prognosis: a meta-analysis of 145 published cases calculated the mean age for i) first symptom-onset (12.1 years in males and 27.9 in females), ii) cardiac transplantation (17.9 years for males, 33.7 for females) and iii) death (19 years in males and 34.6 in females) [7]. Given the early onset of this lethal disease, improving the selection of patients for early intervention by cardiac transplantation is an essential factor in improving clinical management. Yet the parameters used routinely to assess eligibility for this life-saving therapy appear to be poorly developed for application to patients with Danon disease.
As shown in this pedigree, learning difficulties and anxiety can particularly affect the ability of patients with Danon disease to express their so-called “commitment” to the transplant process by dealing with smoking and other addictions as well as maintain weight loss. Their reduced ability to comply places Danon's patients at a disadvantage in accessing their only effective treatment.
3.4. Equity & justice in transplant allocation
A comprehensive review of transplantation criteria is beyond the scope of this paper but briefly, guidelines from the United Kingdom [20], the Netherlands [21] and the United States [22] reveal that coexisting life-threatening medical conditions, abuse of alcohol, smoking, drug abuse, psychiatric history of noncompliance and excess weight are commonly considered as either absolute or relative contraindications to the procedure. However such criteria are based on extrapolation from clinical trials of acquired heart disease [22] and we contend these are inappropriate for inherited diseases without further evidence as they favour patients whose heart disease is not part of multisystem disease such as is the case in Danon's disease.
As an example, one study showed that ambulatory transplant candidates with disorders other than Danon disease and who were not receiving inotropic medication gained little from transplantation [23]. Similarly, patients initially considered too healthy for listing benefited from, and did not have increased mortality with, delayed surgery [24]. But as patients with Danon disease unpredictably and spontaneously deteriorate, they are at first too healthy and soon become unsuitable for such surgery, the criteria may be misleading.
An international survey found little consistency in the application of psychosocial criteria for excluding patients from transplantation [22]. The authors concluded that there was little evidence to link post-operative outcomes with such measures and emphasised the need not to confuse such criteria “with judgments of an individual's social worth” [22]. This might particularly be the case where patients have been involved with police, as in our pedigree, or where alcohol or substance abuse continues. Psychosocial criteria also attempt to evaluate “a commitment to and understanding of the importance of anti-rejection medications” [25] as well as adherence to smoking cessation. Certain transplant centres preclude surgery for smokers; they also mandate urinary checks for substance abuse as well as adherence to dietary advice to ensure weight loss. Persistent obesity and lack of adherence to lifestyle measures - accompanied by a markedly raised pulmonary vascular resistance - precluded priority listing for four of our patients.
Health care systems should - and in the UK are required to - tailor services to ensure that disabilities do not preclude access to treatment and that individual circumstances of patients are taken into account [26]. We discuss elsewhere the ethical and legal arguments to support resource allocation for patients with rare orphan diseases [27] but in brief, ethically, the Harvard philosopher John Rawls argues that the more a disease impairs a person's capacity to pursue their goals, the more urgent it is that their health needs are addressed [28]. A patient group with a reduced cognitive function as compared with other patients eligible for the same treatment should arguably therefore have a stronger, not weaker, claim to the available treatment [26]. Legally, various international declarations and national statutes place the onus on providers to avoid discrimination on the basis of disability and to provide health services to them “specifically because of their disabilities, including early identification and intervention as appropriate, and services designed to minimize and prevent further disabilities”. In this context, access to treatment is demanded because of and not in spite of disabilities such as learning difficulties. Danon disease qualifies as a disability, particularly as defined by the UK Equality Act 2010 as a “mental or physical impairment which substantially affects a person's ability to perform normal day-to-day activities in the long term”. Mental conditions are expressly included, which speaks in favour of treating a patient group with learning disability and progressive conditions also qualify as well as atypical forms of a disease [26]. While it is very likely that our patients would have benefited from earlier cardiac transplantation, given the inherent procedural risks, the need to comply with immunosuppression and rigorous monitoring, as well as the scarcity of donor organs, advancing patients with Danon disease for this potentially life-saving treatment remains a matter of judgement based on individual cases and timing- both requiring intense engagement with the potential recipient.
3.5. The pathogenesis of cardiac hypertrophy in Danon's disease: Implications for treatment
Reduced or impaired ATP production is thought to underlie HCM; the low energy-state initiates a common cellular mechanism leading to proliferation and myocyte hypertrophy. ATP concentrations are altered in cardiac tissue obtained from patients with hypertrophic cardiomyopathy due to sarcomeric mutations [29,30] and also in metabolic causes of HCM. For example, in Fabry disease, impaired mitochondrial energy supply has been documented [31], and deficiency in ATP synthesis occurs in fatty acid oxidation disorders [32], mitochondrial disease [33,34] and Friedreich's ataxia [35–38].
Danon disease shares features with the other “metabolic” causes of hypertrophic cardiomyopathy and accounts for about 5% of patients with HCM in whom conventional genetic testing is unavailing for diagnosis [5]. Danon results from mutations in the gene encoding the LAMP2 glycoprotein [39]. This heavily glycosylated protein is believed to protect the lysosome from its own degradative acid hydrolases [30], which are needed for endocytosis and autophagy [40]. The latter, a form of “self-ingestion”, occurs at basal levels continuously but in times of nutrient deprivation, it allows the cell to simultaneously increase energy supply (from protein degradation) and reduce energy demand (by removing mitochondria and peroxisomes). When LAMP2 function is impaired, autophagosome-lysosome fusion is diminished and autophagic vacuoles therefore accumulate, leading to eventual cell death and tissue injury. While autophagy facilitates cell survival at times of nutritional stress, its impairment in Danon's disease compromises leads to vacuole accumulation and compromise of cellular function.
As a universal and near-atavistic process, dysregulated autophagy has been implicated in a diverse range of diseases such as malignancies and neurodegenerative conditions [41–44]. It has therefore been investigated as a potential therapeutic target with particular focus on mTOR (mammalian target of rapamycin) activity as its activity correlates with autophagy. Several licensed, safe and effective drugs have been found to act on mTOR, such as rapamycin (Sirolimus®) the widely used immunosuppressant for solid organ transplantation.
Rapamycin stimulates the autophagic process, inducing new autophagosome formation. It could therefore prove helpful in conditions where autophagy is impaired but for the present this is entirely speculative for the implicit defect of chaperone-mediated autophagy in Danon's disease and may aggravate the underlying abnormality. Sirolimus is routinely used to prevent cardiac allograft vasculopathy in transplant recipients [44,45], so it should be possible to study its effect in patients with Danon disease after transplantation. Such patients should be scrutinized for any response of the skeletal myopathy and perhaps in the longer-term, cognitive or retinal changes [46] which may justify a trial of rapamycin for Danon disease. It is unclear as to whether pharmacological modulation of the autophagy process is of value in the general HCM population, however Sirolimus® has been shown to reduce left ventricular hypertrophy independently of its antihypertensive and immunosuppressive activity in the cardiac transplant population [47]. Whether this can be attributable to stimulation of autophagy remains to be determined (Supplementary Figs. 1–3).
4. Conclusion
Hypertrophic cardiomyopathy may occur as part of multi-system diseases many of which are rare. Such diagnoses carry additional implications for prognosis, treatment and reproductive planning. We emphasise the importance of searching for systemic features, including those of Danon disease, when assessing patients with HCM, and to refer early to specialist units for clinical review and formal testing where appropriate. Consideration of the clinical diversity of X-linked traits is highly relevant to Danon disease; here we report divergent clinical expression of this condition in patients of the same genders and pedigree, suggesting that other genetic or environmental determinants influence disease expression.
We postulate that defective generation of metabolic energy contributes to the cardiomyopathy and that this immediately suggests an innovative therapeutic approach which may also be applicable to other cardiomyopathies with florid hypertrophic features. Formal clinical trials will be required to determine whether rapamycin is beneficial as a re-purposed orphan drug for mechanistic, as well as therapeutic exploration, in these rare disorders. In the meantime, transplantation criteria for patients with Danon disease should be reconsidered and in the context of emerging data on troponin I as a predictive biomarker for cardiac decompensation. High troponin I may be mistaken for an acute event and patients may wish to wear medical alert bracelets to prevent unnecessary invasive procedures acutely.
Supplementary data related to the cardiac findings to this article can be found online at http://dx.doi.org/10.1016/j.ymgme.2017.06.008.
Supplementary Material
Synopsis.
Danon patients are clinically heterogeneous even within a family and can deteriorate quickly; though further study is needed, serial troponin I measurements may help to inform the current criteria for cardiac transplantation which are arguably unsuitable for this population group.
Acknowledgements
The authors are grateful for the help and assistance of Professor Paul Saftig, Dr. Eeva-Liisa Eskelinen, Dr. Rosemary Rusk, Dr. Andrew Grace, Dr. David Halsall, and Dr. Ichizo Nishino in managing these patients and helping to confirm the clinical diagnosis. TM Cox was supported by a grant in aid from the Cambridge National Institutes for Health Research.
Footnotes
The young female generation VI in Fig. 1, is now aged 8 years. She has a body mass index at the 99th centile (31.9Kg) and behavioural difficulties reminiscent of her late father. Cardiac investigations are currently not abnormal but active monitoring continues.
Contributions
All authors have been involved in analysis, collection and interpretation of data and contributed to the drafting and revision of the manuscript.
Ethics
This study complied with the Declaration of Helsinki and other local and national ethical requirements incumbent on our respective professions.
Conflict of interest
None of the authors has a conflict of interest relating to this disease.
References
- [1].Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352(4):362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- [2].Ashrafian H, Watkins H. New disease taxonomy and therapeutic implications: cardiomyopathies: therapeutics based on molecular phenotype. J Am Coll Cardiol. 2007;49:1251–1264. doi: 10.1016/j.jacc.2006.10.073. [DOI] [PubMed] [Google Scholar]
- [3].Ho CY, Seidman CE. A contemporary approach to hypertrophic cardiomyopathy. Circulation. 2006;113(24):e858–e862. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- [4].Yang Z, McMahon CJ, Smith LR, et al. Danon disease as an under recognized cause of hypertrophic cardiomyopathy in children. Circulation. 2005;112(11):1612–1617. doi: 10.1161/CIRCULATIONAHA.105.546481. [DOI] [PubMed] [Google Scholar]
- [5].Charron P, Villard E, Sébillon P, Laforêt P, Maisonobe T, Duboscq-Bidot L, Romero N, Drouin-Garraud V, Frébourg T, Richard P, Eymard B, et al. Danon's disease as a cause of hypertrophic cardiomyopathy: a systematic survey. Heart. 2004;90(8):842–846. doi: 10.1136/hrt.2003.029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rohrbach M, Clarke JT. Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs. 2007;67(18):2697–2716. doi: 10.2165/00003495-200767180-00005. [DOI] [PubMed] [Google Scholar]
- [7].Boucek D, Jirikowic J, Taylor M. Natural history of Danon disease. Genet Med. 2011;13(6):563–568. doi: 10.1097/GIM.0b013e31820ad795. [DOI] [PubMed] [Google Scholar]
- [8].Maron BJ, Roberts WC, Arad M, et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301(12):1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roberts WC, Podolak MJ. The king of hearts: analysis of 23 patients with hearts weighing 1,000 grams or more. Am J Cardiol. 1985;55(4):485–494. doi: 10.1016/0002-9149(85)90399-6. [DOI] [PubMed] [Google Scholar]
- [10].Danon MJ, Oh SJ, DiMauro S, et al. Lysosomal glycogen storage disease with normal acid maltase. Neurology. 1981;31(1):51–57. doi: 10.1212/wnl.31.1.51. [DOI] [PubMed] [Google Scholar]
- [11].Echaniz-Laguna A, Mohr M, Epailly, et al. Novel Lamp-2 gene mutation and successful treatment with heart transplantation in a large family with Danon disease. Muscle Nerve. 2006;33(3):393–397. doi: 10.1002/mus.20471. [DOI] [PubMed] [Google Scholar]
- [12].Maron BJ, Roberts WC, Arad M, et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA. 2009;301(12):1253–1259. doi: 10.1001/jama.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roos JC, Cox TM. Glycogen storage diseases and cardiomyopathy. N Engl J Med. 2005;352(24):2553. doi: 10.1056/NEJM200506163522417. [DOI] [PubMed] [Google Scholar]
- [14].O'Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur Heart J. 2014;35(30):2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- [15].Kawahara C, Tsutamoto T, Nishiyama K, et al. Prognostic role of high-sensitivity cardiac troponin T in patients with nonischemic dilated cardiomyopathy. Circ J. 2011;75:656–661. doi: 10.1253/circj.cj-10-0837. [DOI] [PubMed] [Google Scholar]
- [16].Kubo T, Kitaoka H, Yamanaka S, Hirota T, Baba Y, Hayashi K, Iiyama T, Kumagai N, Tanioka K, Yamasaki N, Matsumura Y, et al. Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62(14):1252–1259. doi: 10.1016/j.jacc.2013.03.055. [DOI] [PubMed] [Google Scholar]
- [17].Regwan S, Hulten EA, Martinho S, Slim J, Villines TC, Mitchell J, Slim AM. Marathon running as a cause of troponin elevation: a systematic review and meta-analysis. J Interv Cardiol. 2010;23(5):443–450. doi: 10.1111/j.1540-8183.2010.00575.x. [DOI] [PubMed] [Google Scholar]
- [18].Piotrowska-Kownacka D, Kownacki L, Kuch M, Walczak E, Kosieradzka A, Fidzianska A, Krolicki L. Cardiovascular magnetic resonance findings in a case of Danon disease. J Cardiovasc Magn Reson. 2009;11:12. doi: 10.1186/1532-429X-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Katzberg H, Karamchandani J, So YT, Vogel H, Wang CH. End-stage cardiac disease as an initial presentation of systemic myopathies: case series and literature review. J Child Neurol. 2010;25(11):1382–1388. doi: 10.1177/0883073810367683. [DOI] [PubMed] [Google Scholar]
- [20].D'souza RS, Levandowski C, Slavov D, Graw SL, Allen LA, Adler E, Mestroni L, Taylor MR. Danon disease: clinical features, evaluation, and management. Circ Heart Fail. 2014;7(5):843–849. doi: 10.1161/CIRCHEARTFAILURE.114.001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Banner NR, Bonser RS, Clark AL, Clark S, Cowburn PJ, Gardner RS, Kalra PR, McDonagh T, Rogers CA, Swan L, Parameshwar J, et al. UK guidelines for referral and assessment of adults for heart transplantation. Heart. 2011;97(18):1520–1527. doi: 10.1136/heartjnl-2011-300048. [DOI] [PubMed] [Google Scholar]
- [22].de Jonge N, Kirkels JH, Klöpping C, Lahpor JR, Caliskan K, Maat AP, Brügemann J, Erasmus ME, Klautz RJ, Verwey HF, Oomen A, et al. Guidelines for heart transplantation. Neth Hear J. 2008;16(3):79–87. doi: 10.1007/BF03086123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates—2006. J Heart Lung Transplant. 2006;25(9):1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [24].Jimenez J, Bennett EL, Higgins R, Bauerlein J, Pham S, Mallon S. Should stable UNOS status 2 patients be transplanted? J Heart Lung Transplant. 2005;24:178–183. doi: 10.1016/j.healun.2003.10.019. [DOI] [PubMed] [Google Scholar]
- [25].Lewis EF, Tsang SW, Fang JC, et al. Frequency and impact of delayed decisions regarding heart transplantationnon long-term outcomes in patients with advanced heart failure. J Am Coll Cardiol. 2004;43:794–802. doi: 10.1016/j.jacc.2003.10.035. [DOI] [PubMed] [Google Scholar]
- [26].Eisen HJ. Patient information: heart transplantation (beyond the basics) [Last accessed 27/01/16]; Up-to-date. 10-2-14. http://www.uptodate.com/contents/heart-transplantation-beyond-the-basics.
- [27].Hyry HI, Roos JCP, Manuel J, Cox TM. The legal imperative for treating rare disorders. Orphanet J Rare Dis. 2013;8:135. doi: 10.1186/1750-1172-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hyry HI, Roos JCP, Cox TM. Orphan drugs: expensive yet necessary. QJM. 2015;108(4):269–272. doi: 10.1093/qjmed/hcu240. [DOI] [PubMed] [Google Scholar]
- [29].Rawls J. Justice as Fairness: Restatement. Harvard University Press; Cambridge Mass. USA: 2001. [Google Scholar]
- [30].Eskelinen EL. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Asp Med. 2006;27(5–6):495–502. doi: 10.1016/j.mam.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [31].Blair E, Redwood C, Ashrafian H, et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10(11):1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- [32].Crilley JG, Boehm EA, Blair E, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41(10):1776–1782. doi: 10.1016/s0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- [33].Lücke T, Höppner W, Schmidt E, et al. Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab. 2004;82(1):93–97. doi: 10.1016/j.ymgme.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [34].Merante F, Tein I, Benson L, et al. Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNA-glycine gene. Am J Hum Genet. 1994;55:437–446. [PMC free article] [PubMed] [Google Scholar]
- [35].Taylor RW. A homoplasmic mitochondrial transfer ribonucleic acid mutation as a cause of maternally inherited hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:1786–1796. doi: 10.1016/s0735-1097(03)00300-0. [DOI] [PubMed] [Google Scholar]
- [36].Ristow M, Pfister MF, Yee AJ, et al. Frataxin activates mitochondrial energy conversion and oxidative phosphorylation. Proc Natl Acad Sci. 2000;97:12239–12243. doi: 10.1073/pnas.220403797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rötig A, de Lonlay P, Chretien D, et al. Aconitase and mitochondrial iron-sulfur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- [38].Nishino I, Fu J, Tanji K, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- [39].Deretic V, Klionsky DJ. How cells clean house. Sci Am. 2008;298:74–81. doi: 10.1038/scientificamerican0508-74. [DOI] [PubMed] [Google Scholar]
- [40].Rowland TJ, Sweet ME, Mestroni L, Taylor MR. Danon disease – dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J Cell Sci. 2016;129(11):2135–2143. doi: 10.1242/jcs.184770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sugie K, Yamamoto A, Murayama K, et al. Clinicopathological features of genetically confirmed Danon disease. Neurology. 2002;58(12):1773–1778. doi: 10.1212/wnl.58.12.1773. [DOI] [PubMed] [Google Scholar]
- [42].Bradley JL, Blake JC, Chamberlain S, et al. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum Mol Genet. 2000;9:275–282. doi: 10.1093/hmg/9.2.275. [DOI] [PubMed] [Google Scholar]
- [43].Flemming A. Cancer: autophagy presents Achilles heel in melanoma. Nat Rev Drug Discov. 2011;10(7):491. doi: 10.1038/nrd3482. [DOI] [PubMed] [Google Scholar]
- [44].Rubinsztein DC, Gestwicki JE, Murphy LO, et al. potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6(4):304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- [45].Mudge GH. Sirolimus and cardiac transplantation: is it the "magic bullet"? Circulation. 2007;116(23):2666–2668. doi: 10.1161/CIRCULATIONAHA.107.737965. [DOI] [PubMed] [Google Scholar]
- [46].Prall FR, Drack A, Taylor M, Ku L, Olson JL, Gregory D, Mestroni L, Mandava N. Ophthalmic manifestations of Danon disease. Ophthalmology. 2006;113(6):1010–1013. doi: 10.1016/j.ophtha.2006.02.030. [DOI] [PubMed] [Google Scholar]
- [47].Raichlin E, Bae JH, Khalpey Z. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.